Abstract
Background:
In healthy subjects, preparation to move is accompanied by motor cortical disinhibition. Poor control of intracortical inhibitory function in the primary motor cortex (M1) might contribute to persistent abnormal motor behavior in the paretic hand after chronic stroke.
Methods:
Here, we studied GABAergic short intracortical inhibition (SICI) in the ipsilesional M1 in well-recovered chronic stroke patients (n = 14; 63.8 ± 3.0 years) engaged in preparation to move the impaired hand in a reaction time paradigm.
Results:
The main finding was an abnormal persistence of SICI in the ipsilesional M1 during movement preparation that was absent in age-matched controls (n = 14). Additionally, resting SICI was reduced in the patient group relative to controls.
Conclusions:
Our findings document a deficit of dynamic premovement modulation of intracortical inhibition in the ipsilesional primary motor cortex of patients with chronic stroke. This abnormality might contribute to deficits in motor control of the paretic hand, presenting a possible target for correction in the framework of developing novel therapeutic interventions after chronic stroke.
GLOSSARY
- CS
= conditioning magnetic stimulus;
- FDI
= first digital interosseus muscle;
- ISI
= interstimulus interval;
- JTT
= Jebsen-Taylor Hand Function Test;
- M1
= primary motor cortex;
- MEP
= motor evoked potential;
- RC
= recruitment curves;
- RM-ANOVA
= repeated measures analyses of variance;
- rMT
= resting motor threshold;
- RT
= reaction time;
- SICI
= short interval intracortical inhibition;
- TMS
= transcranial magnetic stimulation;
- US
= unconditioned stimulus.
Ischemic stroke results in motor impairment and changes in inhibitory and excitatory intracortical function.1–3 Interactions between intracortical inhibitory and excitatory processes within the primary motor cortex (M1) are crucial for motor control and change in the process of generation of a voluntary movement (at the immediate premovement stage).4–11
GABAergically mediated short intracortical inhibition (SICI) can be studied using a well-established paired pulse transcranial magnetic stimulation (TMS) method.12 Previous work demonstrated premovement modulation of SICI, characterized by a release from inhibition (disinhibition) prior to the onset of a voluntary movement in healthy subjects.7–9 The ability to evaluate SICI in association with performance of a voluntary movement added another perspective to our understanding of intracortical inhibitory circuits and, in fact, seems to enhance the specificity of this technique for some pathologic states of the central motor system. For example, in patients with dystonia, premovement SICI and SICI at rest showed substantially different effects with reduction in SICI at rest and persistent lack of modulation of SICI during movement preparation.7
Previous studies evaluating motor cortex excitability in the acute and subacute stages after stroke1,3,13–18 showed decreased resting SICI in the ipsilesional M1, an abnormality that may normalize in the chronic stage.15,17–19 Movement-related changes in SICI in stroke patients intending to move the paretic hand have not been investigated. Here, we hypothesized that dynamic modulation of premovement SICI, as described in healthy subjects,7–9 would be impaired in the ipsilesional M1 of chronic stroke patients moving the paretic hand.
METHODS
Subjects.
Fourteen right-handed chronic stroke patients (mean age 63.8 ± 3.0, seven women, eight left hemispheric strokes) with a history of a single subcortical ischemic cerebral infarct not involving M1 at least 1 year before the study with good recovery of hand function that allowed them to perform the required motor task were recruited for the study. However, the patients still showed deficits in performance of skilled motor tasks such as the Jebsen-Taylor Hand Function Test (JTT; see Results section20). Upper extremity Fugl-Meyer scores ranged between 54 and 65 points (maximum 66), Medical Research Council between 4.2 and 5, and the Ashworth Spasticity Scale between 0 and 2.
Fourteen healthy right-handed age-matched volunteers (mean age 63.9 ± 2.7, nine women) participated as controls. Written informed consent was obtained from all subjects according to the Declaration of Helsinki21 and from the local Ethics Committee at NIH and Hamburg.
Experimental design.
Initially, subjects familiarized with the reaction time (RT) task. They performed 20 trials to characterize each individual's RT in the absence of TMS. Subsequently, we characterized resting motor thresholds (rMT)22 in each individual as required to determine the settings for TMS stimulation. Then the subjects performed the RT task while paired- and single-pulse TMS was applied.
At the end of the experimental session, recruitment curves and SICI were investigated at rest (for details, see below).
Transcranial magnetic stimulation.
A paired-pulse paradigm was used to assess intracortical inhibition at rest and in the process of the generation of a voluntary movement.7,8,12 In the standard paired pulse paradigm, a subthreshold conditioning stimulus (CS) is followed by a suprathreshold unconditioned stimulus (US). Short interval intracortical inhibition (SICI) takes place at interstimulus intervals (ISIs) of 1–6 msec and reflects the activation of local GABAergic connections.22–25
In our study, SICI was investigated at an ISI of 3 msec based on previous studies.4,22,24 The CS and the US were delivered through a figure-of-eight coil (80 mm wing diameter) connected to two Magstim 200 magnetic stimulators (Magstim Company, Whitland, Dyfed, UK). The coil was placed over the hand motor area of the ipsilesional hemisphere with the handle pointing backwards and laterally approximately 45° to the interhemispheric line. The CS intensity was 80% rMT, a standardized value used in previous reports with a good relation to SICI thresholds,7,22,24,26 which allowed proper comparison of SICI across patient and control groups. Unconditioned TMS pulses were applied at an intensity that evoked unconditioned MEPs of ∼1 mV in both patients and controls. All measurements were evaluated in the ipsilesional M1 of the stroke patients and the corresponding M1 of age-matched controls. The optimal scalp position for eliciting MEPs in the first dorsal interosseous (FDI) muscle of the paretic hand (patients) and corresponding hand (controls) was determined and the position was marked with a pen.
Rest measurements.
Motor threshold (rMT) was defined as the minimal output of the stimulator that produced MEPs of >50 μV in at least 5 out of 10 consecutive trials (figure 1A). Recruitment curves (RC) to systematically increasing TMS stimuli (100%–150% of rMT) and SICI were also measured at rest.
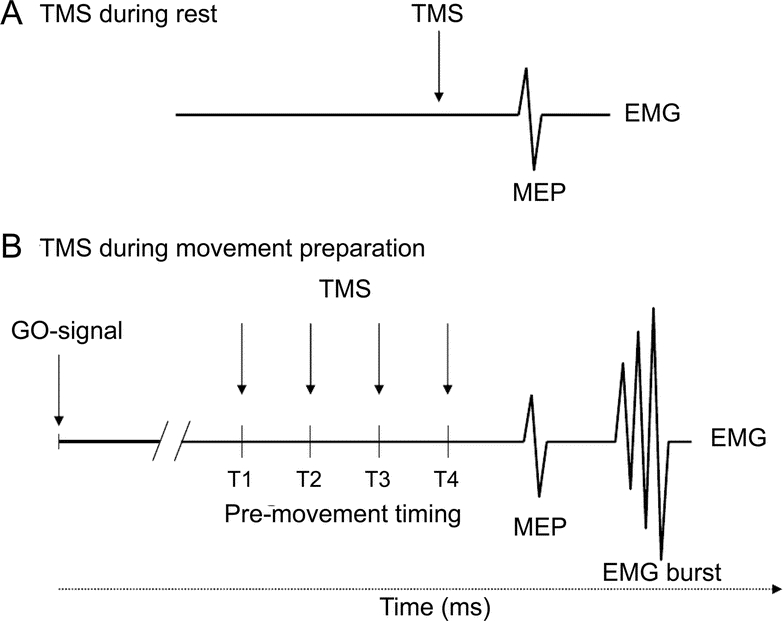
Figure 1 Experimental design
Short interval intracortical inhibition was determined at (A) rest and (B) at four different timings prior to movement onset (T1–T4, T4 being closest to movement onset). Timings were adjusted to the individual reaction times. Mean reaction times were 208.5 msec (±8.3 msec) for stroke patients and 216.9 msec (±9.5 msec) for controls. MEP = motor evoked potential; TMS = transcranial magnetic stimulation.
Premovement measurements.
Paired pulses (US and CS-US) were delivered at different time intervals preceding movement onset in a reaction time paradigm in pseudo-randomized order at four different timings (T1–T4) (figure 1B). T1–T4 were adjusted to each subject's RT according to a well-described procedure,27,28 and ranged between 125 and 240 msec after the go signal (corresponding to an average T1 = 63% of RT, T2 = 75%, T3 = 87%, T4 = 97%). A minimum of 10 trials per condition and timing were recorded (13.4 ± 0.6 trials).
EMG recording.
EMG activity was recorded from the FDI muscle of the paretic hand using surface electrodes positioned in a belly-tendon montage. The amplified and bandpass filtered (50 Hz–1 kHz) EMG signals were digitized and stored on a laptop using a data collection program (CED 1902 amplifier, sampling rate 5 kHz, bandpass filter 50 Hz–1 kHz, Signal Software, Cambridge Electronic Devices, Cambridge, UK). All data were analyzed off-line.
Simple reaction time task.
Subjects were instructed to respond to a visual go signal by performing an index finger abduction motion of the paretic hand as quickly as possible. The go signal appeared at random intervals (6–8 seconds) and the subjects were instructed to avoid anticipation of the go signal and to relax their hand while the fixation point was present. RT was defined as the time interval (in milliseconds) between the go signal and the onset of the EMG-burst in the FDI muscle.
Data analysis.
Premovement measurements.
All trials with EMG activity on visual inspection before TMS were discarded from further analysis. In each subject, MEP amplitudes, measured peak to peak, were sorted according to stimulation condition (SICI, single pulse) and premovement timing (T1–T4). The unconditioned MEP amplitudes at T1–T4 were measured separately to quantify overall corticomotor excitability changes in the premovement period.
SICI was expressed as the percentage of the mean MEP amplitude induced by unconditioned magnetic pulses obtained at that particular timing. In this way, differences in SICI could not be attributed to changes in the unconditioned MEP amplitude. The analysis of data acquired during rest was carried out using the same procedures. All results are given as mean ± SE.
Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS) software (SPSS Inc., Chicago, IL). In line with our a priori hypothesis of a difference in SICI between the two groups (stroke patients, controls) closest to movement onset,7,27,28 unpaired two-samples t test (two-tailed) at the closest premovement timing (T4) was used first to compare the two groups. Additionally, we evaluated the changes of the premovement SICI across the two groups using a repeated measures analyses of variance (RM-ANOVA) with premovement timing(timing 1–4) as a within-subjects factor and group(stroke patients, healthy elderly) as a between-subjects factor. We also expressed the magnitude of SICI at T4 relative to T1 (normalized extent of modulation = SICI at T4/SICI at T1 × 100%) to better characterize changes in SICI relative to movement onset. We also calculated a RM-ANOVA for unconditioned MEP amplitudes (same within- and between-subject factors as mentioned above) to ensure that our results could not solely be attributed to a between-group difference in development of amplitude changes in unconditioned MEP amplitude at T1–T4.
Measurements during rest.
Resting SICI was evaluated using unpaired two-sample t tests (two-tailed). RC were analyzed using RM-ANOVA with the factors group(stroke patients, healthy elderly) and intensity(100–150% MT). RM-ANOVA was Greenhouse-Geisser corrected if necessary. rMT and RT of the stroke patients and controls were compared with unpaired two-samples t tests (two-tailed).
RESULTS
Reaction times.
RT were comparable in the two groups (216.9 msec ± 9.5 msec healthy elderly, 208.5 msec ± 8.3 msec stroke patients; t[26] = 0.67, p = 0.50), consistent with the patient inclusion criteria (patients with substantial initial paralysis but subsequent recovery to the level they were able to carry out the required RT task).
Jebsen-Taylor hand function test.
JTT values in the patients (37.4 ± 2.8 seconds, n = 11) were higher than in controls (27.4 ± 1.6 seconds; t = 3.1, n = 5; p = 0.008). JTT values in patients were also higher relative to a second healthy control group from our laboratory (29.2 ± 1.4 seconds, t = 2.6, n = 10; p = 0.02).
Resting motor thresholds.
Stroke patients and controls had comparable rMTs (42 ± 3% stroke patients, 46 ± 2% healthy elderly, t[26] = 1.232, p = 0.23).
Recruitment curves.
No difference in RC was observed in the stroke patients compared to controls. RM-ANOVA showed an effect of intensity (F = 32.5, p < 0.05), but not group (F = 1.40, p = 0.25) or group(stroke patients, healthy elderly) × intensity(100%–150% MT) interaction (F = 0.58, p = 0.52).
SICI and unconditioned MEP amplitudes at rest.
SICI at rest was reduced in the ipsilesional M1 of the stroke patients (78.2 ± 9.7) compared to controls (49.3 ± 6.0; t[26] = 2.54, p < 0.05; figure 2) in the absence of differences in US MEP amplitudes (0.71 mV ± 0.17 mV stroke patients and 0.83 mV ± 0.22 mV controls, t[26] = −0.41, p = 0.7). There was no significant correlation between SICI at rest and JTT or RT values.
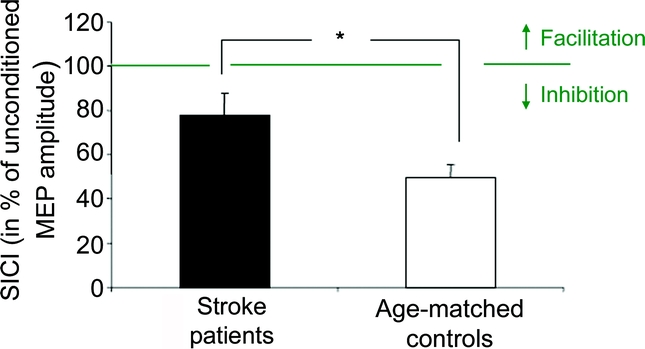
Figure 2 Short interval intracortical inhibition (SICI) during rest
Stroke patients in black, age-matched controls in white. Note the difference between patients and controls, with reduced SICI in patients. The y-axis displays SICI in percentage of the unconditioned motor evoked potential (MEP) amplitude; thus values below 100% correspond to inhibition and values above 100% correspond to facilitation. Asterisk indicates p < 0.05.
Premovement unconditioned MEP amplitude.
As expected, there was an effect of timing(timing 1–4) (F = 5.12, p < 0.05) but not group(stroke patients, healthy elderly) (F = 0.14, p = 0.71) or timing(timing 1–4) × group(stroke, healthy elderly) interaction (F = 1.23, p = 0.28) on US MEP amplitude. A two-sample t test showed no difference in US MEP amplitudes at timing 4 (T4) between patients (2.39 mV ± 0.47) and controls (2.14 mV ± 0.38; t[26] = 0.41, p = 0.7), consistent with a progressive increase in corticospinal recruitment preceding movement onset comparable in patients and controls (figure 3).
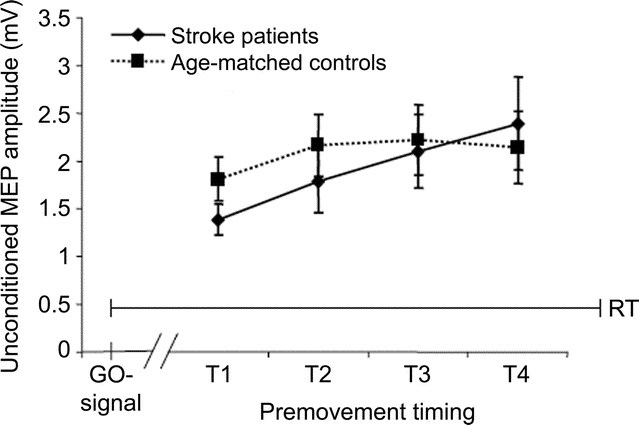
Figure 3 Unconditioned stimulus amplitudes in preparation of movements as measured at timings T1–T4 before movement onset
The x-axis displays the timing of the application of the transcranial magnetic stimulation pulses (T1–T4; T4 is closest to movement onset). The y-axis displays motor evoked potential amplitudes in mV. Note that unconditioned stimulus amplitudes were comparable in both groups.
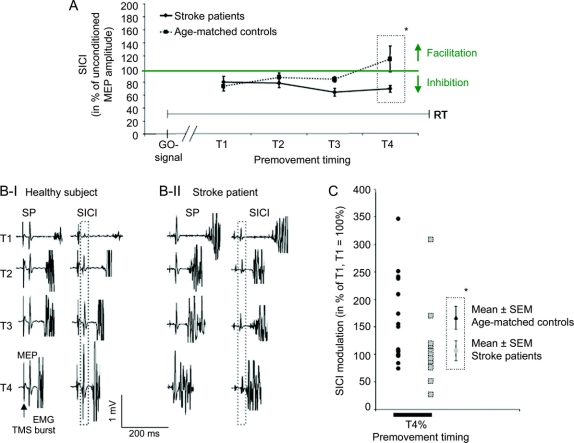
Premovement SICI.
Both groups showed comparable SICI in the early phase of movement preparation, which remained stable and unmodulated in patients and changed to facilitation in controls close to movement onset (figure 4). This group difference was significant at T4 (paired t test T1 vs T4: t[13] = −2.26, p < 0.05; figure 4, A and B). A two-sample t test yielded a difference of SICI between groups at T4 (t[26] = −2.23, p < 0.05). RM-ANOVA revealed an effect of group(stroke patients, healthy elderly) (F = 4.46, p < 0.05) and timing(timing 1–4) × group(stroke, healthy elderly) interaction (F = 3.19, p < 0.05) on premovement SICI. The premovement modulation of SICI (at T4 relative to T1) also differed across groups (figure 4C; t[26] = −2.13, p < 0.05). The factor timing(timing 1–4) (F = 1.73, p = 0.19) did not show effects on SICI. There were no significant correlations between SICI modulation and JTT or RT performance.
Figure 4 SICI in preparation of movements as measured at timings T1–T4 before movement onset
(A) Short interval intracortical inhibition (SICI) in preparation of movements as measured at four timings (T1–T4) before movement onset (mean ± SEM; T4 is closest to movement onset). Mean reaction times were comparable for stroke patients and for controls. Stroke patients are shown with the black line, controls with the dotted line. The x-axis displays the timing of the application of the transcranial magnetic stimulation (TMS) pulses (T1–T4); the y-axis displays SICI in percentage of the unconditioned motor evoked potential (MEP) amplitude; thus values below 100% correspond to inhibition and values above 100% correspond to facilitation. *p < 0.05. (B) Examples of the raw data from one control subject (I) and one stroke patient (II) at timings 1–4 (T1–T4) in the premovement period. MEPs evoked by single pulses (SP) and by paired pulses with an interstimulus interval of 3 msec (SICI) are shown. Note the increase of MEP size from T1 to T4 in the healthy subject which is lacking in the stroke patient (black dotted frame). (C) SICI modulation. Normalized extent of modulation of SICI is shown at timing T4 in percentage of T1 (T1 = 100%) for each subject. Gray squares = stroke patients; black circles = age-matched controls. *p < 0.05.
SICI = short interval intracortical inhibition.
We studied eight patients with a left and six with a right hemisphere stroke. RM-ANOVA did not show significant effects of timing(timing 1-4) (F = 1.44, p = 0.25), HEM(right vs left) (F = 1.08, p = 0.32), or the interaction term (F = 1.99, p = 0.15) on SICI.
Correlation between SICI at rest and modulation of premovement SICI.
There was no significant correlation between the single pulse amplitude at rest and the magnitude of modulation of the single pulse amplitude (R = 0.15; p = 0.63), the rMT and SICI levels at rest (R = −0.23, p = 0.43), or rMT and SICI modulation (R = −0.15; p = 0.62) in patients. There was a correlation in this group between the amount of SICI at rest and the magnitude of modulation of SICI (R = −0.37, p = 0.03, one-tailed; the larger SICI at rest [less inhibition], the smaller the modulation).
DISCUSSION
The main finding of this study was a twofold abnormality of GABAergically mediated SICI in the ipsilesional M1 in a group of well-recovered chronic stroke patients.4,29,30 SICI was reduced at rest and abnormally persistent during paretic hand movement preparation relative to healthy age-matched controls without adequate modulation close to movement onset.
All patients who took part in the study showed an initial paralysis followed by substantial motor recovery. They were all able to perform the required task and generated clear, recordable muscle bursts from the paretic FDI muscle in response to the go signals in the reaction time task. Of note is that reaction times were comparable between patients and controls although the patients still had clear functional deficits particularly obvious during performance of more complex motor tasks, such as, e.g., the JTT (stroke patients: JTT = 37.4 ± 2.8 seconds, vs controls: JTT = 27.4 ± 1.6 seconds).
Disinhibition of the ipsilesional M1 at rest has been reported in the acute and subacute stages after ischemic stroke.1,13,14 We now found that this abnormality may persist into the chronic stage of the disease in patients with good recovery when compared to healthy age-matched controls. A recent report showed no differences in resting SICI in the healthy and affected hemisphere in patients 2 to 96 months after stroke but patients' results in that study were not compared to age-matched controls.16
The mechanisms underlying these findings are not clear. Recent animal data also showed changes in cortical excitability after small cortical lesions associated with functional recovery. In humans, enhanced resting motor cortical excitability (or reduced inhibition) has been proposed as a possible contributing mechanism18 to the recovery process,14,31 possibly influenced by interactions with secondary motor areas, such as the premotor cortex or the supplementary motor area32,33 or even with homologous areas of the opposite hemisphere.27,28
Because motor deficits in patients are most pronounced while performing a motor task, we hypothesized that abnormalities in intracortical function might manifest themselves more prominently in the premovement period, when patients intend to move the paretic hand.
In healthy subjects, SICI decreases and turns into facilitation during voluntary movements, a mechanism thought to facilitate activation of focal motor regions engaged in task performance and adequate fractionation of muscle activity.4,7,8,34 This modulation warrants accurate motor performance. In the patient group, we found an abnormal persistence of SICI in ipsilesional M1 in the period immediately preceding a movement of the paretic hand relative to controls, who demonstrated facilitation.
So far there is not sufficient experimental evidence to determine whether disturbed SICI modulation during movement preparation after stroke represents an adaptive or maladaptive response. However, in healthy subjects physiologic modulation of motor cortical excitability before and during movement has been well characterized and linked to normal motor behavior.7,8 In contrast, abnormal modulation of motor cortical excitability has been linked with abnormal motor function in movement disorders like dystonia and in stroke.7,28 Persistence of SICI is the key feature of abnormal modulation.7 Therefore, we consider it unlikely that the present findings during movement preparation represent adaptation rather than a persisting abnormality.
At rest, however, patients showed disinhibition. It is possible that disinhibition at rest in the chronic stage might contribute to a reduction in the range of operational premovement modulation available when moving the paretic hand. Furthermore, it is possible that after stroke the ipsilesional M1 attempts to compensate for its inability to generate proper voluntary movement by increasing resting motor cortical excitability.
The novel finding of SICI abnormalities after chronic stroke reported in this study may contribute to the understanding of the effects of interventions that modulate the excitability of the ipsilesional M1 in stroke patients.35–39 For example, enhancing the excitability of the ipsilesional M1 by noninvasive cortical stimulation is associated with improved skilled motor functions of the paretic hand.36 In addition, enhanced motor cortical excitability (determined at rest) is correlated with the amount of behavioral improvement.36 Based on the present data it can be speculated that enhancing neuronal activity in the ipsilesional M1, induced by, e.g., cortical stimulation,37 might contribute to normalize SICI (by extending the range of operation for modulation) associated with movements. It remains to be determined if the premovement abnormality reported here with motions of the paretic hand relates in a cause-effect manner with the magnitude of the deficit.
From an electrophysiologic point of view, our findings could not be explained by different recruitment of corticomotoneuronal connections in the premovement period since both groups showed comparable modulation and absolute unconditioned MEP amplitudes close to movement onset, nor by differences in rMT or RC, which did not differ across groups. It would be interesting in the future to adjust the intensity of the CS to induce comparable SICI levels at rest, an issue beyond the goals of our investigation focused on the comparison between patients and controls, and to randomize the presentation of stimulus intensities for determination of RC. As we included patients with dominant and nondominant hemispheric lesions, it remains an open question whether there are differences in SICI modulation between these two groups. In the present sample, we did not find differences between patients with lesions in the dominant vs nondominant hemisphere. Finally, it should be kept in mind that our results apply to a subgroup of patients with good motor recovery after predominantly small subcortical lesions. The situation in patients with more severe deficits or extensive cortical-subcortical lesions might differ. The behavioral relevance of the present finding of deficient SICI modulation in patients has to be determined in detail in upcoming studies.
AUTHOR CONTRIBUTIONS
Statistical analysis was performed by B. Steven and F.C. Hummel.
Address correspondence and reprint requests to Dr. Friedhelm C. Hummel, Department of Neurology, University Medical Center Hamburg-Eppendorf, 20246 Hamburg, Germany f.hummel@uke.uni-hamburg.de
Supported by a grant from the Alexander von Humboldt Foundation (Feodor-Lynen) to F.H., by the Deutsche Forschungsgemeinschaft (SFB 550 A13 to F.H.), by the FFM of the University of Hamburg (NWF04/07 to F.H.), and by the intramural National Institute of Neurological Disorders and Stroke program, NIH.
Disclosure: The authors report no disclosures.
Medical Device: Magstim 200 magnetic stimulator (Magstim Company, Whitland, Dyfed, UK).
Received May 6, 2008. Accepted in final form February 17, 2009.
REFERENCES
- 1.Cicinelli P, Pasqualetti P, Zaccagnini M, Traversa R, Oliveri M, Rossini PM. Interhemispheric asymmetries of motor cortex excitability in the postacute stroke stage: a paired-pulse transcranial magnetic stimulation study. Stroke 2003;34:2653–2658. [DOI] [PubMed] [Google Scholar]
- 2.Manganotti P, Patuzzo S, Cortese F, Palermo A, Smania N, Fiaschi A. Motor disinhibition in affected and unaffected hemisphere in the early period of recovery after stroke. Clin Neurophysiol 2002;113:936–943. [DOI] [PubMed] [Google Scholar]
- 3.Shimizu T, Hosaki A, Hino T, et al. Motor cortical disinhibition in the unaffected hemisphere after unilateral cortical stroke. Brain 2002;125:1896–1907. [DOI] [PubMed] [Google Scholar]
- 4.Chen R. Interactions between inhibitory and excitatory circuits in the human motor cortex. Exp Brain Res 2004;154:1–10. [DOI] [PubMed] [Google Scholar]
- 5.Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD. Interhemispheric inhibition of the human motor cortex. J Physiol 1992;453:525–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hummel FC, Cohen LG. Drivers of brain plasticity. Curr Opin Neurol 2005;18:667–674. [DOI] [PubMed] [Google Scholar]
- 7.Gilio F, Curra A, Inghilleri M, et al. Abnormalities of motor cortex excitability preceding movement in patients with dystonia. Brain 2003;126:1745–1754. [DOI] [PubMed] [Google Scholar]
- 8.Reynolds C, Ashby P. Inhibition in the human motor cortex is reduced just before a voluntary contraction. Neurology 1999;53:730–735. [DOI] [PubMed] [Google Scholar]
- 9.Zaaroor M, Pratt H, Starr A. Time course of motor excitability before and after a task-related movement. Neurophysiol Clin 2003;33:130–137. [DOI] [PubMed] [Google Scholar]
- 10.Muellbacher W, Ziemann U, Boroojerdi B, Cohen L, Hallett M. Role of the human motor cortex in rapid motor learning. Exp Brain Res 2001;136:431–438. [DOI] [PubMed] [Google Scholar]
- 11.Nitsche MA, Schauenburg A, Lang N, et al. Facilitation of implicit motor learning by weak transcranial direct current stimulation of the primary motor cortex in the human. J Cogn Neurosci 2003;15:619–626. [DOI] [PubMed] [Google Scholar]
- 12.Kujirai T, Caramia MD, Rothwell JC, et al. Corticocortical inhibition in human motor cortex. J Physiol 1993;471:501–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butefisch CM, Wessling M, Netz J, Seitz RJ, Homberg V. Relationship between interhemispheric inhibition and motor cortex excitability in subacute stroke patients. Neurorehabil Neural Repair 2008;22:4–21. [DOI] [PubMed] [Google Scholar]
- 14.Liepert J, Storch P, Fritsch A, Weiller C. Motor cortex disinhibition in acute stroke. Clin Neurophysiol 2000;111:671–676. [DOI] [PubMed] [Google Scholar]
- 15.Wittenberg GF, Bastings EP, Fowlkes AM, Morgan TM, Good DC, Pons TP. Dynamic course of intracortical TMS paired-pulse responses during recovery of motor function after stroke. Neurorehabil Neural Repair 2007;21:568–573. [DOI] [PubMed] [Google Scholar]
- 16.Nair DG, Hutchinson S, Fregni F, Alexander M, Pascual-Leone A, Schlaug G. Imaging correlates of motor recovery from cerebral infarction and their physiological significance in well-recovered patients. Neuroimage 2007;34:253–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manganotti P, Acler M, Zanette GP, Smania N, Fiaschi A. Motor cortical disinhibition during early and late recovery after stroke. Neurorehabil Neural Repair 2008;22:396–403. [DOI] [PubMed] [Google Scholar]
- 18.Swayne OB, Rothwell JC, Ward NS, Greenwood RJ. Stages of motor output reorganization after hemispheric stroke suggested by longitudinal studies of cortical physiology. Cereb Cortex 2008;18:1909–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Talelli P, Greenwood RJ, Rothwell JC. Arm function after stroke: neurophysiological correlates and recovery mechanisms assessed by transcranial magnetic stimulation. Clin Neurophysiol 2006;117:1641–1659. [DOI] [PubMed] [Google Scholar]
- 20.Jebsen RH, Taylor N, Trieschmann RB, Trotter MJ, Howard LA. An objective and standardized test of hand function. Arch Phys Med Rehabil 1969;50:311–319. [PubMed] [Google Scholar]
- 21.World Medical Association declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects. JAMA 1997;277:925–926. [PubMed] [Google Scholar]
- 22.Siebner HR, Rothwell J. Transcranial magnetic stimulation: new insights into representational cortical plasticity. Exp Brain Res 2003;148:1–16. [DOI] [PubMed] [Google Scholar]
- 23.Di Lazzaro V, Pilato F, Oliviero A, et al. Origin of facilitation of motor-evoked potentials after paired magnetic stimulation: direct recording of epidural activity in conscious humans. J Neurophysiol 2006;96:1765–1771. [DOI] [PubMed] [Google Scholar]
- 24.Hallett M. Transcranial magnetic stimulation and the human brain. Nature 2000;406:147–150. [DOI] [PubMed] [Google Scholar]
- 25.Ziemann U. TMS and drugs. Clin Neurophysiol 2004;115:1717–1729. [DOI] [PubMed] [Google Scholar]
- 26.Orth M, Snijders AH, Rothwell JC. The variability of intracortical inhibition and facilitation. Clin Neurophysiol 2003;114:2362–2369. [DOI] [PubMed] [Google Scholar]
- 27.Duque J, Hummel F, Celnik P, Murase N, Mazzocchio R, Cohen LG. Transcallosal inhibition in chronic subcortical stroke. Neuroimage 2005;28:940–946. [DOI] [PubMed] [Google Scholar]
- 28.Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol 2004;55:400–409. [DOI] [PubMed] [Google Scholar]
- 29.Brouwer BJ, Schryburt-Brown K. Hand function and motor cortical output poststroke: are they related? Arch Phys Med Rehabil 2006;87:627–634. [DOI] [PubMed] [Google Scholar]
- 30.Carmichael ST, Tatsukawa K, Katsman D, Tsuyuguchi N, Kornblum HI. Evolution of diaschisis in a focal stroke model. Stroke 2004;35:758–763. [DOI] [PubMed] [Google Scholar]
- 31.Nudo RJ. Recovery after damage to motor cortical areas. Curr Opin Neurobiol 1999;9:740–747. [DOI] [PubMed] [Google Scholar]
- 32.Baumer T, Bock F, Koch G, et al. Magnetic stimulation of human premotor or motor cortex produces interhemispheric facilitation through distinct pathways. J Physiol 2006;572:857–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Civardi C, Cantello R, Asselman P, Rothwell JC. Transcranial magnetic stimulation can be used to test connections to primary motor areas from frontal and medial cortex in humans. Neuroimage 2001;14:1444–1453. [DOI] [PubMed] [Google Scholar]
- 34.Ridding MC, Pearce SL, Flavel SC. Modulation of intracortical excitability in human hand motor areas: the effect of cutaneous stimulation and its topographical arrangement. Exp Brain Res 2005;163:335–343. [DOI] [PubMed] [Google Scholar]
- 35.Fregni F, Boggio PS, Mansur CG, et al. Transcranial direct current stimulation of the unaffected hemisphere in stroke patients. Neuroreport 2005;16:1551–1555. [DOI] [PubMed] [Google Scholar]
- 36.Hummel F, Celnik P, Giraux P, et al. Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain 2005;128:490–499. [DOI] [PubMed] [Google Scholar]
- 37.Hummel FC, Cohen LG. Non-invasive brain stimulation: a new strategy to improve neurorehabilitation after stroke? Lancet Neurol 2006;5:708–712. [DOI] [PubMed] [Google Scholar]
- 38.Hummel FC, Voller B, Celnik P, et al. Effects of brain polarization on reaction times and pinch force in chronic stroke. BMC Neurosci 2006;7:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takeuchi N, Chuma T, Matsuo Y, Watanabe I, Ikoma K. Repetitive transcranial magnetic stimulation of contralesional primary motor cortex improves hand function after stroke. Stroke 2005;36:2681–2686. [DOI] [PubMed] [Google Scholar]