Abstract
Objective:
To examine the association between body mass index (BMI), waist circumference (WC), and waist-to-hip ratio (WHR) and risk of dementia and its subtypes in late life.
Methods:
Participants were members of the Kame Project, a population-based prospective cohort study of 1,836 Japanese Americans living in King County, WA, who had a mean age of 71.8 years and were dementia-free at baseline (1992–1994), and were followed for incident dementia through 2001. Cox proportional hazards models were used to estimate the risk of dementia, Alzheimer disease (AD), and vascular dementia (VaD) controlling for demographic and lifestyle characteristics and vascular comorbidities as a function of baseline BMI, WC, and WHR and change in BMI over time.
Results:
Higher baseline BMI was significantly associated with a reduced risk of AD (hazard ratio [HR] = 0.56, 95% confidence interval [CI] = 0.33–0.97) in the fully adjusted model. Slower rate of decline in BMI was associated with a reduced risk of dementia (HR = 0.37, 95% CI = 0.14–0.98), with the association stronger for those who were overweight or obese (HR = 0.18, 95% CI = 0.05–0.58) compared to normal or underweight (HR = 1.00, 95% CI = 0.18–5.66) at baseline.
Conclusion:
Higher baseline body mass index (BMI) and slower declining BMI in late life are associated with a reduced risk of dementia, suggesting that low BMI or a faster decline in BMI in late life may be preclinical indicators of an underlying dementing illness, especially for those who were initially overweight or obese.
GLOSSARY
- AD
= Alzheimer disease;
- BMI
= body mass index;
- CI
= confidence interval;
- DSM-IV
= Diagnostic and Statistical Manual of Mental Disorders, 4th edition;
- HR
= hazard ratio;
- VaD
= vascular dementia;
- WC
= waist circumference;
- WHR
= waist-to-hip ratio.
Evidence suggests that weight loss precedes the diagnosis of dementia1–4 and may be the result of preclinical pathophysiologic changes.5 Increased adiposity also is associated with an increased risk of dementia.6–8 These paradoxical findings are likely related to the long preclinical phase of dementia and the problem that associations between various risk or protective factors and dementia depend upon when they are measured in relation to the clinical onset of disease. In this regard, overweight or obesity in midlife may be more appropriately considered a risk factor, while declining weight in late life may be considered a preclinical indicator of the disease.
The purpose of the current study is to examine the relation between late-life adiposity, measured by body mass index (BMI), waist circumference (WC), and waist-to-hip ratio (WHR), and incident dementia, Alzheimer disease (AD), and vascular dementia (VaD). We used data from the Kame Project, a prospective cohort study of Japanese Americans in King County, WA, to calculate the risk of dementia, AD, and VaD as a function of baseline adiposity, and to further determine whether change in BMI is associated with risk. We hypothesized that higher adiposity at baseline and slower rate of decline in BMI would be associated with decreased risk of dementia and its subtypes.
METHODS
Study population.
Participants were members of the Kame Project, a population-based prospective study of community- and institution-dwelling Japanese Americans 65 years and older living in King County, WA. The study was carried out between May 1992 and December 2001 and consisted of five time points (baseline and follow-ups at approximately 2, 4, 6, and 8 years). The study was approved by the University of Washington Human Subjects Committee and supported by a Japanese American Community Advisory Board, and written informed consent was obtained from all participants. A more detailed description of the study has been presented elsewhere.9
From the 3,045 participants enumerated in a study census of Japanese Americans in King County, WA, in November 1991, 1,985 individuals participated in the baseline examination between May 1992 and September 1994 (65.2%). Of these, 149 were identified as prevalent cases of dementia and 1,836 were dementia-free at baseline and eligible for follow-up to detect incident dementia. Of these 1,836 participants, 1,615 (88.0%) had anthropometric data, of whom 137 were missing follow-up data due to death, loss to follow-up, or refusal to participate, leaving 1,478 (80.5%) participants for the current analysis. During the entire study period (mean = 7.8 years; SD = 0.3), 129 incident dementia cases, 71 incident AD cases, and 22 incident VaD cases were documented in the sample with complete data for this analysis.
Dementia diagnosis.
The diagnosis of dementia was based on a two-stage case ascertainment process consisting of cognitive screening followed by a clinical diagnostic evaluation. Trained interviewers first administered the Cognitive Abilities Screening Instrument10 to assess cognition at baseline and at each biennial. Participants who scored 86 or less of a possible 100 points were referred for full standard clinical and neuropsychological evaluation. The clinical evaluation consisted of physical, neurologic, and laboratory examinations by study physicians9 and informant interviews including the Clinical Dementia Rating Scale.11 Trained psychometrists administered the Consortium to Establish a Registry for AD12 neuropsychological test battery and other tests.9 A consensus committee determined the presence of dementia and its subtypes based on the DSM-IV13 criteria for dementia, the National Institute of Neurological and Communicative Disorders and Related Disorders Association14 criteria for AD, and a number of criteria for VaD.15,16 A more detailed description of the diagnostic procedure can be found elsewhere.9
Anthropometric measures.
At the baseline examination, anthropometric measurements including standing height, weight, WC, and hip circumference were taken by trained interviewers and recorded for each participant. Only weight was measured at each of the follow-up examinations. BMI (weight [kg] over height squared [m2]) was considered the primary index of body weight since it is scaled according to height, and was categorized as obese (BMI ≥25.0), overweight (BMI = 23.0–24.9), normal (BMI = 18.5–22.9), and underweight (BMI <18.5) according to cutoffs proposed by the International Obesity Taskforce for Asian populations17 for descriptive purposes. WC (inches) and WHR (WC [in] over hip circumference [in]) were considered secondary measures of adiposity. Fixed slope parameters for each participant were calculated using random effects modeling and served as the measure of rate of change in BMI across the study period (mean = −0.07, SD = 0.16).
Covariates.
The demographic characteristics of gender (men/women) and education (dichotomized from the original continuous variable as less than high school/high school or greater) were included as covariates. In addition, baseline values were elicited for current or past smoking status (yes/no), current or past alcohol consumption (yes/no), and regular exercise (yes/no). Self-reported history of cardiovascular conditions including hypertension (yes/no), hypercholesterolemia (yes/no), angina pectoris (yes/no), diabetes (yes/no), heart attack (yes/no), TIA (yes/no), and stroke (yes/no) were collected at baseline. Information on ApoE genotype status was available for 1,056 (57.2% [of 1,836]) participants from the first biennial assessment who also had adiposity measures.
Statistical analyses.
The association between the adiposity measures and risk of incident dementia, AD, and VaD was examined using Cox proportional hazard regression models to estimate hazard ratios (HRs) and 95% confidence intervals (CIs), with age at onset as the time scale and age at entry as the truncation variable.18 Three separate models were calculated for continuous baseline BMI, WC, and WHR and continuous change in BMI: 1) adjusting for age, 2) additionally adjusting for gender and education, and 3) additionally adjusting for smoking status, alcohol consumption, regular exercise, hypertension, hypercholesterolemia, angina pectoris, diabetes, heart attack, TIA, and stroke. We also added a quadratic term for BMI when investigating the association between baseline BMI and dementia to account for the nonlinear relation observed graphically, which produced a better fitting model. A multiplicative interaction term between baseline BMI and change in BMI was estimated in fully adjusted proportional hazard regression models for dementia to determine whether the association between change in BMI and dementia depended upon baseline BMI. All analyses were conducted using SAS version 919 with p values less than 0.05 (two-tailed) interpreted as being significant.
RESULTS
The average age of the participants at baseline (n = 1,478) was 71.8 years, 55.3% were women, and 75.8% had at least high school education or greater. The average BMI was 24.3 (range 15.4–47.3), with 39.6% obese, 24.6% overweight, 32.7% normal weight, and 3.1% underweight. In terms of the other covariates at baseline, 48.9% were current or past smokers; 37.1% were current or past drinkers of alcohol; 65.2% reported regular physical activity; 46.8% reported hypertension; 5.3% reported having a coronary artery attack; 3.3% reported having a TIA; 2.6% reported a stroke; 16.6% reported diabetes; 12.3% reported hypercholesterolemia; 5.5% reported angina; and 20.6% were ApoE ɛ4 allele positive. The current sample did not differ from the dementia-free cohort (n = 1,836) with respect to any of the covariates except age and education, where the current sample was 0.9 years younger (t = −2.78; p = 0.004) and 46.0% had greater than or equal to high school education compared to the dementia-free cohort (54.0%; χ2 = 7.13, p = 0.008).
The characteristics of the sample by BMI category at baseline are shown in table 1. Those who were underweight were more likely to be women, less likely to be a current or past smoker or alcohol drinker, and less likely to have high cholesterol or diabetes. Those who were of normal weight were the least likely to have hypertension.
Table 1 Baseline sample characteristics by BMI category among 1,478 participants 65 years and older in the Kame Project (1992–2001)
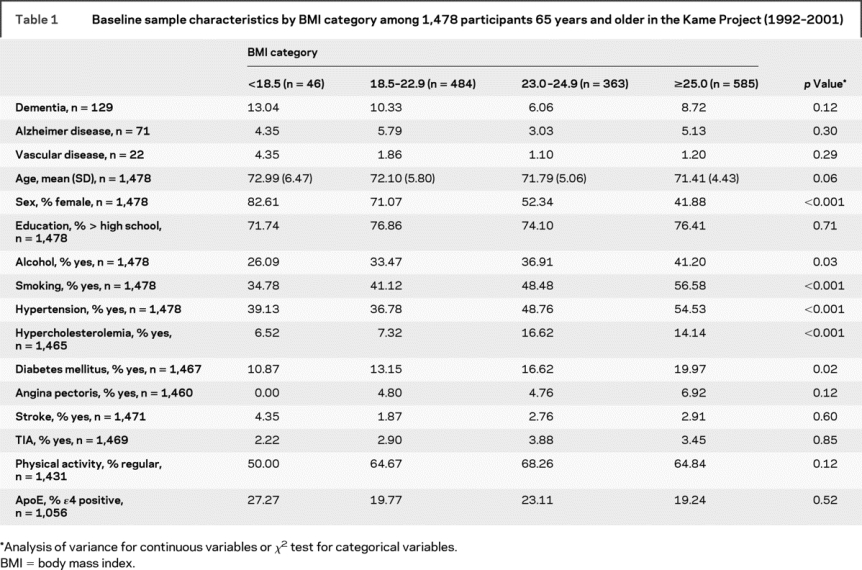
Table 2 shows the associations between baseline and change in BMI and risk of dementia, AD, and VaD. BMI at baseline was inversely associated with dementia, AD, and VaD, but was only significant for AD in the fully adjusted model. We did not find that the risk of dementia, AD, and VaD was associated with WC and WHR at baseline (data not shown). The risk of dementia and AD was reduced with a slower rate of BMI decline during the study period in the fully adjusted model where an average BMI decline of 1.06 units less per year (1.15 for AD) was associated with a 63% reduced risk (68% for AD), although the point estimate for AD was marginally significant. Adjusting for the presence of the ApoE ɛ4 allele did not substantially change the results, but did strengthen the association between rate of change in BMI and the risk of dementia and AD. A significant interaction between baseline BMI and change in BMI was found for dementia (HR = 0.73, 95% CI = 0.53–0.99, p for interaction = 0.048). The reduction in risk of dementia with slower BMI decline was greater with increasing BMI at baseline (figure) where there was a significant reduction in risk for those whose baseline BMI was overweight or obese (HR = 0.18, 95% CI = 0.05–0.58) compared to those who were normal or underweight (HR = 1.00, 95% CI = 0.18–5.66).
Table 2 Hazard ratios for incident dementia by baseline BMI and change in BMI over study period among participants 65 years and older in the Kame Project (1992–2001)
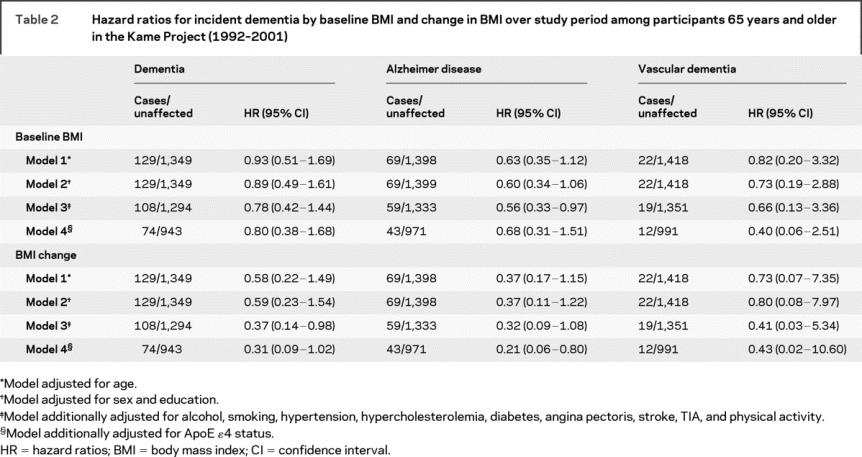
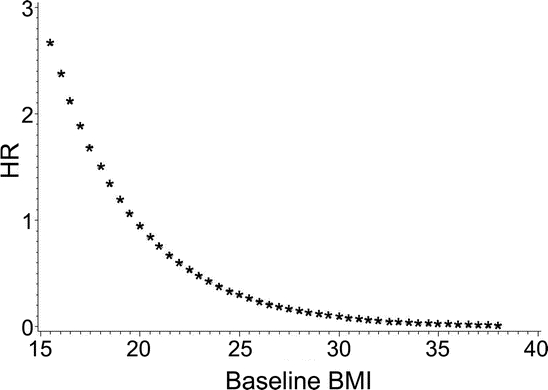
Figure Association between BMI change over the study period and dementia depends on baseline BMI
The x-axis shows baseline body mass index (BMI), and the y-axis shows the hazard ratio (HR) for change in BMI over the study period and dementia controlling for baseline BMI, age, and education.
DISCUSSION
In this analysis from the Kame Project, we report that the risk for AD was reduced with higher late-life BMI at baseline and that the risks of dementia and AD were reduced with a slower rate of BMI decline over a follow-up period of approximately 8 years. More importantly, the extent to which change in BMI was associated with dementia depended upon baseline BMI such that overweight or obese participants at baseline had a more pronounced reduction in risk with slower decline in BMI compared to normal or underweight participants. These findings suggest that late-life adiposity is associated with the risk of dementia, where high and slowly declining BMI reduce the risk; or conversely, that low or fast declining BMI may be preclinical indicators for dementia.
The risk for dementia is believed to develop across the lifespan as the pathologic hallmarks have been detected decades before its clinical presentation. Because of its long prodromal period, assessing the characteristics of a risk factor is time-dependent and the potential for reverse causality exists. Our finding of a reduced risk of AD with higher baseline BMI is suggestive of a protective effect of higher BMI in late life, similar to findings from the Kungsholmen Project6 and the Chicago Health and Aging Project.20 This is different from what has been shown in midlife, where overweight or obesity increased the risk of dementia and AD.8 Our findings are in accord with others who have shown weight loss in later life to be a risk factor for dementia1,2 and for weight loss to precede the diagnosis of dementia.3,4 Hence, it may be that a nonlinear association exists where higher adiposity in midlife increases the risk of dementia and its subtypes, and that pathophysiologic changes associated with dementia then lead to declines in adiposity in late life.5
Measures of central fat distribution, including WC and WHR, are known to increase the risk of coronary heart disease21 and mortality22 more than total body weight. Evidence suggests that central obesity in midlife23 and late life24 increases the risk of dementia and AD. Our findings do not support a link between WC or WHR in late life and dementia risk. These findings are partially in accord with those of the Northern Manhattan study where continuous WC was not associated with dementia, AD, or dementia associated with stroke. The same study did, however, find that the risk of dementia associated with stroke was increased for the largest WC quartile compared to the smallest WC quartile.7 Additional studies at both midlife and late life are needed to further elucidate whether central adiposity is associated with the risk for dementia and its subtypes.
The finding that overweight or obese participants had a reduced risk of dementia with slower decline in BMI compared to those who were normal or underweight may reflect a floor effect. Those who are normal or underweight at baseline have less weight to lose compared to those who are overweight or obese, which would lessen their rate of change in BMI over the course of the study. It may also be that those who were normal or underweight at the beginning of the study and were losing weight at a faster rate were lost to follow-up since this weight loss could affect overall health. Taken together, the results suggest that having a slow rate of a decline in weight if previously overweight or obese may reduce risk more than being overweight or obese alone in late life.
The use of BMI categories for Asian populations in our study resulted in a high number (39.6%) of obese (BMI >25) participants at baseline compared to the United States prevalence of 12% in the early 1990s.25 Using the Caucasian BMI cutpoints reduced this number to 5.0% for obese (BMI >30) with the remaining 3.1% underweight (BMI <18.5), 57.3% normal (BMI = 18.5–25), and 34.6% overweight (BMI = 25–30). Since 96% of the participants in the Kame Project were 100% Japanese,9 and twin studies have shown that genetic influences on BMI are substantial,26 we considered the Asian, rather than Caucasian, categories to be more appropriate for our sample. Furthermore, studies have shown that Asians in general have higher body fat, greater centralized distribution of body fat, and higher WHR than Caucasians with lower or similar BMIs, which highlights the importance of redefining the categories to assess health risks in our sample.17 Despite this descriptive difference, we believe that our main findings are generalizable beyond Japanese American or Asian populations since we used continuous measures of baseline BMI and change in BMI that were independent of BMI categories.
Several biologic processes may explain the association between high and slower decline in BMI and reduced risk of dementia. Higher weight in late life may offer protection by increasing insulin-growth factor I levels,27 increasing leptin hormone levels known to be involved in regulation of synaptic plasticity in the hippocampus,28 and increasing the production of estrogen,29 all of which have been shown to be associated with better cognitive performance.30,31 Slower decline in BMI over the course of the study may indicate that preclinical changes associated with dementia are not occurring. Brain areas that control weight (i.e., mesial temporal cortex)32 are affected during the preclinical dementia phase that may lead to weight loss. Weight loss may also result from predementia apathy,33 reduced olfactory function,34 difficulty in eating,35 or inadequate nutrition36 due to cognitive impairment.
There are both strengths and limitations of this study that should be acknowledged. The first strength is that the association between various measures of adiposity and incident dementia and two of its subtypes were examined. Also, the study included both community and institutionalized individuals, minimizing any selection bias that may result from including only those healthy enough to remain independent in the community. Finally, we adjusted for multiple variables that would likely confound any associations between adiposity and dementia.
Limitations of this study include the relatively short follow-up period, which increases the potential for reverse causality to explain the findings. Also, our sample was limited to Americans who were of Japanese ancestry and thus may limit the generalizability to other populations. We also assumed that height was constant throughout the study period even though studies have shown that women and men lose 0.2 to 0.3 cm per year between the ages of 70 and 90 years37; however, any error introduced would likely nondifferentially overestimate the rate of decline in BMI and not bias the findings. It is also possible that using BMI as our measure of weight may have underestimated adiposity in the elderly who generally have less lean body mass,38 which would have attenuated our findings. Finally, we had insufficient power to detect a relationship between adiposity and VaD, but did find similar point estimates as dementia and AD. Others studies with more power have shown a stronger effect for VaD than AD,7 suggesting that adiposity may exert its influence on dementia with a vascular origin.
Address correspondence and reprint requests to Dr. Tiffany F. Hughes, Department of Psychiatry, University of Pittsburgh School of Medicine, 3811 O’Hara St., Pittsburgh, PA 15213 hughest2@upmc.edu
Disclosure: The authors report no disclosures.
Received November 4, 2008. Accepted in final form February 13, 2009.
REFERENCES
- 1.Buchman AS, Wilson RS, Bienias JL, Shah RC, Evans DA, Bennett DA. Change in body mass index and risk of incident Alzheimer disease. Neurology 2005;65:892–897. [DOI] [PubMed] [Google Scholar]
- 2.Stewart R, Masaki K, Xue Q-L, et al. A 32-year prospective study of change in body weight and incident dementia. Arch Neurol 2005;62:55–60. [DOI] [PubMed] [Google Scholar]
- 3.Johnson DK, Wilkins CH, Morris JC. Accelerated weight loss may precede diagnosis in Alzheimer disease. Arch Neurol 2006;63:1312–1317. [DOI] [PubMed] [Google Scholar]
- 4.Knopman DS, Edland SD, Cha RH, Petersen RC, Rocca WA. Incident dementia in women in preceded by weight loss by at least a decade. Neurology 2007;69:739–746. [DOI] [PubMed] [Google Scholar]
- 5.Buchman AS, Schneider JA, Wilson RS, Bienias JL, Bennett DA. Body mass index in older persons is associated with Alzheimer disease pathology. Neurology 2006;67:1949–1954. [DOI] [PubMed] [Google Scholar]
- 6.Atti AR, Palmer K, Volpato S, Winblad B, De Ronchi D, Fratiglioni L. Late-life body mass index and dementia incidence: nine-year follow-up data from the Kungsholmen Project. J Am Geriatr Soc 2008;56:111–116. [DOI] [PubMed] [Google Scholar]
- 7.Luchsinger JA, Patel B, Tang M-X, Schupf N, Mayeux R. Measures of adiposity and dementia risk in the elderly. Arch Neurol 2007;64:392–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whitmer RA, Gunderson EP, Quesenberry CP, Zhou J, Yaffe K. Body mass index in midlife and risk of Alzheimer disease and vascular dementia. Curr Alzheimer Res 2007;4:103–109. [DOI] [PubMed] [Google Scholar]
- 9.Graves AB, Larson EB, Edland SD, et al. Prevalence of dementia and its subtypes in the Japanese American population of King County, Washington state, The Kame Project. Am J Epidemiol 1996;144:760–771. [DOI] [PubMed] [Google Scholar]
- 10.Teng EL, Hasegawa K, Homma A, et al. The Cognitive Abilities Screening Instrument (CASI): a practical test for cross-cultural epidemiologic studies of dementia. Int Psychogeriatr 1994;6:45–58. [DOI] [PubMed] [Google Scholar]
- 11.Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry 1982;140:566–572. [DOI] [PubMed] [Google Scholar]
- 12.Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD): part I: clinical and neuropsychological assessment of Alzheimer’s disease. Neurology 1989;39:1159–1165. [DOI] [PubMed] [Google Scholar]
- 13.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 14.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of the Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- 15.Chui HC, Victoroff JI, Margolin D, Jagust W, Shankle R, Katzman R. Criteria for the diagnosis of ischemic vascular dementia proposed by the State of California Alzheimer’s Disease Diagnostic and Treatment Centers. Neurology 1992;42:473–480. [DOI] [PubMed] [Google Scholar]
- 16.Roman GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: diagnostic criteria for research studies: report of the NINDS-AIREN International Workshop. Neurology 1993;43:250–260. [DOI] [PubMed] [Google Scholar]
- 17.Kanazawa M, Yoshiike N, Osaka T, Numba Y, Zimmet P, Inoue S. Criteria and classification of obesity in Japan and Asia-Oceania. Asia Pacific J Clin Nutr 2002;11:S732–S737. [DOI] [PubMed] [Google Scholar]
- 18.Korn EL, Graubard BI, Midthune D. Time-to-event analysis of longitudinal follow-up of a survey: choice of the time-scale. Am J Epidemiol 1997;145:72–80. [DOI] [PubMed] [Google Scholar]
- 19.SAS Institute. SAS System for Microsoft Windows (version 9). Cary, NC: SAS Institute Inc; 2003. [Google Scholar]
- 20.Sturman MT, de Leon CF, Bienias JL, Morris MC, Wilson RS, Evans DA. Body mass index and cognitive decline in a biracial community population. Neurology 2008;70:360–367. [DOI] [PubMed] [Google Scholar]
- 21.Yarnell JW, Patterson CC, Thomas HF, Sweetnam PM. Central obesity: predictive value of skinfold measurements for subsequent ischemic heart disease at 14 years follow-up in the Caerphilly Study. Int J Obes Relat Metab Disord 2001;25:1536–1549. [DOI] [PubMed] [Google Scholar]
- 22.Mason C, Craig CL, Katzmarzyk PT. Influence of central and extremity circumferences on all-cause mortality in men and women. Obesity 2008;16:2690–2695. [DOI] [PubMed] [Google Scholar]
- 23.Whitmer RA, Gustafson DR, Barrett-Connor E, Haan MN, Gunderson EP, Yaffe K. Central obesity and increased risk of dementia more than three decades later. Neurology 2008;71:1057–1064. [DOI] [PubMed] [Google Scholar]
- 24.Razay G, Vreugdenhil A, Wilcock G. Obesity, abdominal obesity and Alzheimer disease. Dement Geriatr Cogn Disord 2006;22:173–176. [DOI] [PubMed] [Google Scholar]
- 25.Mokdad AH, Serdula MK, Dietz WH, Bowman BA, Marks JS, Koplan JP. The spread of the obesity epidemic in the United States, 1991–1998. JAMA 1999;282:1519–1522. [DOI] [PubMed] [Google Scholar]
- 26.Stunkard AJ, Harris JR, Pedersen NL, McClearn GE. The body-mass index of twins who have been reared apart. N Engl J Med 1990;322:1483–1487. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto H, Kato Y. Relationship between plasma insulin-growth like factor I (IGF-I) levels and body mass index (BMI) in adults. Endocr J 1993;4:41–45. [DOI] [PubMed] [Google Scholar]
- 28.Harvey J, Solovyova N, Irving A. Leptin and its role in hippocampal synaptic plasticity. Prog Lipid Res 2006;45:369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh M, Dykens JA, Simpkins JW. Novel mechanisms for estrogen-induced neuroprotection. Exp Biol Med 2006;231:514–521. [DOI] [PubMed] [Google Scholar]
- 30.Okereke O, Kang JH, Ma J, Hankinson SE, Pollak MN, Grodstein F. Plasma IGF-I levels and cognitive performance in older women. Neurobiol Aging 2007;28:135–142. [DOI] [PubMed] [Google Scholar]
- 31.Oomura Y, Hori N, Shiraishi T, Fukunaga K, Takeda H, Tsuji M. Leptin facilitates learning and memory performance and enhances hippocampal CA1 long-term potentiation and CaMK II phosphorylation in rats. Peptides 2006;27:2738–2749. [DOI] [PubMed] [Google Scholar]
- 32.Grundman M, Corey-Bloom J, Jerigan T, Archibald S, Thal LJ. Low body weight in Alzheimer’s disease is associated with mesial temporal cortex atrophy. Neurology 1996;46:1585–1591. [DOI] [PubMed] [Google Scholar]
- 33.Friedland RP, Fritsch T, Smyth KA, Koss E, Lerner AJ, Chen CH. Patients with Alzheimer’s disease have reduced activities in midlife compared with healthy control-group members. Proc Natl Acad Sci USA 2001;98:3440–3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Djordjevic J, Jones-Gotman M, De Sousa K, Chertkow H. Olfaction in patients with mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging 2008;29:693–706. [DOI] [PubMed] [Google Scholar]
- 35.Chang CC, Roberts BL. Feeding difficulty in older adults with dementia. J Clin Nurs 2008;17:2266–2274. [DOI] [PubMed] [Google Scholar]
- 36.Shatenstein B, Kergoat MJ, Reid I. Poor nutrient intakes during 1-year follow-up with community-dwelling older adults with early-state Alzheimer dementia compared to cognitively intact matched controls. J Am Diet Assoc 2007;107:2091–2099. [DOI] [PubMed] [Google Scholar]
- 37.Sorkin JD, Muller DC, Andres R. Longitudinal change in the heights of men and women: consequential effects on body mass index. Epidemiol Rev 1999;21:247–260. [DOI] [PubMed] [Google Scholar]
- 38.Elmadfa I, Meyer AL. Body composition, changing physiological functions, and nutrient requirements of the elderly. Ann Nutr Metab 2008; 52 suppl 1: 2–5. [DOI] [PubMed] [Google Scholar]


