Abstract
Introduction
Executive dysfunction (ED) is a prominent and often disabling feature of cognitive impairment in Parkinson’s disease (PD). Few studies have examined treatments. Given the role of noradrenergic pathology in ED, atomoxetine, a norepinephrine reuptake inhibitor indicated for attention deficit hyperactivity disorder (ADHD), may be a potential treatment for PD-related ED.
Methods
12 patients with PD and disabling ED completed an 8-week pilot open-label, flexible dose (25 to 100 mg/day) trial of atomoxetine.
Results
On primary outcome measures, atomoxetine was associated with improved ED based on the Clinical Global Impression-Change Scale (75% positive response rate; 95% CI: 43%-95%, p<.05) and behavioral measures of ED [Frontal Systems Behavior Scale (FrSBE) Executive Dysfunction and Connors Adult ADHD Rating Scale (CAARS) inattention/memory subscales]. Adverse effects included sleep and gastrointestinal disturbances and hypomania.
Conclusion
Atomoxetine is tolerable in PD and may benefit clinical manifestations of ED, warranting further study in controlled trials.
Keywords: Executive Dysfunction, Parkinson’s disease, Cognition, Norepinephrine Reuptake inhibition, Atomoxetine
INTRODUCTION
Cognitive impairment in Parkinson’s disease (PD) is commonly characterized as a progressive dysexecutive syndrome involving deficits in sequencing, planning, set-shifting, response inhibition, working memory, and multitasking.1 As these processes are essential for adaptive functioning, executive dysfunction (ED) is often disabling.2 ED is also associated with transition to dementia,3 but individuals with intact cognitive test performance are also affected negatively.4
Few studies have examined treatments for PD-related ED, but dopaminergic and noradrenergic systems and prefrontal cortex are implicated.5 Accordingly, we conducted a pilot open-label trial on the effectiveness and tolerability of atomoxetine, a selective norepinephrine reuptake inhibitor indicated for attention deficit hyperactivity disorder (ADHD), as a treatment of patients with PD who have ED, but not dementia.
Methods
Subjects were outpatients with idiopathic PD,6 ages 21 to 65 years, recruited through community outreach and clinic sources. In the absence of established diagnostic criteria for ED, clinically significant ED was defined by problems of moderate severity with disorganization, distractibility, task completion, planning or problem solving that affected work or social function, represented a decline from pre-morbid (pre-PD) status, and were confirmed by an informant. Other inclusion criteria were: Mini-Mental State Exam7 ≥ 26; absence of DSM-IV-TR Dementia due to PD; Clinical Dementia Rating Scale Global score8 < 1; Functional Assessment Staging score9 ≤ 4; 21-item Hamilton Depression Rating Scale10 score < 10; stable medications for three months; and, absence of contraindications to atomoxetine use (narrow angle glaucoma, use of monoamine oxidase inhibitor antidepressants), urinary hesitation or retention, hepatic dysfunction, hallucinations without insight, pregnancy, current illicit substance use or alcohol abuse or dependence; and use of concomitant potent CYP2D6 inhibitors, psychostimulants, or wakefulness therapy. Subjects and informants provided informed written consent. The Western Institutional Review Board approved the study.
Dosing for this 8-week open-label, uncontrolled, flexible dose trial consisted of atomoxetine 25 mg/day (Week 1), 50 mg/day (Weeks 2-4), 75 mg/day (Week 5), and 100 mg/day (Weeks 6-8). Dose reductions were allowed to a minimum of 2.5 mg/day for intolerance. Primary outcome measures were the Clinical Global Impression of Change-Clinician rated (CGI-C) score11 and self-rated behavioral measures of ED: the Frontal Systems Behavior Scale (FrSBe)12 Executive Functioning deficits subscore and the Connors Adult ADHD Rating Scale Long form (CAARS-L)13 Inattention/Memory subscore, a primary outcome measure in atomoxetine trials for ADHD.14 Secondary outcomes included a comprehensive neuropsychological and psychiatric battery (see Appendix). Safety assessments included vital signs, spontaneously reported adverse events (AEs), UKU AE checklist,15 Unified Parkinson’s Disease Rating Scale (UPDRS)-Activities of Daily Living, Motor, and Complications of Therapy subscales,16 Hoehn and Yahr Stage,17 changes from baseline laboratory tests, and cardiovascular effects using conventions from previous atomoxetine studies.18
Analyses used STATA Version-9 (StataCorp, College Station, Texas). Efficacy, based on change from Baseline (Day 0) to end of treatment (Day 56), used Wilcoxon signed rank test for continuous variables and chi-square or Fisher exact test for categorical variables. A p-value<0.05 defined significance. There were no corrections for multiple comparisons.
Results
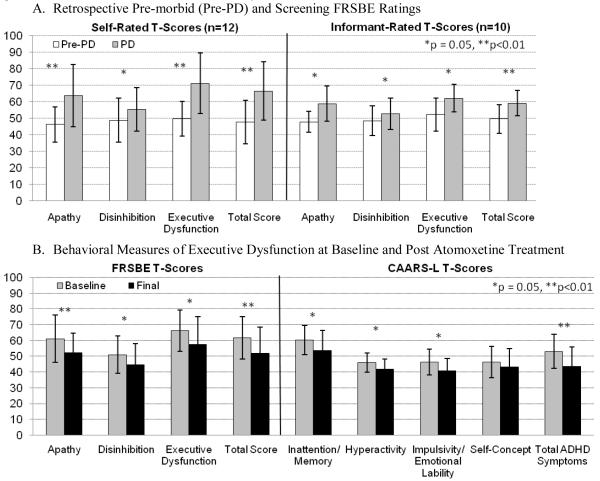
All twelve subjects (Table 1) completed the trial. The mean (SD, range) atomoxetine dose at the final visit was 89.6 (24.9, 25-100) mg/day. CGI-C ratings in nine subjects indicated clinically significant improvement in ED (75% positive response rate, 95% CI:43%-95%) [Three subjects “very much improved” (95% CI:5%-57%); six “much improved” (CI:21% -79%), one “minimally improved” (CI:0%-38%) and two with “no change” (CI:2%-48%)] Atomoxetine was associated with improved scores on the FrSBe Executive Dysfunction and CAARS-L Inattention/Memory subscale and the remaining FrSBe and CAARS-L subscale and total scores, except the CAARS-L Self-Concept subscale (Figure).
Table. Subject Characteristics (n=12) at Baseline.
| Age (years) | 57.3 (7.2, 40-65) |
| Gender (Male/Female) | 5M/7F |
| Education (years) | 18.2 (2.7, 13-24) |
| Age onset PD (years) | 44.5 (9.2) (24-56) |
| Age diagnosis PD (years) | 47.9 (8.2, 32-57) |
| Duration PD (years) | 12.8 (8.4, 3-34) |
| Hoehn & Yahr Stage | 2.1 (0.3) # subjects per stage I½=1, II=8, II½=3 |
| UPDRS ADL Subscore | 14.9 (12.1) |
| Motor Subscore | 23.2 (12.7) |
| Mean (SD, Range) | |
| Concomitant Medications | |||
|---|---|---|---|
| Antiparkinsonian Medications | Psychiatric Medications | ||
| Dopamine agonists only | 2 (17%) | Antidepressants | 7 (58%) |
| l-dopa only | 4 (33%) | Atypical Antipsychotics | 3 (25%) |
| l-dopa + Dopamine agonists | 6 (50%) | Benzodiazepine/hypnotics | 2 (17%) |
| Apomorphine | 1 (8%) | ||
| COMT inhibitor | 3 (25%) | ||
| Anticholinergics | 1 (8%) | ||
| Selegiline | 1 (8%) | ||
| Amantadine | 2 (17%) | ||
Figure.
The Frontal Systems Behavioral Scale (FrSBe), developed for neurological populations, includes current plus retrospective ratings of behaviors pre-illness. The Connors Adult ADHD Rating Scale-Long Form (CAARS-L) and has been a primary outcome measure in trials of atomoxetine for adult ADHD.27 The primary self-rated outcome measures, the FrSBE Executive Dysfunction and CAARS-L Inattention/Memory subscales, measure frequency of behaviors such as task incompletion, disorganization, distractibility, and difficulty planning, multi-tasking, and initiating tasks. A. Change in Self- and Informant-rated FrSBE scores based on behaviors endorsed retrospectively as present before onset of PD compared to behaviors endorsed at screening evaluation. Individual T-scores are based on ratings in a normative sample in which the distribution of T-scores has a mean of 50 and a SD of 10. Group mean (SD) T-scores are presented to allow comparability across gender, age range, and education level within the sample; higher scores indicate greater symptom severity. For all FrSBe scales, T scores ≥ 65 are considered clinically significant and scores of 60 to 64 represent likely borderline impairment.
B. Frontal Systems Behavioral Scale (FrSBe) Subscale and Total scores and Conners’ Adult ADHD Rating Scales Long Form (CAARS-L) Subscales and Total Scores at baseline and final visits. CAARS-L scores are also depicted as group Mean (SD) T scores, derived from comparison to CAARS norms based on gender and age in a normative sample. Similar to the FrSBE, higher T scores are associated with greater symptom severity and T scores above 65 represent symptoms of clinical significance.
Baseline cognitive test performance was within published norms for most subjects. Except for an improved Hopkins Verbal Learning Test-Revised Recognition Discrimination score [10.8(1.7) to 11.9 (0.3), change=1.2(1.8), p<.05], there were no changes in neuropsychological performance. The only changes on psychiatric rating scales were increased Neuropsychiatric Inventory19 sleep difficulties [0.8 (1.5) to 1.8 (2.4), change =1.0 (2.2), p<.05], and improved PDQL Emotional Symptoms Total20 [33.7 (6.0) to 36.9 (5.5), change =3.3 (2.9), p<.01].
Treatment-emergent AEs were mildly to moderately severe. Most common were reduced sleep (n=6, 50%), constipation (n=5, 42%), and nausea/vomiting, tension, confusion, slowed movements, and diaphoresis (each n=3, 25%). AEs endorsed by 2 subjects (17%) were fatigability, depression, agitation, increased dream activity, rigidity, hyperkinetic movements, paresthesias, headaches, dry mouth, tachycardia, rash, weight gain, and dysmenorrhea. Eighteen other AEs were reported in one subject each. Because of AEs, two subjects delayed dose increases and two required dose reductions. One subject developed hypomania on atomoxetine 75 mg/day that remitted with reduction to 25 mg/day. There were no significant motor, vital sign, laboratory test, or EKG changes.
Discussion
This pilot study supports the importance of exploring efficacy of atomoxetine for PD-related ED in future studies, using controlled designs. In 75% of subjects, we observed clinically significant improvement that corresponded to a decline in behavioral symptoms of ED. The majority tolerated atomoxetine and motor function was unaffected, but gastrointestinal effects and hypomania were notable AEs.
Two other studies describe treatment of PD-related ED in non-demented patients. A cognitive training program focusing on working memory showed improved performance on executive tasks, but did not assess ED in daily functioning.21 Consistent with our results, a 16-week open-label study (n=10) of donepezil, an acetylcholinesterase inhibitor, showed improved CGI ratings in the absence of changes in cognitive measures of ED.22
The focus on behavioral aspects of ED is a unique aspect of this study that ensured its clinical relevance. Given the diverse processes involved in ED and heterogeneous neuropsychological deficits of PD, there is no current basis for a single primary cognitive outcome measure.23 Thus, cognitive complaints and behavioral evidence for acquired ED provided a standardized approach for defining appropriate study candidates, a potentially useful strategy for future trials. The CAARS-L Inattention/Memory Problems subscale captures many behaviors described by our subjects, but has not been studied in PD. We also used the FrSBe, a validated measure of behavioral changes associated with frontal systems damage.12 Whereas the CAARS-L, FrSBE, and CGI-C were sensitive tools for evaluating ED severity and treatment response in this study, our subjects were generally unimpaired on psychometric tests. This is not uncommon when evaluating individuals with ED in structured test settings.12 High pre-morbid function in our sample may also limit detection of deficits relative to published norms. Nonetheless, evidence for changes from pre-PD FrSBE ratings (Figure) reflected previously intact executive abilities. The utility of the FrSBe for identifying PD subgroups with ED or predicting cognitive decline needs further investigation; in the future, behavioral changes might serve as a basis for interventions, instead of delaying treatments until declines in cognitive performance are evident.4
The mechanism by which atomoxetine may improve ED is unknown; previous studies emphasize effects on inhibitory control. Atomoxetine acts primarily via presynaptic norepinephrine transporter blockade. It also elevates dopamine in selective cortical regions and has procholinergic effects.24,25 In rats, atomoxetine improved performance on learning, memory consolidation, retrieval, and inhibitory control tasks.25,26 In adult control and ADHD subjects, single atomoxetine doses produced selective effects on response inhibition in the absence of effects on attention or working memory.27,28 Longer-term atomoxetine trials in adults with ADHD also showed improved measures of inhibitory control.29,30
An advantage of atomoxetine is that its long duration of action sustains elevations of prefrontal norepinephrine and dopamine, which may also account for its lack of abuse potential when compared to transient changes seen with psychostimulants.24 In other studies of PD patients, manipulation of the noradrenergic system influences executive functions that rely on attentional resources and subcortical dopaminergic effects.31,32,33 Methylphenidate, a psychostimulant, benefited attention, but only in non-demented patients taking l-dopa, supporting its primary dopaminergic effects.34,35 Furthermore, dopaminergic drugs mainly improve motor function and only minimally benefit or possibly aggravate cognitive deficits, including ED.34,36
Atomoxetine was generally well-tolerated. AEs, especially gastrointestinal symptoms, were consistent with other reports14 and there were no clinically significant motor effects. While pre-existing psychiatric conditions were controlled at baseline, one patient developed hypomania. A previous report describes onset of mania in an adult treated with atomoxetine for depression.37
This study has several limitations. As an open-labeled study, placebo effects cannot be excluded; a blinded, placebo-controlled study with a larger sample is necessary to assess effectiveness and tolerability of atomoxetine for PD-related ED. The small sample size, inter-subject variability, possible inclusion bias, ceiling, and practice effects limit interpretations of neuropsychological data. We did not correct for multiple comparisons and the sample size precludes evaluation of differential effects of atomoxetine relative to baseline cognitive performance. Finally, our sample was restricted to subjects younger than age 65 years.
In this study, atomoxetine was generally tolerable and reduced severity of behavioral symptoms associated with ED. Diagnosis of ED based on clinical symptoms may allow inclusion of patients in trials with disease-related behavioral changes before progression to the point of cognitive deficits on formal testing. Future trials should take into account heterogeneity of ED and cognitive impairment in PD, with deficits and variable progression across multiple domains and involvement of multiple neurotransmitter systems. The noradrenergic system may be an important target, with a favorable role for atomoxetine suggested by the present results, but requiring further study.
Acknowledgements
Primary support for this study was provided by an Eli Lilly, Incorporated Investigator-Initiated Trial Program research grant to Dr. Laura Marsh. Additional support was provided by the Morris K. Udall Parkinson’s Disease Research Center of Excellence at Johns Hopkins (NIH P50-NS-58377), the General Clinical Research Center at Johns Hopkins University School of Medicine (National Center for Research Resources/NIH NIH UL1-RR-025005); the Donna Jean Baumann Fund; the Weldon Hall Trust; Age-Related Cognitive Disorders Training Grant (NIH 5T32-AG-027668, J.R. Williams); and the Parkinson’s Disease Foundation Student Fellowship Program (J.R. Williams). We are grateful to the contributions of Susan Spear Bassett, Ph.D. regarding selection of the neuropsychological test battery.
Appendix
All subjects completed a comprehensive Neuropsychological and Psychiatric Assessment battery. The cognitive battery includes the MMSE, Mattis Dementia Rating Scale, Hopkins Verbal Learning Test-Revised [HVLT-R] and Brief Visuospatial Memory Test [BVMT], visual scanning and set-shifting: Trails A & B, reaction time, attention, and vigilance: Continuous Performance Test-II (CPT-II), planning, sequencing, and problem solving: CANTAB Stockings of Cambridge, generativity: verbal fluency, working memory: Paced Auditory Serial Addition Test (PASAT), letter-number sequencing, and digit span; and inhibition: Stroop. To limit the bias of practice effects on performance at the final visit, the battery was administered at screening and baseline and alternate test forms were used for the HVLT-R, BVMT, and MMSE. Psychiatric assessments included the Neuropsychiatric Inventory (NPI), 21-item Hamilton Depression Rating Scale, the Young Mania Scale, and self-rated measures of depression, anxiety, and quality of life [Beck Depression Inventory, State-Trait Anxiety Inventory, and PD Quality of Life (PDQL) scale.]
References
- 1.Dubois B, Pillon B. Cognitive deficits in Parkinson’s disease. J Neurol. 1997;244(1):2–8. doi: 10.1007/pl00007725. [DOI] [PubMed] [Google Scholar]
- 2.Campbell JJ, Duffy JD, Salloway SP. Treatment strategies for patients with dysexecutive syndromes. In: Salloway SP, Malloy PF, Duffy JD, editors. The frontal lobes and neuropsychiatric illness. American Psychiatric Publishing. Inc.; Washington, D.C.: 2002. pp. 153–163. [Google Scholar]
- 3.Woods SP, Tröster AI. Prodromal frontal/executive dysfunction predicts incident dementia in Parkinson’s disease. J Int Neuropsychol Soc. 2003;9(1):17–24. doi: 10.1017/s1355617703910022. [DOI] [PubMed] [Google Scholar]
- 4.Caviness JN, Driver-Dunckley E, Connor DJ, Sabbagh MN, Hentz JG, Noble B, et al. Defining mild cognitive impairment in Parkinson’s disease. Mov Disord. 2007;22(9):1272–1277. doi: 10.1002/mds.21453. [DOI] [PubMed] [Google Scholar]
- 5.Cools R, Swainson R, Owen AM, Robbins TW. Cognitive dysfunction in non-demented Parkinson’s disease. In: Wolters EC, Sheltens Ph, Berendse HW, editors. Mental dysfunction in Parkinson’s disease II. Academic Pharmaceutical Productions; Utrecht: 1999. pp. 142–164. [Google Scholar]
- 6.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55(3):181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 8.Morris JC. The Clinical Dementia Rating (CDR):current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 9.Reisberg B. Functional assessment staging (FAST) Psychopharm Bull. 1988;24:653–659. [PubMed] [Google Scholar]
- 10.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schneider LS, Olin JT, Doody RS, Clark CM, Morris JC, Reisberg B, et al. Validity and reliability of the Alzheimer’s Disease Cooperative Study-Clinical Global Impression of Change. Alzheimer Disease and Associated Disorders. 1997;11(Suppl 2):S22–S32. doi: 10.1097/00002093-199700112-00004. [DOI] [PubMed] [Google Scholar]
- 12.Grace J, Malloy P. Frontal Systems Behavior Scale (FrSBe) Psychological Assessment Resources, Inc.; Lutz, PL: 2001. [Google Scholar]
- 13.Conners CK, Erhardt D, Sparrow EP. Technical Manual. Multi-Health Systems, Inc; North Tonawanda, NY: 1999. Conners’ Adult ADHD Rating Scales (CAARS) [Google Scholar]
- 14.Michelson D, Adler L, Spencer T, Reimberr FW, West SA, Allen AJ, et al. Atomoxetine in adults with ADHD: two randomized, placebo-controlled studies. Biol Psychiatry. 2003;53:112–120. doi: 10.1016/s0006-3223(02)01671-2. [DOI] [PubMed] [Google Scholar]
- 15.Lingjaerde O, Ahlfors UG, Bech P, Dencker SJ, Elgen K. The Udvalg for Kliniske Undersogelser (UKU) Side Effect Rating Scale: a new comprehensive rating scale for psychotropic drugs, and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr Scand. 1987;(Suppl 76):1–100. doi: 10.1111/j.1600-0447.1987.tb10566.x. [DOI] [PubMed] [Google Scholar]
- 16.Fahn S, Elton RL, Members of the UPDRS Development Committee . Unified Parkinson’s disease rating scale. In: Fahn S, Marsden CD, Goldstein M, editors. Recent developments in Parkinson’s disease II. Macmillan; New York: 1987. pp. 153–163. [Google Scholar]
- 17.Hoehn MM, Yahr MD. Parkinsonism: onset, progression, and mortality. Neurology. 1967;17(5):427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 18.Wernicke JF, Faries D, Girod D, Brown J, Gao H, Kelsey D, et al. Cardiovascular effects of atomoxetine in children, adolescents, and adults. Drug Saf. 2003;26(10):729–740. doi: 10.2165/00002018-200326100-00006. [DOI] [PubMed] [Google Scholar]
- 19.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 20.de Boer AG, Wijker W, Speelman JD, de Haes JC. Quality of life in patients with Parkinson’s disease: development of a questionnaire. Journal of Neurology, Neurosurgery & Psychiatry. 1996;61(1):70–74. doi: 10.1136/jnnp.61.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sammer G, Reuter I, Hullmann K, Kaps M, Vaitl D. Training of executive functions in Parkinson’s disease. J Neurol Sci. 2006;248(12):115–119. doi: 10.1016/j.jns.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 22.Linazasoro G, Lasa A, Van Blercom N. Efficacy and safety of donepezil in the treatment of executive dysfunction in Parkinson disease: a pilot study. Clin Neuropharmacol. 2005;28(4):176–178. doi: 10.1097/01.wnf.0000172498.24770.54. [DOI] [PubMed] [Google Scholar]
- 23.Williams-Gray CH, Foltynie T, Brayne CE, Robbins TW, Barker RA. Evolution of cognitive dysfunction in an incident Parkinson’s disease cohort. Brain. 2007;130(Pt 7):1787–1798. doi: 10.1093/brain/awm111. [DOI] [PubMed] [Google Scholar]
- 24.Sallee FR, Smirnov A. Atomoxetine: novel therapy for attention-deficit/hyperactivity disorder and potential therapeutic implications. Primary Psychiatry. 2003;10(4):41–48. [Google Scholar]
- 25.Tzavara ET, Bymaster FP, Overshiner CD, Davis RJ, Perry KW, Wolff M, et al. Procholinergic and memory enhancing properties of the selective norepinephrine uptake inhibitor atomoxetine. Molecular Psychiatry. 2006;11:187–195. doi: 10.1038/sj.mp.4001763. [DOI] [PubMed] [Google Scholar]
- 26.Robinson ES, Eagle DM, Mar AC, Bari A, Banerjee G, Jiang X, et al. Similar Effects of the Selective Noradrenaline Reuptake Inhibitor Atomoxetine on Three Distinct Forms of Impulsivity in the Rat. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301487. [DOI] [PubMed] [Google Scholar]
- 27.Chamberlain SR, Del Campo N, Dowson J, Muller U, Clark L, Robbins TW, et al. Atomoxetine Improved Response Inhibition in Adults with Attention Deficit/Hyperactivity Disorder. Biol Psychiatry. 2007;62(9):977–84. doi: 10.1016/j.biopsych.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 28.Chamberlain SR, Müller U, Blackwell AD, Clark L, Robbins TW, Sahakian BJ. Neurochemical modulation of response inhibition and probabilistic learning in humans. Science. 2006;311(5762):861–863. doi: 10.1126/science.1121218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spencer T, Biederman J, Wilens T, Prince J, Hatch M, Jones J, et al. Effectiveness and Tolerability of Tomoxetine in Adults with attention deficit hyperactivity disoder. Am J Psychiatry. 1998;155(5):693–695. doi: 10.1176/ajp.155.5.693. [DOI] [PubMed] [Google Scholar]
- 30.Faraone SV, Biederman J, Spencer T, Michelson D, Adler L, Reimherr F, et al. Atomoxetine and stroop task performance in adult attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2005;15(4):664–670. doi: 10.1089/cap.2005.15.664. [DOI] [PubMed] [Google Scholar]
- 31.Riekkinen M, Kejonen K, Jäkälä P, Soininen H, Riekkinen P., Jr Reduction of noradrenaline impairs attention and dopamine depletion slows responses in Parkinson’s disease. European Journal of Neuroscience. 1998;10(4):1429–1439. doi: 10.1046/j.1460-9568.1998.00145.x. [DOI] [PubMed] [Google Scholar]
- 32.Bedard MA, el Massioui F, Malapani C, Dubois B, Pillon B, Renault B, et al. Attentional deficits in Parkinson’s disease: partial reversibility with naphtoxazine (SDZ NV1-085), a selective noradrenergic alpha 1 agonist. Clin Neuropharmacol. 1998;21(2):108–117. [PubMed] [Google Scholar]
- 33.Stern Y, Mayeux R, Côté L. Reaction time and vigilance in Parkinson’s disease. Arch Neurol. 1984;41:1086–1089. doi: 10.1001/archneur.1984.04050210084021. [DOI] [PubMed] [Google Scholar]
- 34.Camicioli R, Lea E, Nutt JG, Sexton G, Oken B. Methylphenidate increases the motor effects of l-dopa in Parkinson’s disease: a pilot study. Clin Neuropharmacol. 2001;24(4):208–213. doi: 10.1097/00002826-200107000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Devos D, Krystkowiak P, Clement F, Dujardin K, Cottencin O, Waucquier N, et al. Improvement of gait by chronic, high doses of methylphenidate in patients with advanced Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2007;78:470–475. doi: 10.1136/jnnp.2006.100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brusa L, Bassi A, Stefani A, Peppe A, Caramia MD, Boffa L, et al. Pramipexole in comparison to l-dopa: a neuropsychological study. J Neural Transm. 2003;(110):373–380. doi: 10.1007/s00702-002-0811-7. [DOI] [PubMed] [Google Scholar]
- 37.Steinberg S, Chouinard G. A case of mania associated with tomoxetine. Am J Psychiatry. 1985;142(12):1517–1518. doi: 10.1176/ajp.142.12.1517. [DOI] [PubMed] [Google Scholar]