Abstract
Schizophrenia patients exhibit deficits in various stages of visual information processing. Despite recent informative efforts to examine visual processing in schizophrenia with functional magnetic resonance imaging (fMRI), much remains unknown about the basic function, structure, and organization of key early visual processing areas in schizophrenia. This study examined magnitude and topography of regional brain activity in three early visual processing areas: early retinotopically organized areas (V1–V4), motion sensitive areas (human area MT, hMT+), and object recognition areas (lateral occipital complex, LO). Using visual stimuli that are known to preferentially activate each respective region, we compared responses in these areas in 22 schizophrenia patients and 19 normal controls. Activity in all three regions was of similar amplitude in schizophrenia patients and normal controls. Activity in retinotopically organized areas and hMT+ showed good spatial overlap between groups. However, activation of LO was more widely distributed in patients compared to normal controls. The findings of abnormal spatial organization of LO in schizophrenia patients may converge with behavioral evidence of deficits in schizophrenia patients for object recognition tasks that are believed to be mediated by LO activity.
Keywords: schizophrenia, visual processing, functional MRI, object processing
1. Introduction
Schizophrenia is associated with dysfunctions in relatively basic aspects of visual processing as reflected in performance on tasks such as visual backward masking, motion coherence detection, and smooth pursuit eye movement. It is important to understand the neural systems underlying deficits in early visual processing in schizophrenia, as a growing body of evidence has shown relationships between these deficits and functional outcome (Butler and Javitt, 2005; Butler et al., 2005; Schechter et al., 2005b; Sergi et al., 2006). The current study aimed to define key visual processing areas in schizophrenia patients and to determine whether these regions are differentially activated or topographically organized compared to healthy individuals.
Among the areas associated with visual processing, we have selected three sets that are important for early and middle levels of visual processing: First, early human visual areas, V1-V4, located in the calcarine sulcus and adjacent cortices (Sereno et al., 1995; DeYoe et al., 1996; Engel et al., 1997). Second, human area MT (hMT+), located bilaterally in the lateral occipital lobe, which is sensitive to motion (Zeki et al., 1991; Tootell et al., 1995). Third, areas of the lateral occipital cortex (LO), responsible for object recognition (Malach et al., 1995; Grill-Spector et al., 2001; Sehatpour et al., 2006). While each of these three regions involves distinct sub-regions, we will consider each region as a single set for the purposes of this study.
Evidence for deficits in early retinotopic regions in schizophrenia patients is mixed, with some studies showing normal activity (e.g., Barch et al., 2003), and others showing abnormal activity (Dakin et al., 2005; Schechter et al., 2005a). Use of different stimuli and tasks in these studies may contribute to these mixed findings.
Deficits among schizophrnenia have also been found in area hMT+, during a smooth pursuit task (Lencer et al., 2005). Behavioral deficits have also been reported for smooth pursuit eye movements (Holzman et al., 1973; Holzman et al., 1974; Levy et al., 1993) and other motion-sensitive tasks (Chen et al., 2004; Chen et al., 2005).
Schizophrenia patients also show deficits in object recognition tasks that activate area LO. An electrophysiological study on object recognition showed that schizophrenia patients demonstrated less activity when viewing a partially seen figure compared to controls, a phenomenon the authors speculate may be due to brain dysfunction in LO in patients (Doniger et al., 2002). Although this study implicated LO, electrophysiology has limited spatial resolution that makes it difficult to know if the activity was LO specifically. No fMRI study to our knowledge has specifically examined activity in LO in schizophrenia patients.
Each of the previous studies that examined key areas of visual processing in schizophrenia using fMRI or electrophysiology examined only one key region. Hence, it has been difficult to assess whether some of the visual processing regions are more or less aberrant than others in schizophrenia. In addition, the previous studies have focused on magnitude, but not topography, of activation. Better specification of visual processing functional activity in key visual regions would guide interpretation of results from other commonly used visual processing tasks (e.g., motion coherence, motion tracking, and backward masking), and allow for a deeper level of understanding to these connections. The aim of this study was to explore differences between schizophrenia patients and normal controls in magnitude and topography of activity in key visual processing areas. We used basic visual tasks that are known to activate key visual processing areas (retinotopically organized areas, hMT+ and LO) and that place minimal reliance on attentional resources. Based on past findings, we expected to see differences in the magnitude and distribution of areas hMT+ and LO, but not in retinotopically organized areas.
2. Methods
2.1 Participants
Twenty-four (5 female) patients with schizophrenia and 19 (5 female) normal control subjects participated in the study. Two patients were excluded from all analyses (one subject had excessive movement artifact due to tardive dyskinesia and the other was excluded due to a scanner malfunction). Subjects were recruited from a larger project on early visual processing (Early Visual Processing in Schizophrenia; PI: Michael F. Green). All subjects had normal or corrected to normal vision. Special lenses were placed within the fMRI goggles which closely approximated a subject’s prescription for those who had corrected vision.
Schizophrenia patients were recruited from outpatient treatment clinics at the Veterans Affairs (VA) Greater Los Angeles Healthcare System and through presentations at local board and care facilities. All patients were administered the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID; First et al., 1997) and met diagnostic criteria for schizophrenia. Patients were medicated, clinically stable at time of testing and exhibited low levels of symptoms (for a more complete characterization of the patient sample, see (Sergi et al., 2006). Patients were between 18 and 60 years of age. Exclusion criteria included: substance abuse or dependence in the last six months, mental retardation, history of loss of consciousness for more than one hour, an identifiable neurological disorder, or not sufficiently fluent in English.
Normal control participants were recruited through flyers posted in the local community and newspaper advertisements in local newspapers. Potential controls were excluded for: any neurological disorder or head injury, schizophrenia or other psychotic disorder in a first-degree relative, not sufficiently fluent in English, history of schizophrenia or other psychotic disorder, bipolar disorder, recurrent depression, history of substance dependence, or any substance abuse in the last 6 months. Normal controls were excluded if they had any of the following Axis II personality disorders: avoidant, borderline, paranoid, schizoid, or schizotypal. Normal controls were between 25 and 55 years of age. Normal control participants were interviewed with the Structured Clinical Interview for DSM-IV (SCID; First et al., 1997) and the Structured Clinical Interview for DSM-IV Axis II Disorders (SCID-II; First et al., 1996). Three controls were diagnosed with a single episode of major depressive disorder, 1 with a history of post-traumatic stress disorder, 3 with a history of alcohol abuse, and 1 with a history of substance abuse. Three patients were diagonsed with a single episode of major depressive disorder, 1 with a history of post-traumatic stress disorder, 1 with a history of alcohol abuse, 4 with a history of alcohol dependence, 3 with a history of substance abuse, and 7 with a history of substance depdendence.
All SCID interviewers were trained to a minimum kappa of 0.75 for key psychotic and mood items through the Treatment Unit of the Department of Veterans Affairs VISN 22 Mental Illness Research, Education, and Clinical Center (MIRECC). All participants were evaluated for the capacity to give informed consent and provided written informed consent after all procedures were fully explained in accordance with Institutional Review Board approval of procedures at UCLA and the VA.
2.2 Procedure
All subjects were administered three localizer scans in a fixed order: LO, retinotopy, motion.
2.3 Localizer Scan Stimuli
2.3.1 Lateral occipital (LO) areas (object processing)
Later-stage object processing in the LO cortex was assessed by contrasting activation to pictures of abstract sculptures with that of pictures of the sculptures randomly scrambled in 10 × 10 blocks (Malach et al., 1995). A fixation cross was displayed in the center of the image for the entire scan. Subjects viewed sculpture images in 12.5 s blocks with alternating scrambled images in 12.5 s blocks, resulting in six blocks per condition (i.e., scrambled vs. unscrambled). Subjects were instructed to press a button whenever the image sequence changed from displaying intact sculptures to scrambled images, and vice versa. Total viewing time was 2.5 minutes.
2.3.2 Retinotopically organized areas
Activity in early visual processing areas was assessed using a rotating contrast reversing checkerboard wedge, that made 5 complete rotations, with a rotation period of 30 s, and contrast reversal rate of 8 Hz. The stimulus subtended 20 deg of visual angle. This checkerboard wedge is known to selectively activate retinotopic brain regions (Sereno et al., 1995; DeYoe et al., 1996; Engel et al., 1997). Subjects fixated on a small black or white square at the center of the display and were instructed to press a button whenever the color of the square changed. Total viewing time was 2.5 minutes.
2.3.3 Motion processing (hMT+) areas (motion processing)
Concentric moving rings were displayed in order to activate hMT+. The spatial frequency of the rings was 0.5 cycles/degree. A fixation cross was displayed in the center of the image for the entire scan. Activity to moving rings was contrasted with that of stationary rings, a method commonly used to activate and identify hMT+ (Tootell et al., 1995). Moving rings were displayed in 15 s blocks with alternating 15 s blocks of stationary rings, resulting in 5 blocks per condition (i.e., moving vs. stationary). Speed and/or direction of moving rings changed randomly every 3 s, with the rings changing direction, changing speed (i.e., moving or stationary), or both. Subjects were instructed to press a button whenever the direction or speed of the rings changed. Total viewing time was 2.5 minutes.
2.4 Functional MRI Acquisition
All scanning was conducted on a 3T Allegra scanner modified for echo-planar imaging (EPI) located in the UCLA Ahmanson Lovelace Brain Mapping Center. For anatomical reference, a high-resolution EPI axial T2-weighted series was obtained for each subject prior to functional scanning (TR = 6000 ms, TE = 54 ms, flip angle 90°, 30 axial slices, FOV 20 cm). A T2-weighted gradient-echo sequence was used to measure blood-oxygen level-dependent (BOLD) signal (TR = 2000ms, TE = 42ms, flip angle=80°, voxel size of 3.125 × 3.125 × 4.00 mm with a 1-mm gap), acquiring 24 slices parallel to the AC-PC plane.
2.5 Functional MRI Analysis
Data were analyzed using FSL (FMRIB Software Library; Smith et al., 2004). The pre-statistics processing included slice-time correction using Fourier-space time-series phase-shifting, motion correction (Jenkinson et al., 2002), non-brain removal (Smith, 2002), spatial smoothing using a Gaussian kernel of FWMH 5mm, high pass temporal filtering (Gaussian weighted LSF straight line fitting with sigma = 50.0s).
To facilitate multisubject analysis, a single common space brain for all subjects, comprising the average of the patients and controls, was defined which approximated the average size, shape, and orientation of each subject’s higher-resolution T2-weighted image (Woods et al., 1999). Based on the parameters created from the higher-resolution image, statistical images created for each subject were normalized into this common space (12-parameter model).
LO was defined as the region with a significant activation for the abstract sculpture vs. the scrambled pictures. Retinotopically organized areas were defined based on the results of scans on which subjects viewed slowly rotating wedges of contrast-reversing checkerboard (Engel et al., 1997). hMT+ was defined as the region with significant preferential activation for moving rings vs. stationary rings. For hMT+, for a minority of subjects (six patients and four controls) the implemented block duration was either longer or shorter from the desired 15 s (approximately +/− 4 s) due to a programming error. To address this problem, we conducted probabilistic independent component analysis (PICA) on all of the hMT+ scans using MELODIC (Multivariate Exploratory Linear Optimised Decomposition into Independent Components), a part of FSL (Beckman and Smith, 2004). PICA allowed us to identify the actual block duration; the largest component returned by the analysis showed temporal variation at the actual block duration. In situations in which the PICA indicated a change in stimulus timing (n = 10) we adjusted the block length accordingly in subsequent analyses to define the ROI.
In defining each ROI, any activated areas outside the inferior temporal lobe and occipital lobe were removed based on previous published findings with similar activation stimuli (e.g., Zeki et al., 1991; Watson et al., 1993; Malach et al., 1995; Tootell et al., 1995). Each scan was thresholded on the basis of the magnitude (Z > 2.3) and extent (cluster significance P < .01) of activation (Forman et al., 1995; Poline et al., 1997) in each individual. To determine areas activated in each group (e.g., group average), we used FLAME (FMRIB’s Local Analysis of Mixed Effects) for each ROI (Beckmann et al., 2003). A threshold of cluster significance of P <0.0025 was used to obtain the group average for each ROI in each group.
ROI data were analyzed in three ways. First, we compared the magnitude of activation (i.e., z statistics) in each ROI between the patients and the controls. To examine group differences in magnitude of activation, we performed a t-test on the mean activation level of voxels (i.e., average z statistics of voxels activated in each ROI) in each ROI. Second, we compared the extent (i.e., volume) of activation in ROIs between the patients and controls. The extent of activity was examined by between-group t-tests on the mean number of voxels activated in each ROI.
Third, we examined the overlap of activity between the patients and control groups in each ROI separately. To do this, we identified the activated voxels separately for each patient. Next we determined the voxels activated by the control group (i.e., control average), and this area was then transferred to each individual patient’s brain. We next calculated an overlap ratio, which was defined as the proportion of activated voxels for each patient that overlapped with the control average. We derived a comparable overlap ratio for the controls, with one notable difference: the control average was defined as the average of all the controls, excluding the particular control subject under examination. This exclusion was done so that the amount of overlap would be determined with a control average that was independent of the particular control subject under consideration.
3. Results
Table 1 shows the demographic data for both groups as well as the symptom ratings for the schizophrenia patients. There were no significant differences in age or education between the two groups. The symptom ratings are comprised of the positive (unusual thought content, hallucinations, conceptual disorganization) and negative (emotional withdrawal, blunted affect, motor retardation) factors of the BPRS (Overall et al., 1967) as well as the total score for the BPRS. Schizophrenia patients exhibited low to mild levels of symptomatology.
Table 1.
Group demographics and symptom ratings for schizophrenia patients
| Mean | SD | |
|---|---|---|
| Normal Controls (14 male/5 female) | ||
| Age | 42.8 | 9.0 |
| Education | 13.2 | 1.4 |
| Schizophrenia Patients (17 male/5 female) | ||
| Age | 38.8 | 10.9 |
| Education | 13.6 | 1.6 |
| Total BPRS Score | 36.3 | 7.0 |
| BPRS Subscales | ||
| Positive Symptoms | 2.2 | 1.0 |
| Negative Symptoms | 1.7 | 0.5 |
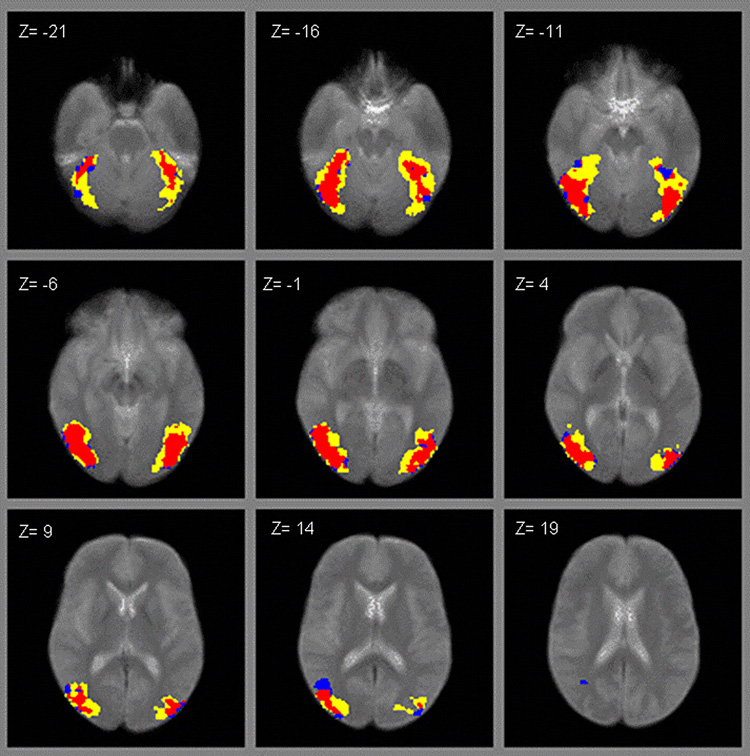
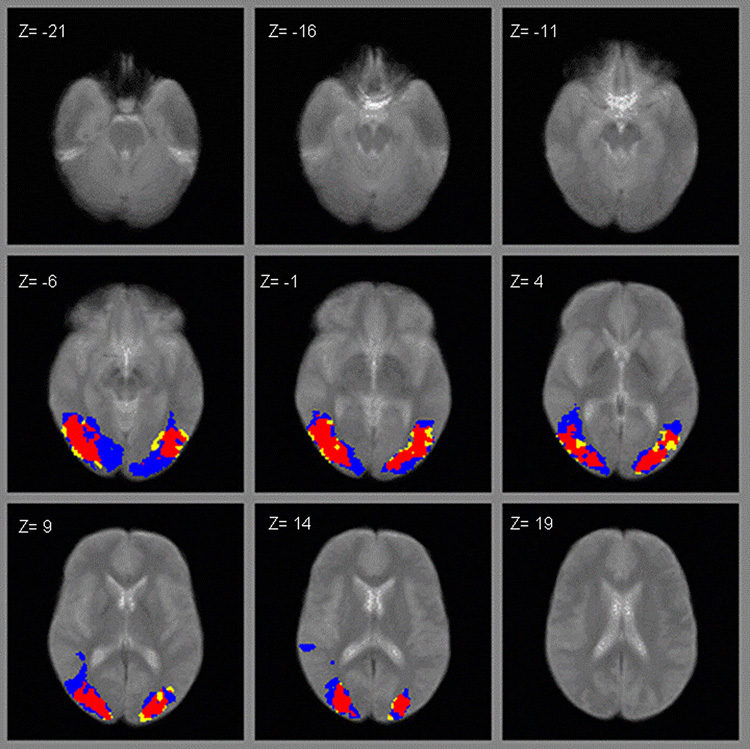
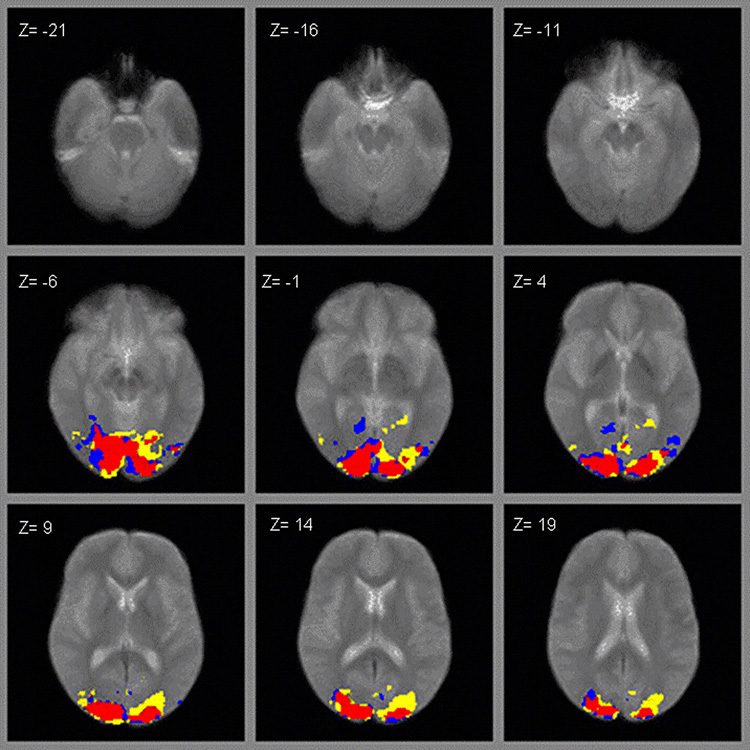
For each activation condition, a small number of subjects failed to produce any supra-threshold activation (3 controls for the LO scan; 2 patients and 1 control for the retinotopy scan; 1 patient and 1 control for hMT+). These subjects were not included in the respective regional analyses. Figure 1–Figure 3 show the regional activation for the LO, retinotopy and hMT+ activation tasks, respectively. In each figure, patients’ activation is denoted by yellow, controls’ by blue, and overlapping activity between both groups in red.
Figure 1.
ROI activation to images of objects vs. scrambled objects (LO scan; yellow = patients, blue = controls, red = overlapping).
Figure 3.
ROI activation to moving concentric rings (hMT+ scan; yellow = patients, blue = controls, red = overlapping).
To evaluate group differences in magnitude of activation, we examined peak pixel activation for each predefined visual processing area. The results showed that schizophrenia patients did not significantly differ from normal controls in the peak pixel activation in any of the predefined visual processing areas to any of the three visual tasks. The mean (standard deviation) of activation (z-statistics) for LO, retinotopy, and hMT+, respectively, were: patients = 3.15 (0.18), controls = 3.12 (0.22); patients = 3.63 (0.37), controls = 3.50 (0.45); patients = 4.97 (0.90), controls = 4.56 (0.88).
As can be seen for data in the LO scan (Figure 1), it appears that patients had a wider distribution of activation bilaterally (denoted by yellow) compared to controls. However, patients and controls had comparable amounts of activation in retinotopical organized areas (Figure 2) and in hMT+ (Figure 3), with patients and controls showing relatively high overlap (areas in red). As suggested by the fMRI images, schizophrenia patients activated a significantly larger area in LO compared to normal controls (Figure 1), with patients activating 309 voxels and controls 185, t (35) = 2.16, P < 0.05. There were no significant differences between groups in the number of voxels activated in retinopic (t (36) = 1.63, P < 0.12) or hMT+ (t (37) = 0.13, P < 0.90). In terms of effect sizes, the difference in LO was largest, with Cohen’s d’ of 0.73, whereas the differences in retinotopy and hMT+ were 0.53 and 0.04 respectively.
Figure 2.
ROI activation to rotating checkerboard (retinotopic scan; yellow = patients, blue = controls, red = overlapping).
We next examined the spatial location of the ROIs by examining the overlap of activity between the patients and controls in each ROI, with the results summarized in Table 2. The table includes: 1) the mean number of activated voxels by group, 2) the mean number overlapping voxels with the control group separately for each ROI, and 3) the mean number of voxels in the control average (this value differs between groups as described above). The final column in the table contains the ratio of overlapping voxels. The patients had a high level of overlapping activation with controls in each activation task, with ratios ranging from 0.48–0.68, comparable to that in the control group (0.50–0.67). These data indicate that the ROIs in patients were fairly consistent in their spatial location and extent of activation compared to the controls.
Table 2.
Number of voxels activated in each task
| Mean # Activated Voxels | Mean # Overlapping Voxels | Mean # Voxels in Control Average | Ratio | |
|---|---|---|---|---|
| Retinotopy | ||||
| Patients mean (SD) | 579 (215)* | 275 (92) | 571 (40) | 0.48 (0.10) |
| Controls mean (SD) | 465 (216) | 230 (91) | 557 (90) | 0.50 (0.15) |
| hMT+ | ||||
| Patients mean (SD) | 440 (258)† | 297 (161) | 954 (71) | 0.68 (0.15) |
| Controls mean (SD) | 452 (281) | 305 (157) | 941 (115) | 0.67 (0.12) |
| LO | ||||
| Patients mean (SD) | 309 (190)‡ | 163 (90) | 738 (52) | 0.53 (0.12) |
| Controls mean (SD) | 186 (143) | 104 (70) | 681 (102) | 0.56 (0.16) |
Differences between groups in mean number of activated voxles: P < 0.12
P < 0.90
P < 0.05
4. Discussion
The results of this study showed that schizophrenia patients and controls do not differ in the magnitude of activation, or in their degree of overlap, in LO, retinotopically organized areas, or hMT+ when processing basic visual stimuli. However, the topography for LO was more wide-spread in patients compared to controls, while the topography in retinotopically organized areas and hMT+ were comparable between groups. The finding of wide-spread topography in LO was unexpected, and the results should be interpreted cautiously until the effect is replicated in further studies.
The relative broad topography of LO in schizophrenia patients could possibly be explained by less specialization of cortex or compensatory activation within LO. It may be possible that neurons in LO are are less specialized, so more of them respond to any given localizer stimulus (but not at a high level, which is why we did not see amplitude differences). Alternatively, responding neurons are just as specilaized, but not as segregated spatially, so that the same number are responding, but they are spread out more. Future studies will need to be conducted to fully explore these possibilities.
Another possible explanation for the broad topography in LO in schizophrenia patients may be our use of spatial smoothing. Spatial smoothing may result in an artifically high volume of activation in areas of high magnitude of activation. However, given that hemodynamic blurring of magnitude and volume are never truly independent and we used a relatively small spatial smoothing filter (only 5 mm FWHM), we do not believe that these results are do to our spatial smoothing.
The finding of no significant differences in activation in retinotopically organized areas is fairly consistent with the literature (Barch et al., 2002; Braus et al., 2002), though some studies have found functional deficits in retinotopically organized areas (e.g., (Dakin et al., 2005; Schechter et al., 2005a). The differences may be due to the differences in stimuli and task used in our study vs. other studies. Furthermore, other studies have found structural deficits in retinotopic regions. For example, Dorph-Petersen et al. (2007) in a post-morten study found reduced volume and neuron number in primary visual cortex in schizophrenia patients. Butler et al. (2006), using diffusion tensor imaging, found reduced white matter integrity in optic radiations. While our study found normal activation in primary visual areas we cannot conclude that structural deficits do not exist. It is possible that structural deficits result in abnormal functioning, though structural deficits were not assessed in the present study.
The lack of activation magnitude differences in hMT+ was contrary to our expectations, as reports in the functional imaging literature (Lencer et al., 2005) as well as psychophysical literature (e.g., Chen et al., 2004; Chen et al., 2005) implicate hMT+ deficits in schizophrenia. The use of a simple moving ring pattern with little attentional demand in our study may explain this discrepancy. The use of more demanding tasks that engage attentional resources to a greater degree may better detect patient-control differences (e.g., Curtis et al., 1999; Ross et al., 2000).
The finding of broader topographical organization in LO in schizophrenia patients may help explain their poorer performance on tasks that involve object recognition, such as visual backward masking tasks. We have shown in normal controls that area LO may be a key region mediating backward masking (Green et al., 2005) as LO was more sensitive to longer intervals between the target and mask (i.e., weaker masking). If activity in LO is diffuse and not well-organized in schizophrenia, schizophrenia patients may require longer intervals between the target and mask, compared to normal controls, to fully process the target. Alternatively, neurons in LO may be less selective and respond equally to both the target and the mask, thus resulting in increased masking effects. We are currently examining activity in LO during a backward masking task in schizophrenia patients which might be able to futher elucidate the impact of diffuse or less-specialize neurons in LO on object recognition tasks.
The goal of this study was to define key regions of interest (retinotopically organized areas, hMT+ and LO) in early and middle stages of visual processing in schizophrenia to basic visual stimuli and explore regional brain activation. No previous study to our knowledge examined the functional integrity of the all three visual processing areas in schizophrenia patients. This study provides evidence that two key regions, retinotopically organized areas and hMT+, have normal magnitude of activation and topography in schizophrenia, at least with the basic visual stimuli used in the current study. The finding of diffuse topography in area LO in schizophrenia patients is convergent with poorer performance on specialized object recognition tasks. These findings can help guide fMRI studies in visual processing in schizophrenia.
Acknowledgements
Support for this study came from NIMH Grant MH43292 (PI: Michael F. Green, Ph.D) and NIMH Grant MH065707 (PI: Michael F. Green, Ph.D.). For generous support, we also thank the Brain Mapping Medical Research Organization, Brain Mapping Support Foundation, Pierson-Lovelace Foundation, The Ahmanson Foundation, William M. and Linda R. Dietel Philanthropic Fund at the Northern Piedmont Community Foundation, Tamkin Foundation, Jennifer Jones-Simon Foundation, Capital Group Companies Charitable Foundation, Robson Family, and Northstar Fund. The authors wish to thank Poorang Nori and Alisa Malin for assistance in data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barch DM, Mathews JR, Buckner RL, Maccotta L, Csernansky JG, Snyder AZ. Hemodynamic responses in visual, motor, and somatosensory cortices in schizophrenia. NeuroImage. 2003;20:1884–1893. doi: 10.1016/s1053-8119(03)00449-x. [DOI] [PubMed] [Google Scholar]
- Beckman CF, Smith SM. Probabilistic independent component analysis for function magnetic resonance imaging. IEEE Transactions on Medical Imaging. 2004;23:137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. Neuroimage. 2003;20:1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Braus DF, Weber-Fahr W, Tost H, Ruf M, Henn FA. Sensory information processing in neuroleptic-naive first-episde schizophrenic patients. Archives of General Psychiatry. 2002;59:696–701. doi: 10.1001/archpsyc.59.8.696. [DOI] [PubMed] [Google Scholar]
- Butler PD, Hoptman MJ, Nierenberg J, Foxe JJ, Javitt DC, Lim KO. Visual white matter integrity in schizophrenia. American Journal of Psychiatry. 2006;163:2011–2013. doi: 10.1176/appi.ajp.163.11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler PD, Javitt DC. Early-stage visual processing deficits in schizophrenia. Current Opinion in Psychiatry. 2005;18:151–157. doi: 10.1097/00001504-200503000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler PD, Zemon V, Schechter I, Saperstein AM, Hoptman MJ, Lim KO, Revheim N, Silipo G, Javitt DC. Early-stage visual processing and cortical amplification deficits in schizophrenia. Archives of General Psychiatry. 2005;62:495–504. doi: 10.1001/archpsyc.62.5.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Bidwell LC, Holzman PS. Visual motion integration in schizophrenia patients, their first-degree relatives, and patients with bipolar disorder. Schizophrenia Research. 2005;74:271–281. doi: 10.1016/j.schres.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Chen Y, Levy DL, Sheremata S, Holzman PS. Compromised late-stage motion processing in schizophrneia. Biological Psychiatry. 2004;55:834–841. doi: 10.1016/j.biopsych.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Curtis VA, Bullmore ET, Morris RG, Brammer MJ, Williams SC, Sharma T, Murray RM, McGuire PK. Attenuated frontal activation in schizophrenia may be task dependent. Schizophrenia Research. 1999;37:35–44. doi: 10.1016/s0920-9964(98)00141-8. [DOI] [PubMed] [Google Scholar]
- Dakin S, Carlin P, Hemsley D. Weak suppression of visual context in chronic schizophrenia. Current Biology. 2005;15:822–824. doi: 10.1016/j.cub.2005.10.015. [DOI] [PubMed] [Google Scholar]
- DeYoe EA, Carman GJ, Bandettini P, Glickman S, Wieser J, Cox R, Miller D, Neitz J. Mapping striate and extrastriate visual areas in human cerebral cortex. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:2382–2386. doi: 10.1073/pnas.93.6.2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doniger GM, Foxe JJ, Murray MM, Higgins BA, Javitt DC. Impaired visual object recognition and dorsal/ventral stream interaction in schizophrenia. Archives of General Psychiatry. 2002;59:1011–1020. doi: 10.1001/archpsyc.59.11.1011. [DOI] [PubMed] [Google Scholar]
- Dorph-Petersen K-A, Pierri JP, Wu Q, Sampson AR, Lewis DA. Primary visual cortex volume and total neuron number are reduced in schizophrenia. The Journal of Comparative Neurology. 2007;501:290–301. doi: 10.1002/cne.21243. [DOI] [PubMed] [Google Scholar]
- Engel SA, Glover GH, Wandell BA. Retinotopic organization in human visual cortex and the spatial precision of functional MRI. Cerebral Cortex. 1997;7:181–192. doi: 10.1093/cercor/7.2.181. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbons M, Spitzer RL, Williams JBW. Structured Clinical Interview for DSM-IV Axis II Personality Disorders. Biometrics Research Department. 1996 [Google Scholar]
- First MB, Spitzer RL, Gibbons M, Williams JBW. The Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition. Biometrics Research. 1997 [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI); Use of a cluster-sized threshold. Magnetic Resonance in Medicine. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Green MF, Glahn D, Engel SA, Nuechterlein KH, Sabb F, Strojwas M, Cohen MS. Regional brain activity associated with visual backward masking. J Cogn Neurosci. 2005;17:13–23. doi: 10.1162/0898929052880011. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Kourtzi Z, Kanwisher N. The lateral occipital complex and its role in object recogniton. Vision Research. 2001;41:1409–1422. doi: 10.1016/s0042-6989(01)00073-6. [DOI] [PubMed] [Google Scholar]
- Holzman PS, Proctor LR, Hughes DW. Eye-tracking patterns in schizophrenia. Science. 1973;181:179–181. doi: 10.1126/science.181.4095.179. [DOI] [PubMed] [Google Scholar]
- Holzman PS, Proctor LR, Levy DL, Yasillo NJ, Meltzer HY, Hurt SW. Eye-tracking dysfunctions in schizophrenic patients and their relatives. Archives of General Psychiatry. 1974;31:143–151. doi: 10.1001/archpsyc.1974.01760140005001. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister PR, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Lencer R, Nagel M, Sprenger A, Heide W, Binkofski F. Reduced neuronal activity in the V5 complex underlies smooth-pursuit deficit in schizophrenia: Evidence from an fMRI study. NeuroImage. 2005;24:1256–1259. doi: 10.1016/j.neuroimage.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Levy DL, Holzman PS, Matthysse S, Mendell NR. Eye tracking dysfunction and schizophrenia: A critical perspective. Schizophrenia Bulletin. 1993;19:461–536. doi: 10.1093/schbul/19.3.461. [DOI] [PubMed] [Google Scholar]
- Malach R, Reppas JB, Benson RR, Kwong KK, Jiang H, Kennedy WA, Ledden PJ, Brady TJ, Rosen BR, Tootell RB. Object-related activity revealed by functional magnetic resonance imaging in human occipital cortex. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:8135–8139. doi: 10.1073/pnas.92.18.8135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall JE, Hollister LE, Pichot P. Major psychiatric disorders: A four-dimensional model. Archives of General Psychiatry. 1967;16:146–151. doi: 10.1001/archpsyc.1967.01730200014003. [DOI] [PubMed] [Google Scholar]
- Poline JB, Worsley KJ, Evans AC, Friston KJ. Combining spatial extent and peak intensity to test for activations in functional imaging. NeuroImage. 1997;5:83–96. doi: 10.1006/nimg.1996.0248. [DOI] [PubMed] [Google Scholar]
- Ross RG, Olincy A, Harris JG, Sullivan B, Radant A. Smooth pursuit eye movements in schizophrenia and attentional dysfunction: Adults with schizophrenia, ADHD, and a normal comparison group. Biological Psychiatry. 2000;48:197–203. doi: 10.1016/s0006-3223(00)00825-8. [DOI] [PubMed] [Google Scholar]
- Schechter I, Butler PD, Zemon V, Revheim N, Saperstein AM, Jalbrzikowski M, Pasternak R, Silipo G, Javitt DC. Impairments of early-stage transient visual evoked potentials to magno- and parvocellular-selective stimuli in schizophrenia. Clinical Neurophysiology. 2005a;116:2204–2215. doi: 10.1016/j.clinph.2005.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter I, Butler PD, Zemon VM, Revheim N, Saperstein AM, Jalbrzikowski M, Pasternak R, Silipo G, Javitt DC. Impairments in generation of early-stage transient visual evoked potentials to magno- and parvocellular-selective stimuli in schizophrenia. Clinical Neurophysiology. 2005b;116:2204–2215. doi: 10.1016/j.clinph.2005.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehatpour P, Molholm S, Javitt DC, Foxe JJ. Spatiotemporal dynamics of human object recognition processing: An integrated high-density electrical mapping and functional imaging study of "closure" processes. NeuroImage. 2006;29:605–618. doi: 10.1016/j.neuroimage.2005.07.049. [DOI] [PubMed] [Google Scholar]
- Sereno M, Dale A, Reppas J, Kwong K, Belliveau J, Brady T, Rosen B, Tootell R. Borders of multiple visual areas in humans revealed by functional magnetic resonance imaging. Science. 1995;268:889–893. doi: 10.1126/science.7754376. [DOI] [PubMed] [Google Scholar]
- Sergi MJ, Rassovsky Y, Nuechterlein KH, Green MF. Social perception as a mediator of the influence of early visual processing on functional status in schizophrenia. American Journal of Psychiatry. 2006;163:356–358. doi: 10.1176/appi.ajp.163.3.448. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckman CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Tootell RBH, Reppas JB, Kwong KK, Malach R, Born RT, Brady TJ, Rosen BR, Belliveau JW. Functional analysis of human MT and related visual cortical areas using magnetic resonance imaging. Journal of Neuroscience. 1995;15:3215–3230. doi: 10.1523/JNEUROSCI.15-04-03215.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson JD, Myers R, Frackowiak RS, Hajnal JV, Woods RP, Mazziotta JC, Shipp S, Zeki S. Area V5 of the human brain: Evidence from a combined study using positron emission tomography and magnetic resonance imaging. Cerebral Cortex. 1993;3:79–94. doi: 10.1093/cercor/3.2.79. [DOI] [PubMed] [Google Scholar]
- Woods RP, Dapretto M, Sicotte NL, Toga AW, Mazziotta JC. Creation and use of a Talaraich-compatible atlas for accurate, automated, nonlinear intersubject registration, and analysis of functional imaging data. Human Brain Mapping. 1999;8:73–79. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<73::AID-HBM1>3.0.CO;2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki S, Watson JDG, Lueck CJ, Friston KJ, Kennard C, Frackowiak RSJ. A direct demonstration of functional specialization in human visual cortex. Journal of Neuroscience. 1991;11:641–649. doi: 10.1523/JNEUROSCI.11-03-00641.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]