Abstract
The human liver fluke Opisthorchis viverrini is endemic in Thailand, Laos and Cambodia where long standing infection is associated with cancer of the bile ducts, cholangiocarcinoma. Here we describe a cathepsin D-like aspartic protease from the gut and other tissues in O. viverrini. Phylogenetic analysis indicated that Ov-APR-1 is cathepsin D-like, conforming with Clan AA, Family A1 of the MEROPS classification. Ov-APR-1 is expressed in the gut of the mature hermaphroditic parasite, in the reproductive tissues including the testis and immature spermatids, and the developing miracidium within the eggshell. The enzyme was also detected in the excretory/secretory products of cultured adult flukes, indicating a role in host-parasite relationships. A recombinant form of the enzyme expressed in Escherichia coli and refolded from denatured inclusion bodies underwent autocatalytic activation and demonstrated hydrolytic activity against the peptide substrate 7-methoxycoumarin-4-acetyl-GKPILFFRLK(DNP)-D-Arg-amide with a kcat/Km = 1.7 × 104 M−1s−1 and a pH optimum around pH 2.5–3.0. The recombinant enzyme digested hemoglobin and bovine serum albumin. Forty-six serum albumin peptides were detected after digestion with recombinant Ov-APR-1 and sequenced. Like many other aspartic proteases, Ov-APR-1 displayed promiscuous preferences for residues accommodated at the key subsites of the binding pocket although hydrophobic (Leu, Ala, Ile), positively charged (Lys) and bulky aromatic (Phe) residues, in that order, were preferred at P1. Similar residues were accommodated at P1′ although even less selectivity was exerted at this position.
Keywords: liver fluke, cholangiocarcinoma, aspartic protease, cathepsin D, Opisthorchis
Introduction
The liver fluke, Opisthorchis viverrini, infects more than 6 million people in Thailand alone and the infection has spread into surrounding countries in South-East Asia including Laos and Vietnam (Sripa et al., 2007). People become infected by ingesting raw or fermented fish which form a staple of the diet in rural areas where poor sanitation practices and inadequate sewerage infrastructures prevail. Adult worms reside in the bile ducts, and sequelae of chronic infection can include the hepatobiliary abnormalities cholangitis, hepatomegaly and periportal fibrosis. The most insidious complication of chronic opisthorchiasis, however, is cholangiocarcinoma (CCA), cancer of the bile ducts (Sripa et al., 2007).
The mechanisms by which this liver fluke causes cancer are unknown, but may be multi-factorial. One mechanism by which biliary epithelial cells are thought to become neoplastic is due to excessive and unchecked proliferation stimulated by mitogenic proteins secreted by adult parasites in the biliary tree (Thuwajit et al., 2004; Suttiprapa et al., 2008). To gain a better understanding of the host-parasite interactions underlying molecular pathogenesis of opisthorchiasis, we undertook a shotgun approach to gene discovery for O. viverrini and identified numerous secreted proteins with known roles in other host-parasite systems, including proteases (Laha et al., 2007). Liver fluke proteases, particularly those involved in the digestion of host tissues, are of interest for several reasons including (1) their functional characterization sheds light on their roles in molecular pathogenesis and (2) digestive proteases from other parasitic helminths are efficacious vaccines and drug targets. Indeed, one of the most efficacious vaccine antigens described thus far from the liver fluke of sheep and cattle, Fasciola hepatica, is a cysteine protease expressed in the intestine of the adult fluke (Dalton et al., 2003). Moreover, vaccination of rats with plasmid DNA encoding a cysteine protease from Clonorchis sinensis, a close relative of O. viverrini that infects 30 million people throughout East Asia (Lun et al., 2005), resulted in partial protection against challenge infection (Lee et al., 2006). Cysteine protease inhibitors have shown promise as drugs for fasciolosis (Alcala-Canto et al., 2007) and other parasitic flukes (Abdulla et al., 2007), further highlighting the essential roles these enzymes play in parasite survival.
Almost all of the literature describing proteases from the human liver flukes deals with the C1, and to a lesser extent the C13, families of clan CA cysteine proteases. Extensive investigations have been conducted on the phylogeny (Tort et al., 1999; Robinson et al., 2008), structural biology/enzymology (Stack et al., 2008) and vaccinology (Dalton et al., 2003; Hillyer, 2005; McManus and Dalton, 2006) of clan CA proteases from F. hepatica, but substantially less is known about this and other protease families from the human liver flukes (Kang et al., 2004; Kaewpitoon et al., 2008; Laha et al., 2008; Na et al., 2008).
Aspartic proteases belonging to clan AA are less well represented than the cysteine proteases in terms of gene diversity and abundance in parasitic helminths. Their roles have been relatively well characterized in the blood-feeding nematodes (Williamson et al., 2003; Williamson et al., 2004) and trematodes (the schistosomes) (Brindley et al., 2001; Caffrey et al., 2004; Delcroix et al., 2006; Morales et al., 2008), but to our knowledge, there are no reports of aspartic proteases from any species of liver fluke. Here we describe the cloning of a clan AA, family A1 aspartic protease from O. viverrini, its functional expression in E. coli, immunolocalization and detailed characterization of its catalytic activity against blood proteins and its substrate subsite preferences.
Materials and Methods
Parasites
O. viverrini metacercariae were obtained from naturally infected cyprinoid fish caught by commercial fishermen in fresh water reservoirs in the endemic area of Khon Kaen province, Thailand. The fish were digested by pepsin-HCl. After several washes with normal saline, metacercariae were collected, identified under a dissecting microscope, and viable metacercariae were used to infect hamsters (Mesocricetus auratas) by stomach intubation. The hamsters were maintained at the animal research facility of the Khon Kaen University Faculty of Medicine; protocols approved by the Khon Kaen University Animal Ethics Committee were used for all vertebrate animal research in this study. The hamsters were euthanized 2–3 months after infection, necropsied, and adult O. viverrini flukes recovered from the bile ducts. Subsequently, adult O. viverrini were cultured to collect eggs and excretory-secretory (ES) products as described (Suttiprapa et al., 2008). Egg, metacercariae and adult worm extracts were prepared as described (Sripa and Kaewkes, 2000). Protein concentrations of O. viverrini extracts were determined by the Bradford protein assay (Bio-Rad, Hercules, CA, USA) after which parasite extracts were stored at −80°C until required.
RNA extraction and RT-PCR
Total RNA from each stage of O. viverrini was extracted with TRIzol (Invitrogen) according to the manufacturer’s instructions. Contaminating genomic DNA was removed by treatment of RNA with DNase I (Promega). For reverse transcription-PCR (RT-PCR), first-strand cDNA was produced with an oligo (dT) primer from 1.0 μg of total RNA using avian myeloblastosis virus reverse transcriptase (Promega) at 42°C for 60 min. A 1.0 μl aliquot of the resultant cDNA was amplified using primers specific for β-actin (Fwd - 5′-CGAGGTATCCTCACCCTCAA-3′; Rev - 5′-GCGACTCGCAACTCATTGTA-3′) and Ov-apr-1 (Fwd - 5′-CATAGGAACACCGCCTCAGT -3′; Rev - 5′-GTGATGCAGCCAACAAGCTA-3′) based on the following conditions: 30 sec denaturation at 94°C, 30 sec annealing at 55°C, and 30 sec extension at 72°C for 30 cycles. Control RT-PCR reactions were performed without reverse transcriptase to ensure that amplified products were derived from cDNA and not contaminating genomic DNA. PCR products were sized by electrophoresis through 1% agarose and visualized under UV light after staining with ethidium bromide.
Amplification of Ov-apr-1 and sequence analysis
A cDNA encoding a putative family A1 aspartic protease from C. sinensis was identified in GenBank (accession number AF420068). A series of oligonucleotides corresponding to this cDNA sequence were synthesized and used to amplify an orthologous cDNA fragment from an O. viverrini adult worm cDNA library (Laha et al., 2007). The forward primer (Ov-apr-f1: 5′ - CGGATTCAAAAATGTGAGACGC - 3′) spanned nt 88–109 of C. sinensis AF420068, and generated a band of the expected size when coupled with two different reverse primers, Ov-apr-r4 (5′ - TTGCCAAACCAGGTCATTCG - 3′) spanning nt 998–1017 and Ov-apr-r2 (5′ - TCCCGCTTATGCTGAACTGGAC - 3′) spanning nt 941–962. Full length 5′ and 3′ends were obtained for Ov-apr-1 by 5′ and 3′ rapid amplification of cDNA ends (RACE) using a Generacer kit (Invitrogen). Sequences were edited and analyzed with assistance from the MacVector software package. Homology searches were performed using Blast search at NCBI (http://www.ncbi.nlm.nih.go/Blast/). ORFs were analyzed for signal peptides/anchors using SignalP-NN prediction and SignalP-HMM prediction at http://www.cbs.dtu.dk/services/SignalP/.
Phylogenetic analysis
The phylogenetic relationship of Ov-APR-1 (GenBank accession no. AF420068) with other members of the A1 family was inferred using PHYLIP (version 3.67) (Felsenstein, 1989). Bootstrap values were obtained with the SEQBOOT program (1000 data sets were generated) and distance matrices were established with the PRODIST program (Kimura formula; analysis of 1000 data sets). Where bootstrap values were below 50%, clades were collapsed to form polytomies. Neighbor-joining analysis was carried out with the NEIGHBOR program (Neighbor-Joining method; analysis of 1000 data sets). The phylogenetic tree was drawn with TreeView software (version 1.6.6) (Page, 1996).
Expression, purification and refolding of Ov-APR-1
The Ov-apr-1 coding sequence (without the predicted signal peptide) was inferred using primers, APR41PF (5′- GCGCGCCATATGAGCGTGATTCGGATTCCT - 3′) and APR41PR (5′- GCGCGCCTCGAGCTGTCCGACTCCGAGCAA - 3′). The amplicon was sequenced to confirm its identity and ligated into the expression vector pET-41a(+) (Novagen). E. coli BL21 (DE3) cells were transformed with the ligation products and recombinant clones were obtained used antibiotic selection of the transformed E. coli. Expression of recombinant protein was induced by addition of isopropyl β-D-1-thiogalactopyranoside (IPTG) to 1 mM. Target protein expression was monitored by SDS-PAGE followed by staining with Coomassie Brilliant Blue. The recombinant protein, Ov-APR-1, was located in inclusion bodies, dissolved in 8 M urea, and affinity purified under denaturing conditions on Ni-NTA resin (QIAGEN). The denatured Ov-APR-1 was refolded in 50 mM Tris pH 8.0 containing 240 mM NaCl, 2 mM KCl, 2 mM MgCl2, 2 mM CaCl2 and 0.5 M L-arginine, and dialyzed into PBS. Yields of refolded Ov-APR-1 were quantified using the Bradford assay and recombinant Ov-APR-1 was stored at −80°C.
Auto-activation and protease assays
One microgram (1 μg/μl) of refolded Ov-APR-1 was added to 20 μl of 50 mM sodium acetate at 0.5 pH unit increments from pH 2.0 – 5.0 at 37°C for 18 h. Activation of the enzyme was monitored by SDS-PAGE and hydrolysis of the diagnostic fluorescent peptide, 7-Methoxycoumarin-4-Acetyl-GKPILF↓FRLK(DNP)-D-Arg-Amide (MoCAc-GKPILFFRLK; Sigma) (Yasuda et al., 1999).
Enzymatic activity of Ov-APR-1
Hydrolysis of MoCAc-GKPILFFRLK by Ov-APR-1 was monitored using a Fluostar Optima microplate reader (BMG Labtech). Rates of hydrolysis were recorded by monitoring the increase in fluorescence measured in arbitrary units (relative fluorescence units – rfu) at excitation and emission wavelengths of 330 nm and 390 nm, respectively. The pH optimum for enzyme activity was determined by assaying in 50 mM sodium acetate at half-unit pH increments from pH 2–6 at 37°C. The final substrate concentration was 1.0 μM and the final volume of each reaction was 100 μl. Enzyme (2 nM) efficiency was assessed at pH 2.5 by measuring initial rates over a range of substrate concentrations (0.2–25 μM). The catalytic constants kcat, Km and kcat/Km were established from the resulting Michaelis-Menten plot.
Digestion of blood proteins by Ov-APR-1
The ability of recombinant Ov-APR-1 to digest human hemoglobin (Hb) and bovine serum albumin (BSA) was assessed. Hb was prepared by lysing human red blood cells (which had been washed three times to remove the plasma). Cell debris was removed by centrifugation at 14,000 g for 30 min at 4°C and the supernatant was used as Hb. One hundred micrograms of Hb was incubated with 1.0 μg of refolded Ov-APR-1 in 200 μl of 50 mM sodium acetate pH 2.0–5.0 at 37°C for up to 18 h. Hydrolysis of Hb was assayed by SDS-PAGE and visual analysis of the gels. For digestion of BSA, 10 μg of BSA was incubated with 1.0 μg of refolded Ov-APR-1 in 20 μl of 50 mM sodium acetate pH 2.0–5.0 at 37°C for up to 18 h. Digestion of BSA was monitored by SDS-PAGE and liquid chromatography tandem mass spectrometry (LC-MS/MS) as described below.
LC-MS and MS/MS analysis of BSA hydrolysates
After incubation with Ov-APR-1, BSA hydrolysates were fractionated by RP-HPLC employing a Vydac column, monomeric C18 300 Å 3 μm150 μm x 150 mm, at a flow rate of 1.2 μl/min. Subsequently, eluted BSA-derived peptides were identified by LC-MS/MS. LC-MS/MS analysis was performed using an Ultimate 3000 nanoLC system (Dionex) with a CAP-LC flow splitter and a variable wavelength UV-VIS detector scanning at 214 nm coupled to a quadrupole time-of-flight mass spectrometer (MicrOTOFq, Bruker) operated with a low flow electrospray needle. For the RP-HPLC, the mobile phase buffers used for the gradient were (A) water with 0.1% formic acid and (B) acetonitrile:water (4:1) with 0.1% formic acid; the gradient program consisted of 5% B for 5 minutes, linear ramping to 55% B over 29 min, linear ramping to 90% B over 1 min, holding at 90% B for 9 min, ramping back to 5% B over 1 min, and holding at 5% B for 20 min. The mass spectrometer scanned 50–3000 m/z and acquired data for 50 mins of each analysis. Data acquisition was facilitated using Hystar (Bruker) and data were processed using Data Analysis (Bruker). The mass spectrometer used an autoMSn methodology that collected MS2 spectra for the two most intense ions in each full scan spectrum. The scan time was 0.5 seconds for the Survey Scan and the MS2 spectra were recorded were the result of 2 microscans, giving an overall duty cycle of 2.5 seconds. In addition, dynamic exclusion was used such that after 2 MS2 spectra, the precursor would be added to an exclusion list for 1 min. This allowed the collection of the maximum number of MS2 spectra during the analysis. Calibration was performed immediately prior to the analysis. The data were prepared into a format suitable for mascot database searching using Data Analysis, and Biotools (Bruker) was used to store and further scrutinize the data and search results. Mascot searches were performed using Swiss-Prot database and a 20 ppm tolerance on the precursor, 0.2 Da tolerance on the product ions, methionine oxidation as a variable modification, and charge states 1, 2 and 3. Searches were performed using the “no enzyme” setting so that novel protease cleavage sites for the fluke protease could be determined. Data were entirely dependent on mascot ID and an individual ion score of >30 was used as a threshold to elucidate false IDs. The relative entropy between the observed and background distributions of each amino acid at the P4-P4′ subsites for each enzyme was calculated using the WebLogo program (Crooks et al., 2004).
Preparation of anti-Ov-APR-1 antibody
Five mice were immunized subcutaneously with purified Ov-APR-1 (25 μg per immunization). The first immunization was carried out with recombinant protein formulated with Freund’s complete adjuvant; the second and third immunizations were carried out with recombinant protein formulated with Freund’s incomplete adjuvant. Immunizations were conducted on days 1, 15 and 29. Blood was collected two weeks after the third immunization and sera were separated.
Western blot analysis
Soluble extracts from O. viverrini eggs, metacercariae, adult worms, and ES products were prepared as described (Laha et al., 2008). Ten micrograms of each extract and 1.0 μg of recombinant Ov-APR-1 were separated by SDS-PAGE and transferred to nitrocellulose membrane. The membrane was blocked with 5% skimmed milk in PBS/0.05% Tween-20 (PBS-T) for 2 h. The membrane was incubated in pooled mouse anti-Ov-APR-1 serum diluted 1:500 (v/v) in 2% skimmed milk in PBS-T for 2 h at room temperature. After washing 3×5 mins with PBS-T, the membrane was incubated in horseradish peroxidase (HRP)-conjugated goat anti-mouse antibody (Zymed Laboratories) for 1 h. The membrane was washed again 3×5 mins with PBS-T followed by a final 10 min rinse with PBS. Reactive bands were visualized with a chemiluminescence detection procedure (ECL Plus™, GE Healthcare) following the manufacturer’s instructions.
Immunohistochemistry
O. viverrini adult worms or liver tissue from hamsters infected with O. viverrini (weeks 1–24) were fixed and cut into sections of 4 μm with a microtome as described (Sripa and Kaewkes, 2000). The sections were deparaffinized in xylene, hydrated in a series of ethanol and distilled water, respectively. The endogenous peroxidase was eliminated by treating sectioned tissues with absolute methanol containing 5% H2O2 for 30 min. The sections were then washed in water and PBS. Non-specific staining was blocked by treating slides with 5% normal mouse serum in PBS for 30 min. The thin sections were probed with pooled mouse anti-Ov-APR-1 serum diluted 1:1000 (v/v) in PBS was applied to the sections and incubated for 2 h at room temperature. After rinsing 3×5 mins with PBS the sections were incubated with HRP-conjugated goat anti-mouse IgG for 1 h. Sections were rinsed with PBS 2×10 mins, after which the immunolocalization was developed with diaminobenzidine (DAB). The sections were counterstained with Mayer’s hematoxylin, dehydrated, cleared in xylene, mounted in Permount®. The sections were examined by light microscopy and images captured with a digital camera.
Results
An aspartic protease from O. viverrini
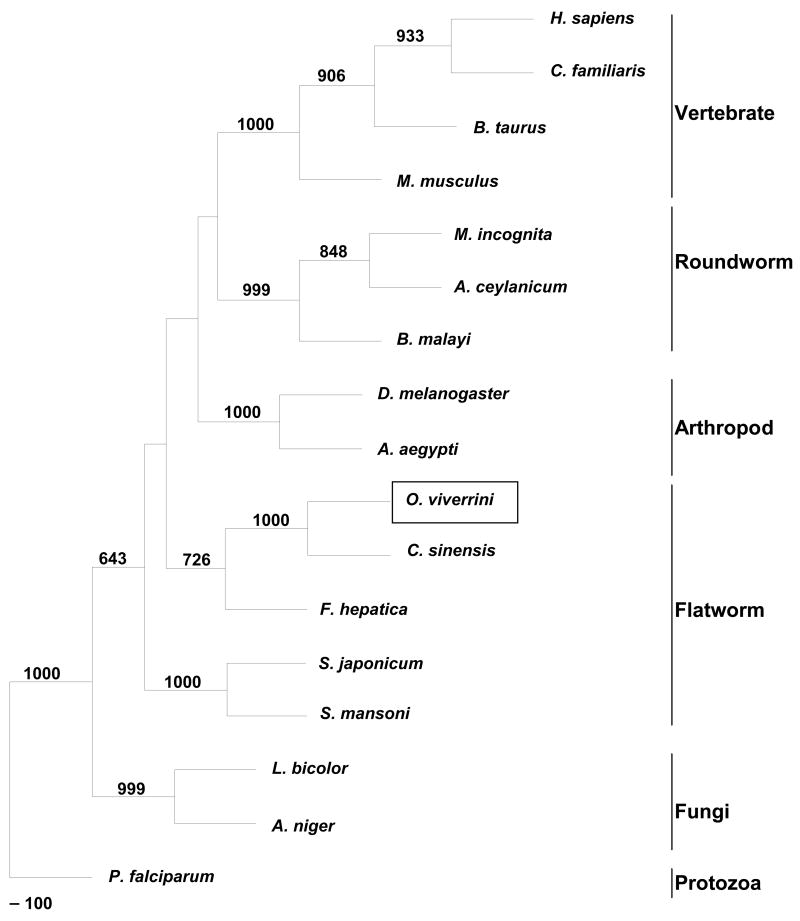
The cDNA sequence was 1,562 nt in length and encoded a pre-pro-enzyme of 425 amino acids. A signal peptide was present with a cleavage site between Cys-17 and Ser-18 was predicted. Five potential N-glycosylation sites were apparent, two in the pro-region at Asn-45 and -54, and three in the mature protease at Asn-124, -155 and -215. The Ov-apr-1 cDNA sequence has been assigned GenBank accession number DQ131585. The ORF shared 83% identity with the cathepsin D-like sequence from C. sinensis (AF420068) and 56% identity with S. mansoni cathepsin D (U60995) (Fig. S1). Phylogenetic analysis of Ov-APR-1 and other A1 family proteases showed the proteases grouping mostly by taxa. The liver fluke (O. viverrini, C. sinensis and F. hepatica) proteases clustered together to form a clade with 72% bootstrap support; within this clade, Ov-APR-1 and C. sinensis cathepsin D grouped together with 100% support. However, the liver fluke enzymes were no more closely related to the A1 proteases from other platyhelminth parasites (schistosomes) than they were to homologues from other phyla (Fig. 1). Ov-APR-1, like some other helminth family A1 proteases (Becker et al., 1995), but unlike human pepsin and cathepsin D, contained a C-terminal extension of about 30 amino acid residues in length (Fig. S1). The Ov-apr-1 mRNA was detected in all intra-mammalian developmental stages of the parasite examined - eggs, metacercariae, immature and mature adult worms (Fig. 2). PCR without reverse transcriptase did not produce amplicons (not shown), indicating that the RNA preparations were free of contaminating genomic DNA.
Figure 1.
Phylogenetic tree of aspartic proteases, including Ov-APR-1 from Opisthorchis viverrini (GenBank protein accession no. AAZ39883); protozoa, Plasmodium falciparum (plasmepsin1, P39898): fungi, Laccaria bicolor (XP_001877133), Aspergillus niger (XP_001399855); flatworm, Clonorchis sinensis (AAL14708) Fasciola hepatica (ABJ97285), Schistosoma japonicum (AAC37302), Schistosoma mansoni (AAB63442); arthropod, Aedes aegypti (XP_001657556), Drosophila melanogaster (NP_652013); roundworm, Ancylostoma ceylanicum (AAO22152), Meloidogyne incognita (ABC88426); vertebrates, Homo sapiens (NP_001900), Canis familiaris (NP_001020792), Mus musculus (NP_034113), Bos taurus (BAB21620). The number on each branch represents the bootstrap value from 1000 replicates.
Figure 2.
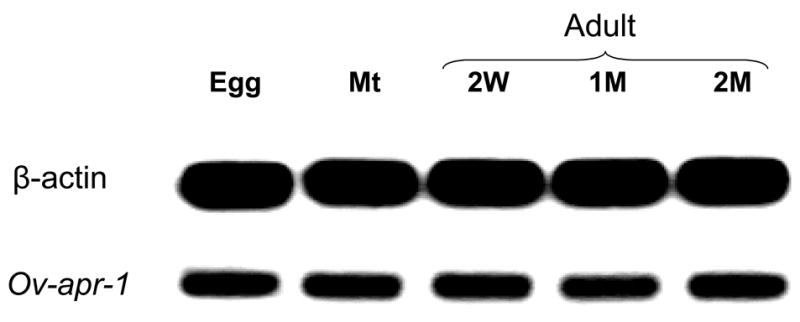
Expression of Ov-apr-1 mRNA in different developmental stages of O. viverrini, as determined by RT-PCR. The following cDNA templates were included: lane 1, eggs; lane 2, metacercariae; lane 3, 2 week old juvenile worms; lane 4, one month old juvenile worms; lane 5, 2 month old adult worms. The upper panel shows expression of the β-actin mRNA in the different developmental stages, included here as a constitutively expressed control transcript. The lower panel shows expression of Ov-apr-1 mRNA. Control reactions where reverse transcriptase was omitted did not result in detectable amplicons (not shown).
Recombinant Ov-APR-1 is expressed in inclusion bodies but can be refolded
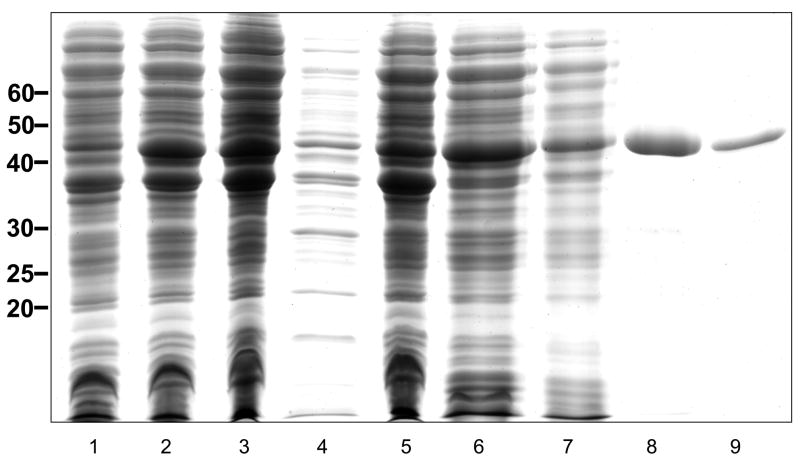
Recombinant Ov-APR-1 was expressed at high yield in E. coli but formed insoluble inclusion bodies. Inclusion bodies were solubilized with 8 M urea and the recombinant protein was purified using nickel-NTA chromatography under denaturing conditions at a yield of 5 mg/L. After refolding and centrifugation, approximately 10% of the pro-enzyme was recovered in soluble form as determined by SDS-PAGE followed by densitometry (Fig. 3).
Figure 3.
Expression of Ov-APR-1 in Escherichia coli as insoluble inclusion bodies and refolding of the purified denatured protein. SDS-PAGE gel stained with Coomassie Brilliant Blue showing non-induced cell pellet (lane 1), induced cell pellet (2), insoluble protein remaining in the pellet after sonication of the bacteria under native conditions (3), soluble protein after sonicating bacteria under native conditions (4), insoluble material after resuspending the pellet shown in lane 3 with 8 M urea (5), soluble material after resuspending the pellet shown in lane 3 with 8 M urea (6), flow-through from a nickel-NTA column loaded with material from lane 6 under denaturing conditions (7), eluate from the nickel-NTA column loaded with material from lane 6 under denaturing conditions (8), equal loading of soluble protein from lane 7 after refolding into PBS (9).
Refolded Ov-APR-1 auto-activates and is catalytically active
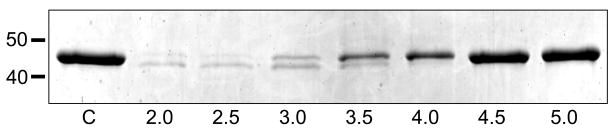
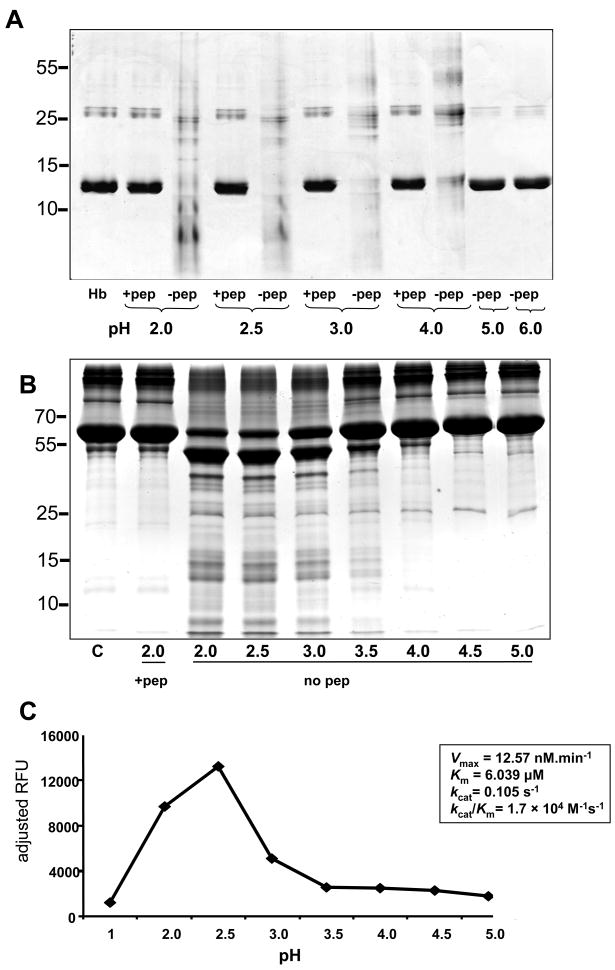
Refolded Ov-APR-1 underwent a pH dependent auto-processing from its pro-form to a mature enzyme. Auto-activation was optimal between pH 2.0 – 3.5 but was not apparent at pH 4.5 or higher. This was evident from a shift in molecular mass from 45 to 41 kDa. Most, but not all, of the zymogen converted to active enzyme at the lowest pH values assessed (pH 2.0 – 2.5), approximately 50% underwent activation at pH 3.0 and the majority of protein remained inactivated from pH 4.0 onwards (Fig. 4).
Figure 4.
Auto-activation of Ov-APR-1 from a pro-enzyme to its mature form at acidic pH. Recombinant Ov-APR-1 (pro-enzyme) was incubated in 50 mM sodium acetate at half-unit pH increments from pH 2–5 at 37°C for 18 h. C – control Ov-APR-1 protein in PBS, pH 7.2; 2.0 – 5.0 - pH values at which Ov-APR-1 was incubated to observe auto-activation from pro- (upper band) to mature form (lower band).
Ov-APR-1 is expressed in intra-mammalian and free-living stages of O. viverrini
Anti-Ov-APR-1 pooled serum was employed to investigate expression of the aspartic protease in different developmental stages of the O. viverrini liver fluke. Protein of the expected molecular mass was detected by Western blotting in soluble extracts of eggs, metacercariae and adult worms. Bands detected were weak in egg extract compared to all other extracts. The enzyme was readily detected in ES products of the adult worm (Fig. 5). A doublet of ~ 41–45 kDa was detected in extracts from metacercariae and adult worms, but only the upper band was detected in ES products, suggesting that the lower band corresponded to material that had yet to enter the secretory pathway and undergo glycosylation. Alternatively, the lower band might represent Ov-APR-1 that has undergone further autodigestion or cleavage by another parasite protease during or after the preparation of soluble extracts, and this protease is not released into ES products when worms are cultured in vitro.
Figure 5.
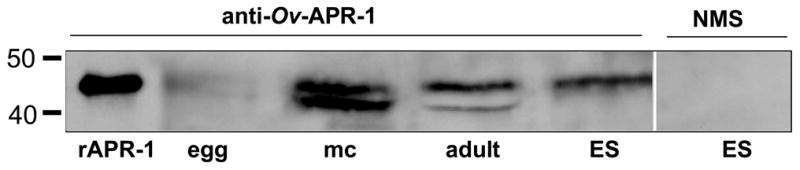
Recognition of recombinant Ov-APR-1 and parasites extracts from different developmental stages of O. viverrini. Western blot of recombinant Ov-APR-1 (rAPR-1), soluble egg extract (egg), soluble metacercariae extract (MC), soluble adult somatic extract (adult) and adult worm excretory/secretory proteins (ES) probed with pooled serum from mice immunized with recombinant Ov-APR-1. ES products were also probed with normal mouse serum (NMS - pre-vaccination with APR-1) and did not reveal any bands.
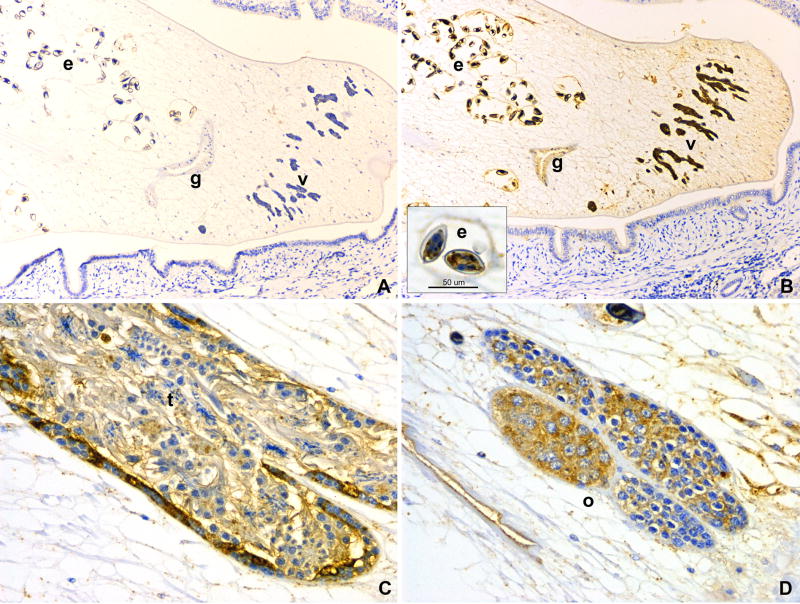
Immunohistochemical localization of Ov-APR-1 in adult O. viverrini revealed strong expression in the testis, ovary, vitelline glands and, to a lesser extent, in the gut epithelium (Fig. 6). Within the testis, intense staining with anti-Ov-APR-1 serum was observed in the primary spermatogonia while the more mature sperm showed less staining (Fig. 6C). Mature sperm in the seminal receptacle were negative for staining with anti-Ov-APR-1 antibody (not shown). All O. viverrini eggs showed Ov-APR-1 expression, particularly in the developing miracidium within the eggshell (inset, Fig. 6B). Some parenchymal cells of the flukes stained weakly, while other organs and tissues showed very low or no Ov-APR-1 expression. Unlike some other fluke proteins that are known to be secreted by O. viverrini adult worms (Suttiprapa et al., 2008), Ov-APR-1 was not detected in epithelia of bile ducts of O. viverrini infected hamsters (not shown).
Figure 6.
Immunohistochemistry of Ov-APR-1 in adult O. viverrini and infected hamster bile ducts. Negative control using pre-immune mouse serum shows no staining in the fluke and bile duct epithelium (A). Ov-APR-1 expresses in the vitelline glands (v), gut (g) and parasite eggs (e) (B). The miracidia in the eggs show intense staining (inset). Strong expression of Ov-APR-1 is observed in the fluke testis (t) (C) and ovary (o) (D). Immunoperoxidase staining, original magnification (A–B, 100×; C-D, 200×).
Ov-APR-1 digests blood proteins
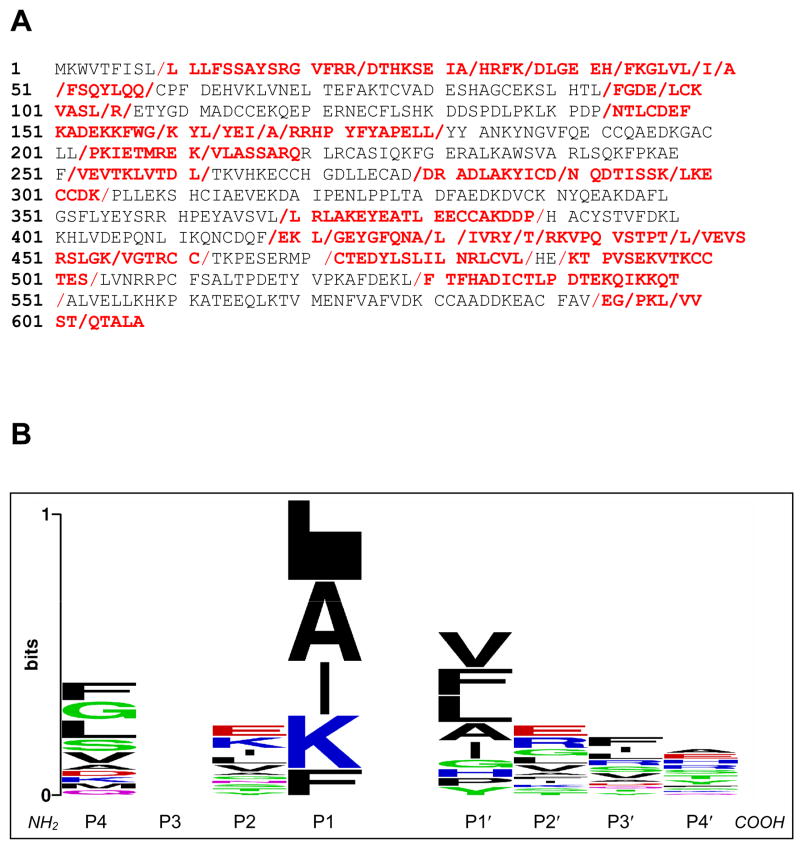
Recombinant Ov-APR-1 readily digested both Hb and BSA. Digestion of Hb was detected between pH 2.0 – 4.0 but was not readily detected at pH 5.0 by visual inspection of SDS-PAGE gels (Fig. 7A). Proteolytic activity was completely inhibited by pepstatin A. Digestion of BSA by Ov-APR-1, like Hb, was detected between pH 2.0–4.0 and optimal at ~ pH 2.5 (Fig. 7B). These findings were supported by the relative catalytic activities observed against the peptide substrate MoCAc-GKPILFFRLK, where optimal hydrolysis was evident at pH 2.0 to pH 3.0 (Fig. 7C). At pH 3.0, Ov-APR-1 cleaved the substrate with a kcat/Km = 1.7 × 104 M−1s−1. To further explore the digestion of BSA by recombinant Ov-APR-1 and infer subsite preferences (P4–P4′- denoting the four N- and C-terminal amino acids respectively of the substrate peptide that flank the scissile bond cleaved by the protease) from the data, Ov-APR-1 generated hydrolysates of BSA were investigated by LC-MS-MS. Forty-six peptides were detected (Fig. 8A). Like many other aspartic proteases, Ov-APR-1 displayed, in general, a promiscuity in substrate residue preferences within its binding pocket. Nonetheless, the enzyme preferred hydrophobic (Leu, Ala, Ile), positively charged (Lys) and bulky aromatic (Phe) residues at the P1 position (the residue immediately N-terminal to the scissile bond), in that order (Fig. 8B). Similar residues were accommodated at P1′ (Val, Phe, Leu, Ala, Ile in that order), although less selectivity was evident at this site.
Figure 7.
Refolded recombinant Ov-APR-1 digests human hemoglobin and serum albumin. Cleavage of human hemoglobin (Hb) by Ov-APR-1 at pH 2.0 - 4.0 but not at pH >5.0, and inhibition of hemoglobinolytic activity by pepstatin A (A). Cleavage of serum albumin by Ov-APR-1 at pH 2.0 - 3.5 but not at pH >4.0, and inhibition of catalytic activity by pepstatin A (B). Catalytic activity of refolded Ov-APR-1 against the peptidyl substrate 7-Methoxycoumarin-4-Acetyl-GKPILF↓FRLK(DNP)-D-Arg-Amide at different pH values (C). The final substrate concentration was 1.0 μM and the final volume of each reaction was 100 μl. Enzyme (2 nM) efficiency was assessed at pH 2.5 by measuring initial rates over a range of substrate concentrations (0.2–25 μM). The catalytic constants kcat, Km and kcat/Km were established from the resulting Michaelis-Menten plot.
Figure 8.
Sites within serum albumin where Ov-APR-1 digested the protein as determined by liquid chromatography tandem mass spectrometry (LC-MS-MS). Peptides detected by LC-MS-MS are shown in red – forward slash (/) denotes the cleavage sites (A). P4–P4′ subsite specificities of Ov-APR-1. MS/MS data compiled from Mascot searches were used to create an alignment of peptide cleavage sites within serum albumin which was submitted to the WebLogo program to generate the image (B). Plots depict relative entropy between the observed and background distributions of amino acids at each subsite. The larger the letter denoting each amino acid, the more frequently that residue occurred. Hydrophobic residues are black, polar are green, basic are blue and acidic are red.
Discussion
Here we report the sequence, phylogeny, tissue localization and substrate specificity of Ov-APR-1 from O. viverrini, the first aspartic protease to be described from any species of liver fluke. Ov-apr-1 mRNA was expressed in all intra-mammalian stages and in the infective stage derived from fish, the metacercaria. The enzyme was localized in the intestine, male and female reproductive organs, and in the miracidium within the developing egg within the uterus of the adult fluke. The widespread distribution of the mRNA and protease suggests multiple roles for this hydrolase in the physiology of the parasite. Not all of the tissues where APR-1 is expressed are considered to be particularly acidic. For example, the developing miracidium within the egg and the immature spermatids are not thought to be acidic tissues, however these cells likely contain lysosomes, and Ov-APR-1 might function in protein turnover and remodeling in these cells. An extracellular role for Ov-APR-1 in the digestion of host proteins can also be inferred from its location in the gut epithelium and ES products of adult worms, as well as the ability of the recombinant protease to digest blood proteins that are abundant in host plasma and erythrocytes. McKerrow and co-workers have hypothesized, based on findings with other parasitic helminths, that early metazoans evolved a successful digestive network of proteases comprising cathepsins D, B, L, C and legumain that predated the evolution of the pancreas and the subsequent primacy of serine proteases as digestive enzymes in vertebrates (Delcroix et al., 2006). The findings reported here with Ov-APR-1 support these notions.
From a recent survey of the O. viverrini transcriptome, representatives from the four major mechanistic classes of protease were identified (Laha et al., 2007). From only 5,000 ESTs representing 1,932 distinct genes, 11 contigs representing cysteine proteases but only one contig representing aspartic proteases (Ov-apr-1) were identified. A similar distribution of protease mRNAs is found in the genomes of the human schistosomes (Hu et al., 2003; Verjovski-Almeida et al., 2003), where cysteine proteases are more abundantly represented than aspartic proteases. This dichotomy in the sizes of protease gene families is even more apparent in blood-feeding parasitic nematodes, where most of the protease-encoding mRNAs are expressed in gut tissue (Jasmer et al., 2001; Ranjit et al., 2008).
Unlike aspartic proteases from other many other helminth parasites, Ov-APR-1 was detected in different developmental stages of the parasite, and in multiple tissues within the same developmental stage. Ov-APR-1 was found in the gut and ES products of the adult worm, indicating a role in digestion of host tissues, possibly for nutrient acquisition. Ov-APR-1 was also detected in the testis, ovary and vitelline glands. Within the testis, intense staining with anti-Ov-APR-1 serum was observed in the primary spermatogonia while the mature sperm in the seminal receptacle did not express APR-1, implying a role in the sperm maturation process. All O. viverrini eggs showed Ov-APR-1 expression, mainly in the developing miracidium inside the egg, again suggesting a role for the enzyme in growth and development. Cathepsin D from the filarial parasite, Onchocerca volvulus (Nematoda), the causative agent of River Blindness, displayed similar anatomic distribution in the lysosomes of the hypodermis and epithelia of the intestine, reproductive organs and mature oocytes and early morulae (Jolodar et al., 2004). However, aspartic proteases from most other helminths, including schistosomes (Brindley et al., 2001) and blood-feeding parasitic nematodes such as hookworms (Williamson et al., 2002; Williamson et al., 2003) and Haemonchus contortus (Longbottom et al., 1997; Smith et al., 2003), are expressed exclusively in the intestinal epithelia where they degrade Hb and serum proteins. We cannot, of course, exclude the possibility that antibodies to Ov-APR-1 cross-reacted with aspartic proteases other than APR-1, but to date only one mRNA encoding an aspartic protease has been identified from adult O. viverrini (Laha et al., 2007).
S. mansoni cathepsin D (which shares 56 % sequence identity with Ov-APR-1) is the initial enzyme that cleaves Hb in the schistosome gastrodermis (Brindley et al., 2001; Delcroix et al., 2006), and is essential for parasite survival. Indeed, larval schistosomes transformed by treatment with cathepsin D double stranded RNA do not mature to adulthood when injected into mice (Morales et al., 2008). Ov-APR-1 is expressed in the gut of adult O. viverrini and digests erythrocyte and serum proteins, supporting its role in nutrient acquisition; however its expression in the miracidium inside mature oocytes suggests additional functions, such as escape from the egg and/or regulation of embryogenesis. Opisthorchis ingests biliary epithelial cells, mucins and bile proteins such as albumin, and red blood cells have been observed in the gut lumen of O. viverrini recovered from infected hamsters (Sripa, unpublished). Moreover, host-derived Hb has been observed in the gut of another liver fluke, F. hepatica, which is thought to predominantly feed on blood (Todd and Ross, 1966), supporting the role of Ov-APR-1 in food acquisition.
Ov-APR-1, like other A1 family aspartic proteases, displayed broad substrate preferences, cleaving between hydrophobic and aromatic P1 and P1′ residues. However, APR-1 also readily cleaved at sites with lysine at P1. Numerous fungal aspartic proteases prefer P1 Lys, typified by their ability to convert trypsinogen into trypsin at the Lys6-Ile7 junction, but mammalian homologues including pepsin and cathepsin D do not readily cleave P1 Lys residues (Shintani et al., 1997). The basis for the selective cleavage of P1 Lys by fungal enzymes may be due to replacement of Ser/Thr-77 (pepsin numbering) in the active site flap of mammalian enzymes with Asp-77 in fungal proteases, and the insertion of Ser between Gly-78 and Ser-79 in the fungal enzymes (Shintani et al., 1997). Indeed, a double mutant of porcine pepsin engineered with these substitutions cleaved substrates with a P1 Lys while wild type enzyme did not (Shintani et al., 1997). Interestingly, Ov-APR-1 had an active site flap that resembled mammalian enzymes (Ser-77 and the absence of Ser between Gly-78 and Ser-79) yet it readily cleaved serum albumin at sites with a P1 Lys, indicating that residues in the S1 binding pocket other than those described above are important in determining substrate specificities at P1.
Unlike some other intestinal proteases of liver flukes (Rokni et al., 2003; Laha et al., 2008), Ov-APR-1 was not strongly recognized by sera from people infected with O. viverrini (not shown). The O. viverrini asparaginyl endopeptidase, Ov-AEP-1, is expressed in the same developmental stages of the parasite as Ov-APR-1, but Ov-AEP-1 recombinant protein was strongly recognized by IgG from infected subjects, and shows promise as a diagnostic antigen (Laha et al., 2008). Similar scenarios exist for the hookworm (Ac-APR-1 and Na-APR-1) and S. mansoni cathepsin D-like enzymes – these orthologous proteases are expressed in the gut of hookworms (Williamson et al., 2002) and schistosomes (Brindley et al., 2001) and they are not strongly recognized by sera from infected individuals, but both show promise as cryptic vaccine antigens (Verity et al., 2001; Loukas et al., 2005). Vaccine trials with O. viverrini antigens have not been reported, but the hamster, Mesocricetus auratus, is a permissive laboratory host for O. viverrini. Indeed, hamsters infected with O. viverrini while being maintained on a diet that is high in nitrosamines develop cholangiocarcinoma (Thamavit et al., 1978), implying that this is an admirable model for studying the pathogenesis of human opisthorchiasis, as well as a means by which to assess the efficacy of recombinant antigens in a pre-clinical setting. Using the hamster model, we now aim to assess the vaccine efficacy of recombinant Ov-APR-1, as well as other proteases expressed in the gastrodermis of adult worms. If efficacious, these enzymes will be assessed as single antigens and as components of multivalent recombinant vaccines targeting the different developmental stages of O. viverrini found within the mammalian host. Such a vaccine would be a welcome tool in terms of controlling liver fluke infections and preventing one of the most fatal human cancers in southeast Asia.
Acknowledgments
This research was supported by award number UO1AI065871 from the National Institute of Allergy and Infectious Diseases (the content is solely the responsibility of the authors and does not necessarily represent the official views of the NIAID or the NIH), the Sandler Family Foundation, the Thailand-Tropical Diseases Research Program (T-2, grant number ID02-2-HEL-05-054) and the National Health and Medical Research Council, Australia (NHMRC). SS is a Royal Golden Jubilee PhD scholar in the laboratory of BS. AL is supported by a senior research fellowship from NHMRC. JM is supported by a Peter Doherty training award from NHMRC. We thank Professor John Dalton for helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdulla MH, Lim KC, Sajid M, McKerrow JH, Caffrey CR. Schistosomiasis mansoni: novel chemotherapy using a cysteine protease inhibitor. PLoS Med. 2007;4:e14. doi: 10.1371/journal.pmed.0040014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcala-Canto Y, Ibarra-Velarde F, Sumano-Lopez H, Gracia-Mora J, Alberti-Navarro A. Effect of a cysteine protease inhibitor on Fasciola hepatica (liver fluke) fecundity, egg viability, parasite burden, and size in experimentally infected sheep. Parasitol Res. 2007;100:461–5. doi: 10.1007/s00436-006-0308-7. [DOI] [PubMed] [Google Scholar]
- Becker MM, Harrop SA, Dalton JP, Kalinna BH, McManus DP, Brindley PJ. Cloning and characterization of the Schistosoma japonicum aspartic proteinase involved in hemoglobin degradation. J Biol Chem. 1995;270:24496–501. doi: 10.1074/jbc.270.41.24496. [DOI] [PubMed] [Google Scholar]
- Brindley PJ, Kalinna BH, Wong JY, Bogitsh BJ, King LT, Smyth DJ, et al. Proteolysis of human hemoglobin by schistosome cathepsin D. Mol Biochem Parasitol. 2001;112:103–12. doi: 10.1016/s0166-6851(00)00351-0. [DOI] [PubMed] [Google Scholar]
- Caffrey CR, McKerrow JH, Salter JP, Sajid M. Blood ‘n’ guts: an update on schistosome digestive peptidases. Trends Parasitol. 2004;20:241–8. doi: 10.1016/j.pt.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Crooks GE, Hon G, Chandonia JM, Brenner SE. WebLogo: a sequence logo generator. Genome Res. 2004;14:1188–90. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton JP, Neill SO, Stack C, Collins P, Walshe A, Sekiya M, et al. Fasciola hepatica cathepsin L-like proteases: biology, function, and potential in the development of first generation liver fluke vaccines. Int J Parasitol. 2003;33:1173–81. doi: 10.1016/s0020-7519(03)00171-1. [DOI] [PubMed] [Google Scholar]
- Delcroix M, Sajid M, Caffrey CR, Lim KC, Dvorak J, Hsieh I, et al. A multienzyme network functions in intestinal protein digestion by a platyhelminth parasite. J Biol Chem. 2006;281:39316–29. doi: 10.1074/jbc.M607128200. [DOI] [PubMed] [Google Scholar]
- Felsenstein J. Mathematics vs. Evolution: Mathematical Evolutionary Theory. Science. 1989;246:941–2. doi: 10.1126/science.246.4932.941. [DOI] [PubMed] [Google Scholar]
- Hillyer GV. Fasciola antigens as vaccines against fascioliasis and schistosomiasis. J Helminthol. 2005;79:241–7. doi: 10.1079/joh2005304. [DOI] [PubMed] [Google Scholar]
- Hu W, Yan Q, Shen DK, Liu F, Zhu ZD, Song HD, et al. Evolutionary and biomedical implications of a Schistosoma japonicum complementary DNA resource. Nat Genet. 2003;35:139–47. doi: 10.1038/ng1236. [DOI] [PubMed] [Google Scholar]
- Jasmer DP, Roth J, Myler PJ. Cathepsin B-like cysteine proteases and Caenorhabditis elegans homologues dominate gene products expressed in adult Haemonchus contortus intestine. Mol Biochem Parasitol. 2001;116:159–69. doi: 10.1016/s0166-6851(01)00312-7. [DOI] [PubMed] [Google Scholar]
- Jolodar A, Fischer P, Buttner DW, Miller DJ, Schmetz C, Brattig NW. Onchocerca volvulus: expression and immunolocalization of a nematode cathepsin D-like lysosomal aspartic protease. Exp Parasitol. 2004;107:145–56. doi: 10.1016/j.exppara.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Kaewpitoon N, Laha T, Kaewkes S, Yongvanit P, Brindley PJ, Loukas A, et al. Characterization of cysteine proteases from the carcinogenic liver fluke, Opisthorchis viverrini. Parasitol Res. 2008;102:757–64. doi: 10.1007/s00436-007-0831-1. [DOI] [PubMed] [Google Scholar]
- Kang TH, Yun DH, Lee EH, Chung YB, Bae YA, Chung JY, et al. A cathepsin F of adult Clonorchis sinensis and its phylogenetic conservation in trematodes. Parasitology. 2004;128:195–207. doi: 10.1017/s0031182003004335. [DOI] [PubMed] [Google Scholar]
- Laha T, Pinlaor P, Mulvenna J, Sripa B, Sripa M, Smout MJ, et al. Gene discovery for the carcinogenic human liver fluke, Opisthorchis viverrini. BMC Genomics. 2007;8:189. doi: 10.1186/1471-2164-8-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laha T, Sripa J, Sripa B, Pearson M, Tribolet L, Kaewkes S, et al. Asparaginyl endopeptidase from the carcinogenic liver fluke, Opisthorchis viverrini, and its potential for serodiagnosis. Int J Infect Dis. 2008 doi: 10.1016/j.ijid.2008.03.033. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Kim IS, Sohn WM, Lee J, Yong TS. Vaccination with DNA encoding cysteine proteinase confers protective immune response to rats infected with Clonorchis sinensis. Vaccine. 2006;24:2358–66. doi: 10.1016/j.vaccine.2005.11.062. [DOI] [PubMed] [Google Scholar]
- Longbottom D, Redmond DL, Russell M, Liddell S, Smith WD, Knox DP. Molecular cloning and characterisation of a putative aspartate proteinase associated with a gut membrane protein complex from adult Haemonchus contortus. Mol Biochem Parasitol. 1997;88:63–72. doi: 10.1016/s0166-6851(97)00074-1. [DOI] [PubMed] [Google Scholar]
- Loukas A, Bethony JM, Mendez S, Fujiwara RT, Goud GN, Ranjit N, et al. Vaccination with recombinant aspartic hemoglobinase reduces parasite load and blood loss after hookworm infection in dogs. PLoS Med. 2005;2:e295. doi: 10.1371/journal.pmed.0020295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lun ZR, Gasser RB, Lai DH, Li AX, Zhu XQ, Yu XB, et al. Clonorchiasis: a key foodborne zoonosis in China. Lancet Infect Dis. 2005;5:31–41. doi: 10.1016/S1473-3099(04)01252-6. [DOI] [PubMed] [Google Scholar]
- McManus DP, Dalton JP. Vaccines against the zoonotic trematodes Schistosoma japonicum, Fasciola hepatica and Fasciola gigantica. Parasitology. 2006;133(Suppl):S43–61. doi: 10.1017/S0031182006001806. [DOI] [PubMed] [Google Scholar]
- Morales ME, Rinaldi G, Gobert GN, Kines KJ, Tort JF, Brindley PJ. RNA interference of Schistosoma mansoni cathepsin D, the apical enzyme of the hemoglobin proteolysis cascade. Mol Biochem Parasitol. 2008;157:160–8. doi: 10.1016/j.molbiopara.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na BK, Kang JM, Sohn WM. CsCF-6, a novel cathepsin F-like cysteine protease for nutrient uptake of Clonorchis sinensis. Int J Parasitol. 2008;38:493–502. doi: 10.1016/j.ijpara.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Page RD. TreeView: an application to display phylogenetic trees on personal computers. Comput Appl Biosci. 1996;12:357–8. doi: 10.1093/bioinformatics/12.4.357. [DOI] [PubMed] [Google Scholar]
- Ranjit N, Zhan B, Stenzel DJ, Mulvenna J, Fujiwara R, Hotez PJ, et al. A family of cathepsin B cysteine proteases expressed in the gut of the human hookworm, Necator americanus. Mol Biochem Parasitol. 2008;160:90–9. doi: 10.1016/j.molbiopara.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Robinson MW, Tort JF, Lowther J, Donnelly SM, Wong E, Xu W, et al. Proteomic and phylogenetic analysis of the cathepsin L protease family of the helminth pathogen, Fasciola hepatica: expansion of a repertoire of virulence-associated factors. Mol Cell Proteomics. 2008;7:1111–23. doi: 10.1074/mcp.M700560-MCP200. [DOI] [PubMed] [Google Scholar]
- Rokni MB, Massoud J, Hanilo A. Comparison of adult somatic and cysteine proteinase antigens of Fasciola gigantica in enzyme linked immunosorbent assay for serodiagnosis of human fasciolosis. Acta Trop. 2003;88:69–75. doi: 10.1016/s0001-706x(03)00175-x. [DOI] [PubMed] [Google Scholar]
- Shintani T, Nomura K, Ichishima E. Engineering of porcine pepsin. Alteration of S1 substrate specificity of pepsin to those of fungal aspartic proteinases by site-directed mutagenesis. J Biol Chem. 1997;272:18855–61. doi: 10.1074/jbc.272.30.18855. [DOI] [PubMed] [Google Scholar]
- Smith WD, Skuce PJ, Newlands GF, Smith SK, Pettit D. Aspartyl proteases from the intestinal brush border of Haemonchus contortus as protective antigens for sheep. Parasite Immunol. 2003;25:521–30. doi: 10.1111/j.0141-9838.2004.00667.x. [DOI] [PubMed] [Google Scholar]
- Sripa B, Kaewkes S. Localisation of parasite antigens and inflammatory responses in experimental opisthorchiasis. Int J Parasitol. 2000;30:735–40. doi: 10.1016/s0020-7519(00)00054-0. [DOI] [PubMed] [Google Scholar]
- Sripa B, Kaewkes S, Sithithaworn P, Mairiang E, Laha T, Smout, et al. Liver Fluke Induces Cholangiocarcinoma. PLoS Med. 2007;4:e201. doi: 10.1371/journal.pmed.0040201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack CM, Caffrey CR, Donnelly SM, Seshaadri A, Lowther J, Tort JF, et al. Structural and Functional Relationships in the Virulence-associated Cathepsin L Proteases of the Parasitic Liver Fluke, Fasciola hepatica. J Biol Chem. 2008;283:9896–908. doi: 10.1074/jbc.M708521200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttiprapa S, Loukas A, Laha T, Wongkham S, Kaewkes S, Gaze S, et al. Characterization of the antioxidant enzyme, thioredoxin peroxidase, from the carcinogenic human liver fluke, Opisthorchis viverrini. Mol Biochem Parasitol. 2008;160:16–22. doi: 10.1016/j.molbiopara.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thamavit W, Bhamarapravati N, Sahaphong S, Vajrasthira S, Angsubhakorn S. Effects of dimethylnitrosamine on induction of cholangiocarcinoma in Opisthorchis viverrini-infected Syrian golden hamsters. Cancer Res. 1978;38:4634–9. [PubMed] [Google Scholar]
- Thuwajit C, Thuwajit P, Kaewkes S, Sripa B, Uchida K, Miwa M, et al. Increased cell proliferation of mouse fibroblast NIH-3T3 in vitro induced by excretory/secretory product(s) from Opisthorchis viverrini. Parasitology. 2004;129:455–64. doi: 10.1017/s0031182004005815. [DOI] [PubMed] [Google Scholar]
- Todd JR, Ross JG. Origin of hemoglobin in the cecal contents of Fasciola hepatica. Exp Parasitol. 1966;19:151–4. doi: 10.1016/0014-4894(66)90063-4. [DOI] [PubMed] [Google Scholar]
- Tort J, Brindley PJ, Knox D, Wolfe KH, Dalton JP. Proteinases and associated genes of parasitic helminths. Adv Parasitol. 1999;43:161–266. doi: 10.1016/s0065-308x(08)60243-2. [DOI] [PubMed] [Google Scholar]
- Verity CK, McManus DP, Brindley PJ. Vaccine efficacy of recombinant cathepsin D aspartic protease from Schistosoma japonicum. Parasite Immunol. 2001;23:153–62. doi: 10.1046/j.1365-3024.2001.00369.x. [DOI] [PubMed] [Google Scholar]
- Verjovski-Almeida S, DeMarco R, Martins EA, Guimaraes PE, Ojopi EP, Paquola AC, et al. Transcriptome analysis of the acoelomate human parasite Schistosoma mansoni. Nat Genet. 2003;35:148–57. doi: 10.1038/ng1237. [DOI] [PubMed] [Google Scholar]
- Williamson AL, Brindley PJ, Abbenante G, Prociv P, Berry C, Girdwood K, et al. Cleavage of hemoglobin by hookworm cathepsin D aspartic proteases and its potential contribution to host specificity. FASEB J. 2002;16:1458–60. doi: 10.1096/fj.02-0181fje. [DOI] [PubMed] [Google Scholar]
- Williamson AL, Brindley PJ, Knox DP, Hotez PJ, Loukas A. Digestive proteases of blood-feeding nematodes. Trends Parasitol. 2003;19:417–23. doi: 10.1016/s1471-4922(03)00189-2. [DOI] [PubMed] [Google Scholar]
- Williamson AL, Lecchi P, Turk BE, Choe Y, Hotez PJ, McKerrow JH, et al. A multi-enzyme cascade of hemoglobin proteolysis in the intestine of blood-feeding hookworms. J Biol Chem. 2004;279:35950–7. doi: 10.1074/jbc.M405842200. [DOI] [PubMed] [Google Scholar]
- Yasuda Y, Kageyama T, Akamine A, Shibata M, Kominami E, Uchiyama Y, et al. Characterization of new fluorogenic substrates for the rapid and sensitive assay of cathepsin E and cathepsin D. J Biochem. 1999;125:1137–43. doi: 10.1093/oxfordjournals.jbchem.a022396. [DOI] [PubMed] [Google Scholar]