Abstract
The functional coupling of T cell receptor (TCR)-mediated signaling events in primary human T cells remains undefined. We demonstrate here that alterations in the expression of proximal TCR-coupled signaling subunits are associated with distinct effector capacities in differentiated human CD4 T cells. Analysis of proximal signaling profiles using biochemical and single cell approaches reveals decreased CD3ζ and ZAP-70 expression correlating with functional anergy, with increased CD3ζ/ZAP-70 expression and phosphorylation connoting acquisition of effector capacity. By contrast, the FcRγ signaling subunit known to be expressed in human effector cells and in T cells from the autoimmune disease SLE, is up-regulated upon activation, yet does not correlate with functional capacity in effector cells, and does not alter signaling or function in primary FcRγ transfectants. Our results have implications for targeting signaling molecules in immunotherapy and evaluating the functional consequence of signaling alterations associated with autoimmunity and chronic diseases.
Keywords: signal transduction, tyrosine kinase, T cell differentiation, cytokines, T cell receptor
INTRODUCTION
Activation of T lymphocytes through the T cell antigen receptor (TCR) triggers a series of intracellular signaling events culminating in IL-2 gene transcription in the nucleus (for reviews see [1, 2]), commencing a differentiation process leading to the generation of effector T cells. Effector T cells can be distinguished from naive or unprimed T cells by the expression of specific cell surface activation and differentiation markers [3], by enhanced activation kinetics and lower activation threshold [4, 5], by the acquisition of effector function including the ability to rapidly produce effector cytokines [6, 7], and by an increased propensity for activation-induced cell death [8]. While the biochemical pathways coupled to TCR engagement in resting T cells and T cell lines have been extensively characterized [9], little is known regarding how TCR-mediated signals are transduced in effector T cells. Moreover, the TCR-coupled signaling processes that control activation and function in effector T cells remain largely unknown, and are critical parameters to design strategies to modulate T cell function in autoimmunity and chronic infection where effector T cells can predominate.
Proximal TCR-mediated signaling events coupled directly to TCR engagement involve the phosphorylation on tyrosine residues of the TCR-associated CD3ε and CD3ζ subunits by the p56lck tyrosine kinase, resulting in the recruitment, phosphorylation and activation of the 70 kDa SH2-containing ZAP-70 tyrosine kinase [10, 11]. Activated ZAP-70 subsequently phosphorylates linker adapter molecules such as SLP-76 and LAT leading to activation of distal MAP kinases and ultimately to the activation and mobilization of nuclear transcription factors for IL-2 gene transcription [1]. While these proximal and distal signaling processes are operable in resting T cells, we previously identified differences in TCR-coupled proximal signaling in primary human effector CD4 T cells marked by decreased CD3ζ protein expression and up-regulation of the related ITAM-containing signaling subunit FcRγ [12, 13], typically associated with the high affinity IgE FcR [14]. In effector cells, the FcRγ subunit formed a new TCR/CD3ε/FcRγ complex contrasting the conventional TCR/CD3ε/CD3ζ complex expressed by resting T cells and T cell lines [15]. Effector cell-associated signaling changes including decreased CD3ζ expression have been found in T cells in cancer, autoimmunity and chronic viral infections [12, 16, 17] and upregulation of FcRγ expression has been also found in the peripheral T cells of patients with systemic lupus erythematosus (SLE) [18]. It is not known whether these signaling differences common to effector cells and disease-associated T cells reflect alterations in functional capacity or represent changes occurring during the course of T cell differentiation.
In this study, we investigated how the expression of the proximal signaling molecules, CD3ζ and FcRγ, in human effector CD4 T cells was coupled to functional regulation using biochemical and single cell analyses of primary human CD4 T cells and transfectants. We found that sustained activation of human CD4+CD25− T cells by anti-CD3/anti-CD28 antibodies generated effector cells producing IFN-γ and IL-2 whereas stimulation with anti-CD3 and autologous monocytes resulted in functionally hyporesponsive effector cells, with only a small proportion producing cytokines. Analysis of proximal signaling profiles using biochemical and single cell approaches reveals decreased CD3ζ and ZAP-70 expression correlating with functional anergy, with increased CD3ζ/ZAP-70 expression and phosphorylation connoting acquisition of effector capacity. By contrast, the FcRγ signaling subunit known to be expressed in human effector cells and in T cells from the autoimmune disease SLE, is up-regulated upon activation in both effector types, yet does not correlate with functional capacity, nor does it drive signaling or functional alterations in FcRγ transfectants. Our results have implications for targeting signaling molecules in immunotherapy and for identifying the functional consequence of signaling alterations associated with autoimmunity and chronic diseases.
MATERIALS AND METHODS
Human Cells
Heparinized peripheral venous blood was obtained from consenting healthy adult volunteers, or as Leukopacs purchased from BRT Laboratories (Baltimore, MD).
Antibodies
IgM anti-CD3 (2Ad2A2) was generously provided by Dr. Robert Siliciano (Johns Hopkins University, Baltimore, MD). Anti-FcRγ and anti-phosphotyrosine (4G10) were purchased from Upstate Biotechnology (Charlottesburg, VA). PE-conjugated anti-TCRζ (2H2D9) was purchased from Immunotech (Marseille, France), and anti-human/mouse ZAP-70 from Invitrogen (Carlsbad, CA). Anti-CD3 (UCHT1) and the following fluorochrome-conjugated antibodies to CD14 (M5E2), CD69 (FN50), CD25 (M-A251), CD152 (BN13), CD86 (FUN-1), IL-2, IFN-γ, and CD4 (RPA-T4) were purchased from BD-Pharmingen (San Jose, CA). For confocal analysis, anti-CD3 (UCHT1; Sigma), isotype control antibodies, FITC- and TRITC-conjugated secondary antibodies were purchased from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA).
Cell Isolation and stimulation
Human CD4 T cells were purified from peripheral blood mononuclear cells (PBMC) by negative selection using the CD4 T cell isolation kit and autoMACS™ as per the manufacturer’s recommendations (Miltenyi Biotec, Auburn, CA), and subsequently depleted of CD4+CD25+ T cells using anti-CD25 conjugated microbeads (Miltenyi Biotec), yielding >98% pure CD4+CD25− T cells. For antigen presenting cells (APC), peripheral blood monocytes were purified by positive selection with anti-CD14 magnetic microbeads (Miltenyi Biotec) and uniformly expressed CD86 (B7-2).
CD4+CD25− T cells (1×106/well in a 24 well plate) were activated either with Dynabeads® CD3/CD28 T cell expander (Invitrogen, Carlsbad, CA) at a 1:1 cell:bead ratio, or as previously described [13, 15]with soluble anti-CD3 antibody (4μg/ml UCHT1) and APC (2×106/well) for 48 h at 37°C in complete RPMI medium supplemented with 50U/ml of recombinant human IL-2 (hIL-2) (Peprotech, Rocky Hill, NJ). The resultant activated CD4 T cells were purified by centrifugation through Ficoll (LSM, ICN/Cappel, Aurora, OH), and residual monocytes depleted using anti-CD14-coupled magnetic Dynabeads (Invitrogen), yielding 99% purity [15].
Western blotting
For western blot analyses, T cells (2×106) were left untreated or activated by anti-CD3 IgM antibody for 2 min at 37°C before lysing in 1% NP40 lysis buffer with protease/phosphatase inhibitors as previously described [13]. Lysates were resolved using 10 or 12% NuPAGE® Bis-Tris gels (Invitrogen), transferred to nitrocellulose and blots incubated with antibodies to phosphotyrosine and actin followed by HRP-conjugated goat anti-mouse (Biorad, Hercules, CA) as described [13].
Primary T cell transfection
CD4+CD25− T cells were stimulated with Dynabeads® CD3/CD28 and 50U/ml hIL-2 for 24 hr, washed and transfected by nucleofection (Human T cell Kit; Amaxa, Gaithersburg, MD) with 20μg of pMG-FcRγ [19] or a control CMV expression vector and cultured for 18 hr in complete RPMI media, prior to biochemical and functional analyses.
Intracellular cytokine staining
For intracellular cytokine staining, resting and effector cell populations were stimulated either with Dynabeads® CD3/CD28 or PMA/ionomycin for 6 hr in the presence of Golgistop (BD Pharmingen). Cells were harvested, stained for surface markers, fixed in Cytofix buffer (BD Pharmingen), permeabilized and incubated with anti-cytokine antibodies or their isotype controls, and analyzed using the FACScalibur™ or LSRII (Becton Dickinson) with Cellquest™ or FACSdiva™ software, respectively.
Confocal microscopy
Control, FcRγ plasmid transfectants and differentiated effectors were adhered to slides, incubated with IgM anti-CD3 for capping, fixed, and stained for CD3ε and FcRγ as previously described [15]. Cells were analyzed with a confocal microscope (Zeiss Axiovert 100M Scope with LSM 510 SP1 software; Jena, Germany).
RESULTS
Generation of effector CD4 T cells with distinct functional capacity
Primary human effector cells were generated by culturing purified CD4+CD25− T cells in vitro with anti-CD3 to provide the TCR/CD3 stimulus, and either anti-CD28 (CD3/CD28 stimulation) or monocytic-derived APC bearing B7-2 ligands (Fig. 1A) (CD3/mc stimulation) to provide the second signal. Both stimulation conditions yielded an expanded population of activated T cells with efficient up-regulation of activation markers CD69, CD25 (IL-2Rα) and CTLA4 (CD152) (Fig. 1A), compared to unstimulated CD4+CD25− T cells (Fig. 1A), indicating that each stimulation condition generated activated/effector phenotypes. However, when the functional capacity of the resultant effector cells was examined, striking differences were noted. Short term restimulation of each effector cell type with anti-CD3/anti-CD28 antibodies resulted in a large proportion of IL-2 and IFN-γ producers from CD3/CD28 effector cells, with much lower proportions (3–4 fold lower) of CD3/mc effector cells producing IL-2 or IFN-γ (Fig. 1B), with minimal levels of IL-4 and IL-10 produced from either effector populations (data not shown). In response to PMA/I stimulation that bypasses the TCR, however, similar proportions of both effector types produced IL-2, and IFN-γ (Fig. 1B, fourth and fifth columns), indicating that functional differences were being transduced through the TCR/CD3 complex. This difference in cytokine production between CD3/mc and CD3/CD28 effector cells was apparent at all time-points examined (24–72 hours, data not shown) indicating that the refractoriness of CD3/mc cells to TCR-stimulation was not due to kinetic differences.
Figure 1. Generation of effector CD4 T cells with distinct functional capacity.
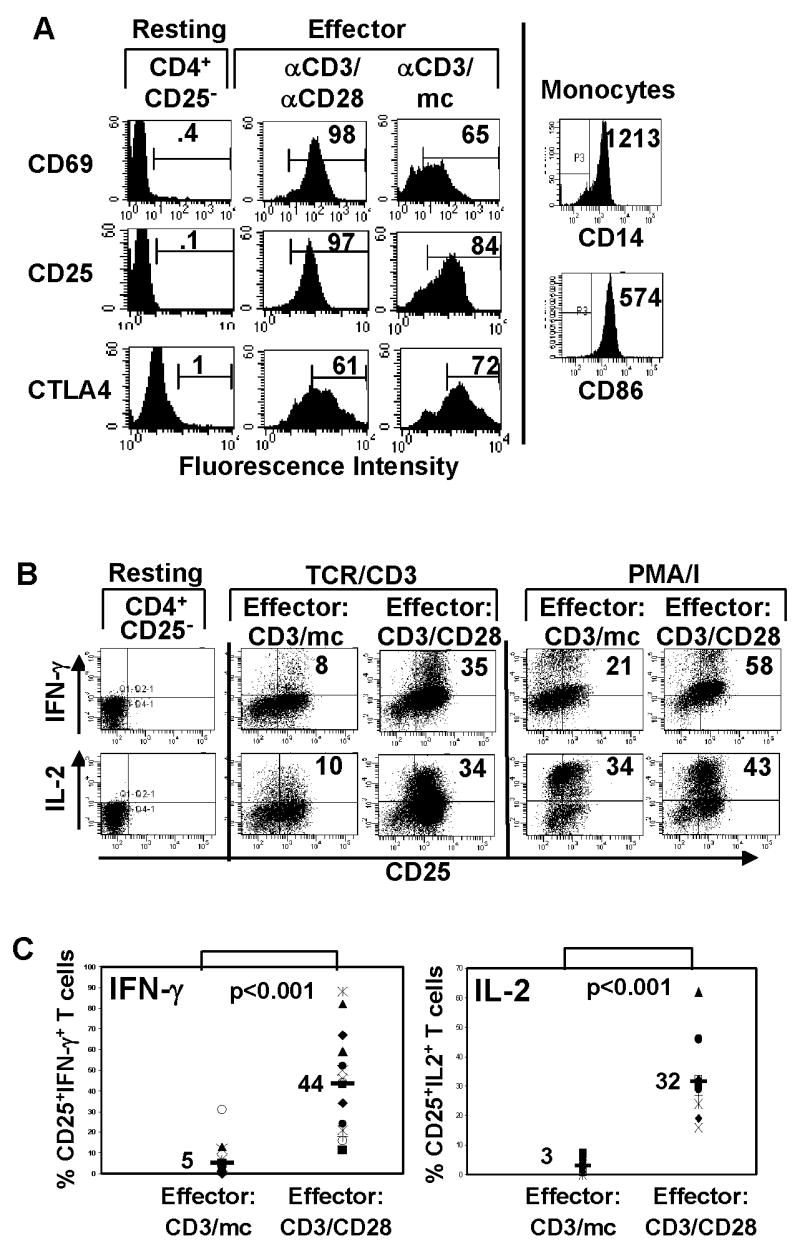
(A) Cell surface CD25, CD69 and intracellular CTLA-4 phenotype of resting CD4+CD25− T cells and those stimulated with anti-CD3/anti-CD28 antibodies (CD3/CD28) or anti-CD3 and monocytes (CD3/mc) gated on live CD4+ T cells. Isolated monocytes were surfaced stained for CD14 and CD86 (B7-2) expression. Data from a single donor are representative of 25 donors. (B) Freshly isolated CD4+CD25− T cells were assessed for IFN-γ and IL-2 production. Activated cells were restimulated with anti-CD3/CD28 antibodies (TCR/CD3) or PMA/ionomycin (PMA/I) for 6 hours in the presence of Golgistop and IFN-γ and IL-2 production determined by ICS. Dot plots show CD25 versus IFN-γ (upper row) or IL-2 expression (lower row) by CD3/mc effector cells and CD3/CD28 effector cells gated on live CD4+ T cells. Number in the upper right quadrant indicates percentage of CD25+ IFN-γ+ or CD25+IL-2+ CD4 T cells. (C) Data from multiple donors (N=15) showing proportion of IFN-γ producers in CD3/mc and CD3/CD28 activated cells. Number in top graph indicates mean IFN-γ production (5±8) for CD3/mc and (44±24) for CD3/CD28 differing with high significance (p<0.001). Bottom graph contains data from multiple donors (N=10) showing proportion of IL-2 producers in CD3/mc and CD3/CD28−activated cells. Number indicates mean IL-2 production (3±2.5) for CD3/mc and (32±13) for CD3/CD28 cells differing with high significance (p<0.001).
Because the human population exhibits variability between individuals, we performed a large scale analysis of signaling and functional parameters from in vitro activated CD4+CD25− T cells from 25 healthy donors. Functional data compiled from multiple donors shows that CD3/mc-primed effector T cells are hypo-responsive in their ability to produce either IFN-γ or IL-2, compared to high proportions of IFN-γ (Fig. 1C left, average 44%) and IL-2 producers (Fig. 1C right, average 32%) from CD3/CD28-primed effector cells in all donors analyzed. These results indicate that CD3/mc-primed effector cells are impaired in their ability to produce cytokines when triggered through the TCR, whereas CD3/CD28 effector cells remain fully competent for TCR-mediated cytokine production.
Distinct TCR-coupled signaling in functional and hyporesponsive effector cells
To assess the TCR signaling profile of these differentially generated effector T cells, we purified each CD4 T cell population, removing contaminating monocytes from CD3/mc activated cells ([15], and see methods) and analyzed total tyrosine phosphorylation in lysates from resting CD4+CD25−, CD3/CD28 or CD3/mc effector cells directly or following additional TCR/CD3 crosslinking. We found that the basal level of tyrosine phosphorylation in both effector cell types was higher than in resting counterparts as we previously observed in diverse systems [13, 20], with TCR/CD3 crosslinking inducing the phosphorylation of specific protein species in all cell types (Fig. 2A, lanes 2, 4, 6). Notably, phosphorylation of a 21 kDa protein corresponding to phospho-CD3ζ was strongly induced following TCR/CD3 crosslinking of resting CD4+CD25− T cells as expected (Fig. 2A, lanes 1,2), was poorly expressed in effector cells generated by anti-CD3/mc stimulation (lanes 5,6) as we previously reported [13], yet was expressed in effector cells generated by anti-CD3/CD28 stimulation comparable to levels detected in resting CD4+CD25− T cells (lanes 3,4). These results indicate that CD3ζ phosphorylation in effector cells varies according to the type of stimulation.
Figure 2. Biochemical analysis of resting and differentially activated CD4+CD25− T cells.
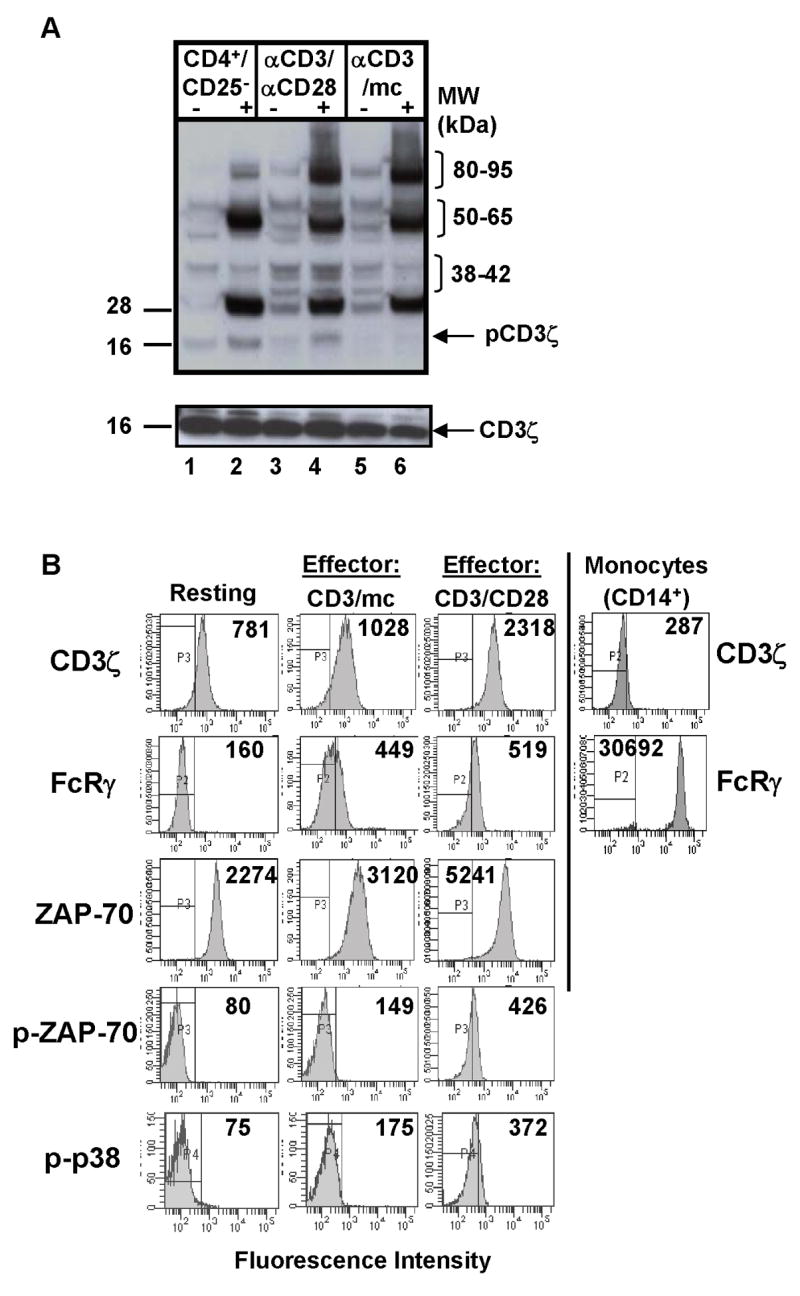
(A) Phosphotyrosine immunoblot of resting CD4+CD25− T cells, CD3/CD28 cells, and CD3/APC cells (2×106 cell equivalents/lane) either unstimulated (“−”) or crosslinked (“+”) with IgM anti-CD3 antibody. Blot was stripped and reprobed for total CD3ζ protein (lower blot). (B) Intracellular analysis of TCR-coupled signaling in resting and effector CD4 T cells. Human CD4+CD25− cells were stimulated with anti-CD3/anti-CD28 antibodies or anti-CD3/mc for 48 hours (see methods) and cells were stained intracellularly for CD3ζ, FcRγ ZAP-70, phosphorylated ZAP-70 (pZAP-70) and phosphorylated p38 kinase (p-p38) expression. Isolated monocytes were surface stained for CD14 expression and intracellularly stained with anti-CD3ζ and anti-FcRγ antibody followed by anti-rabbit-FITC-conjugated antibody as a secondary stain for FcRγ expression. Boundaries are drawn based on isotype-matched controls and numbers in histograms indicate mean fluorescence intensity.
To further dissect how proximal signaling alterations in hyporesponsive and functional effector cells were coupled to downstream function, we used intracellular analysis of signaling molecules by multiparameter flow-cytometry. In contrast to analyses of total cellular lysates, flow cytometric analysis of biochemical pathways enables quantitative measurements of native proteins and protein modifications, simultaneous observations of multiple signaling molecules at the single cell level or gated on specific cell subsets [21, 22], and characterization of signaling molecules altered in response to a given perturbation [21]. Using this approach, we analyzed the expression of proximal TCR-associated molecules and downstream phosphorylation in resting and effector human CD4 T cells, including expression of CD3ζ, the FcRγ subunit that we previously found to be up-regulated following activation of human CD4 T cells [15], the expression and phosphorylation of the proximal kinase ZAP-70, and phosphorylation of distal p38 MAP kinase.
We found differences in the expression level and phosphorylation of specific signaling molecules in effector versus resting CD4 T cells as well as differences between the two effector cell types. There was a three-fold increase in expression of the CD3ζ protein in CD3/CD28-generated effector cells compared to resting CD4 T cells, whereas CD3ζ expression in CD3/mc-generated effector cells was comparable to resting T cells (Fig. 2B, top row). Compared to resting CD4 T cells that did not express the FcRγ subunit, both CD3/mc and CD3/CD28 effector cells up-regulated FcRγ to similar extents manifested by a 2–4-fold increase in MFI in FcRγ staining in effectors versus resting CD4+CD25− T cells (Fig. 2B, second row, and data not shown). When analyzed by intracellular staining, higher levels of FcRγ expression are apparent in monocytes compared to activated T cells (Fig. 2B, second row and [15]). Analysis of FcRγ and TCR/CD3 expression by confocal microscopy likewise demonstrated up-regulation of FcRγ in both effector cell types relative to resting CD4 T cells, with regions of association of FcRγ and CD3ε observed in CD3-capped effector cells (Fig. 3). These results demonstrate FcRγ up-regulation as a feature of human T cell activation.
Figure 3. Co-localization of FcRγ-chain with the CD3ε subunit of the TCR/CD3 complex in effector CD4 T cells.
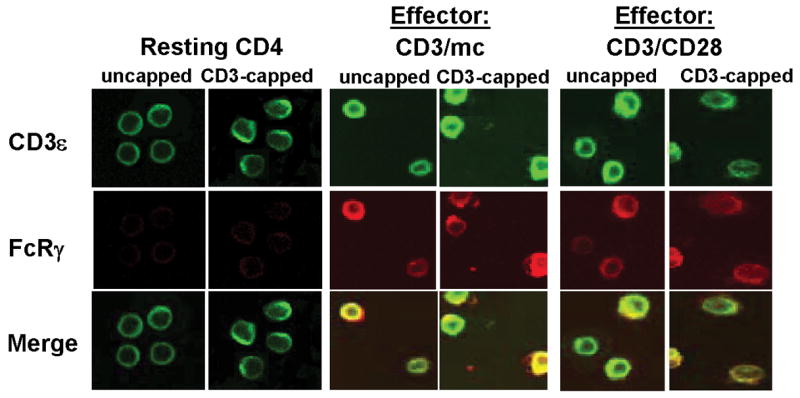
Resting CD4 T cells and differentially activated CD4 T cells were directly fixed (uncapped) or activated with IgM-anti-CD3ε Ab at 37°C to induce CD3ε cap formation before fixation (CD3–capped). Cells were surface stained with anti-CD3ε-FITC (green) and intracellularly stained with anti-FcRγ and its TRITC-conjugated secondary anti-rabbit antibody (red). The merged images (yellow) indicate co-localization of CD3ε with FcRγ. Controls with secondary Ab alone did not show red or green fluorescence (data not shown). The magnification is 100X. Results are representative of three experiments.
Differences in the expression and/or phosphorylation of the proximal ZAP-70 kinase and the distal p38 MAP kinase were also observed in the two effector cell types. Compared to resting CD4+CD25− T cells, there was a notable increase in ZAP-70 protein expression, with CD3/CD28 activated cells consistently showing higher levels of ZAP-70 compared to CD3/mc activated cells and resting cells (Fig. 2B, third row). In addition, only CD3/CD28-generated effector cells exhibited significant expression of the phosphorylated form of ZAP-70 and phosphorylated p38 MAP kinase (Figure 2B, fourth and fifth rows), compared to resting and CD3/mc-generated hyporesponsive effector cells that did not express phospho-ZAP-70 or -p38 by this analysis. The lack of ZAP-70 and p38 phosphorylation in CD3/mc effector cells is consistent with phosphotyrosine analysis of cell lysates by western blot showing decreased phosphorylation in the 38–40 and 70 kDa range of CD3/mc compared to CD3/CD28 effector cells (Fig. 2A). Together, the flow cytometry results of signaling molecule expression reveal that FcRγ is upregulated in all effector cell types, whereas increased CD3ζ and ZAP-70 expression are associated with increased downstream phosphorylation and cytokine production.
Dynamic alterations in proximal signaling during effector differentiation
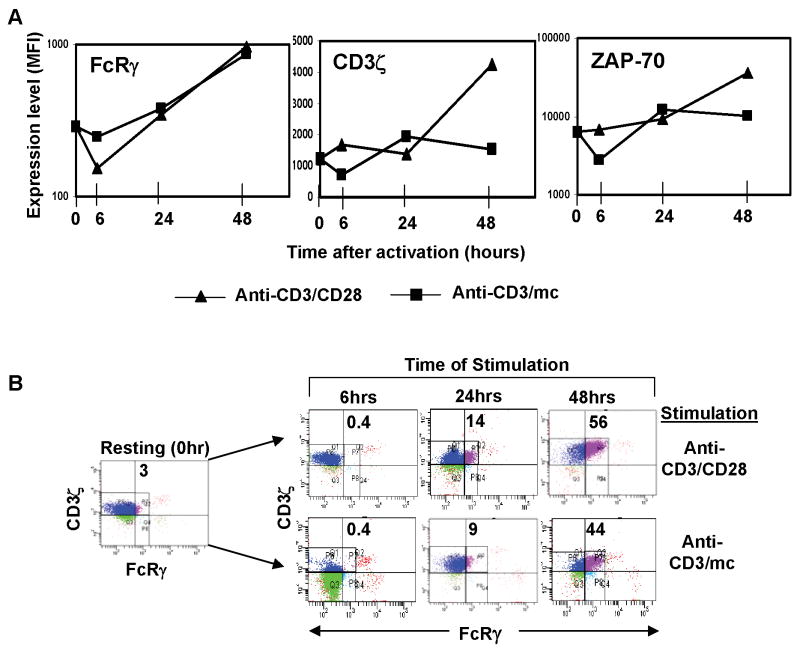
To further define the alterations in TCR-mediated signaling that accompany differential generation of effector T cells, we performed a kinetic analysis of CD3ζ, FcRγ, and ZAP-70 expression in CD4+CD25− T cells activated with CD3/CD28 or CD3/mc for 6–48 hours, with results expressed as mean fluorescence intensity of each signaling intermediate over time (Fig. 4A). We found that FcRγ up-regulation occurred after sustained (>24 hr) T cell stimulation in both effector types (Fig. 4A, left), whereas both qualitative and quantitative differences in the levels of CD3ζ and ZAP-70 expression occurred in the two activation conditions (Fig. 4A, middle and right panels). The expression of both CD3ζ and ZAP-70 protein in functional effector cells (CD3/CD28-generated) increased substantially with prolonged stimulation manifested by a three-fold increase in CD3ζ expression and a five-fold increase in ZAP-70 expression in CD3/CD28 effectors versus resting CD4 T cells (Fig. 4A, middle and right panels). By contrast, hyporesponsive effector cells did not exhibit as extensive up-regulation of CD3ζ and ZAP-70 expression from the resting state, and there was an early downregulation of both CD3ζ and ZAP-70 expression in CD3/mc effector T cells after 6 hours of activation (Fig. 4A).
Figure 4. Dynamic alterations in proximal signaling during effector differentiation.
Human CD4+CD25− cells stimulated with anti-CD3/anti-CD28 or anti-CD3/mc were harvested at indicated time-points, surfaced stained for CD4 and CD25 and stained intracellularly for FcRγ, CD3ζ, and ZAP-70 expression. (A) Graphs show change in the mean fluorescence intensity (MFI) depicted on a linear scale for FcRγ and CD3ζ expression and on a log scale for ZAP-70 expression over time following activation with anti-CD3/anti-CD28 antibodies (triangle) or anti-CD3/mc (square). Cells are gated on CD4+ T cells. (B) CD3ζ and FcRγ expression in CD4 T cells activated for 6–48 hours in the anti-CD3/CD28 activated (top panel) and anti-CD3/mc activated cells (bottom panel of dot blots). Number in quadrant indicates percentage of CD3ζ+FcRγ+ cells at each time-point, with quadrants drawn based on isotype-matched controls.
Because FcRγ was comparably up-regulated in the two effector cell types, we asked whether there were differences in the coordinate expression of CD3ζ and FcRγ in the two effector populations. Anti-CD3/CD28 stimulation resulted in the appearance of a CD3ζ+/FcRγ+ population at 24 hours, increasing in proportion by 48 hrs where CD3ζ+/FcRγ+ and CD3ζ+/FcRγ−populations are generated (Fig. 4B, upper row). Stimulation with anti-CD3/mc also resulted in similar proportions of CD3ζ+/FcRγ+ and CD3ζ+/FcRγ− populations at 48hrs although there was an overall lower level of CD3ζ, such that the CD3ζ+/FcRγ+ fraction contained reduced levels of CD3ζ (Fig. 4B, lower). Analysis of cytokine production from CD3ζ+/FcRγ+ and CD3ζ+/FcRγ−populations revealed similar IFN-γ production from each signaling subset for CD3/CD28 effectors (data not shown), indicating that the presence of FcRγ did not correlate with differences in functional capacity. Together, these results illustrate the dynamic changes in proximal signaling that accompany T cell activation and differentiation, and that coordinate expression of CD3ζ and FcRγ occurred in both functional and hyporesponsive effector cells.
Coupling of proximal FcRγ expression to downstream effector function
While the flow cytometry results suggested that FcRγ was not directing T cell function and signaling, it was necessary to modulate FcRγ expression to definitively establish or rule out causality. We used gene transfer to express the FcRγ subunit in activated CD4 T cells, prior to endogenous up-regulation of FcRγ. Compared to control transfectants, a substantial proportion of FcRγ transfectants expressed FcRγ protein (61%) (Fig. 5A), which we also found was associated with surface TCR/CD3 complex by confocal analysis (data not shown). Expression of FcRγ did not appear to alter the level of proximal signaling molecules CD3ζ or ZAP-70, as expression of these molecules by intracellular staining was comparable in FcRγ+ and FcRγ−fractions of the FcRγ transfectants and also in control transfectants (Fig. 5B, histograms). To assess whether FcRγ expression altered functional coupling through TCR/CD3 ligation, we stimulated control and FcRγ transfectants with anti-CD3/anti-CD28 antibodies, and analyzed the production of IFN-γ (Fig. 5B, bar graph). We found comparable IFN-γ production from the FcRγ− and FcRγ+ subsets of FcRγ transfectants that was likewise similar to IFN-γ production from control transfectants (Fig. 5B, bar graph). These results indicate that FcRγ expression does not alter expression of CD3ζ nor does it affect the functional coupling through the TCR/CD3 complex in effector T cells.
Figure 5. Over-expression of FcRγ protein does not alter the expression of proximal signaling molecules or IFN-γ production.
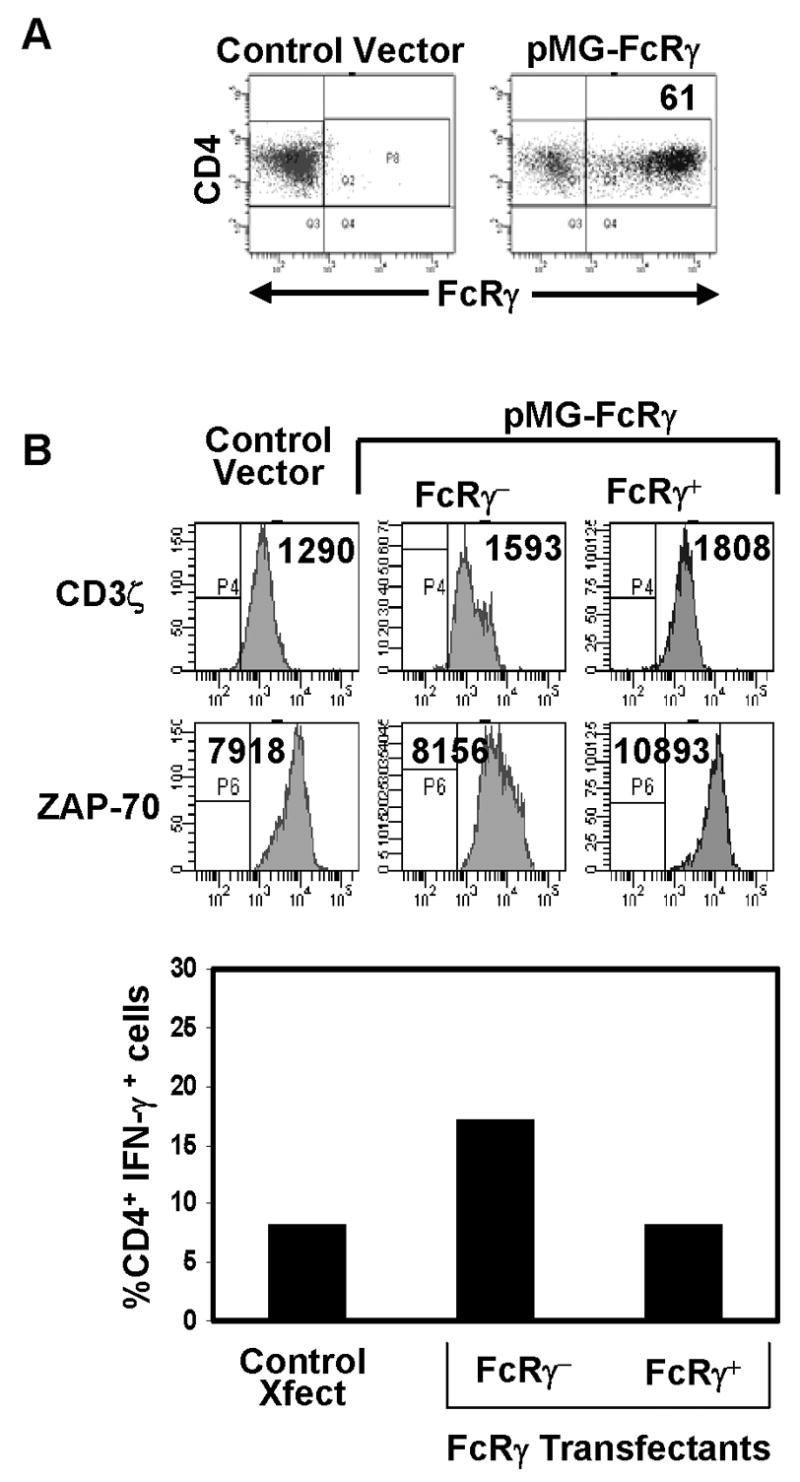
(A) FcRγ expression in activated CD4+ T cells transfected with control or FcRγ-expressing plasmids (see methods), as assessed by intracellular staining using anti-FcRγ antiserum, gated on CD4+ T cells. (B) Histograms: Expression of CD3ζ and ZAP-70 in FcRγ and control transfectants as assessed by intracellular staining. Boundaries are drawn based on isotype-matched controls and number in histograms indicates mean fluorescence intensity. Graph: Production of IFN-γ by control and PMG-FcRγ transfectants was assessed by intracellular staining and expressed as percent of CD4 T cells producing IFN-γ representative of three experiments.
Functional recovery of hyporesponsive effector cells is associated with increased CD3ζ expression
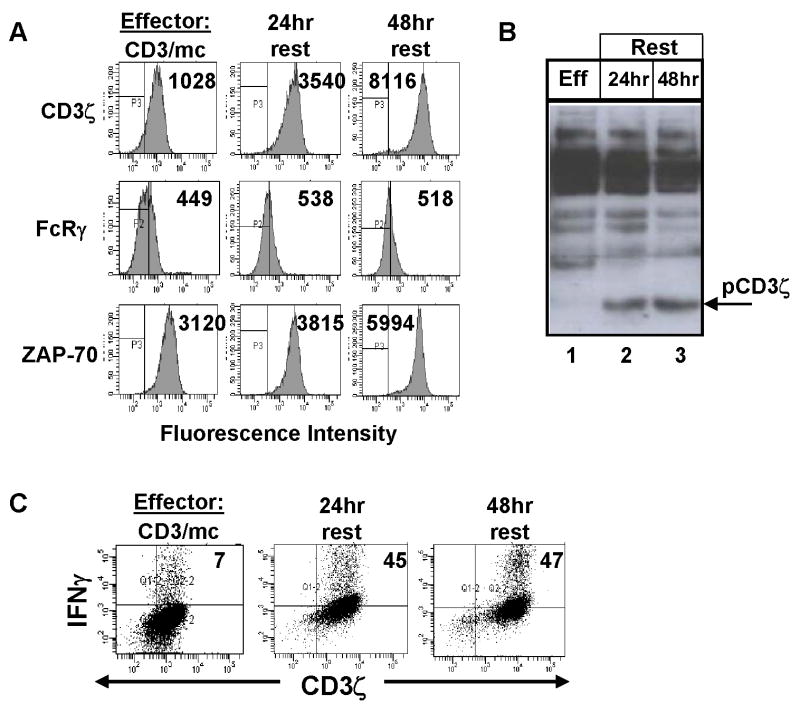
The above results demonstrated that up-regulation of FcRγ expression in effector cells does not affect the level of CD3ζ and ZAP-70 expression in effector T cells. Our previous results using western blot analyses had suggested that CD3ζ expression in CD3ζlo effector cells could be restored upon removal of the activating stimulus [12, 15]. We therefore asked whether prolonged culture of hypo-responsive effector cells without further stimulation could result in alterations in proximal signaling and/or functional capacity. As shown in Figure 6A, culture of CD3/mc-generated effector cells in media alone for 24 and 48 hours resulted in marked increases in CD3ζ expression, (eight-fold increase after 48hrs) and increases in ZAP-70 expression, although to a lesser extent than CD3ζ (Fig. 6A, third and first row, respectively). By contrast, there was no change in FcRγ expression in effector cells following prolonged culture, providing further evidence that fluctuations of CD3ζ expression are uncoupled from FcRγ expression (Fig. 6A, second row). Phosphorylation of CD3ζ was likewise observed in 24- and 48hr-rested effector cells, demonstrating a restoration of proximal signaling through CD3ζ (Fig. 6B, lanes 2, 3). Functionally, 24hr and 48hr-rested effector cells exhibited a 5–7-fold increase in capacity to produce IFN-γ in response to TCR/CD3 triggering, which occurred from T cells expressing higher levels of CD3ζ than the initial CD3/mc effector cells (Fig. 6C). These results demonstrate that the functional capacity of effector cells is associated with alterations in CD3ζ expression; low CD3ζ expression and lack of CD3ζ phosphorylation correlates with low level downstream signaling and functional hyporesponsiveness through the TCR, whereas high level CD3ζ expression and phosphorylation correlates with increased effector function and signaling through the TCR.
Figure 6. Functional recovery of hyporesponsive effector cells occurs in the presence of upregulated CD3ζ expression.
Purified anti-CD3 effector T cells were depleted of monocytes and cultured in media alone for 24 and 48 hours. (A) Expression of CD3ζ FcRγ and ZAP-70 in CD3/mc effectors prior to, or following 24hr and 48hr culture. Boundaries are drawn based on isotype-matched controls for each antibody and number in histograms indicates mean fluorescence intensity. (B) Phosphotyrosine immunoblot of lysates from 2×106 CD3/mc effector cells and equivalent numbers of 24- and 48 hour-rested effectors. Results are representative of two separate experiments. (C) CD3/mc, and rested effector cells were stimulated for 6hrs with anti-CD3/anti-CD28 antibodies, harvested and stained intracellularly for expression of CD3ζ and IFN-γ. Quadrants are drawn based on the isotype-matched controls and each number in the upper right quadrant represents cells producing IFN-γ, and are representative of four experiments.
DISCUSSION
In this study, we took multiple approaches to analyze TCR-coupled signaling changes and their functional coupling during activation and differentiation to human effector CD4 T cells. We demonstrate that differential activation of resting T cells resulted in the generation of effector cells with distinct biochemical signaling profiles and functional capacities. T cell differentiation led to up-regulation and association of the proximal signaling subunit FcRγ with the TCR/CD3ε complex. However, neither endogenous expression of FcRγ in effector T cells nor over-expression of FcRγ in activated T cells resulted in significant signaling or functional outcomes. By contrast, increased expression of CD3ζ correlated with effector function, as high levels of IFN-γ and IL-2 were produced by CD3ζhi effector cells; whereas, CD3ζlo effectors were hyporesponsive to TCR triggering. Increased CD3ζ and ZAP-70 expression in hyporesponsive effector cells resulted in enhanced capacity to produce IFN-γ upon TCR stimulation. Together, our results suggest that the effector T cell function can be controlled at the proximal level of TCR-mediated signaling, with CD3ζ playing a dominant role in this process.
FcRγ up-regulation occurred after sustained (>24 hr) T cell stimulation and was independent of the mode of activation. We found that endogenous up-regulation of FcRγ in effector T cells was heterogeneous; however, FcRγ expression did not distinguish effector T cells with different functional capacities and forced over expression of FcRγ did not alter the expression of proximal signaling molecules CD3ζ and ZAP-70, or affect functional coupling leading to IFN-γ production. While we did not detect a primary role for the FcRγ subunit in modulating TCR-mediated signaling or functional responses, we propose that FcRγ in a setting where CD3ζ expression is very low may have more dominant effects on function or downstream signaling. In support of this idea, it has been shown that T cells isolated from CD3−/− mice expressing an FcRγ transgene (FcRγTG/ζKO mice) exhibit a marked polarity towards IFN-γ production when compared to wildtype T cells [23]. In addition, FcRγ+/CD3ζlo T cells have been identified in the periphery of patients with the autoimmune disease SLE, [18], and SLE T cells likewise exhibit defects in IL-2 production [24]--both features similar to the hyporesponsive FcRγ+/CD3ζlo effectors examined here. However, the defect in CD3ζ expression in SLE T cells is at the transcriptional and genetic level [25–27], suggests that certain TCR-mediated signaling alterations in SLE T cells [28] could derive directly from FcRγ signaling [29]. In healthy T cells that we found to exhibit transient alterations in CD3ζ expression ([13], and this study), FcRγ may play more of a structural role as it is known to stabilize surface TCR expression, albeit less efficiently than CD3ζ [30–32]. It is possible that up-regulation of FcRγ after T cell activation is important for restoring TCR expression during transient CD3ζ downregulation, and enable sustained TCR signaling necessary for effector cell development, but its expression may be less critical in differentiated T cells.
Our results demonstrate that high levels of IL-2 and IFN-γ were produced by effectors exhibiting increased expression and phosphorylation of CD3ζ conversely; low CD3ζ expression and lack of phospho-CD3ζ were biochemical features of hyporesponsive effectors. We summarize the phenotype, function and signaling profile of resting, functional and hyporesponsive effector cells in Table I. While functional effector cells with CD3ζ expression and phosphorylation are associated with productive immune responses, loss of CD3ζ expression in CD4 T cells has been reported in multiple chronic diseases characterized by T cell dysfunctions, such as cancer, viral infections, and autoimmune conditions [12, 16, 17]. Our findings that differential activation of resting T cells from healthy donors could also result in effector T cells exhibiting downregulation of CD3ζ show that loss of CD3ζ expression is a signaling change that occurs during the course of T cell differentiation. Variations in the level of CD3ζ expression resulting from the two types of activation suggests that CD3ζ can serve as a potential target for fine-tuned modulation of T cell functional capacity.
Table I.
Summary of signaling profiles associated with functional subsets of human CD4 T cells and their correlations to in vivo immune responses and disease states
| Cell Type | Phenotype | Cytokine production | Signaling Intermediatesa | Disease Implications |
|---|---|---|---|---|
|
Resting
CD4+CD25− T cells |
CD25lo
CD69lo CD62Lhi CTLA-4lo |
IL-2 | CD3ζ+
pCD3ζ++ FcRγ− ZAP-70+ pZAP-70+ |
--- |
|
Functional
Effector T cells (anti-CD3/CD28) |
CD25hi
CD69hi CD62Llo CTLA-4hi |
IL-2 (high)
IFN-γ (high) |
CD3ζ++
pCD3ζ++ FcRγ+ ZAP-70++ pZAP-70++ |
Productive immune response |
|
Hyporesponsive
Effector T cells (anti-CD3/mc) |
CD25hi
CD69hi CD62Llo CTLA-4hi |
IL-2 (low)
IFN-γ (low) |
CD3ζlo
pCD3ζlo FcRγ+ ZAP-70+ PZAP-70− |
TILs
Chronic viral infections SLE |
p = tyrosine phosphorylated; lo = low level expression or phosphorylation
Indicates protein expression or phosphorylation
We also demonstrate here that expression of the ZAP-70 protein tyrosine kinase is likewise associated with differences in effector capacity. ZAP-70 expression increased with activation by anti-CD3/CD28, with functional effector cells exhibiting higher levels of ZAP-70 expression and phosphorylation compared to hyporesponsive effector cells. These results suggest that CD3ζ expression and phosphorylation in turn, affects the expression and signaling role of its associated ZAP-70 tyrosine kinase. We have recently found that ZAP-70 expression remains elevated in memory CD4 T cells and is associated with rapid effector function (Chandok, M. and Farber, D.L., submitted for publication), indicating a potentially important role for modulation of ZAP-70 kinase expression in T cell differentiation.
In conclusion, these findings suggest that primary activated/differentiated T cell function is intrinsically regulated at the level of the TCR-proximal signaling complex, specifically through the level of CD3ζ expression and signaling, and that up-regulation of FcRγ and transient modulation of CD3ζ expression are signaling changes that occur during the course of differentiation or activation. Our results have implications for modulation of T cell responses in vivo by altering the activity or expression of TCR-coupled signaling molecules acting at the most proximal level of T cell activation.
Acknowledgments
We wish to thank members of the Farber and Vogel laboratory for serving as blood donors for these studies. This work was supported by NIH AI42092 awarded to D.L.F.; F.O. was supported by NIH F31 AI058924; G.C.T. by NIH AI042269
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jordan MS, Singer AL, Koretzky GA. Adaptors as central mediators of signal transduction in immune cells. Nat Immunol. 2003;4:110–116. doi: 10.1038/ni0203-110. [DOI] [PubMed] [Google Scholar]
- 2.Kane LP, Lin J, Weiss A. Signal transduction by the TCR for antigen. Curr Opin Immunol. 2000;12:242–249. doi: 10.1016/s0952-7915(00)00083-2. [DOI] [PubMed] [Google Scholar]
- 3.Bradley LM, Duncan DD, Tonkonogy SL, Swain SL. Characterization of antigen-specific CD4+ effector T cells in vivo: Immunization results in a transient population of MEL14-, CD45RB- helper cells that secretes IL-2, IL-3, and IL-4. J Exp Med. 1991;174:547–559. doi: 10.1084/jem.174.3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iezzi G, Karjalainen K, Lanzavecchia A. The duration of antigenic stimulation determines the fate of naive and effector T cells. Immunity. 1998;8:89–95. doi: 10.1016/s1074-7613(00)80461-6. [DOI] [PubMed] [Google Scholar]
- 5.Dubey C, Croft M, Swain SL. Naive and effector CD4 T cells differ in their requirements for T cell receptor versus costimulatory signals. J Immunol. 1996;157:3280–3289. [PubMed] [Google Scholar]
- 6.Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2:251–262. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- 7.Kaech SM, Hemby S, Kersh E, Ahmed R. Molecular and functional profiling of memory CD8 T cell differentiation. Cell. 2002;111:837–851. doi: 10.1016/s0092-8674(02)01139-x. [DOI] [PubMed] [Google Scholar]
- 8.Zhang X, Giangreco L, Broome HE, Dargan CM, Swain SL. Control of CD4 effector fate: transforming growth factor beta 1 and interleukin 2 synergize to prevent apoptosis and promote effector expansion. J Exp Med. 1995;182:699–709. doi: 10.1084/jem.182.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kane LP, Lin J, Weiss A. Signal transduction by the TCR for antigen. Curr Opin Immunol. 2000;12:242–249. doi: 10.1016/s0952-7915(00)00083-2. [DOI] [PubMed] [Google Scholar]
- 10.Chan AC, Iwashima M, Turck CW, Weiss A. ZAP-70: A 70 kd Protein-Tyrosine Kinase that associates with the TCR ζ chain. Cell. 1992;71:649–662. doi: 10.1016/0092-8674(92)90598-7. [DOI] [PubMed] [Google Scholar]
- 11.Iwashima M, Irving BA, van Oers NS, Chan AC, Weiss A. Sequential interactions of the TCR with two distinct cytoplasmic tyrosine kinases. Science. 1994;263:1136–1139. doi: 10.1126/science.7509083. [DOI] [PubMed] [Google Scholar]
- 12.Krishnan S, Farber DL, Tsokos GC. T cell rewiring in differentiation and disease. J Immunol. 2003;171:3325–3331. doi: 10.4049/jimmunol.171.7.3325. [DOI] [PubMed] [Google Scholar]
- 13.Krishnan S, Warke VG, Nambiar MP, Wong HK, Tsokos GC, Farber DL. Generation and biochemical analysis of human effector CD4 T cells: alterations in tyrosine phosphorylation and loss of CD3ζ expression. Blood. 2001;97:3851–3859. doi: 10.1182/blood.v97.12.3851. [DOI] [PubMed] [Google Scholar]
- 14.Blank U, Ra C, Miller L, White K, Metzger H, Kinet JP. Complete structure and expression in transfected cells of high affinity IgE receptor. Nature. 1989;337:187–189. doi: 10.1038/337187a0. [DOI] [PubMed] [Google Scholar]
- 15.Krishnan S, Warke VG, Nambiar MP, Tsokos GC, Farber DL. The FcRgamma subunit and Syk kinase replace the CD3zeta-chain and ZAP-70 kinase in the TCR signaling complex of human effector CD4 T cells. J Immunol. 2003;170:4189–4195. doi: 10.4049/jimmunol.170.8.4189. [DOI] [PubMed] [Google Scholar]
- 16.Whiteside TL. Down-regulation of zeta-chain expression in T cells: a biomarker of prognosis in cancer? Cancer Immunol Immunother. 2004;53:865–878. doi: 10.1007/s00262-004-0521-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baniyash M. TCR zeta-chain downregulation: curtailing an excessive inflammatory immune response. Nat Rev Immunol. 2004;4:675–687. doi: 10.1038/nri1434. [DOI] [PubMed] [Google Scholar]
- 18.Enyedy EJ, Nambiar MP, Liossis SN, Dennis G, Kammer GM, Tsokos GC. Fc epsilon receptor type I gamma chain replaces the deficient T cell receptor zeta chain in T cells of patients with systemic lupus erythematosus. Arthritis Rheum. 2001;44:1114–1121. doi: 10.1002/1529-0131(200105)44:5<1114::AID-ANR192>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 19.Bori-Sanz T, Inoue KS, Berndt MC, Watson SP, Tulasne D. Delineation of the region in the glycoprotein VI tail required for association with the Fc receptor gamma-chain. J Biol Chem. 2003;278:35914–35922. doi: 10.1074/jbc.M301826200. [DOI] [PubMed] [Google Scholar]
- 20.Hussain SF, Anderson CF, Farber DL. Differential SLP-76 Expression and TCR-Mediated Signaling in Effector and Memory CD4 T Cells. J Immunol. 2002;168:1557–1565. doi: 10.4049/jimmunol.168.4.1557. [DOI] [PubMed] [Google Scholar]
- 21.Sachs K, Perez O, Pe’er D, Lauffenburger DA, Nolan GP. Causal protein-signaling networks derived from multiparameter single-cell data. Science. 2005;308:523–529. doi: 10.1126/science.1105809. [DOI] [PubMed] [Google Scholar]
- 22.Perez OD, Nolan GP. Simultaneous measurement of multiple active kinase states using polychromatic flow cytometry. Nat Biotechnol. 2002;20:155–162. doi: 10.1038/nbt0202-155. [DOI] [PubMed] [Google Scholar]
- 23.Krymskaya L, Lee WH, Zhong L, Liu CP. Polarized development of memory cell-like IFN-gamma-producing cells in the absence of TCR zeta-chain. J Immunol. 2005;174:1188–1195. doi: 10.4049/jimmunol.174.3.1188. [DOI] [PubMed] [Google Scholar]
- 24.Linker-Israeli M, Bakke AC, Kitridou RC, Gendler S, Gillis S, Horwitz DA. Defective production of interleukin 1 and interleukin 2 in patients with systemic lupus erythematosus (SLE) J Immunol. 1983;130:2651–2655. [PubMed] [Google Scholar]
- 25.Nambiar MP, Enyedy EJ, Warke VG, Krishnan S, Dennis G, Kammer GM, Tsokos GC. Polymorphisms/Mutations of TCR-zeta-Chain Promoter and 3′ Untranslated Region and Selective Expression of TCR zeta-Chain with an Alternatively Spliced 3′ Untranslated Region in Patients with Systemic Lupus Erythematosus. J Autoimmun. 2001;16:133–142. doi: 10.1006/jaut.2000.0475. [DOI] [PubMed] [Google Scholar]
- 26.Chowdhury B, Tsokos CG, Krishnan S, Robertson J, Fisher CU, Warke RG, Warke VG, Nambiar MP, Tsokos GC. Decreased stability and translation of T cell receptor zeta chain mRNA with alternatively spliced 3′ untranslated region contributes to zeta chain down-regulation in patients with systemic lupus erythematosus. J Biol Chem. 2005 doi: 10.1074/jbc.M501048200. [DOI] [PubMed] [Google Scholar]
- 27.Liossis SN, Ding XZ, Dennis GJ, Tsokos GC. Altered pattern of TCR/CD3-mediated protein-tyrosyl phosphorylation in T cells from patients with systemic lupus erythematosus. Deficient expression of the T cell receptor zeta chain. J Clin Invest. 1998;101:1448–1457. doi: 10.1172/JCI1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong HK, Kammer GM, Dennis G, Tsokos GC. Abnormal NF-kappa B activity in T lymphocytes from patients with systemic lupus erythematosus is associated with decreased p65-RelA protein expression. J Immunol. 1999;163:1682–1689. [PubMed] [Google Scholar]
- 29.Nambiar MP, Fisher CU, Kumar A, Tsokos CG, Warke VG, Tsokos GC. Forced Expression of the Fc Receptor gamma-Chain Renders Human T Cells Hyperresponsive to TCR/CD3 Stimulation. J Immunol. 2003;170:2871–2876. doi: 10.4049/jimmunol.170.6.2871. [DOI] [PubMed] [Google Scholar]
- 30.Rodewald HR, Arulanandam AR, Koyasu S, Reinherz EL. The high affinity Fc epsilon receptor gamma subunit (Fc epsilon RI gamma) facilitates T cell receptor expression and antigen/major histocompatibility complex-driven signaling in the absence of CD3 zeta and CD3 eta. J Biol Chem. 1991;266:15974–15978. [PubMed] [Google Scholar]
- 31.Ohno H, Aoe T, Ra C, Yamamoto T, Saito T. TCR isoform containing the Fc receptor gamma chain exhibits structural and functional differences from isoform containing CD3 zeta. Int Immunol. 1993;5:1403–1411. doi: 10.1093/intimm/5.11.1403. [DOI] [PubMed] [Google Scholar]
- 32.Liu CP, Lin WJ, Huang M, Kappler JW, Marrack P. Development and function of T cells in T cell antigen receptor/CD3 zeta knockout mice reconstituted with Fc epsilon RI gamma. Proc Natl Acad Sci U S A. 1997;94:616–621. doi: 10.1073/pnas.94.2.616. [DOI] [PMC free article] [PubMed] [Google Scholar]