Abstract
A2E, an important constituent of lipofuscin in human retinal pigment epithelium (RPE), is thought to mediate light-induced oxidative damage associated with aging and other ocular disorders. Ocular carotenoids in overlying retinal tissues were measured by HPLC and mass spectrometry and were correlated with levels of RPE A2E. We observed a statistically significant increase in total A2E levels in human RPE/choroid with age, and A2E levels in macular regions were approximately 1/3 lower than in peripheral retinal regions of the same size. There was a statistically significant inverse correlation between peripheral retina carotenoids and peripheral RPE/choroid A2E. Prospective carotenoid supplementation studies in Japanese quail demonstrated nearly complete inhibition of A2E formation and oxidation. These findings support current recommendations to increase dietary intake of xanthophyll carotenoids in individuals at risk for macular degeneration and highlight a new potential mechanism for their protective effects--inhibition of A2E formation and oxidation in the eye.
Keywords: Lutein, Zeaxanthin, Lipofuscin, A2E, Retinal Pigment Epithelium (RPE), Retina, Age-Related Macular Degeneration (AMD), Photooxidation
Introduction
Age-related macular degeneration (AMD) is the major cause of irreversible blindness in developed countries, yet its molecular pathophysiology remains inadequately understood [1]. Cellular damage due to high levels of oxidative stress appears to be one of the main pathological explanations for age-related ocular diseases including AMD [2], and cellular accumulation of lipofuscin, a complex mixture of highly fluorescent retinoid and phospholipid metabolites, is considered to be a primary pathogenic biomarker of aging in the retinal pigment epithelium (RPE) [3].
A2E is the one of the important constituents of lipofuscin in the RPE. Chemically, it is a combination of two all-trans-retinal molecules and one ethanolamine molecule [4]. Levels of A2E and other lipofuscin components rise with age, with light exposure, and with development of AMD, and early onset macular disorders such as Stargardt and Best diseases are notable for unusually high levels of A2E in humans and in animal models [5–9]. Studies have demonstrated that lipofuscin components such as A2E and its cis isomers can act as blue-light-mediated photosensitizers for the generation of reactive oxygen species that could cause damage and cell death in the macula, potentially leading to loss of central vision [10–11].
On the other hand, the dietary xanthophyll carotenoids lutein and zeaxanthin are concentrated at very high levels in the human macula and to a lesser extent in the peripheral retina where they are believed to limit retinal oxidative damage by absorbing incoming blue light and/or by quenching reactive oxygen intermediates [12–13]. In vitro studies have suggested that the ocular carotenoids may alleviate A2E-mediated oxidative damage either by direct quenching or by screening phototoxic blue light [14], but in vivo evidence is notably lacking, in part due to the difficulty in obtaining human ocular tissues and the rarity of non-primate small animal models that accumulate significant levels of both A2E and ocular carotenoids. Here we report the relationship of A2E and carotenoids in the macula and peripheral retina of a large collection of human eyes, and we study the inhibition of A2E formation by dietary carotenoids in the Japanese quail Coturnix japonica, a bird that has substantial ocular levels of both A2E and carotenoids.
Materials and methods
Chemicals
Organic solvents were HPLC grade from Fisher Scientific (Hampton, NH). Standards of A2E and iso-A2E were prepared and column purified in the laboratory of Dr. Heidi R. Volmer-Snarr, Department of Chemistry and Biochemistry, Brigham Young University, Provo, Utah. They were dissolved in methanol (MeOH) at a concentration of 1 μg/ml, stored at −70 °C, and brought to room temperature before use. The concentration of the stock solution of A2E was confirmed spectroscopically [E (1M) at 439 nm = 36900] using a published extinction coefficient [4]. Similar standard stock solutions of lutein (Kemin Health, Des Moines, IA) and zeaxanthin (DSM, Kaiseraugst, Switzerland) were prepared, and concentrations were confirmed using published extinction coefficients [15].
Tissue procurement and processing
Human donor human eyes were obtained from the Utah Lions Eye Bank within 24 hours after death after corneas had been harvested for transplantation. None of the donors had a known history of eye disease. Tissue procurement and distribution complied with the tenets of the Declaration of Helsinki. The time between donor death and enucleation was not more than four hours. Dissections were carried out 6–24 hours after donor death in a dim light environment. These data exclude outliers with unusually high levels of ocular carotenoids who had been consuming high-dose lutein supplements regularly prior to death because of potential confounding effects on data interpretation since exact dosage and duration of supplementation were unknown [16]. All eyecups were visually inspected with a handheld magnifier to exclude the presence of obvious ocular pathology such as intermediate or large drusen, hemorrhages, or scars. After carefully removing adherent vitreous, macular and mid-peripheral retinal tissues were excised with an 8-mm circular trephine. The underlying RPE/choroid layer was then carefully excised using the same trephine. Diverse non-human mammalian eyes were obtained from neighboring laboratories or local slaughterhouses. Since non-primate animal eyes do not have a macula, the entire retina and RPE/choroid were isolated and processed. Wet weights were recorded for all collected tissues after blotting excess moisture. Total protein levels and protein separation patterns determined by Bradford assay and by one-dimensional SDS gel electrophoresis, respectively, were similar in both macular and retinal RPE/choroid punches from humans (~250 μg/8-mm of tissue).
Extraction of A2E from RPE/choroid
A2E and its isomers were extracted and isolated from RPE/choroid using a previously described method [8]. RPE samples were homogenized in 1:1 CHCl3/MeOH (2 ml) and 0.01 M phosphate-buffered saline (PBS) (1 ml). The homogenizer was washed with 1:1 CHCl3/MeOH(2 ml), 0.01 M PBS (1 ml), and then CHCl3 (2 ml) and CH2Cl2 (2 ml) were added to remove any remaining material. All solutions were combined, and the organic layer was extracted from the aqueous layer. The combined organic extracts were evaporated to dryness under vacuum at room temperature. The residue was dissolved in MeOH for HPLC. The vials were centrifuged at approximately 2000 g to remove the minor amounts of insoluble solid particles prior to analysis.
Extraction of carotenoids from retina
Tissues were homogenized and extracted three times with tetrahydrofuran containing 0.1% butylated hydroxytoluene by sonication at 5°C to 10°C for 30 minutes each time. The combined organic extracts were evaporated to dryness under vacuum at room temperature. The dried residue was redissolved in one ml of HPLC mobile phase and centrifuged at approximately 2000 g for 10 minutes to remove the minor amounts of insoluble solid particles prior to analysis. The majority of carotenoids in bird retinas are esterified [17], so after the initial extraction, the dried carotenoid residue was redissolved in hexane and subjected to saponification in 1.8 % (w/v) methanolic potassium hydroxide (KOH) for two hours at room temperature. After saponification, the samples were washed with water until the samples achieved neutral pH. The vials were centrifuged at approximately 2000 g to remove the minor amounts of insoluble solid particles. The solution was evaporated to dryness on a rotary evaporator under reduced pressure at room temperature and reconstituted in the appropriate HPLC solvents.
HPLC conditions
HPLC analysis was performed on a Thermo Separations (San Jose, CA) HPLC system with binary gradient pumps, a refrigerated autosampler, a UV6000 photodiode-array detector (PDA), and an MSQ single quadrupole mass spectrometer. Peak identities were confirmed by PDA and mass spectra and by co-elution with authentic standards as necessary. Calibration was by external standardization curves with authentic standards. We do not routinely use internal standards because they may interfere with low-level analytes in small biological samples [18]. Typical reproducibility with external standardization in our laboratory is ± 5%.
A2E HPLC analysis
The dried A2E samples were re-dissolved in 100 μl of MeOH. A gradient of 84–100% acetonitrile (A) with 0.05% trifluoroacetic acid in H2O (B) over 35 minutes was used to separate A2E at a flow rate of 1.0 ml min−1 on a reverse-phase C18 column (4.6 × 250 mm, Phenomenex, Atlanta, GA). The column was maintained at room temperature, and the HPLC PDA detector was operated at 440 nm.
Carotenoid HPLC analysis
The dried extracts were re-dissolved in 100 μl of HPLC mobile phase [hexane: dichloromethane: methanol: N,N′-di-isopropylethylamine (80:19.2:0.7:0.1 v v−1)]. HPLC separation was carried out at a flow rate of 1.0 ml min−1 on a cyano column (Microsorb 25 cm length × 4.6 mm id, particle size 5 μm, Rainin Instrument Co., Woburn, MA). The column was maintained at room temperature, and the HPLC PDA detector was operated at 450 nm.
Mass spectrometry (MS) equipment and conditions
MS analysis was performed using a Thermo Separations (San Jose, CA) MSQ single quadrupole mass spectrometer, equipped with an electron spray ionization (ESI) source and an atmospheric pressure chemical ionization (APCI) source. A2E and carotenoids were ionized in positive ion ESI and APCI modes, respectively. To avoid overloading of eluted solvent molecules in the mass spectrometer and to optimize ionization conditions, 50 % of the eluate was directed to waste with the help of a diverter valve after the PDA detector. The delay time from PDA to MS was 0.13 minutes. The protonated precursor molecular ions were initially acquired in full-scan mode from 300–1000 Da with 0.2 step size, revealing the molecular masses of the components. Selected ion monitoring (SIM) was performed using dwell time of 200 ms for each channel. In SIM mode, the m/z channels 592 ± 3, 608± 1.5, 624± 1.5, and 640 ± 1.5 were used for A2E and its oxidative products. Typical detection conditions for A2E were: RF lens bias voltage 0.1V, cone voltage 80V and heater temperature 550 °C. The ion source and tuning lens parameters were optimized automatically by infusing A2E samples via the injector. For carotenoids, the m/z channels 551 ± 0.7 and 569 ± 0.7 were used for lutein, and 569 ± 0.8 for zeaxanthin. Typical detection conditions were: corona discharge current 5 μA, cone voltage 80V, and probe temperature 500 °C.
Xanthophyll supplementation studies in Japanese quail
Adult Japanese quail (Coturnix japonica) were procured from B&D Farms (Harrah, Oklahoma), and all experiments were reviewed and approved by the University of Utah’s Institutional Animal Care and Use Committee. The birds were kept under ambient room light on a 12-hour light-dark cycle, and they were divided into three groups of four birds: lutein supplemented, zeaxanthin supplemented, and unsupplemented. All birds were fed daily with a standard turkey diet (~20 g/day) (Purina Mills, St. Louis, Missouri) that was low in carotenoids (1–10 ng/g). The supplemented birds were gavaged daily with 0.5 ml of a microbial extract rich in lutein or zeaxanthin for 16 weeks (0.2 mg of carotenoid per bird per day). Lutein was prepared from the freshwater alga Chlorella protothecoides CS 41 (Microalgae Supply Service, CSIRO, Hobart Australia) under conditions described earlier [19]. Zeaxanthin was obtained from the non-pathogenic bacteria Flavobacterium multivorum ATCC 55238 grown on liquid growth medium as detailed earlier [20]. After the experiments, the birds were sacrificed, and the A2E and carotenoid content of the RPE/choroid and retina, respectively, were measured as described above.
Statistics
Reported values are mean ± standard deviation (SD). Statistical analysis was done using Microcal Origin version 6.0 (Northampton, MA). In most cases, a two-population (independent) t-test was performed with significance level set at 0.05.
Results
A2E and carotenoid levels in human RPE/choroid and retina
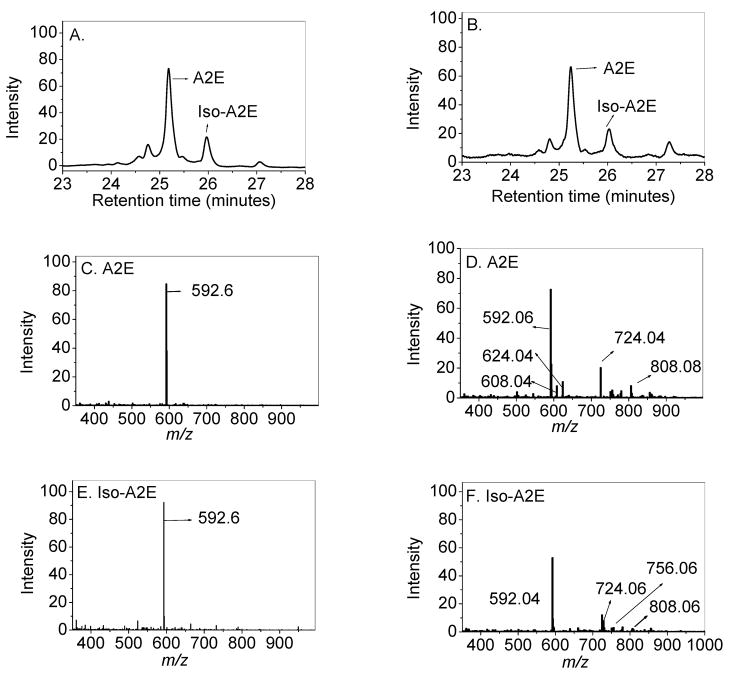
We used photodiode array (PDA) and mass-spectral (MS) detection for convenient in-line HPLC quantitation and identification of A2E and its isomers from human RPE/choroid. Younger human eyes (<50 years old; N=22) always had a single major molecular ion peak for A2E and iso-A2E at m/z 592.6, while eyes older than 50 (N=31) were more likely to exhibit detectable higher molecular weight ions of A2E, two of which are consistent with previously reported oxidation products at m/z 608 and 624 (N=24) [21–22], as well as unidentified higher m/z ions between 724 and 808 (Figure 1). Single ion monitoring (SIM) of these ions allowed the quantification these peaks relative to the A2E peak at m/z 592 ± 3, 608 ± 1.5, and 624± 1.5. The ratio of the m/z 608 and 624 peaks present in donors above 50 years relative to unoxidized A2E was 0.027 ± 0.008 (N=24), while these oxidized peaks were absent in the donors below age 50.
Fig 1.
HPLC PDA-chromatograms (A and B) and full scan mass spectra (C, D, E, F) of the A2E and iso-A2E peaks from the RPE/choroids of a 14-year-old (A, C, & E) and a 74-year-old (B, D, & F) donor.
Initial experiments with whole RPE/choroid from human eyes between ages 20 to 88 revealed a linear increase of A2E with age (N=66, P<0.0001) (Figure 2A). This same significant increase with age was present in both the macula and the peripheral retina, although A2E levels were typically 4-fold higher in the periphery (Figures 2B and 2C).
Fig 2.
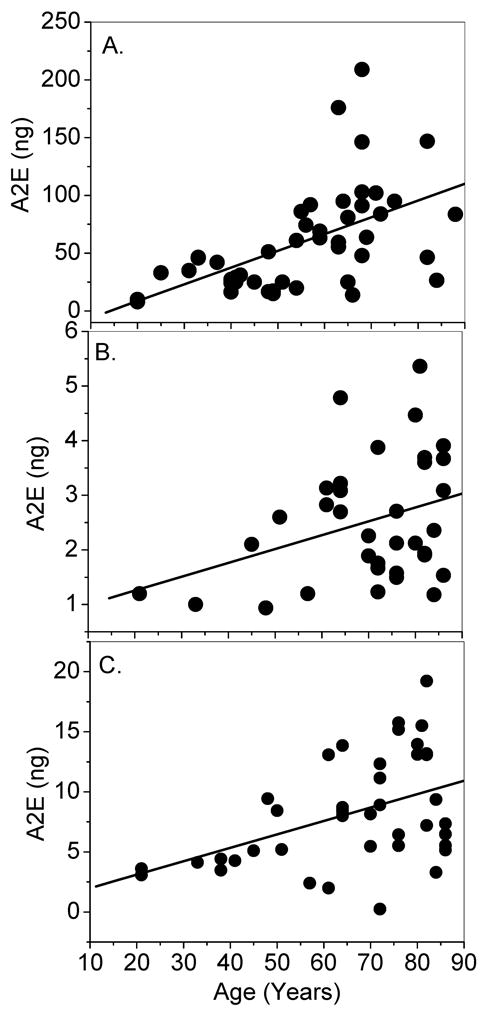
Agewise distribution of A2E levels in human whole RPE/choroid (A); 8-mm macular punches (B); and 8-mm peripheral retina punches (C). There was a significant increase observed with age (P < 0.001) in all cases.
We speculated that lower levels of macular A2E in comparison to peripheral retina might be related to higher levels of carotenoids in the overlying retina. Table 1 shows that this was indeed the case with 10-fold higher levels of total carotenoids in the macula versus the periphery and greater than 3-fold higher levels of A2E in the periphery versus the macula in young and old eyes. Retinal carotenoid levels were inversely correlated with A2E levels in the underlying RPE/choroid in both the macula (R=− 0.28; N= 35; P= 0.10) and the peripheral retina (R= −0.36, N=40, P=0.02), but only the peripheral retina reached statistical significance (Figure 3).
Table 1.
A2E levels in the RPE/choroid and carotenoids in the overlying retina in 8-mm macular and peripheral punches from human eyes (mean ± SD)*.
| Donors <50 years old (N=22) | Donors >50 years old (N=31) | Statistical significance | |
|---|---|---|---|
| Age (Years) | 37 ± 10 | 74 ± 9 | P<0.0001 |
| A2E in macula (ng) | 1.3 ± 0.5 | 2.7 ± 1.1 | P<0.001 |
| A2E in periphery (ng) | 5.1 ± 2.6 | 9.1 ± 4.6 | P<0.0001 |
| Iso-A2E in macula (ng) | 0.1 ± 0.1 | 0.8 ± 0.4 | P<0.01 |
| Iso-A2E in periphery (ng) | 0.5 ± 0.2 | 1.4 ± 0.9 | P<0.0001 |
| Total carotenoids in macula (ng) | 43.6 ± 14.6 | 38 ± 15.1 | P<0.001 |
| Total carotenoids in periphery (ng) | 2.6 ± 2.2 | 2.8 ± 2.8 | Not significant |
These data exclude outliers with unusually high levels of ocular carotenoids who had been consuming high-dose lutein supplements regularly prior to death because of potential confounding effects on data interpretation since exact dosage and duration of supplementation were unknown [16].
Fig 3.
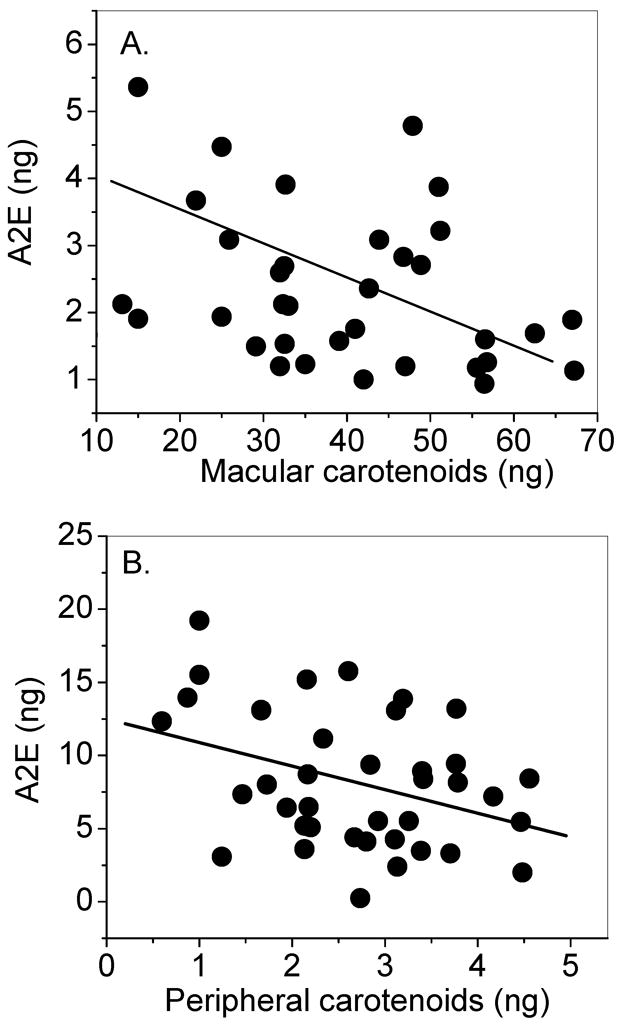
Distribution of RPE/choroid A2E levels in relation to overlying retinal carotenoid levels in 8-mm macular punches (A) and 8-mm peripheral retina punches (B). There was a statistically significant inverse correlation between carotenoids in the peripheral retina and A2E levels in the underlying RPE-choroid (R= −0.36, N=40, P=0.02); however, this inverse relationship was not statistically significant between macular carotenoids and macular A2E (R= −0.28; N= 35; P= 0.10).
A2E and carotenoid levels in animal RPE/choroid and retina
The human eye is not easily amenable to experimental manipulation of carotenoid and A2E levels due to the slow changes of macular carotenoid levels in response to dietary manipulations and the invasive nature of the A2E analytical measurements, so we surveyed the eyes of a variety of higher vertebrates and compared ocular carotenoid and A2E levels on a wet weight basis (Table 2). As expected, monkey eyes most closely resembled human eyes with respect to A2E and carotenoid content, but these laboratory animals are expensive to procure and difficult to manage and handle. Young rat and mouse eyes contain barely detectable levels of carotenoids, and there is no published literature demonstrating that high-dose carotenoid supplementation can alter these very low levels. Moreover, their A2E levels relative to tissue wet weight are orders of magnitude lower than the other animals listed. Generally, rodents have high levels of A2E only when they are appropriately genetically modified, as in the Abca4 knockout and Elovl4 transgenic mice [7–8]. Cows and, to a lesser extent, pigs have reasonable levels of A2E and carotenoids suitable for dietary manipulation, but these animals are quite large. The female Japanese quail possesses the best combination of small size and reasonable levels of ocular A2E and carotenoids, so they were selected for further study.
Table 2.
A2E and carotenoid levels in the RPE/choroid and overlying retina in a variety of higher vertebrates.
| Animal | Age | N (eyes) | RPE/choroid A2E content (ng/g wet weight ± SD) | Total retinal carotenoid content (ng/g wet weight ± SD) |
|---|---|---|---|---|
| Young human (<50 years old) | ||||
| a. macula (8-mm punch) | 23 ± 5 years | 22 | 26 ± 12 | 611.1 ± 19.6 |
| b. periphery (8-mm punch) | 102 ± 16 | 91.1 ± 15 | ||
|
| ||||
| Older human (>50 years old) | ||||
| a. macula (8-mm punch) | 60 ± 6 years | 31 | 53.4 ± 13 | 716.6 ± 12.9 |
| b. periphery (8-mm punch) | 182 ± 23 | 118.5 ± 43 | ||
|
| ||||
| Young monkey Macaca fascicularis | ||||
| a. macula (8-mm punch) | 2 ± 2 years | 8 | 73 ± 5 | 401.1 ± 15.1 |
| b. periphery (8-mm punch) | 53 ± 85 | 9.5 ± 3.2 | ||
|
| ||||
| Older monkey Macaca fascicularis | ||||
| a. macula (8-mm punch) | 9 years | 4 | 86 ± 15 | 323.1 ± 33.1 |
| b. periphery (8-mm punch) | 93 ± 85 | 3.5 ± 5.2 | ||
|
| ||||
| Marmoset Callithrix jacchus | 2 ± 1 years | 8 | 187.5 ± 1.2 | 211.1 ± 24.2 |
|
| ||||
| Cow | Adult | 2 | 16 ± 2.8 | 30.3 ± 12.1 |
| Pig | Adult | 2 | 23 ± 3.2 | 2.3 ± 2.5 |
|
| ||||
| Rat* | 3 months | 12 | 0.029 ± 0.007 | 0.002 ± 0.004 |
| Mouse* | 3 ± 3 months | 16 | 0.034 ± 0.002 | 0.006 ± 0.0019 |
|
| ||||
| Young Japanese quail | ||||
| a. female | 8 ± 2 weeks | 8 | 19.3 ± 3.2 | 43.2 ± 10 |
| b. male | 8 | 24.2 ± 7.2 | 66.2 ± 12 | |
|
| ||||
| Older Japanese quail | ||||
| a. female | 26 ± 4 weeks | 8 | 38 ± 4.6 | 32.4 ± 11.0 |
| b. male | 8 | 42 ± 2.3 | 33.7 ± 12.0 | |
For rats and mice, pooled samples of n= 6 and n=8 were used for extraction and detection of both carotenoids and A2E.
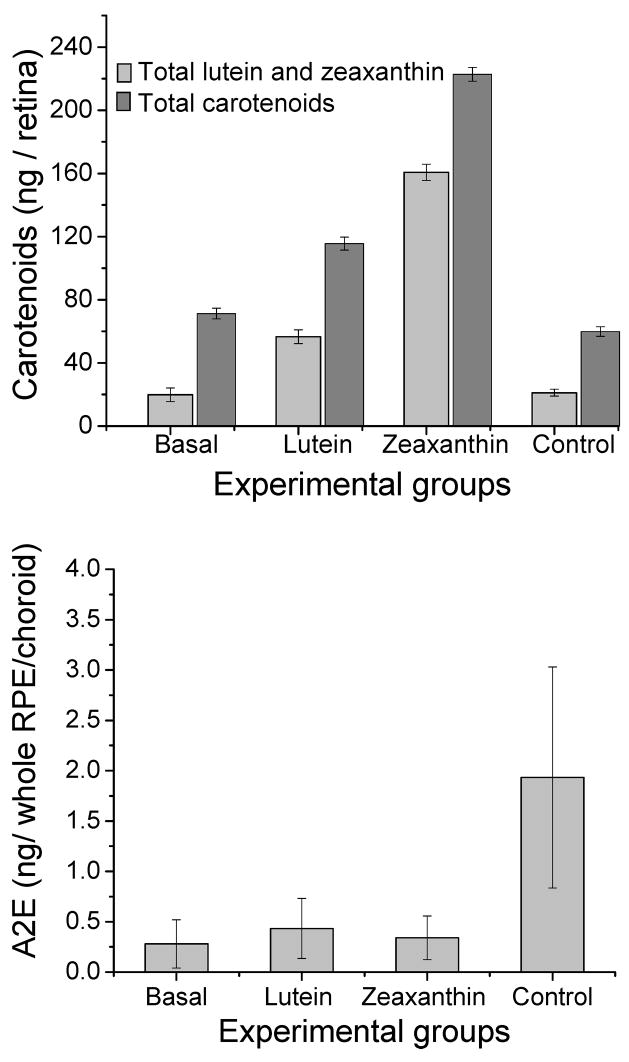
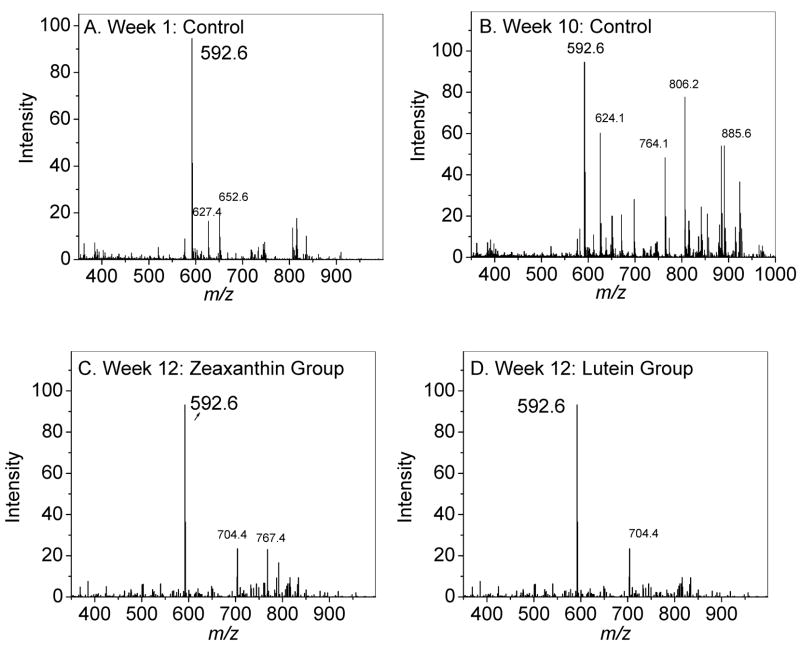
Young adult female Japanese quail were supplemented with (3R, 3′R, 6′R)-lutein or (3R, 3′R)-zeaxanthin for a 16-week period and compared to unsupplemented quail. As shown in Figure 4 and Table 3, total retinal carotenoid levels rose 1.6-fold in the lutein-supplemented group and 3.1-fold in the zeaxanthin supplemented group relative to baseline, while control diet birds decreased 16%. Total lutein and zeaxanthin levels increased 2.8-fold in the lutein supplemented group and 8.1-fold in the zeaxanthin supplemented groups relative to baseline, while control diet birds increased 6.5%. All of these increases in supplemented birds were statistically significant (P<0.05). Supplementation of lutein and zeaxanthin also led to significant increases in the levels of lutein, zeaxanthin and their carotenoid metabolites in the RPE in comparison to basal and control groups (P<0.05) (Table 3). A2E levels rose more than six-fold relative to basal levels (P<0.001) in the unsupplemented control group in a 16-week period, while the lutein and zeaxanthin supplemented birds registered barely any rise from baseline levels (P=0.1). Mass spectra of A2E from the control group had higher levels of oxidation products in comparison to mass spectra from the lutein and zeaxanthin supplemented groups (Figure 5).
Fig 4.
Manipulation of ocular carotenoid and A2E levels in response to dietary supplementation with lutein or zeaxanthin in Japanese quail for 16 weeks (N=4 for all groups). In the upper panel, dark grey bars represent total carotenoids, and light grey bars represent total lutein and zeaxanthin content in the respective groups. Control animals were on a low carotenoid diet for 16 weeks. The A2E levels in the RPE were significantly higher for the control group relative to the other three groups (P < 0.001). The A2E levels of other groups were not significantly different from each other.
Table 3.
Carotenoid content in the RPE/choroid and retina of Japanese quail at the beginning and end of the supplementation experiments.
| Carotenoids in RPE/choroid at baseline (ng/tissue ± SD; N=4) | Carotenoids in RPE/choroid at 16 weeks (ng/tissue ± SD; N=2) | Carotenoids in retina at baseline (ng/tissue ± SD; N=4) | Carotenoids in retina at 16 weeks (ng/tissue ± SD; N=4) | |||||
|---|---|---|---|---|---|---|---|---|
| Basal | Control | Lutein | Zeaxanthin | Basal | Control | Lutein | Zeaxanthin | |
| Adonirubin | 0.23 ±0.42 | 0.3 ±0.51 | 0.32 ±0.21 | 0.61 ±0.07 | 3.4 ±3.1 | 1.9 ±1.5 | 2.4 ±2.1 | 6.4 ±3.9 |
| 3′-Oxolutein | 0.31±0.09 | - | 0.15±0.12 | 1.6±1.0 | 3.8 ±3.2 | 3.1 ±1.1 | 3.8 ±3.1 | 5.9 ±2.3 |
| Lutein | 2.8±1.0 | 0.25±0.1 | 1.2±0.3 | 1.5±0.31 | 5.1±3.2 | 3.4±3.2 | 32.2±6.3 | 3.9±2.6 |
| Astaxanthin | 0.2±0.5 | 5.1±0.6 | 5.2±0.9 | 1.6±15 | 18.2±1.5 | 10.6±2.3 | 31.2±4.5 | 16.2±3.5 |
| Zeaxanthin | - | 1.72±0.3 | 3.1±0.4 | 3.8±0.9 | 14.8±5.1 | 17.8±1.1 | 24.4±2.1 | 156.8±1.1 |
| β-apo-2′-carotenol | - | - | - | - | 6.5±3.5 | 9.6±4.5 | 6.7±4.5 | 8.7±6.5 |
| Unidentified | - | - | - | - | 2.9±2.2 | 4.2±2.4 | 3.9±2.4 | - |
| ε,ε,-Carotene | - | - | - | 11.5±4.3 | 3.5±6.3 | 14.5±6.5 | 12.5±6.3 | |
| Galloxanthin | 0.2 ±5.1 | - | 2.8±2.9 | 1.2±1.2 | 9.2±4.1 | 9.2±3.2 | 03.2±6.5 | 25.2±3.1 |
| Total carotenoids | 3.5±3.1 | 7.4±0.35 | 12.8±0.2 | 10.3±0.8 | 75.4 ±3.6 | 63.3±3.2 | 122.3±4.3 | 235.6±4.6 |
Fig 5.
Full scan mass spectra of the A2E extracted from RPE of experimental birds. Week 1 control diet (A); Week 10 control diet (B); Week 12 zeaxanthin-supplemented diet (C); Week 12 lutein-supplemented diet (D).
Discussion
Ever since its identification as a major fluorophore of ocular lipofuscin, A2E has been considered an important mediator of pathological processes involved in aging and retinal degenerations [3–6]. Clinically, autofluorescent compounds in the human retinal pigment epithelium increase with age when measured with autofluorescence imaging [23–24], and early onset macular dystrophies such as Stargardt and Best diseases likewise exhibit high levels of lipofuscin deposition [7–9]. In dry age-related macular degeneration, intense autofluorescence is often seen at the border of geographic atrophy in regions in which further expansion of atrophy is likely to occur [25]. Increased levels of A2E have been confirmed biochemically in autopsy eyes of elderly individuals and in donor eyes from individuals with macular dystrophies [4–9]. Animal models of dominant and recessive Stargardt disease have been developed that accumulate A2E such as the Abca4 knockout mouse (heterozygous and homozygous) as well as the Elovl4 transgenic mouse [7–8]. In vitro studies indicate that A2E may exert its toxic effects on the RPE through blue-light-mediated free radical generation or by induction of lysosomal dysfunction through detergent-like and pH altering effects [10–11, 26–27].
There is general consensus that limiting A2E formation is a worthwhile target for pharmacological interventions against AMD and related diseases, and a number of groups in academia and industry have dedicated themselves to this task. A2E formation has been linked to intense light exposure which causes high degrees of throughput of retinoids in the visual cycle, facilitating formation of elevated amounts of various Schiff base adducts of all-trans-retinal with phosphatidylethanolamine, the precursors of A2E [28–29]. Although severe light restriction inhibits A2E formation in animal models [28], this approach is probably not practical in humans. Therefore, inhibitors of the visual cycle have been a primary focus. These include 13-cis-retinoic acid, an FDA approved acne medicine that inhibits dark adaptation through alcohol dehydrogenase and/or isomerase inhibition [30], fenretinide, a retinoid analogue that induces a moderate systemic deficiency of vitamin A [31], and RPE-65 antagonists targeted to inhibit a key step of the vertebrate mechanism which isomerizes all-trans-retinoids to 11-cis-retinoids [32]. All of these agents will cause some degree of night blindness which may be uncomfortable for the patients, and retinoid-based compounds when used chronically may cause significant systemic side effects and teratogenicity. Non-retinoid RPE-65 antagonists appear to be well tolerated in animal models, but there is little, if any, human experience with these compounds.
The macular carotenoids lutein and zeaxanthin have also been considered as possible antagonists against the formation and the toxic effects of A2E [14]. Multiple epidemiological studies have demonstrated an inverse correlation between high dietary intakes, blood levels, and macular levels of these xanthophylls and risk of AMD [33–36], and the AREDS 2 study is currently evaluating their efficacy against AMD in a large, randomized, placebo-controlled, prospective manner. The macular carotenoids efficiently absorb blue light, the region of the visible spectrum that is most likely to produce free radicals from A2E, and in vitro they can inhibit photo-oxidation of A2E and its precursors [14].
In this study, we first examined the relationship between retinal carotenoids and A2E in human donor eyes. We confirmed that A2E rises with age in both the macula and the peripheral retina, and that it becomes more oxidized with age. Interestingly, macular A2E levels in these 8-mm punches were about three-fold lower in the macula relative to the periphery despite its focused light exposure and high metabolic activity. In both the macula and the periphery, there was an inverse correlation between A2E levels and total carotenoids, although statistical significance was reached only in the periphery. Taken in sum, these human cadaver eye data are consistent with the hypothesis that retinal carotenoids inhibit formation and oxidation of A2E in the underlying RPE, but proof of this hypothesis requires either prospective clinical studies or appropriate animal experiments. Human studies would be challenging because noninvasive quantitative A2E measurements are difficult to execute with adequate reliability, and it is not possible to measure peripheral carotenoids in living humans. Therefore, we turned to animal models for further study.
Rodents have been used most frequently to study inhibitors of A2E formation, but their low endogenous ocular levels of A2E and carotenoids and their inability to incorporate carotenoids into the retina in response to supplementation make further study impossible. We surveyed a diverse array of other potential study animals and determined that the Japanese quail was the best small nonprimate animal model to study further since it had baseline levels of carotenoids and A2E comparable to a human on a wet weight basis, and it is amenable to dietary manipulation of ocular carotenoid levels. [17,37]. Our experiments demonstrated a profound inhibition of A2E formation in birds supplemented with high dose lutein or zeaxanthin relative to birds fed a control diet low in carotenoids. Since most carotenoids in the quail retina are sequestered as fatty acid esters in oil droplets, it is more likely that inhibition of A2E formation by ingested carotenoids is mediated by a light filtering effect rather than a direct antioxidant mechanism because although there was a significant increase in RPE carotenoids in response to supplementation, levels still remained about ten times lower than the overlying retina.
Our findings provide evidence for a new mechanism for the potential protective effect of lutein and zeaxanthin in degenerative eye disorders—inhibition of A2E formation and oxidation. As noninvasive quantitation of ocular lipofuscin improves, this hypothesis can be directly tested in living humans. Lutein and zeaxanthin are particularly attractive agents for clinical use as A2E formation inhibitors because, unlike the other inhibitors currently under investigation, they are already extensively used in humans as dietary supplements, and no toxicity has ever been reported. Moreover, our investigations point out the value of non-rodent small animal models for testing A2E formation antagonists. At 150–200 grams, an adult Japanese quail is the size of a rat, is easy to care for, and forms several-fold increased levels of A2E over a several month period when fed a control diet low in carotenoids. Thus, rapid screening of novel pharmacological inhibitors of A2E formation should be quite feasible.
Acknowledgments
This work was supported by National Institute of Health Grant EY-11600, The Ruth and Milton Steinbach Foundation (New York, NY), and by Research to Prevent Blindness, Inc. (New York, NY). The authors thank Dr. Heidi R. Volmer-Snarr, Department of Chemistry and Biochemistry, Brigham Young University, Provo, Utah for providing us with A2E and iso-A2E standards. The technical assistance from Da You Zhao, MD is greatly appreciated. The Utah Lions Eye Bank generously provided human donor eyes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rattner A, Nathans J. Nat Rev Neurosci. 2006;7:860–872. doi: 10.1038/nrn2007. [DOI] [PubMed] [Google Scholar]
- 2.Beatty S, Koh H, Phil M, Henson D, Boulton M. Surv Ophthalmol. 2000;45:115–134. doi: 10.1016/s0039-6257(00)00140-5. [DOI] [PubMed] [Google Scholar]
- 3.Dorey CK, Wu G, Ebenstein D, Garsd A, Weiter JJ. Invest Ophthalmol Vis Sci. 1989;30:1691–1699. [PubMed] [Google Scholar]
- 4.Parish CA, Hashimoto M, Nakanishi K, Dillon J, Sparrow JR. Proc Natl Acad Sci USA. 1998;95:14609–14613. doi: 10.1073/pnas.95.25.14609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lamb LE, Simon JD. Photochem and Photobiol. 2004;79:127–136. doi: 10.1562/0031-8655(2004)079<0127:aacool>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 6.Sparrow JR, Boulton M. Exp Eye Res. 2005;80:595–606. doi: 10.1016/j.exer.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Mata NL, Weng J, Travis GH. Proc Natl Acad Sci USA. 2000;97:7154–7159. doi: 10.1073/pnas.130110497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karan G, Lillo C, Yang Z, Cameron DJ, Locke KG, Zhao Y. Proc Natl Acad Sci USA. 2005;102:4164–4169. doi: 10.1073/pnas.0407698102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bakall B, Radu RA, Stanton JB, Burke JM, McKay BS, Wadelius C, Mullins RF, Stone EM, Travis GH, Marmorstein AD. Exp Eye Res. 2007;85:34–43. doi: 10.1016/j.exer.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 10.Rozanowska M, Wessels J, Boulton M, Burke JM, Rodgers MA, Truscott TG, Sarna T. Free Radic Biol Med. 1998;24:1107–1112. doi: 10.1016/s0891-5849(97)00395-x. [DOI] [PubMed] [Google Scholar]
- 11.Sparrow JR, Zhou J, Ben-Shabat S, Vollmer H, Itagaki Y, Nakanishi K. Invest Ophthalmol Vis Sci. 2002;43:1222–1227. [PubMed] [Google Scholar]
- 12.Moeller SM, Jacques PF, Blumberg JB. J Am Coll Nut. 2000;19:522S–527S. doi: 10.1080/07315724.2000.10718975. [DOI] [PubMed] [Google Scholar]
- 13.Krinsky NI, Landrum JT, Bone RA. Annu Rev Nutr. 2003;23:171–201. doi: 10.1146/annurev.nutr.23.011702.073307. [DOI] [PubMed] [Google Scholar]
- 14.Kim SR, Nakanishi K, Itagaki Y, Sparrow JR. Exp Eye Res. 2006;82:828–839. doi: 10.1016/j.exer.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Britton G. UV/Visible Spectroscopy. In: Britton G, Liaaen-Jenson S, Pfander H, editors. Carotenoids. 1B. Birkhaeuser; Basel, Switzerland: 1995. pp. 13–62. [Google Scholar]
- 16.Bhosale P, Zhao DY, Bernstein PS. Invest Ophthalmol Vis Sci. 2007;48:543–549. doi: 10.1167/iovs.06-0558. [DOI] [PubMed] [Google Scholar]
- 17.Bhosale P, Serban B, Zhao DY, Bernstein PS. Biochemistry. 2007;46:9050–9057. doi: 10.1021/bi700558f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhosale P, Zhao DY, Serban B, Bernstein PS. Invest Ophthalmol Vis Sci. 2007;48:1435–1440. doi: 10.1167/iovs.06-1046. [DOI] [PubMed] [Google Scholar]
- 19.Bhosale P, Serban B, Bernstein PS. Biotechnol Lett. 2006;17:1371–1375. doi: 10.1007/s10529-006-9105-8. [DOI] [PubMed] [Google Scholar]
- 20.Bhosale P, Teredesai PV, Lihong J, Ermakov IV, Gellermann W, Bernstein PS. Biotechnol Lett. 2005;21:1719–1723. doi: 10.1007/s10529-005-2737-2. [DOI] [PubMed] [Google Scholar]
- 21.Jang YP, Matsuda H, Itagaki Y, Nakanishi K, Sparrow JR. J Biol Chem. 2005;280:39732–39739. doi: 10.1074/jbc.M504933200. [DOI] [PubMed] [Google Scholar]
- 22.Radu RA, Mata NL, Bagla A, Travis GH. Proc Natl Acad Sci USA. 2004;101:5928–5933. doi: 10.1073/pnas.0308302101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delori FC. Arch Biochem Biophys. 2004;430:156–162. doi: 10.1016/j.abb.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 24.Sharifzadeh M, Bernstein PS, Gellermann W. Opt Soc Am A. 2006;10:2373–2387. doi: 10.1364/josaa.23.002373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holz FG, Bindewald-Wittich A, Fleckenstein M, Dreyhaupt J, Scholl HP, Schmitz-Valckenberg S FAM-Study Group. Am J Ophthalmol. 2007;143:463–472. doi: 10.1016/j.ajo.2006.11.041. [DOI] [PubMed] [Google Scholar]
- 26.Sparrow JR, Parish CA, Hashimoto M, Nakanishi K. J Gen Physiol. 1999;120:147–157. [Google Scholar]
- 27.De TP, Sakmar S. J Gen Physiol. 2002;120:147–157. doi: 10.1085/jgp.20028566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mata NL, Tzekov RT, Liu X, Weng J, Birch DG, Travis GH. Invest Ophthalmol Vis Sci. 2001;42:1685–1590. [PubMed] [Google Scholar]
- 29.Kim SR, Fishkin N, Kong J, Nakanishi K, Allikmets R, Sparrow JR. Proc Natl Acad Sci USA. 2004;101:11668–11672. doi: 10.1073/pnas.0403499101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Radu RA, Mata NL, Nusinowitz S, Liu X, Sieving PA, Travis GH. Proc Natl Acad Sci USA. 2003;100:4742–4747. doi: 10.1073/pnas.0737855100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Radu RA, Han Y, Bui TV, Nusinowitz S, Bok D, Lichter J, Widder K, Travis GH, Mata NL. Invest Ophthalmol Vis Sci. 2005;46:4393–4401. doi: 10.1167/iovs.05-0820. [DOI] [PubMed] [Google Scholar]
- 32.Maiti P, Kong J, Kim SR, Sparrow JR, Allikmets R, Rando RR. Biochemistry. 2006;45:852–860. doi: 10.1021/bi0518545. [DOI] [PubMed] [Google Scholar]
- 33.Eye Disease Case-Control Study Group. Arch Ophthalmol. 1993;111:104–109. doi: 10.1001/archopht.1993.01090010108035. [DOI] [PubMed] [Google Scholar]
- 34.Seddon JM, Sperduto RD, Hiller R, Blair N, Burton TC, Farber MD, Gragoudas ES, Haller J, Miller DT. JAMA. 1994;272:1413–1420. [PubMed] [Google Scholar]
- 35.SanGiovanni JP, Chew EY, Clemons TE, Ferris FL, 3rd, Gensler G, Lindblad AS, Milton RC, Seddon JM, Sperduto RD. AREDS Report No 22. Arch Ophthalmol. 2007;125:1225–1232. doi: 10.1001/archopht.125.9.1225. [DOI] [PubMed] [Google Scholar]
- 36.Bernstein PS, Zhao DY, Wintch SW, Ermakov IV, McClane RW, Gellermann W. Ophthalmology. 2002;109:1780–1787. doi: 10.1016/s0161-6420(02)01173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toyoda Y, Thomson LR, Langner A, Craft NE, Garnett KM, Nichols CR, Cheng KM, Dorey CK. Invest Ophthalmol Vis Sci. 2002;43:1210–1221. [PubMed] [Google Scholar]