Abstract
Protease-activated receptor (PAR)-2 and PAR-4 are implicated in nonhistaminergic itch. We investigated dose dependence, tachyphylaxis, and cross-tachyphylaxis of itch-associated scratching elicited by intradermal injections of PAR-2 and PAR-4 agonists, serotonin (5-hydroxytryptamine, 5-HT), and histamine in ICR mice, as well as μ-opioid modulation of PAR-2 agonist-evoked scratching. Each agent elicited dose-related increases in scratch bouts. Scratching elicited by the PAR-4 agonist and histamine both exhibited significant tachyphylaxis but no cross-tachyphylaxis with each other. Scratching evoked by 5-HT did not exhibit significant tachyphylaxis but did exhibit significant cross-tachyphylaxis to scratching evoked by the PAR-2 and PAR-4 agonists and histamine. Naltrexone and high-dose morphine (10 mg/kg) attenuated PAR-2 agonist-evoked scratching, whereas lower dose morphine (1 mg/kg) had no effect. High-dose morphine also significantly increased circling behavior, which may have interfered with scratching. The PAR-2 agonist and 5-HT produced overlapping distributions of Fos-like immunoreactivity in the superficial dorsal horn. These results indicate that PAR-2 and PAR-4 agonists, histamine, and 5-HT elicit itch-related scratching and activate superficial dorsal horn neurons that may participate in scratch reflex and ascending itch signaling pathways.
Chronic itch occurs in a variety of dermatologic conditions and systemic diseases and is often resistant to antihistamine treatment (Twycross et al., 2003; Ikoma et al., 2006). The protease-activated receptor (PAR)-2 has recently been implicated in pain and inflammation (Cottrell et al., 2003) and itch (Steinhoff et al., 2003). Intradermal injection of PAR-2 agonists elicits dose-related scratching in mice (Shimada et al., 2006; Ui et al., 2006; Tsujii et al., 2008), and PAR-2 may represent a nonhistaminergic transduction mechanism for itch. Pods from the bean plant Mucuna pruriens (cowhage) have spicules or trichomes that contain the protease mucunain, which elicits itch with no accompanying flare when inserted into the epidermis (Johanek et al., 2007). Mucunain was recently characterized and shown to evoke itch via PAR-2 and PAR-4 (Reddy et al., 2008) and intradermal injections of PAR-1, PAR-2, and PAR-4 agonists elicit scratching in ICR mice (Tsujii et al., 2008). Cowhage excites C- and A-fiber mechanosensitive nociceptors (Johanek et al., 2008; Namer et al., 2008; Schepers et al., 2008). In contrast, intradermal histamine induces a local flare and itch sensation by exciting a different population of mechanically insensitive C-fiber afferents (Schmelz et al., 1997), as well as mechanosensitive C-fiber nociceptors to varying degrees (Handwerker et al., 1991; Johanek et al., 2008). Histamine also elicits dose-related scratching behavior in ICR mice and variable (or no) scratching in other mouse strains (Kuraishi et al., 1995; Inagaki et al., 2001; Green et al., 2006; Shimada and LaMotte, 2008). Histamine excites lamina I spinothalamic tract neurons in cats over a time course consistent with itch sensation (Andrew and Craig, 2001). It is noteworthy that histamine and cowhage were recently shown to excite largely separate populations of primate spinothalamic tract neurons (Davidson et al., 2007), suggesting that there may be distinct pathways for transmission of histaminergic and nonhistaminergic itch.
Serotonin (5-hydroxytryptamine, 5-HT) is another inflammatory mediator that elicits weak itch sensation in humans (Fjellner and Hägermark, 1979; Schmelz et al., 2003) but robust dose-dependent scratching behavior in rats (Thomsen et al., 2001; Jinks and Carstens, 2002; Nojima and Carstens, 2003; Nojima et al., 2003) and mice (Yamaguchi et al., 1999; Inagaki et al., 2001; Cuellar et al., 2003). 5-HT excites superficial dorsal horn neurons in the rat over a prolonged time course consistent with 5-HT-evoked scratching behavior (Jinks and Carstens, 2002), suggesting a role in itch. In the present study, we wanted to directly compare the scratch-evoking capacity of 5-HT, histamine, and PAR-2 and PAR-4 agonists and to investigate whether they exhibit tachyphylaxis and cross-tachyphylaxis. Because PAR-2 represents a particularly attractive target for development of nonhistaminergic antipruritics, we wanted to further investigate whether scratching elicited by the PAR-2 agonist SLIGRL-NH2 is affected by opioids in a manner consistent with itch (i.e., reduced by μ-opioid antagonists but not agonists) (Nojima et al., 2003). Finally, we used the method of Fos immunohistochemistry to investigate whether the PAR-2 agonist and 5-HT activate overlapping populations of neurons in the mouse superficial dorsal horn consistent with the proposed role for such neurons in signaling itch (Andrew and Craig, 2001; Jinks and Carstens, 2002).
Materials and Methods
Experiments were conducted using adult male ICR mice (Harlan, Oxnard, CA) under a protocol approved by the University of California Davis Animal Care and Use Committee.
Chemicals. Drugs used were PAR-2 agonist SLIGRL-NH2 (Quality Controlled Biochemicals, Hopkinton, MA, and GenScript, Piscataway, NJ; Shimada et al., 2006), PAR-4 agonist AYPGKF-NH2 (GenScript) (Tsujii et al., 2008), 5-HT HCl (Sigma-Aldrich, St. Louis, MO), histamine (Sigma-Aldrich), capsaicin (Sigma-Aldrich), morphine sulfate (Sigma-Aldrich), and naltrexone (DuPont, Garden City, NJ). All chemicals were dissolved in sterile isotonic saline except capsaicin, which was dissolved in 7% Tween 80.
Behavioral Scratching Studies. Scratching experiments followed procedures described in our previous report (Cuellar et al., 2003). In brief, the fur on the rostral back was shaved, and mice were habituated to the Plexiglas recording arena 1 week before testing. Microinjections were made intradermally in the nape of the neck using a 30-gauge needle attached to a Hamilton microsyringe (Hamilton Co., Reno, NV) by polyethylene-50 tubing. The injection site was marked so a second injection could be made at the same location in experiments testing for tachyphylaxis and cross-tachyphylaxis (Table 1, groups 7–22). Immediately after the injection, the mouse was placed into the arena and videotaped from above for 40 to 60 min. In general, three to four mice were injected and videotaped simultaneously. Immediately after commencing videotaping, all investigators left the room.
TABLE 1.
Experimental groups to investigate dose dependency, tachyphylaxis, and cross-tachyphylaxis, and opioid modulation of scratching behavior Column denoted “first” indicates which drug was applied first, and column denoted “second” indicates which agent (if any) was applied next in the same session. For groups 7 to 22, after the first agent was injected, mice were videotaped for 40 min, at which time a second injection of the same or a different mediator was made at the same site and mice were videotaped for another 40 min. For groups 23 to 29, the first agent was administered systemically (i.e., intraperitoneally or subcutaneously) 10 min before intradermal injection of the second agent. All treatments are intradermal unless otherwise specified.
| Group | First | Dose/Route | Second | Dose/Route |
|---|---|---|---|---|
| 1 | None (spontaneous) | None | ||
| 2 | Vehicle (saline) | 10 μl | None | |
| 3a, b, c | SLIGRL-NH2 | 35, 50, or 100 μg/10 μl | None | |
| 4a, b, c | AYPGKF-NH2 | 35, 50, or 100 μg/10 μl | None | |
| 5a, b, c | 5-HT | 14, 47, and 141 nmol/10 μl | None | |
| 6 | Histamine | 35, 50, 100 μg/10 μl | None | |
| 7 | SLIGRL-NH2 | 50 μg/10 μl | SLIGRL-NH2 | 50 μg/10 μl |
| 8 | SLIGRL-NH2 | 50 μg/10 μl | AYPGKF-NH2 | 50 μg/10 μl |
| 9 | SLIGRL-NH2 | 50 μg/10 μl | Histamine | 50 μg/10 μl |
| 10 | SLIGRL-NH2 | 50 μg/10 μl | 5-HT | 47 nmol/10 μl |
| 11 | AYPGKF-NH2 | 50 μg/10 μl | AYPGKF-NH2 | 50 μg/10 μl |
| 12 | AYPGKF-NH2 | 50 μg/10 μl | SLIGRL-NH2 | 50 μg/10 μl |
| 13 | AYPGKF-NH2 | 50 μg/10 μl | Histamine | 50 μg/10 μl |
| 14 | AYPGKF-NH2 | 50 μg/10 μl | 5-HT | 47 nmol/10 μl |
| 15 | Histamine | 50 μg/10 μl | Histamine | 50 μg/10 μl |
| 16 | Histamine | 50 μg/10 μl | SLIGRL-NH2 | 50 μg/10 μl |
| 17 | Histamine | 50 μg/10 μl | AYPGKF-NH2 | 50 μg/10 μl |
| 18 | Histamine | 50 μg/10 μl | 5-HT | 47 nmol/10 μl |
| 19 | 5-HT | 47 nmol/10 μl | 5-HT | 47 nmol/10 μl |
| 20 | 5-HT | 47 nmol/10 μl | SLIGRL-NH2 | 50 μg/10 μl |
| 21 | 5-HT | 47 nmol/10 μl | AYPGKF-NH2 | 50 μg/10 μl |
| 22 | 5-HT | 47 nmol/10 μl | Histamine | 50 μg/10 μl |
| 23 | Saline | 0.1 ml/kg i.p. | SLIGRL-NH2 | 50 μg/10 μl |
| 24 | Naltrexone | 1 mg/kg s.c. | SLIGRL-NH2 | 50 μg/10 μl |
| 25 | Morphine | 1 mg/kg i.p. | SLIGRL-NH2 | 50 μg/10 μl |
| 26 | Morphine | 3 mg/kg i.p. | SLIGRL-NH2 | 50 μg/10 μl |
| 27 | Morphine | 10 mg/kg i.p. | SLIGRL-NH2 | 50 μg/10 μl |
| 28 | Saline | 0.1 ml/kg i.p. | Capsaicin | 10 μg/10 μl |
| 29 | Naltrexone | 1 mg/kg s.c. | Capsaicin | 10 μg/10 μl |
i.d., intradermal
The various treatment conditions are listed in Table 1. Groups 1 to 6 were studies of dose-related scratching behavior elicited by each pruritogen (5–8 mice/group). For studies of tachyphylaxis and cross-tachyphylaxis, a 4 × 4 design was used to test the effects of an injection of each mediator followed by subsequent injection of that same mediator or one of the other three mediators, 40 min after the first injection. The 16 combinations are represented in groups 7 to 22 in Table 1. For studies of opioid modulation of scratching (Table 1, groups 23–29), either vehicle (saline; groups 23 and 28), naltrexone (groups 24 and 29), or morphine at three different doses (groups 25–27) was administered systemically 10 min before intradermal injection of either SLIGRL-NH2 (groups 23–27) or capsaicin (groups 28 and 29). For some studies (e.g., dose-related scratching elicited by a given agent), the same cohort of mice (6–8/group) was used, with a minimum of 4 days between successive test sessions to avoid any carryover effects.
Videotapes were reviewed by investigators blinded as to treatment, and the number of scratch bouts was recorded at 5-min intervals. A scratch bout was defined as one or more rapid back-and-fort hind paw motions directed toward and contacting the injection site and ending with licking or biting of the toes and/or placement the hind paw on the floor. Hind paw movements directed away from the injection site (e.g., ear-scratching) and grooming movements were not counted. Dose-related scratching for each agent (including the vehicle trial) was analyzed by analysis of variance, with dose as the main effect. For studies of tachyphylaxis, a paired t test was used to compare the total number of scratch bouts/40 min elicited by the first versus the second injection. For studies of cross-tachyphylaxis, scratch counts over the four sessions in which animals received a given mediator first were compared with scratch counts evoked by the same mediator when it was applied second using t tests. ANOVA was used to compare the total number of scratch bouts across morphine and naltrexone treatment groups. Animals receiving morphine (Table 1, groups 25–27) exhibited rotational (circling) behavior so we additionally counted the total number of 360° rotations over the 45-min period. ANOVA followed by post hoc least significant difference test was used to compare numbers of rotations across morphine concentrations. In all cases, p < 0.05 was considered to be significant.
Fos Immunohistochemistry. These experiments used mice that had completed behavioral testing. They were anesthetized with sodium pentobarbital (80 mg/kg i.p.) and received an intradermal microinjection of SLIGRL-NH2 (50 μg/5 μl), 5-HT (47 nmol/5 μl), or saline in the nape of the neck as in the behavioral experiments. After 2 h, the mice were perfused transcardially with phosphate-buffered saline followed by 4% paraformaldehyde as described previously (Merrill et al., 2006). The cervical spinal cord was postfixed, transferred to 30% sucrose, and cut in 50-μm sections that were collected in 24-well containers. The sections were processed for Fos immunohistochemistry as described previously (Merrill et al., 2006). In brief, every third section (150-μm intervals) was blocked in goat serum (3%) and then incubated in primary c-fos antibody (1:50,000) for 2 days. Sections were subsequently exposed to a secondary biotinylated (goat anti-rabbit) antibody and then subjected to an avidinbiotin-peroxidase complex reaction enhanced with biotinyl tyramide/H2O2. The reaction product was visualized as black through a nickel-enhanced diaminobenzidine reaction. Finally, sections were collected onto glass microscope slides, covered with a coverslip, and examined under the light microscope by an investigator blinded as to treatment. The number of cell nuclei in superficial laminae of the cervical dorsal horn exhibiting Fos-like immunoreactivity (FLI) was counted in five representative sections from each mouse. Between-treatment counts of FLI were analyzed by ANOVA.
Results
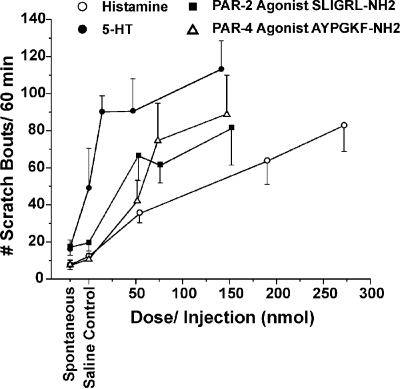
Dose-Dependent Scratching. Intradermal injection of 5-HT, histamine, and agonists of PAR-2 and PAR-4 each evoked dose-related scratching, as summarized in Fig. 1 for the absolute doses in nanomoles. For each agent, there was a significant overall effect of dose (ANOVA; p < 0.05).
Fig. 1.
Dose-dependent scratching elicited by various itch mediators. Graph plots mean number of scratch bouts/60 min versus dose of PAR-2 agonist SLIGRL-NH2, PAR-4 agonist AYPGKF-NH2, 5-HT, and histamine. Total injected dose calculated as nanomoles in a 10-μl volume (n = 6–8/group). There was a significant overall effect of dose for each agent (ANOVA; p < 0.05). Error bars are S.E.M.
The time course of scratching elicited by the initial intradermal injection is shown in Figs. 2, 3, 4, 5 for the PAR-2 agonist SLIGRL-NH2, the PAR-4 agonist AYPGKF-NH2, histamine, and 5-HT, respectively (open symbols). In each case, maximal scratching occurred within the initial 5 to 10 min after the injection and decreased within 20 to 30 min.
Fig. 2.
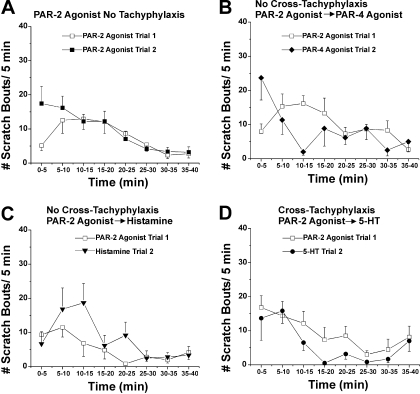
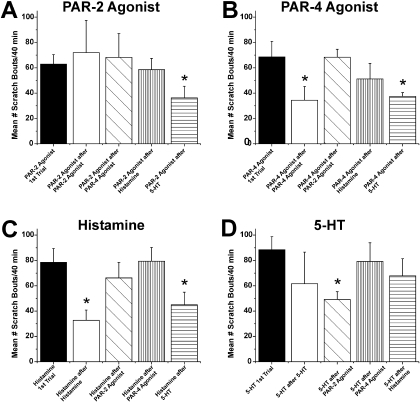
Time course, tachyphylaxis, and cross-tachyphylaxis for hind limb scratching elicited by intradermal microinjection of PAR-2 agonist. In each panel, the PAR-2 agonist was injected first, and scratch bouts (□) were counted over 40 min, followed by injection of a second agent (filled symbols), and scratch bouts were counted again over 40 min. A, graph plots mean number of scratch bouts/5-min intervals after first injection of PAR-2 agonist SLIGRL-NH2 (□; trial 1; 50 μg/10 μl) and after second injection of the same dose of SLIGRL-NH2 at the same site (▪; trial 2). B, as in A for PAR-2 agonist followed by PAR-4 agonist AYPGKF-NH2 (50 μg/10 μl). C, as in A for PAR-2 agonist followed by 5-HT (47 mM/10 μl). D, as in A for PAR-2 agonist followed by histamine (50 μg/10 μl). Error bars are S.E.M. (n = 5–8/group).
Fig. 3.
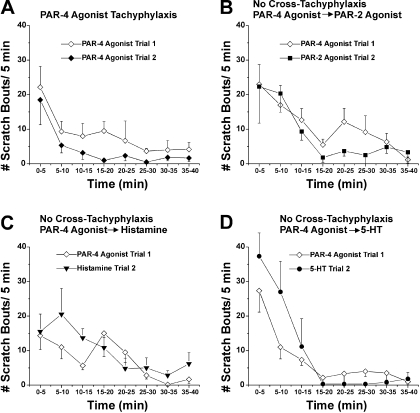
PAR-4 agonist. Graphs as in Fig. 2 for experiments in which the PAR-4 agonist AYPGKF-NH2 was injected first, followed 40 min later by injection of either the PAR-4 agonist again (A), the PAR-2 agonist (B), histamine (C), or 5-HT (D).
Fig. 4.
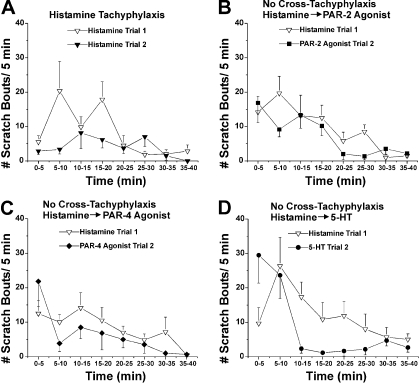
Histamine. Graphs as in Fig. 2 for experiments in which histamine was injected first, followed 40 min later by injection of either histamine again (A), the PAR-2 agonist (B), the PAR-4 agonist (C), or 5-HT (D).
Fig. 5.
5-HT. Graphs as in Figs. 2, 3, 4 for experiments in which 5-HT was injected first, followed 40 min later by injection of either 5-HT again (A), the PAR-2 agonist (B), the PAR-4 agonist (C), or histamine (D).
Tachyphylaxis. After the initial intradermal injection of a given agent, it was reinjected at the identical site 40 min later to test for tachyphylaxis. Figure 2A shows that the first and second injections of SLIGRL-NH2 (50 μg/10 μl) elicited an equivalent amount of scratching over a similar time course. The summary data in Fig. 6A show that the total number of scratch bouts/40 min did not differ significantly between the first and second injections (Fig. 6A, ▪ versus □), indicating lack of tachyphylaxis.
Fig. 6.
Summary of tachyphylaxis and cross-tachyphylaxis results. A, bar graph plots mean number of scratch bouts/40 min elicited by intradermal microinjection of the PAR-2 agonist when it was given first (left-hand bar) and when it was injected 40 min after a prior injection of the same PAR-2 agonist, the PAR-4 agonist, histamine, or 5-HT (second–fifth bars to right). *, p < 0.05, significantly different from first trial of PAR-2 agonist. B, as in A for PAR-4 agonist. *, p < 0.05, significantly different from first trial of PAR-4 agonist. C, as in A for scratch bouts elicited by histamine. *, p < 0.05, significantly different from first trial of histamine. D, as in A for 5-HT. *, p < 0.05, significantly different from first trial of 5-HT.
A second injection of the PAR-4 agonist AYPGKF-NH2 elicited less scratching compared with the first (Fig. 3A), with the total scratch count being significantly less after the second injection compared with the first injection (Fig. 6B, ▪ versus □). Histamine-evoked scratching also exhibited tachyphylaxis, with the second injection evoking a significantly lower total scratch count compared with the first injection (Figs. 4A and 6C).
For 5-HT, the second injection elicited significantly (p < 0.01) less scratching compared with the first (Fig. 5A), although this value did not differ significantly compared with the mean number of 5-HT-evoked scratch bouts averaged across all four experiments in which 5-HT was tested first (Fig. 6D, ▪ versus □).
Cross-Tachyphylaxis. We also investigated the effect of each
mediator to reduce (cross-tachyphylaxis) or otherwise affect scratching
elicited by subsequent application of a different mediator. The PAR-2 agonist
SLIGRL-NH2 did not significantly affect scratching elicited by
subsequent injection of the PAR-4 agonist AYPGKF-NH2 (Figs.
2B and
6B,
 ) or histamine (Figs.
2C and
6C,
) or histamine (Figs.
2C and
6C,
 ) but resulted in a reduction
in scratching elicited by subsequent injection of 5-HT (Figs.
2D and
6D,
) but resulted in a reduction
in scratching elicited by subsequent injection of 5-HT (Figs.
2D and
6D,
 ). Although the PAR-4 agonist
AYPGKF-NH2 exhibited tachyphylaxis, it did not exhibit
cross-tachyphylaxis to any other mediator (Figs.
3, B–D;
6A,
). Although the PAR-4 agonist
AYPGKF-NH2 exhibited tachyphylaxis, it did not exhibit
cross-tachyphylaxis to any other mediator (Figs.
3, B–D;
6A,
 ; and C and D,
; and C and D,
 ).
).
Histamine also exhibited tachyphylaxis but no cross-tachyphylaxis to the
other mediators (Figs. 4,
B–D; and 6, A and
B,  ; and D,
; and D,
 ).
).
5-HT exhibited significant cross-tachyphylaxis to each of the other
mediators (Figs. 5, B–D;
and 6, A–C,
 ).
).
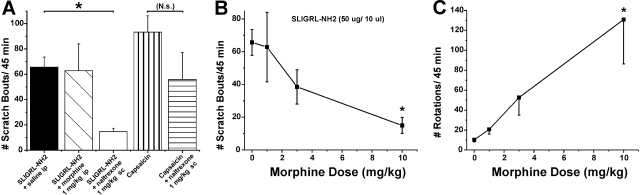
μ-Opioid Modulation of Scratching. The μ-agonist morphine (1
mg/kg) had no effect on scratching elicited by intradermal injection of the
PAR-2 agonist SLIGRL-NH2 (Fig.
7A, ▪ versus
 ), whereas naltrexone
significantly attenuated scratching (Fig.
7A, □). Figure
7B plots mean scratching versus dose of morphine, which
significantly reduced scratching only at the highest dose (10 mg/kg). In
addition, we tested capsaicin, which was recently reported to elicit hind limb
scratching in ICR mice (Shimada and
LaMotte, 2008), although not in ddY mice
(Kuraishi et al., 1995). The
present data confirm that intradermal capsaicin elicits scratching in ICR mice
(Fig. 7A,
), whereas naltrexone
significantly attenuated scratching (Fig.
7A, □). Figure
7B plots mean scratching versus dose of morphine, which
significantly reduced scratching only at the highest dose (10 mg/kg). In
addition, we tested capsaicin, which was recently reported to elicit hind limb
scratching in ICR mice (Shimada and
LaMotte, 2008), although not in ddY mice
(Kuraishi et al., 1995). The
present data confirm that intradermal capsaicin elicits scratching in ICR mice
(Fig. 7A,
 ) comparable with scratching
elicited by pruritogens (Fig.
1). Naltrexone did not significantly reduce capsaicin-evoked
scratching (Fig. 7A,
) comparable with scratching
elicited by pruritogens (Fig.
1). Naltrexone did not significantly reduce capsaicin-evoked
scratching (Fig. 7A,
 versus
versus
 ).
).
Fig. 7.
Opioid modulation of PAR-2 agonist-evoked scratching. A, bar graph plots
mean number of scratch bouts/45 min. First three bars show, from left to
right, number of scratch bouts evoked PAR-2 agonist (SLIGRL-NH2;50
μg/10 μl) when preceded by intraperitoneal saline (▪; control),
morphine (1 mg/kg;  ), or
naltrexone (1 mg/kg; □), respectively. *, p < 0.01,
significant difference between saline and naltrexone groups (n =
5–6/group). Bars to right show scratching elicited by intradermal
capsaicin (10 μg/10 μl;
), or
naltrexone (1 mg/kg; □), respectively. *, p < 0.01,
significant difference between saline and naltrexone groups (n =
5–6/group). Bars to right show scratching elicited by intradermal
capsaicin (10 μg/10 μl;  )
and lack of significant effect of naltrexone
(
)
and lack of significant effect of naltrexone
( ). B, graph plots total number
of scratch bouts/45 min elicited by the PAR-2 agonist SLIGRL-NH2
versus dose of morphine. The number of scratch bouts at 1- and 3-mg/kg doses
of morphine was not significantly different from vehicle but was significantly
lower (*, p < 0.05) at the highest morphine dose (10 mg/kg). C,
graph plots mean number of rotations (circling)/45 min versus dose of
morphine. *, p < 0.05 for all, significantly different from 0-,
1-, and 3-mg/kg doses.
). B, graph plots total number
of scratch bouts/45 min elicited by the PAR-2 agonist SLIGRL-NH2
versus dose of morphine. The number of scratch bouts at 1- and 3-mg/kg doses
of morphine was not significantly different from vehicle but was significantly
lower (*, p < 0.05) at the highest morphine dose (10 mg/kg). C,
graph plots mean number of rotations (circling)/45 min versus dose of
morphine. *, p < 0.05 for all, significantly different from 0-,
1-, and 3-mg/kg doses.
Observation of mice receiving morphine revealed that they exhibited rotational (circling) behavior. Counts of rotations increased with morphine dose (Fig. 7C), confirming a previous study (Morihisa and Glick, 1977).
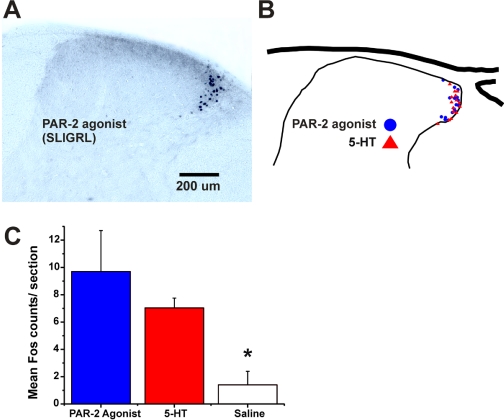
Fos Expression. Intradermal microinjection of the PAR-2 agonist SLIGRL-NH2 resulted in FLI that was distributed in the lateral aspect of the cervical superficial dorsal horn (Fig. 8A) similar to that observed previously (Nojima et al., 2003; Nakano et al., 2008). 5-HT resulted in a similar, overlapping distribution of FLI (Fig. 8B). Both SLIGRL-NH2 and 5-HT resulted in a significant increase in FLI compared with intradermal vehicle (saline) injections (Fig. 8C).
Fig. 8.
Overlapping distributions of FLI in cervical spinal cord after intradermal injections of SLIGRL-NH2 (50 μg/5 μl) or 5-HT (47 nmol/5 μl) in nape of neck. A, photomicrograph of midcervical section showing FLI elicited by PAR-2 agonist. B, drawing of cervical dorsal horn compiling FLI from one section each from mice injected with PAR-2 agonist (•) or 5-HT (▴) in nape of neck. C, mean counts of FLI from mice receiving intradermal PAR-2 agonist (blue bar; n = 6), 5-HT (red bar; n = 6), or saline (white bar; n = 4). *, p < 0.05 significantly different versus groups receiving PAR-2 agonist and 5-HT (counts for PAR-2 agonist versus 5-HT not significantly different).
Discussion
The present results confirm dose-dependent scratching behavior and show tachyphylaxis and cross-tachyphylaxis of scratching for some of the itch mediators tested. Scratching elicited by the PAR-2 agonist was not decreased by low or intermediate doses of morphine but was attenuated by the μ-opioid antagonist, consistent with itch sensation. Moreover, the PAR-2 agonist and 5-HT activated neurons in the superficial dorsal horn in an overlapping distribution. The results are discussed in terms of roles for each of these mediators in itch and the central mechanisms involved.
PARs. Both PAR-2 and PAR-4 agonists elicited equivalent dose-related scratching (Fig. 1). Our data confirm recent reports that the PAR-2 agonist SLIGRL-NH2 evoked dose-related scratching in ICR mice over a 10- to 100-μg range (Shimada et al., 2006; Tsujii et al., 2008), as did the PAR-4 agonist AYPGKF-NH2 (Tsujii et al., 2008). Tryptase, another PAR-2 agonist, elicited scratching in a manner that was antagonized by a PAR-2 antagonist and antibody (Ui et al., 2006). PAR-2 agonist-evoked scratching was not reduced by antihistamines (Shimada et al., 2006; Tsujii et al., 2008). We did not presently observe cross-tachyphylaxis between the PAR-2 agonist and histamine, in further support that these mediators do not share a common transduction mechanism.
Opioid antagonists reduce experimentally evoked itch in humans (Heyer et al., 1997), and μ-opioid agonists are commonly known to induce itch while reducing pain. It is therefore assumed that μ-opioid antagonists should reduce, whereas μ-agonists should enhance or not affect, itch-related scratching. This was presently borne out, because PAR-2 agonist-evoked scratching was significantly attenuated by naltrexone but was not significantly affected by morphine at 1- and 3-mg/kg doses (Fig. 7). This confirms our previous findings showing suppression of 5-HT-evoked scratching in rats by naltrexone but not morphine (Nojima et al., 2003). At the highest morphine dose tested (10 mg/kg), PAR-2 agonist-evoked scratching was significantly attenuated. However, mice exhibited significant dose-dependent circling behavior (Fig. 7C). This confirms a previous study showing dose-dependent circling behavior in mice that was virtually identical to our data over the 1- to 10-mg/kg dose range (Morihisa and Glick, 1977). We speculate that the marked circling behavior at the 10 mg/kg morphine dose may have interfered with scratching behavior. It is conceivable that the strong locomotor drive induced by morphine may have prevented scratching by overriding the urge of the animals to stop and scratch at the PAR-2 agonist injection site.
Capsaicin, which normally elicits burning pain sensation in humans (LaMotte et al., 1991), presently elicited robust scratching that was not significantly affected by naltrexone (Fig. 7A). Capsaicin-evoked scratching might conceivably reflect itch or some irritant sensation that compels the animal to scratch. In support of this idea, topical capsaicin was reported to elicit a sensation of itch in >50% of human subjects tested (Green and Shaffer, 1993). Alternatively, capsaicin may evoke burning pain that induces scratching as a means of counterirritation, despite the general assumption that scratching would exacerbate the pain and thus be avoided. It has been shown that capsaicin injections into the cheek elicited an ipsilateral forelimb wiping response but little or no hind limb scratching directed to the injection site, whereas injection of histamine evoked hind limb scratching but little forelimb wiping (Shimada and LaMotte, 2008). The authors suggested that hind limb scratching and forelimb wiping responses reflect facial itch and pain, respectively. In behavioral studies of itch, chemicals are usually injected in the nape of the neck. However, the response repertoire of the animal is biomechanically limited because the injection site can only be accessed by hind limb scratch movements. We believe that naltrexone-sensitive, morphine-insensitive scratching evoked by the PAR-2 agonist probably reflected itch sensation, whereas naltrexone-insensitive scratching elicited by capsaicin probably reflected pain.
The present data with the PAR-4 agonist AYPGKF-NH2 confirm a recent report that this agent elicited dose-related scratching in ICR mice over a 10- to 100-μg dose range (Tsujii et al., 2008). In the latter study, the PAR-4 agonist was less efficacious than the PAR-2 agonist, whereas we observed an equivalent degree of scratching in response to these two agonists (Fig. 1). It is noteworthy that the antihistamine terfenadine significantly reduced scratching elicited by the PAR-4 but not PAR-2 agonist, suggesting that the former might act partly via degranulation of cutaneous mast cells to release histamine (Tsujii et al., 2008). However, we did not presently observe cross-tachyphylaxis of scratching elicited by histamine when given after a prior injection of the PAR-4 agonist. We did observe significant tachyphylaxis to PAR-4, but not PAR-2, agonist-evoked scratching, and no cross-tachyphylaxis between them, suggesting that these two agents may not share a common mechanism of action. A PAR-1 agonist also evoked histamine-dependent scratching (Tsujii et al., 2008), suggesting two or more mechanisms of action for the participation of PAR-1, -2, and -4 in itch.
Histamine. Histamine elicited dose-dependent scratching (Fig. 1), confirming previous studies showing robust scratching in ICR mice with variable efficacy in other strains (Inagaki et al., 2001; Green et al., 2006; Shimada and LaMotte, 2008) that is mediated via H1 and H4 histamine receptors (Ohtsuka et al., 2001; Bell et al., 2004). Histamine-evoked scratching exhibited significant tachyphylaxis, consistent with reduced itch sensation upon repeated challenge with histamine in humans (Ståhle-Bäckdahl et al., 1988).
5-HT. 5-HT elicits itch or sometimes pain in human skin when injected (Fjellner and Hägermark, 1979) or applied by iontophoresis (Weisshaar et al., 1997) or microdialysis (Schmelz et al., 2003). 5-HT-evoked scratching in mice was attenuated by naloxone (Yamaguchi et al., 1999). The degree of 5-HT-evoked scratching observed presently in ICR mice was comparable with that reported previously in this and other mouse strains (Yamaguchi et al., 1999; Inagaki et al., 2001; Cuellar et al., 2003). Pharmacological studies indicate that scratching is mediated via peripheral 5-HT2 receptors in mice (Yamaguchi et al., 1999) and rats (Nojima and Carstens, 2003). 5-HT-evoked scratching did not exhibit significant tachyphylaxis, although there was a numeric reduction in the number of scratch bouts evoked by the second compared with the first 5-HT injection. It is noteworthy that 5-HT induced significant cross-tachyphylaxis to scratching elicited by histamine and the PAR-2 and PAR-4 agonists (Figs. 5 and 6), suggesting that 5-HT may exert a depressant effect on peripheral transduction of these two mediators. If itch sensations mediated via PAR-2/4 versus histamine H1/H4 receptors are signaled separately by C-fiber polymodal nociceptors and mechanically insensitive C-afferents, respectively (see Introduction), then it may be speculated that 5-HT acts at sensory nerve endings of both types of C-fiber. It would be of interest to determine the cellular mechanisms of 5-HT cross-interactions with C-fiber responses to histamine or the PAR-2/4 agonists.
Central Transmission of Itch. Intradermal injection of both the PAR-2 agonist and 5-HT elicited overlapping distributions of FLI in the lateral aspect of the cervical superficial dorsal horn (Fig. 8). The distribution of 5-HT-evoked FLI was similar to that reported previously in the rat (Nojima et al., 2003). Intradermal injection of the PAR-2 agonist SLIGRL-NH2 was recently reported to evoke FLI mainly in laminae I and outer II of the cervical dorsal horn in ICR mice, whereas histamine elicited FLI in inner lamina II within an isolectin-B4 positive region (Nakano et al., 2008), suggesting that synaptic input from primary afferents activated by these two agents may be spatially segregated. Additional studies are needed to determine whether 5-HT and histamine elicit overlapping distributions of FLI.
A limitation of anatomical Fos studies is that they do not reveal whether a given neuron is excited by multiple agents, and an electrophysiological approach is needed to answer this question. In rats, the majority of superficial dorsal horn neurons were excited by both histamine and 5-HT, as well as algogens including capsaicin and mustard oil (Jinks and Carstens, 2002). Superficial dorsal horn neurons in mouse responded to intradermal injection of the PAR-2 agonist SLIGRL-NH2, and most of these additionally responded to 5-HT, capsaicin, and mustard oil (Akiyama et al., 2009). These data are consistent with the distributions of FLI elicited by the PAR-2 agonist and 5-HT. However, cowhage and histamine seem to excite largely separate populations of C-fiber afferents (Johanek et al., 2008; Namer et al., 2008) and primate spinothalamic tract neurons (Davidson et al., 2007). We are currently investigating whether the PAR-2 agonist SLIGRL-NH2 and histamine excite separate populations of superficial dorsal horn neurons in the mouse, as would be consistent with the differing distributions of FLI elicited by these agents (Nakano et al., 2008). If this turns out to be the case, it will be of further interest to determine whether these separate itch-signaling pathways use different neurotransmitters such as substance P or gastrin-releasing peptide and its receptor that has recently been implicated in the central transmission of itch (Sun and Chen, 2007).
This work was supported by the National Institutes of Health Institute of Dental and Craniofacial Research [Grant DE013685]; and by the National Institutes of Health National Institute of Arthritis and Musculoskeletal and Skin Diseases [Grant AR057194].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.152256.
ABBREVIATIONS: PAR, protease-activated receptor 2; 5-HT, 5-hydroxytryptamine; ANOVA, analysis of variance; FLI, Fos-like immunoreactivity.
References
- Akiyama T, Merrill AW, Carstens MI, and Carstens E (2009) Activation of superficial dorsal horn neurons in the mouse by a PAR-2 agonist and 5-HT: potential role in itch. J Neurosci doi: 10.1523/jneurosci.6103-08. [DOI] [PMC free article] [PubMed]
- Andrew D and Craig AD (2001) Spinothalamic lamina I neurons selectively sensitive to histamine: a central neural pathway for itch. Nat Neurosci 4 72-77. [DOI] [PubMed] [Google Scholar]
- Bell JK, McQueen DS, and Rees JL (2004) Involvement of histamine H4 and H1 receptors in scratching induced by histamine receptor agonists in Balb C mice. Br J Pharmacol 142 374-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell GS, Amadesi S, Schmidlin F, and Bunnett N (2003) Protease-activated receptor 2: activation, signalling and function. Biochem Soc Trans 31 1191-1197. [DOI] [PubMed] [Google Scholar]
- Cuellar JM, Jinks SL, Simons CT, and Carstens E (2003) Deletion of the preprotachykinin A gene in mice does not reduce scratching behavior elicited by intradermal serotonin. Neurosci Lett 339 72-76. [DOI] [PubMed] [Google Scholar]
- Davidson S, Zhang X, Yoon CH, Khasabov SG, Simone DA, and Giesler GJ Jr (2007) The itch-producing agents histamine and cowhage activate separate populations of primate spinothalamic tract neurons. J Neurosci 27 10007-10014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjellner B and Hägermark O (1979) Pruritus in polycythemia vera: treatment with aspirin and possibility of platelet involvement. Acta Derm Venereol 59 505-512. [DOI] [PubMed] [Google Scholar]
- Green BG and Shaffer GS (1993) The sensory response to capsaicin during repeated topical exposures: differential effects on sensations of itching and pungency. Pain 53 323-334. [DOI] [PubMed] [Google Scholar]
- Green AD, Young KK, Lehto SG, Smith SB, and Mogil JS (2006) Influence of genotype, dose and sex on pruritogen-induced scratching behavior in the mouse. Pain 124 50-58. [DOI] [PubMed] [Google Scholar]
- Handwerker HO, Forster C, and Kirchhoff C (1991) Discharge patterns of human C-fibers induced by itching and burning stimuli. J Neurophysiol 66 307-315. [DOI] [PubMed] [Google Scholar]
- Heyer G, Dotzer M, Diepgen TL, and Handwerker HO (1997) Opiate and H1 antagonist effects on histamine induced pruritus and allokinesis. Pain 73 239-243. [DOI] [PubMed] [Google Scholar]
- Ikoma A, Steinhoff M, Ständer S, Yosipovitch G, and Schmelz M (2006) The neurobiology of itch. Nat Rev Neurosci 7 535-547. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Nagao M, Igeta K, Kawasaki H, Kim JF, and Nagai H (2001) Scratching behavior in various strains of mice. Skin Pharmacol Appl Skin Physiol 14 87-96. [DOI] [PubMed] [Google Scholar]
- Jinks SL and Carstens E (2002) Responses of superficial dorsal horn neurons to intradermal serotonin and other irritants: comparison with scratching behavior. J Neurophysiol 87 1280-1289. [DOI] [PubMed] [Google Scholar]
- Johanek LM, Meyer RA, Friedman RM, Greenquist KW, Shim B, Borzan J, Hartke T, LaMotte RH, and Ringkamp M (2008) A role for polymodal C-fiber afferents in nonhistaminergic itch. J Neurosci 28 7659-7669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanek LM, Meyer RA, Hartke T, Hobelmann JG, Maine DN, LaMotte RH, and Ringkamp M (2007) Psychophysical and physiological evidence for parallel afferent pathways mediating the sensation of itch. J Neurosci 27 7490-7497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuraishi Y, Nagasawa T, Hayashi K, and Satoh M (1995) Scratching behavior induced by pruritogenic but not algesiogenic agents in mice. Eur J Pharmacol 275 229-233. [DOI] [PubMed] [Google Scholar]
- LaMotte RH, Shain CN, Simone DA, and Tsai EF (1991) Neurogenic hyperalgesia: psychophysical studies of underlying mechanisms. J Neurophysiol 66 190-211. [DOI] [PubMed] [Google Scholar]
- Merrill AW, Barter LS, Rudolph U, Eger EI 2nd, Antognini JF, Carstens MI, and Carstens E (2006) Propofol's effects on nociceptive behavior and spinal c-fos expression after intraplantar formalin injection in mice with a mutation in the gamma-aminobutyric acid-type(A) receptor beta3 subunit. Anesth Analg 103 478-483. [DOI] [PubMed] [Google Scholar]
- Morihisa JM and Glick SK (1977) Morphine-induced rotation (circling behavior) in rats and mice: species differences, persistence of withdrawal-induced rotation and antagonism by naloxone. Brain Res 123 180-187. [DOI] [PubMed] [Google Scholar]
- Nakano T, Andoh T, Lee JB, and Kuraishi Y (2008) Different dorsal horn neurons responding to histamine and allergic itch stimuli. Neuroreport 19 723-726. [DOI] [PubMed] [Google Scholar]
- Namer B, Carr R, Johanek LM, Schmelz M, Handwerker HO, and Ringkamp M (2008) Separate peripheral pathways for pruritus in man. J Neurophysiol 100 2062-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nojima H and Carstens E (2003) 5-Hydroxytryptamine (5-HT)2 receptor involvement in acute 5-HT-evoked scratching but not in allergic pruritus induced by dinitrofluorobenzene in rats. J Pharmacol Exp Ther 306 245-252. [DOI] [PubMed] [Google Scholar]
- Nojima H, Simons CT, Cuellar JM, Carstens MI, Moore JA, and Carstens E (2003) Opioid modulation of scratching and spinal c-fos expression evoked by intradermal serotonin. J Neurosci 23 10784-10790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka E, Kawai S, Ichikawa T, Nojima H, Kitagawa K, Shirai Y, Kamimura K, and Kuraishi Y (2001) Roles of mast cells and histamine in mosquito bite-induced allergic itch-associated responses in mice. Jpn J Pharmacol 86 97-105. [DOI] [PubMed] [Google Scholar]
- Reddy VB, Iuga AO, Shimada SG, LaMotte RH, and Lerner EA (2008) Cowhage-evoked itch is mediated by a novel cysteine protease: a ligand of protease-activated receptors. J Neurosci 28 4331-4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepers RJ, Johanek LM, Hartke TV, Shim B, Borzan J, Meyer RA, and Ringkamp M (2008) A subpopulation of A-delta nociceptors in monkey is vigorously activated by cowhage spicules. Soc Neurosci Abstr 34 170.3. [Google Scholar]
- Schmelz M, Schmidt R, Bickel A, Handwerker HO, and Torebjörk HE (1997) Specific C-receptors for itch in human skin. J Neurosci 17 8003-8008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelz M, Schmidt R, Weidner C, Hilliges M, Torebjork HE, and Handwerker HO (2003) Chemical response pattern of different classes of C-nociceptors to pruritogens and algogens. J Neurophysiol 89 2441-2448. [DOI] [PubMed] [Google Scholar]
- Shimada SG and Lamotte RH (2008) Behavioral differentiation between itch and pain in mouse. Pain 139 681-687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada SG, Shimada KA, and Collins JG (2006) Scratching behavior in mice induced by the proteinase-activated receptor-2 agonist, SLIGRL-NH2. Eur J Pharmacol 530 281-283. [DOI] [PubMed] [Google Scholar]
- Ståhle-Bäckdahl M, Hägermark O, and Lins LE (1988) The sensitivity of uremic and normal human skin to histamine. Acta Derm Venereol 68 230-235. [PubMed] [Google Scholar]
- Steinhoff M, Neisius U, Ikoma A, Fartasch M, Heyer G, Skov PS, Luger TA, and Schmelz M (2003) Proteinase-activated receptor-2 mediates itch: a novel pathway for pruritus in human skin. J Neurosci 23 6176-6180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YG and Chen ZF (2007) A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature 448 700-703. [DOI] [PubMed] [Google Scholar]
- Thomsen JS, Petersen MB, Benfeldt E, Jensen SB, and Serup J (2001) Scratch induction in the rat by intradermal serotonin: a model for pruritus. Acta Derm Venereol 81 250-254. [DOI] [PubMed] [Google Scholar]
- Tsujii K, Andoh T, Lee JB, and Kuraishi Y (2008) Activation of proteinase-activated receptors induces itch-associated response through histamine-dependent and -independent pathways in mice. J Pharmacol Sci 108 385-388. [DOI] [PubMed] [Google Scholar]
- Twycross R, Greaves MW, Handwerker H, Jones EA, Libretto SE, Szepietowski JC, and Zylicz Z (2003) Itch: scratching more than the surface. QJM 96 7-26. [DOI] [PubMed] [Google Scholar]
- Ui H, Andoh T, Lee JB, Nojima H, and Kuraishi Y (2006) Potent pruritogenic action of tryptase mediated by PAR-2 receptor and its involvement in anti-pruritic effect of nafamostat mesilate in mice. Eur J Pharmacol 530 172-178. [DOI] [PubMed] [Google Scholar]
- Weisshaar E, Ziethen B, and Gollnick H (1997) Can a serotonin type 3 (5-HT3) receptor antagonist reduce experimentally-induced itch. Inflamm Res 46 412-416. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Nagasawa T, Satoh M, and Kuraishi Y (1999) Itch-associated response induced by intradermal serotonin through 5-HT2 receptors in mice. Neurosci Res 35 77-83. [DOI] [PubMed] [Google Scholar]