Abstract
Flavonoids have poor bioavailabilities largely because of metabolism via UDP-glucuronosyltransferases (UGTs). This study aims to further understand the functions of UGT in metabolizing genistein and apigenin, two compounds metabolized more extensively in the gut than in the liver. Because Gunn rats are deficient in UGT1As, we determined whether this deficiency would result in less flavonoid glucuronidation, using rat intestinal perfusion model and microsomes prepared from rat liver and intestine. In yeast-expressed rat UGT isoforms, rat UGT1A isoforms (especially UGT1A7) were mainly responsible for flavonoid metabolism. In perfusion studies, the two flavonoids were rapidly absorbed at comparable rates, but the intestinal excretions of glucuronides in Gunn rats compared with Wistar rats were not only comparable for genistein but also were higher (p < 0.05) for apigenin, suggesting up-regulation of UGT isoforms in Gunn rats. To determine the possible compensatory UGT isoforms, we first verified that UGT1A activities were significantly lower (p < 0.05) in Gunn rats by using UGT1A-specific probes 7-ethyl-10-hydroxycamptothecin (SN-38) and prunetin. We then demonstrated using UGT2B probes testosterone, ezetimibe, and indomethacin that UGT2B activities were usually significantly higher in Gunn rats. In addition, testosterone was metabolized much faster in liver microsomes than in intestinal microsomes, and in microsomes prepared from Gunn rats compared with Wistar rats. In conclusion, flavonoids are efficiently metabolized by UGT1A-deficient Gunn rats because of compensatory up-regulation of intestinal UGT2Bs and hepatic anion efflux transporters, which increases their disposition and limits their oral bioavailabilities.
Flavonoids hold great promise as agents to improve human health because epidemiological studies have shown protective effects associated with flavonoid consumption against coronary heart diseases (Arts and Hollman, 2005), stroke (Keli et al., 1996), and lung and colorectal cancers (Hirvonen et al., 2001; Bobe et al., 2008). Currently, several clinical trials are ongoing to test the anticancer efficacy of soy isoflavones and green tea, both of which contain flavonoids as the active ingredients (Crowell, 2005). A major impediment to the development of flavonoids is their poor bioavailabilities, which are usually approximately 3 to 5% (Coldham et al., 2002). Poor bioavailabilities make it more difficult to test flavonoids in a clinical setting because it requires a large number of subjects because exposure levels are going to be highly variable across the population. Poor bioavailabilities also make it more challenging to get a sufficient amount of flavonoids into humans from dietary sources (e.g., fruits and vegetables) or in the form of a pill (e.g., soy isoflavone tablets).
Improving our understanding of the processes that govern flavonoid bioavailabilities, hence, is of great significance if we were to identify means to improve flavonoid bioavailabilities. Researchers from several laboratories, including ours, have been studying these processes and have shown that flavonoid aglycones are well absorbed but that absorbed flavonoids are rapidly metabolized via phase II conjugation, resulting in poor bioavailabilities (Busby et al., 2002; Chen et al., 2003, 2005a; Hu et al., 2003; Setchell et al., 2003; Walle, 2004). Because flavonoid conjugation is predominantly carried out by UDP-glucuronosyltransferases (UGTs) and sulfotransferases, the metabolites formed are highly hydrophilic organic anions (Chen et al., 2003, 2005a; Jeong et al., 2005c). These organic anions are then eliminated by active efflux transporters because of their inabilities to passively diffuse across the cell membranes (Liu and Hu, 2007).
A coupling mechanism in which UGTs and efflux transporters (i.e., MRP2) work in concert is considered to be necessary to enable rapid elimination of flavonoid conjugates (Jeong et al., 2005b). This coupling mechanism enables efficient metabolism of flavonoids, resulting in poor oral bioavailabilities. It also allows efficient enteric and enterohepatic recycling, which allows the excreted flavonoid glucuronides (sulfates) to return as aglycones after their hydrolysis by microflora, thereby increasing their apparent half-lives. The enterohepatic recycling has long been documented as the mechanism responsible for the apparent inconsistency of low bioavailabilities (3–5%) but long (3–7 h) apparent half-lives of flavonoids (Cassidy, 2006; Moon et al., 2006). Our laboratory has shown recently that enteric recycling contributes to and further enhances the flavonoid recycling (Chen et al., 2003, 2005b; Jeong et al., 2005a; Wang et al., 2006).
Previous studies from our laboratory showed that glucuronides, not sulfates (usually not detected in rats), were the predominant metabolites of flavonoids such as genistein and apigenin and that rat intestinal UGTs play a more important role than liver UGTs (Chen et al., 2003, 2005b; Wang et al., 2006). However, it was unknown which rat UGT isoforms were responsible for the metabolism of flavonoids and whether deficiency in major rat UGT isoforms will result in changes in metabolic phenotypes. The latter is important if we aim to increase flavonoid bioavailabilities, because less flavonoid metabolism in Gunn rats would mean that it is feasible to use selective chemical inhibitors of UGT1As to increase flavonoid bioavailabilities. It is also important because certain UGT1A polymorphisms could affect the potential beneficial effects of flavonoids, because UGT polymorphism was shown to affect toxicities and efficacies of SN-38 as well as androgen signaling (Ando and Hasegawa, 2005; Chouinard et al., 2008).
The current study 1) determined the consequences of UGT1As deficiency (as in Gunn rats) in the overall glucuronidation of flavonoids and 2) identified mechanisms that may compensate the functional absence of UGT1As in Gunn rats.
Materials and Methods
Materials. Genistein and apigenin were purchased from Indofine Chemicals (Hillsborough, NJ). Testosterone, indomethacin, β-glucuronidase with or without sulfatase, uridine diphosphoglucuronic acid, alamethicin, d-saccharic-1,4-lactone monohydrate, magnesium chloride, Tris, and Hanks' balanced salt solution (powder form) were purchased from Sigma-Aldrich (St. Louis, MO). All other materials (typically analytical grade or better) were used without further purification.
Animals. Male Gunn rats [10–11 weeks old; verified as homozygous (j/j) by the supplier] and their controls (i.e., Wistar rats) weighing between 300 and 324 g were purchased from Harlan Teklad (Madison, WI). Gunn rats are deficient in UGT1As because of a recessive gene (Chowdhury et al., 1993). The rats were fed with Teklad F6 rodent diet (W) from Harlan Teklad. The rats were fasted overnight before the day of the experiment. No flavonoids were found in perfusate obtained by perfusing blank Hanks' balanced salt solution, pH 7.4, buffer through a segment of jejunum, indicating minimal presence of dietary flavonoids in the intestine.
Rat Intestinal Microsomes and Liver Microsomes Preparation. Rat intestinal microsomes and male rat liver microsomes were prepared as described previously (Chen et al., 2003). The resulting microsomes were suspended in 250 mM sucrose solution, separated into microcentrifuge tubes, and stored at -80°C until use.
Measurement of Protein Concentration. Protein concentrations of microsomes were determined by a protein assay kit (Bio-Rad, Hercules, CA) using bovine serum albumin as the standard.
Expression of UDP-Glucuronosyltransferase Isoforms in Yeast AH22 Cells. The procedures for the preparation of UGT isoforms were described previously, and the same yeast cell lysates were used in the present study (Daidoji et al., 2005). All UGT isoforms expressed by yeast microsomes were confirmed using Western blots (data not shown) and subsequently used for glucuronidation reactions using procedures described below.
Measurement of UGT Activities Using Microsomes. The incubation procedures for measuring UGT activities using microsomes were as follows: 1) mix microsomes (final concentration, ≈0.05 mg/ml protein), magnesium chloride (0.88 mM), saccharolactone (4.4 mM), and alamethicin (0.022 mg/ml); different concentrations of substrates in a 50 mM potassium phosphate buffer, pH 7.4; and uridine diphosphoglucuronic acid (3.5 mM; add last); 2) incubate the mixture (final volume, 200 μl) at 37°C for 30 or 60 min; and 3) stop the reaction by addition of 50 μl of 94% acetonitrile/6% glacial acetic acid containing 100 μM testosterone as the internal standard.
Sample Extraction. To confirm the formation of a particular conjugate, samples were selectively extracted with methylene chloride to remove >90% aglycones. The resulting sample was then divided into two parts, one of which was analyzed after incubating with water (same volume as the enzyme), and the other was analyzed after hydrolysis by glucuronidase. The difference in amount of aglycones found in these two samples was the amount of metabolite formed. The relationship between the peak areas of the metabolites before hydrolysis and the peak areas of aglycones after the hydrolysis is used to establish the conversion factor used to quantify the amounts of isoflavone glucuronides as described previously (Liu and Hu, 2002).
Animal Surgery. The procedures were approved by the University of Houston's Institutional Animal Care and Uses Committee. The intestinal surgical procedures were modified from our previous publication (Hu et al., 1998), in that four segments of the intestine were cannulated simultaneously (a “four-site model”) along with a bile duct cannulation (Liu and Hu, 2002; Chen et al., 2003; Wang et al., 2006). The circulation to the liver and intestine was not disrupted in this model. To keep the temperature of the perfusate constant, the inlet cannulate was insulated and kept warm by a 37°C circulating water bath.
Transport and Metabolism Experiments in Perfused Rat Intestinal Model. This is a single-pass perfusion method. Four segments of the intestine (duodenum, upper jejunum, terminal ileum, and colon) were perfused simultaneously with a perfusate containing the compound of interest using an infusion pump (model PHD2000; Harvard Apparatus Inc., Holliston, MA) at a flow rate of 0.191 ml/min. After a 30-min washout period, which was usually sufficient to achieve the steady-state absorption, four samples were collected from the outlet cannulae every 30 min. Bile samples (approximately 0.4 ml) were collected before perfusion started and every 30 min afterward. After perfusion, the length of the intestine was measured as described previously (Hu et al., 1988, 1998). The outlet concentrations of test compounds in the perfusate were determined by HPLC. Bile samples (without detectable aglycones) were diluted (1:10) with buffer, added to glucuronidase + sulfatase, and reacted for2hto release the aglycones for HPLC measurement.
HPLC Analysis of Flavonoids/Isoflavonoids and Their Conjugates. The conditions for analyzing genistein, apigenin, testosterone, indomethacin, and ezetimibe and their conjugates were as follows: system, Agilent 1090 liquid chromatograph with diode array detector and ChemStation (Agilent Technologies, Santa Clara, CA); column, Aqua (Phenomenex, Gilroy, CA), 5 μm, 150 × 0.45 cm; mobile phase A, water (0.04% H3PO4 and 0.09% triethylamine or C6H15N, pH 6.0); mobile phase B, 100% acetonitrile; gradient, 0 to 3 min, 15% B, 3 to 15 min, 15 to 19% B, and 15 to 52 min, 19 to 41% B; wavelength, 254 nm (for the isoflavones and the internal standard); and injection volume, 200 μl. There was a 4-min interval between the end of the run and the next injection to allow the column to be re-equilibrated with 15% mobile phase B. The detection limit was 0.25 μM, and the tested linear range was 0.5 to 100 μM. The precision is typically better than 5%, and accuracy is better than 10%.
UPLC Analysis of Flavonoids/Isoflavonoids and Their Conjugates. Selected samples were also run on ultraperformance liquid chromatography (UPLC) that was much more time efficient than HPLC. The conditions for analyzing isoflavones using UPLC were as follows: system, AcQuity UPLC (Waters, Milford, MA) with photodiode array detector and Empower software; column, AcQuity UPLC BEH C18 column (Waters), 1.7 μm, 2.1 × 50 mm; mobile phase A, 100% acetonitrile; mobile phase B, aqueous solution (0.06% triethylamine and 0.045% formic acid/methanol, 90:10); gradient, 0 to 0.3 min, 0% B, flow rate, 1 ml/min; 0.3 to 1.80 min, 0 to 50% B, flow rate, 0.925 ml/min; 1.80 to 2.10 min, 50 to 100% B, flow rate, 0.925 ml/min; 2.10 to 2.40 min, 100% B, flow rate, 0.925 ml/min; and 2.40 to 2.50, 100 to 0% B, flow rate, 1 ml/min; wavelength, 254 nm; and injection volume, 10 μl. We have recently started to use UPLC method to measure the concentration of flavonoids. The detection limit is 0.2 μM and the tested linear range is from 0.38 to 50 μM. The precision is typically within 5% and accuracy is within 9%.
Data Analysis. Amounts of isoflavones absorbed (Mab), amounts of conjugated isoflavones excreted into the intestinal lumen (Mgut), amounts of conjugated isoflavones excreted via the bile (Mbile), and the percentage of absorbed and percentage of metabolized values were calculated as described previously (Chen et al., 2003; Wang et al., 2006). In brief, Mab was expressed as follows:
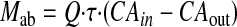 |
(1) |
where Q is the flow rate (milliliters per minute); τ is the sampling interval (30 min); and CAin and CAout are the inlet and outlet concentrations of aglycones corrected for water flux, respectively. Mgut was expressed as follows:
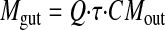 |
(2) |
where CMout is the outlet concentrations (nanomoles per milliliter) of metabolites corrected for water flux. Mbile was expressed as follows:
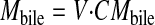 |
(3) |
where CMbile is the bile concentrations (nanomoles per milliliter) of metabolites, and V is the volume of bile collected over a 30-min period.
Percentage absorbed and percentage metabolized were calculated as follows:
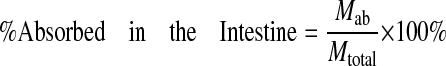 |
(4) |
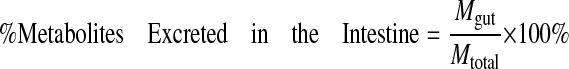 |
(5) |
where Mtotal is the total amount of compound perfused over a 30-min period.
Rates of metabolism in intestinal or liver microsomes were expressed as amounts of metabolites formed per minute per milligram of protein or nanomoles per minute per milligram. Detailed procedures and models used to determine the kinetic parameters were published previously (Chen et al., 2005b; Wang et al., 2006). In brief, assuming that the glucuronidation rates versus concentration curves follow simple Michaelis-Menten kinetics, eq. 6 is used to derive Km, Vmax, and the intrinsic clearance (Vmax /Km) values:
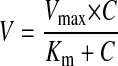 |
(6) |
Statistical Analysis. General linear model analysis of variance, two-sample t tests, one-way analysis of variance, and subsequential Tukey-Kramer multiple comparison tests (critical value = 4.1987) were used to analyze the data using the 2001 version of the NCSS software. The prior level of significance was set at 5%, or p < 0.05.
Results
Metabolism of Flavonoids by Expressed Rat UGT Isoforms. The main conjugation reaction of genistein and apigenin in rats is glucuronidation, which is expected to be catalyzed by multiple UGT isoforms (Doerge et al., 2000; Jia et al., 2004; Chen et al., 2005b; Wang et al., 2006).
Because microsomes prepared from both intestine and liver are composed of pooled UGT isoforms, it is impossible to determine the UGT isoform(s) responsible without specific chemical or macromolecular (monoclonal) inhibitors. Therefore, AH22 yeast-expressed rat UGT isoforms were used to determine which isoforms(s) is responsible for flavonoid glucuronidation at 50 μM. A high concentration of substrate is used because it is more likely to produce quantifiable amounts of glucuronides when glucuronidation reaction occurs.
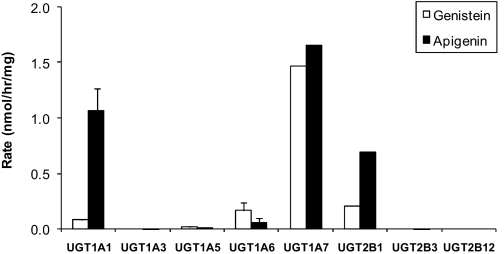
The results indicated that glucuronidation of 50 μM genistein and apigenin was largely carried out via UGT1A isoforms (Fig. 1). UGT1A7 was the main isoform responsible for the glucuronidation of 50 μM genistein (1.47 ± 0.001 nmol/h/mg) and apigenin (1.65 ± 0.002 nmol/h/mg). For genistein, UGT1A7 rates was followed by UGT2B1, UGT1A6, UGT1A1, and UGT1A5 (0.21 ± 0.002, 0.17 ± 0.06, 0.083 ± 0.008, and 0.017 ± 0.003 nmol/min/mg, respectively) (Fig. 1). For apigenin, the UGT1A7 rates were followed closely by UGT2B1 (0.69 ± 0.001 nmol/h/mg) and then by UGT1A6, UGT1A5, UGT2B3, and UGT1A3 (0.06 ± 0.038, 0.02 ± 0.002, 0.004 ± 0.001, and 0.002 ± 0.003 nmol/min/mg, respectively) (Fig. 1). One isoform (i.e., UGT2B12) did not metabolize these two flavonoids.
Fig. 1.
Metabolism of genistein (open columns) and apigenin (solid columns) using rat UGT isoforms expressed in yeast strain AH22. From left to right, UGT1A1, UGT1A3, UGT1A5, UGT1A6, UGT1A7, UGT2B1, UGT2B3, and UGT2B12 were tested with optimal incubation with either 50 μM genistein or apigenin (number of replicates, n = 3).
The kinetic parameters of UGT1A7-catalyzed glucuronidation of genistein and apigenin were determined using a concentration range of 0.78 to 50 μM, and glucuronidation kinetics of both compounds followed a simple Michaelis-Menten kinetics (data not shown). Genistein displayed an intrinsic clearance value of 1.26 ml/min/mg, a Vmax value of 4.188 nmol/h/mg, and a Km value of 3.318 μM. Apigenin, in contrast, displayed an intrinsic clearance value of 8.67 ml/min/mg, a Vmax value of 3.887 nmol/h/mg, and a Km value of 0.45 μM.
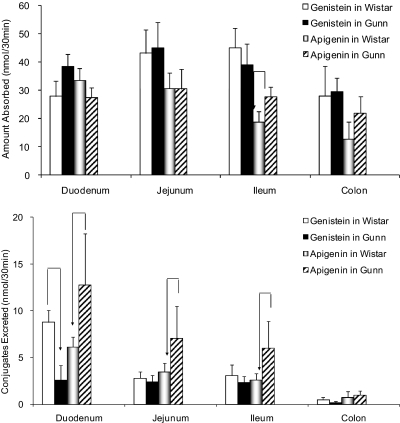
Absorption of Genistein and Apigenin from Different Regions of the Wistar and Gunn Rat Intestine. Given the ability of UGT1A isoforms to metabolize apigenin and genistein, it was of interest to investigate whether metabolism of these flavonoids would be different in rats lacking UGT1As. Perfusion studies were performed to compare the absorption of genistein and apigenin (10 μM) in four regions of the intestine between Wistar and Gunn rats, because Gunn rats are derived from Wistar rats but lack UGT1A isoforms (Chowdhury et al., 1993). In all four sites of the intestine (duodenum, jejunum, ileum, and colon), significant amounts of genistein and apigenin were absorbed at generally comparable rates in these two rat strains (Fig. 2, top).
Fig. 2.
Disposition of genistein and apigenin in a four-site rat intestinal perfusion model (number of replicates, n = 4). Four segments of the intestine (i.e., duodenum, upper jejunum, terminal ileum, and colon) were perfused simultaneously at a flow rate of 0.191 ml/min using perfusate containing 10 μM genistein in Wistar control (solid white columns) or Gunn rats (solid black columns) or using perfusate containing 10 μM apigenin in Wistar control (light graded columns) or Gunn rats (dark diagonal slashed columns). Amounts absorbed (top) or excreted (bottom) were normalized to 10-cm intestinal length using eqs. 1, 2, 3. At a flow rate of 0.191 ml/min, the amount perfused was 57.3 nmol/10 cm/30 min for each segment or 229 nmol/30 min for all four intestinal segments. According, absorption of 27 nmol from one perfused segment represents 47% of the perfused amount. The arrow indicated a statistically significant difference between two strains of rats using the same flavonoid. Each column represents the average of four determinations, and the error bar is the S.D.
Excretion of Genistein and Apigenin Metabolites from Different Regions of the Wistar and Gunn Rat Intestine. Luminal glucuronides but not sulfates were found in the outlet intestinal perfusate, and their concentrations were quantified as described under Materials and Methods. For genistein, only in the duodenum of Gunn rats was the excretion of metabolites significantly (p < 0.05) lower than that of Wistar control rats (Fig. 2, bottom). In the jejunum, ileum, and colon, excretion rates between Gunn and Wistar control were comparable (Fig. 2, bottom). For apigenin, the intestinal excretion of its metabolites in Gunn rats was significantly (p < 0.05) higher than for the Wistar control for all three small intestinal segments (Fig. 2, bottom), although it was only marginally higher in the colon (35%; p > 0.05).
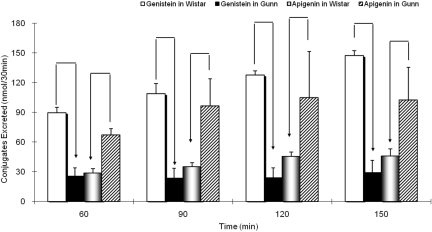
Biliary Excretion of Genistein and Apigenin Metabolites in Wistar and Gunn Rats. Amounts of genistein and apigenin metabolites excreted in the bile were measured after the initiation of perfusion, and samples were taken every 30 min from 60 to 150 min. Only glucuronides were quantifiable, and sulfates were not found. For genistein, amounts of its glucuronides in Gunn rat bile were significantly (p < 0.05) lower than for their wild-type Wistar rats (Fig. 3). This was consistent for the duration of the experiment (from 60-min sample time to 150-min sample time). For apigenin, the results were exactly the opposite. The amount of apigenin glucuronides excreted in the bile of Gunn rats was significantly (p < 0.05) higher than their Wistar control (Fig. 3), which was also consistent for the duration of the experiment.
Fig. 3.
Biliary excretion of flavonoid glucuronides in Wistar and Gunn rats. Biliary excretion samples (at 30-min sample interval) (n = 4) were collected when conducting perfusion experiments using 10 μM genistein in Wistar control (solid white columns) or Gunn rats (solid black columns) as well as using 10 μM apigenin in Wistar control rats (light graded columns) or Gunn rats (dark diagonal slashed columns) (number of replicates, n = 4). Significant difference (p < 0.05) in biliary excretion between two rat strains using either genistein or apigenin was indicated with an arrow in the figure. Each column represents the average of four determinations, and the error bar is the S.D.
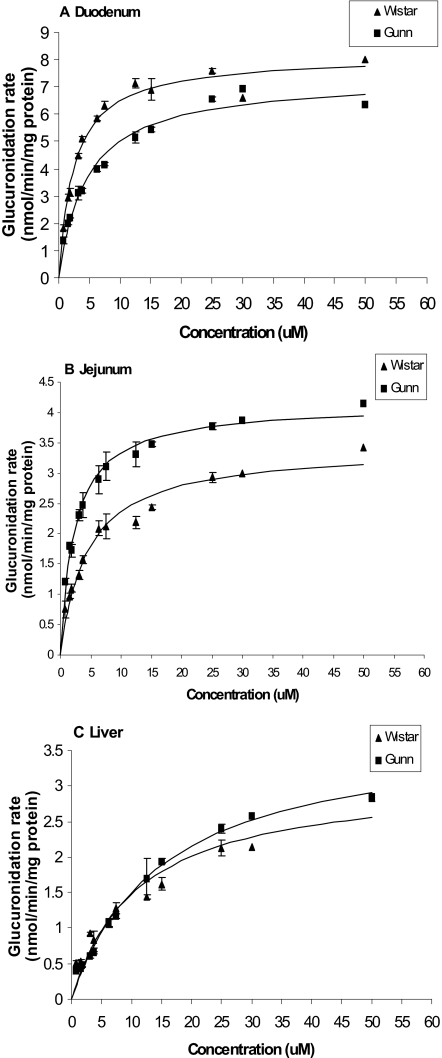
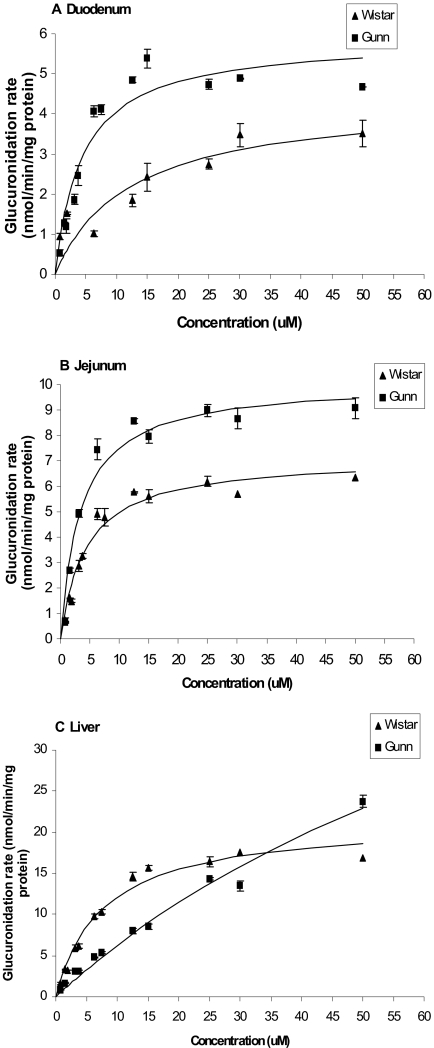
Metabolism of Genistein and Apigenin by Rat Intestinal and Liver Microsomes. We found saturable glucuronidation of genistein and apigenin in microsomes prepared from tissues of both Gunn and Wistar rats (Figs. 4 and 5). This was the only similarity between apigenin and genistein metabolism. For genistein, its glucuronidation in liver microsomes of both strains was nearly identical (Fig. 4C; Table 1). In contrast, its glucuronidation in duodenal microsomes of Gunn rats was lower than in Wistar rats, but its glucuronidation in jejunum microsomes of Gunn rats was higher than Wistar rats (Fig. 4, A and B). For apigenin, the glucuronidation rates of apigenin in Gunn rat microsomes were generally higher than that in Wistar rat microsomes (Fig. 5), except for liver microsomes (Fig. 5C). In liver microsomes prepared from Gunn rats, the glucuronidation rates were slower (than in Wistar rat liver microsomes) at lower concentrations (<30 μM) but faster at higher concentrations (>35 μM) (Fig. 5C). Kinetic parameters for the glucuronidation of flavonoids by intestinal and liver microsomes for both strains are listed in Table 1.
Fig. 4.
Apparent glucuronidation rates of genistein in rat duodenal (A), jejunal (B), and liver (C) microsomes of Gunn and Wistar as a function of concentration (n = 3). Rates of metabolism were determined from 0.625 to 50 μM, and reaction time was for 30 min. The metabolism rate is the average of three determinations and the error bar represents the standard deviation of the mean. The points were observed genistein glucuronide formation rates, and the curves were estimated based on fitted parameters using a simple Michaelis-Menten equation (eq. 6).
Fig. 5.
Apparent glucuronidation rates of apigenin in rat duodenal (A), jejunal (B), and liver (C) microsomes of Gunn and Wistar as a function of concentration (n = 3). Rates of metabolism were determined from 0.625 to 50 μM, and reaction time was for 30 min. The metabolism rate is the average of three determinations and the error bar represents the S.D. of the mean. The points were observed apigenin glucuronide formation rates, and the curves were estimated based on fitted parameters generated by a simple Michaelis-Menten equation (eq. 6).
TABLE 1.
Apparent kinetic parameters of genistein and apigenin glucuronidation in different microsomes Data analyzed using simple Michaelis-Menten equation and nonlinear regression.
|
Flavonoid
|
Species
|
Duodenum Kinetic Parameter
|
Jejunum Kinetic Parameter
|
Liver Kinetic Parameter
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Km | Vmax | CLint | Km | Vmax | CLint | Km | Vmax | CLint | ||
| μM | nmol / min / mg protein | ml / min / mg | μM | nmol / min / mg protein | ml / min / mg | μM | nmol / min / mg protein | ml / min / mg | ||
| Genistein | Wistar | 2.5 | 8.1 | 3.2 | 4.5 | 3.4 | 0.76 | 11.1 | 3.1 | 0.28 |
| Gunn | 4.6 | 7.3 | 1.6 | 2.4 | 4.1 | 1.8 | 15.3 | 3.8 | 0.25 | |
| Apigenin | Wistar | 12.2 | 4.4 | 0.36 | 4.4 | 7.2 | 1.6 | 7.7 | 21.4 | 2.8 |
| Gunn | 4.5 | 5.9 | 1.3 | 3.5 | 10.1 | 2.9 | 99.7 | 66.8 | 0.69 | |
| Prunetin | Wistar | 0.48 | 4.3 | 8.9 | 0.51 | 0.56 | 0.91 | |||
| Gunn | 0.81 | 2.2 | 2.7 | 0.77 | 0.19 | 0.25 | ||||
Metabolism of UGT1A Probe Substrates by Rat Intestinal and Liver Microsomes. The above-mentioned differences in glucuronidation between Gunn and Wistar control rats were unexpected in that glucuronidation was either lower (for genistein) or became elevated (for apigenin) in Gunn rats. Because both compounds may be metabolized by UGT2B, UGT1A-specific substrates were used to verify that the UGT1A activities were significantly lower in Gunn rat microsomes. The two compounds we used were SN-38 and prunetin, both of which have been shown to be mainly the substrates of human UGT1As (Paoluzzi et al., 2004; Joseph et al., 2007). In addition, SN-38 glucuronide formation was slower in Gunn rats and expression of UGT1A1 gene in Gunn rats through gene therapy increased glucuronidation of SN-38 (Tallman et al., 2007).
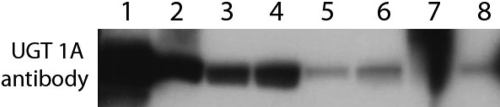
Our results indicated that the metabolism of both of these compounds was significantly less (p < 0.05) in Gunn rat liver microsomes. For prunetin, this decrease in metabolism was clearly reflected in the decreased Vmax values (Table 1), consistent with a lack of expression of UGT1As. For SN-38, this slower metabolism was shown at all three tested concentrations (Fig. 6). Use of jejunal microsomes yielded results consistent with those derived from liver (Fig. 6). A human UGT1A1-specific antibody was then used to probe the expression level of UGT1A in rats using the Western blot, and the results indicated a reduced expression of rat UGT1A in Gunn rats (Fig. 7), consistent with the results of the kinetic studies shown above. Unfortunately, the antibody used is not highly reactive with rat UGT1A and reacts weakly with human UGT2B7.
Fig. 6.
Apparent glucuronidation rates of SN-38 in Gunn and Wistar rat liver and jejunal microsomes at three concentrations. SN-38 is a specific probe substrate for UGT1A1, and formation of SN-38 glucuronide was impaired in Gunn rats (Tallman et al., 2007). A higher concentration of SN-38 was not used because it is not soluble in the current reaction system. Each column bar represents an average of three determinations, and the error bar represents the S.D. of the mean.
Fig. 7.
Western blot analysis of UGT1A and UGT2B expression. Lane 1, 0.5 μg of expressed human UGT1A1; lane 2, 100 μg of yeast lysate expressing rat UGT1A7; lane 3, 10 μg of Wistar rat liver microsomes; lane 4, 20 μg of Wistar rat liver microsomes; lane 5, 10 μg of Gunn rat liver microsomes; lane 6, 20 μg of Gunn rat liver microsomes; lane 7, defective; and lane 8, 0.5 μg of expressed human UGT2B7. The antibody (sc-25847; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) seems to be highly selective for the human UGT1A1 over human UGT2B7, although it reacts weakly with UGT2Bs. As such, the antibody was expected to be selective with rat UGT1As over rat UGT2Bs.
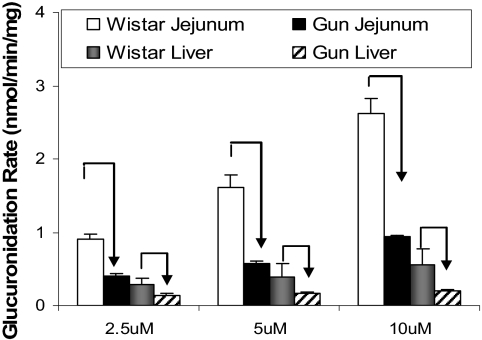
Metabolism of UGT2B Probe Substrates by Rat Intestinal and Liver Microsomes. The results from earlier perfusion and microsome studies using Gunn and Wistar rats indicated the possibility of compensatory mechanisms by which alternative UGT enzyme isoforms have been up-regulated because of decreased UGT1A activities in Gunn rats.
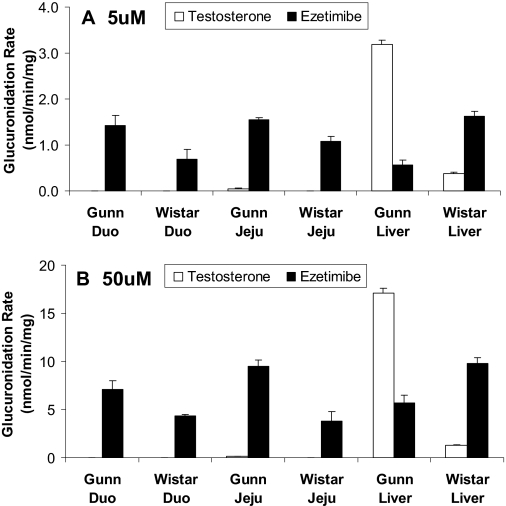
To determine which UGT2B isoform(s) were responsible, the metabolic profile of testosterone (a common UGT2B1, 2B3, and 2B6 metabolic probe substrate) was determined using two concentrations and three different (tissue) microsomes. The results indicated that little or no metabolism occurred in the intestine (Fig. 8), but glucuronidation in liver microsomes of Gunn rats was rapid and much faster (10–13-fold) than in Wistar rats at both 5 and 50 μM concentrations.
Fig. 8.
Apparent glucuronidation rates of testosterone and ezetimibe at 5 μM (A) and 50 μM (B). The reaction was proceeded at an optimal incubation time of 1 h using duodenal, jejunal, and liver microsomes prepared from Wistar and Gunn rats (n = 3). Each column represents the average of three determinations, and the error bar is the S.D.
The second UGT2B probe used was ezetimibe [or 1-(4-fluorophenyl)-3(R)-4(S)-(4-hydroxyphenyl)-2-azetidinone] to confirm the testosterone glucuronidation results. Ezetimibe was shown to be metabolized by recombinant human UGT2B7 and UGT2B15 (Ghosal et al., 2004). At 5 μM, Gunn rat duodenum and jejunum microsomes had a significantly (p < 0.05) higher ezetimibe glucuronidation rate than Wistar rat microsomes (Fig. 8A). Conversely, Gunn rat liver microsomes had a significantly (p < 0.05) lower glucuronidation rate (0.56 ± 0.11 nmol/min/mg) than Wistar rat microsomes (1.62 ± 0.11 nmol/min/mg). Metabolic patterns at 50 μM concentration observed for both rat strains (Fig. 8B) were similar to the pattern observed at 5 μM (Fig. 8A).
The third UGT2B probe used was indomethacin because it is a substrate for intestinal UGT2B7. At 5 μM, Gunn rat jejunal microsomes metabolized indomethacin slower (33% less; p > 0.05) than those in Wistar rats (≈0.05 nmol/min/mg protein), its liver microsomes metabolized indomethacin at comparable rates (≈0.09 nmol/min/mg protein) as in Wistar rats, and its duodenal microsomes metabolized indomethacin at a faster rate (50%; p < 0.05) than in Wistar rats. At 50 μM, Gunn rat liver and jejunal microsomes metabolized indomethacin at a rate (≈0.35 nmol/min/mg protein) comparable with that of Wistar rats, in which its duodenal microsomes metabolized indomethacin slightly slower (30% less or ≈0.025 nmol/min/mg protein; p > 0.05).
Discussion
We have performed the present study using the UGT1A-deficient Gunn rats to determine the roles of UGT1As in rendering poor oral bioavailabilities of flavonoids. The latter is an important problem because bioavailability challenges have limited the potential of flavonoids as chemopreventive agents (Setchell et al., 2003).
We have chosen the Gunn rat model for the present study because it lacks expression of UGT1A, as the result of recessive genes. This animal model was used frequently to determine the consequence of UGT1A deficiency in vivo, which usually resulted in much slower excretion of glucuronides (Chowdhury et al., 1993; Watanabe et al., 2000). A recent search of PubMed using the keyword combo “Gunn rats” generated more than 800 hits, indicating that this animal model is well received by biomedical researchers. The results of present study were therefore important because this would be the first report to show that activities of other UGT isoforms had changed (i.e., elevated) to compensate for UGT1A deficiency.
We observed consistent but unexpected results: similar or higher intestinal excretion of both flavonoid glucuronides and higher biliary excretion of apigenin glucuronides (Figs. 2 and 3). They were unexpected because absorption was similar in both rat strains (Fig. 2). We expected much slower glucuronidation of apigenin and genistein because previous studies from various research groups have shown the importance of UGT1As for metabolism phenolic and polyphenolic compounds (Strassburg et al., 1997; Galijatovic et al., 2001; Malfatti and Felton, 2004; Tian et al., 2005). Our own results also did not predict this observation because both genistein and apigenin (at 50 μM) were mainly metabolized by UGT1As, especially UGT1A7 (Fig. 1). Although UGT2B subfamily (specifically UGT2B1) participated in the glucuronidation, they seemed to be much less efficient than UGT1A7 (Fig. 1). In addition, combined levels of UGT2B mRNAs were shown to be much lower (severalfold) than combined levels of UGT1A mRNAs (Shelby et al., 2003).
To explain the observed excretion pattern, we first performed microsomal glucuronidation experiments, and the results suggested that glucuronidation of flavonoids in Gunn rat microsomes was usually faster than in Wistar rat microsomes (Figs. 4 and 5). The only exceptions were the duodenal glucuronidation of genistein and liver glucuronidation of apigenin at low concentrations (<25 μM) (Figs. 4 and 5). Therefore, the slower intestinal excretion of genistein glucuronides in duodenum of Gunn rats and faster intestinal excretion of apigenin glucuronides in the duodenum and jejunum of Gunn rats observed in perfusion studies were probably caused by changes in relevant UGT activities in the gut. However, the same statement cannot be made for biliary excretion of flavonoid glucuronides (discussed below).
We then determined the possible mechanisms by which flavonoid glucuronidation became faster in the intestine of Gunn rats. To do this, we first verified that UGT1A activities in Gunn rat microsomes were indeed lower by using two UGT1A-specific substrates, SN-38 and prunetin. The results confirmed significantly slower glucuronidation rates in Gunn rat intestinal microsomes (Fig. 6; Table 1). We then conducted glucuronidation studies using UGT2B-specific substrates testosterone, ezetimibe, and indomethacin, and the results showed that their metabolism rates in Gunn rat intestinal microsomes were usually higher or the same (Fig. 8). Together, these results suggested that higher UGT2B activities in Gunn rats, as the result of compensation for UGT1A deficiency, were the main reason for the observed pattern of flavonoid glucuronide excretion observed in the Gunn rat gut. Based on known expression pattern of six rat UGT2Bs (Shelby et al., 2003), the possible isoforms responsible for enhanced flavonoid glucuronidation in the gut are UGT2B6 and 2B8 because UGT2B3 and 2B12 did not metabolize either flavonoids (Fig. 1), and UGT2B1 and 2B2 were not expressed in rat intestine (Shelby et al., 2003). Because UGT2B8 is only expressed in the duodenum (Shelby et al., 2003) and genistein was glucuronidated less in the Gunn rat duodenum, it is probably not the isoform responsible. Therefore, UGT2B6 is the only known intestinal isoform that could have been up-regulated in the gut, although yet-to-be-defined new UGT2Bs or other UGTs could also be up-regulated. The latter was recognized here as a possible source of uncertainty because of limitations imposed by available methods.
We also determined the possible mechanisms by which excretion of flavonoid glucuronides in the bile of Gunn rats was lower (as for genistein) or higher (as for apigenin) than the control Wistar rats. The use of two UGT1A-specific substrates, SN-38 and prunetin, in microsomal studies confirmed lower glucuronidation rates in Gunn rat liver microsomes (Fig. 6; Table 1). The latter was also consistent with the lower UGT1A expression in Western blot of Gunn rat liver microsomes (as opposed to Wistar rats) (Fig. 7). However, the biliary excretion pattern of genistein glucuronides was not consistent with its microsomal reaction pattern, because a big drop in biliary excretion was not consistent with a lack of change in the microsomal rates of genistein metabolism between the two strains (Fig. 4C). Biliary excretion pattern of apigenin glucuronides was also surprising, because glucuronidation rates of Gunn rat microsomes were only higher at concentrations greater than the maximal expected plasma level of 0.25 μM (Fig. 5C). Therefore, in the liver, changes in excretion of flavonoids glucuronides were related not only to changes in UGT activities but also to changes to efflux transporter activities, although the exact transporters involved in the excretion remained to be determined. Previously, organic anion efflux transporter MRP3 (located at the bile canicular membrane) was shown to be up-regulated in Gunn rats (Ogawa et al., 2000), but it was unknown whether MRP3 can selectively efflux apigenin glucuronides over genistein glucuronides. As for the UGT isoforms that were up-regulated, we believe that the higher than expected hepatic UGT activities against flavonoids in Gunn rats probably came from elevated UGT2B1 activities, because only UGT2B1 and 2B2 are highly expressed in liver (Shelby et al., 2003), but UGT2B2 was not a testosterone-conjugating enzyme (Tian et al., 2005). Last, glucuronidation of testosterone was higher only in Gunn rat liver microsomes (Fig. 8). Additional evidence in support of our analysis is that UGT2B8 was not expressed in liver (Shelby et al., 2003) and UGT2B3 and 2B12 were not active against flavonoids (Fig. 1). However, we could not role out the contribution of 2B6 isoforms because they are also expressed in liver, although an equal up-regulation of UGT2B6 at both intestine and liver would have resulted in much higher glucuronidation of testosterone in the Gunn rat intestinal microsomes, which did not occur. As stated, unknown UGT2Bs and other UGTs could also contribute.
The results of this study showed that UGT1A deficiency had limited negative impact on the intestinal excretion of flavonoid glucuronides, in which only duodenal glucuronidation of genistein was significantly slowed. UGT1A deficiency in Gunn rats had variable impact on biliary conjugate excretion because only excretion of genistein glucuronides was lowered, whereas the excretion of apigenin glucuronides was elevated. Because intestinal excretion of flavonoid glucuronides usually changed with intestinal UGT activities and biliary excretion did not, hepatic efflux transporters probably played a more important role in affecting flavonoid disposition than intestinal efflux transporters. Taken together, we have demonstrated strong compensatory responses to UGT1A deficiency in Gunn rats. These responses have resulted in no suppression or even elevation in flavonoid disposition. Therefore, the enteric and enterohepatic recycling are highly robust in vivo processes that would be difficult to suppress. The inability to suppress the flavonoid disposition as demonstrated here will make it more difficult to increase the bioavailabilities of flavonoids via the use of UGT inhibitors. Thus, it would be more difficult to overcome the bioavailability challenges associated with flavonoids because viable in vivo mechanisms are ready to compensate for any functional deficiency in metabolism of flavonoids. Looking at this point positively would suggest that flavonoids are going to be safe in humans because only small amounts of these compounds are able to reach the systemic circulation.
In conclusion, our study using expressed rat UGT isoforms indicates that UGT1As are responsible for the glucuronidation of flavonoids such as genistein and apigenin. Systematic investigation of glucuronidation in Gunn rats suggests that deficiency in UGT1As is compensated by up-regulation of intestinal UGT2Bs, and hepatic UGT2Bs and efflux transporters. The up-regulation of several UGT2Bs, probably including UGT2B6 in the gut and UGT2B1 in the liver, compensates effectively for UGT1A deficiencies in intestine and liver of Gunn rats.
This work was supported by National Institutes of Health National Institute of General Medical Sciences [Grant GM070737].
The work was submitted as part of dissertation for Wang SWJ (2009) Investigation into the role and functional capacity of MRP2 efflux transporter and the predominant UDP-glucuronosyltransferases isoform(s) in the intestinal disposition of isoflavones. Ph.D. thesis, University of Houston, Houston, TX.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.108.147371.
ABBREVIATIONS: UGT, uridine 5′-diphosphate glucuronosyltransferase; MRP, multidrug resistance-associated protein; HPLC, high-performance liquid chromatography; UPLC, ultraperformance liquid chromatography; SN-38, 7-ethyl-10-hydroxycamptothecin.
References
- Ando Y and Hasegawa Y (2005) Clinical pharmacogenetics of irinotecan (CPT-11). Drug Metab Rev 37 565-574. [DOI] [PubMed] [Google Scholar]
- Arts IC and Hollman PC (2005) Polyphenols and disease risk in epidemiologic studies. Am J Clin Nutr 81 317S-325S. [DOI] [PubMed] [Google Scholar]
- Bobe G, Weinstein SJ, Albanes D, Hirvonen T, Ashby J, Taylor PR, Virtamo J, and Stolzenberg-Solomon RZ (2008) Flavonoid intake and risk of pancreatic cancer in male smokers (Finland). Cancer Epidemiol Biomarkers Prev 17 553-562. [DOI] [PubMed] [Google Scholar]
- Busby MG, Jeffcoat AR, Bloedon LT, Koch MA, Black T, Dix KJ, Heizer WD, Thomas BF, Hill JM, Crowell JA, et al. (2002) Clinical characteristics and pharmacokinetics of purified soy isoflavones: single-dose administration to healthy men. Am J Clin Nutr 75 126-136. [DOI] [PubMed] [Google Scholar]
- Cassidy A (2006) Factors affecting the bioavailability of soy isoflavones in humans. J AOAC Int 89 1182-1188. [PubMed] [Google Scholar]
- Chen J, Lin H, and Hu M (2003) Metabolism of flavonoids via enteric recycling: role of intestinal disposition. J Pharmacol Exp Ther 304 1228-1235. [DOI] [PubMed] [Google Scholar]
- Chen J, Lin H, and Hu M (2005a) Absorption and metabolism of genistein and its five isoflavone analogs in the human intestinal Caco-2 model. Cancer Chemother Pharmacol 55 159-169. [DOI] [PubMed] [Google Scholar]
- Chen J, Wang S, Jia X, Bajimaya S, Lin H, Tam VH, and Hu M (2005b) Disposition of flavonoids via recycling: comparison of intestinal versus hepatic disposition. Drug Metab Dispos 33 1777-1784. [DOI] [PubMed] [Google Scholar]
- Chouinard S, Yueh MF, Tukey RH, Giton F, Fiet J, Pelletier G, Barbier O, and Bélanger A (2008) Inactivation by UDP-glucuronosyltransferase enzymes: the end of androgen signaling. J Steroid Biochem Mol Biol 109 247-253. [DOI] [PubMed] [Google Scholar]
- Chowdhury JR, Kondapalli R, and Chowdhury NR (1993) Gunn rat: a model for inherited deficiency of bilirubin glucuronidation. Adv Vet Sci Comp Med 37 149-173. [PubMed] [Google Scholar]
- Coldham NG, Zhang AQ, Key P, and Sauer MJ (2002) Absolute bioavailability of [14C] genistein in the rat; plasma pharmacokinetics of parent compound, genistein glucuronide and total radioactivity. Eur J Drug Metab Pharmacokinet 27 249-258. [DOI] [PubMed] [Google Scholar]
- Crowell JA (2005) The chemopreventive agent development research program in the Division of Cancer Prevention of the US National Cancer Institute: an overview. Eur J Cancer 41 1889-1910. [DOI] [PubMed] [Google Scholar]
- Daidoji T, Gozu K, Iwano H, Inoue H, and Yokota H (2005) UDP-glucuronosyltransferase isoforms catalyzing glucuronidation of hydroxy-polychlorinated biphenyls in rat. Drug Metab Dispos 33 1466-1476. [DOI] [PubMed] [Google Scholar]
- Doerge DR, Chang HC, Churchwell MI, and Holder CL (2000) Analysis of soy isoflavone conjugation in vitro and in human blood using liquid chromatographymass spectrometry. Drug Metab Dispos 28 298-307. [PubMed] [Google Scholar]
- Galijatovic A, Otake Y, Walle UK, and Walle T (2001) Induction of UDP-glucuronosyltransferase UGT1A1 by the flavonoid chrysin in Caco-2 cells–potential role in carcinogen bioinactivation. Pharm Res 18 374-379. [DOI] [PubMed] [Google Scholar]
- Ghosal A, Hapangama N, Yuan Y, Achanfuo-Yeboah J, Iannucci R, Chowdhury S, Alton K, Patrick JE, and Zbaida S (2004) Identification of human UDP-glucuronosyltransferase enzyme(s) responsible for the glucuronidation of ezetimibe (Zetia). Drug Metab Dispos 32 314-320. [DOI] [PubMed] [Google Scholar]
- Hirvonen T, Pietinen P, Virtanen M, Ovaskainen ML, Häkkinen S, Albanes D, and Virtamo J (2001) Intake of flavonols and flavones and risk of coronary heart disease in male smokers. Epidemiology 12 62-67. [DOI] [PubMed] [Google Scholar]
- Hu M, Chen J, and Lin H (2003) Metabolism of flavonoids via enteric recycling: mechanistic studies of disposition of apigenin in the Caco-2 cell culture model. J Pharmacol Exp Ther 307 314-321. [DOI] [PubMed] [Google Scholar]
- Hu M, Roland K, Ge L, Chen J, Li Y, Tyle P, and Roy S (1998) Determination of absorption characteristics of AG337, a novel thymidylate synthase inhibitor, using a perfused rat intestinal model. J Pharm Sci 87 886-890. [DOI] [PubMed] [Google Scholar]
- Hu M, Sinko PJ, deMeere AL, Johnson DA, and Amidon GL (1988) Membrane permeability parameters for some amino acids and beta-lactam antibiotics: application of the boundary layer approach. J Theor Biol 131 107-114. [DOI] [PubMed] [Google Scholar]
- Jeong EJ, Jia X, and Hu M (2005a) Disposition of formononetin via enteric recycling: metabolism and excretion in mouse intestinal perfusion and Caco-2 cell models. Mol Pharm 2 319-328. [DOI] [PubMed] [Google Scholar]
- Jeong EJ, Liu X, Jia X, Chen J, and Hu M (2005b) Coupling of conjugating enzymes and efflux transporters: impact on bioavailability and drug interactions. Curr Drug Metab 6 455-468. [DOI] [PubMed] [Google Scholar]
- Jeong EJ, Liu Y, Lin H, and Hu M (2005c) Species- and disposition model-dependent metabolism of raloxifene in gut and liver: role of ugt1a10. Drug Metab Dispos 33 785-794. [DOI] [PubMed] [Google Scholar]
- Jia X, Chen J, Lin H, and Hu M (2004) Disposition of flavonoids via enteric recycling: enzyme-transporter coupling affects metabolism of biochanin A and formononetin and excretion of their phase II conjugates. J Pharmacol Exp Ther 310 1103-1113. [DOI] [PubMed] [Google Scholar]
- Keli SO, Hertog MG, Feskens EJ, and Kromhout D (1996) Dietary flavonoids, antioxidant vitamins, and incidence of stroke: the Zutphen study. Arch Intern Med 156 637-642. [PubMed] [Google Scholar]
- Liu Y and Hu M (2002) Absorption and metabolism of flavonoids in the caco-2 cell culture model and a perused rat intestinal model. Drug Metab Dispos 30 370-377. [DOI] [PubMed] [Google Scholar]
- Liu Z and Hu M (2007) Natural polyphenol disposition via coupled metabolic pathways. Expert Opin Drug Metab Toxicol 3 389-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malfatti MA and Felton JS (2004) Human UDP-glucuronosyltransferase 1A1 is the primary enzyme responsible for the N-glucuronidation of N-hydroxy-PhIP in vitro. Chem Res Toxicol 17 1137-1144. [DOI] [PubMed] [Google Scholar]
- Moon YJ, Sagawa K, Frederick K, Zhang S, and Morris ME (2006) Pharmacokinetics and bioavailability of the isoflavone biochanin A in rats. AAPS J 8 E433-E442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa K, Suzuki H, Hirohashi T, Ishikawa T, Meier PJ, Hirose K, Akizawa T, Yoshioka M, and Sugiyama Y (2000) Characterization of inducible nature of MRP3 in rat liver. Am J Physiol Gastrointest Liver Physiol 278 G438-G446. [DOI] [PubMed] [Google Scholar]
- Paoluzzi L, Singh AS, Price DK, Danesi R, Mathijssen RH, Verweij J, Figg WD, and Sparreboom A (2004) Influence of genetic variants in UGT1A1 and UGT1A9 on the in vivo glucuronidation of SN-38. J Clin Pharmacol 44 854-860. [DOI] [PubMed] [Google Scholar]
- Setchell KD, Brown NM, Desai PB, Zimmer-Nechimias L, Wolfe B, Jakate AS, Creutzinger V, and Heubi JE (2003) Bioavailability, disposition, and dose-response effects of soy isoflavones when consumed by healthy women at physiologically typical dietary intakes. J Nutr 133 1027-1035. [DOI] [PubMed] [Google Scholar]
- Shelby MK, Cherrington NJ, Vansell NR, and Klaassen CD (2003) Tissue mRNA expression of the rat UDP-glucuronosyltransferase gene family. Drug Metab Dispos 31 326-333. [DOI] [PubMed] [Google Scholar]
- Strassburg CP, Oldhafer K, Manns MP, and Tukey RH (1997) Differential expression of the UGT1A locus in human liver, biliary, and gastric tissue: identification of UGT1A7 and UGT1A10 transcripts in extrahepatic tissue. Mol Pharmacol 52 212-220. [DOI] [PubMed] [Google Scholar]
- Tallman MN, Miles KK, Kessler FK, Nielsen JN, Tian X, Ritter JK, and Smith PC (2007) The contribution of intestinal UDP-glucuronosyltransferases in modulating 7-ethyl-10-hydroxy-camptothecin (SN-38)-induced gastrointestinal toxicity in rats. J Pharmacol Exp Ther 320 29-37. [DOI] [PubMed] [Google Scholar]
- Tian H, Ou J, Strom SC, and Venkataramanan R (2005) Activity and expression of various isoforms of uridine diphosphate glucuronosyltransferase are differentially regulated during hepatic regeneration in rats. Pharm Res 22 2007-2015. [DOI] [PubMed] [Google Scholar]
- Walle T (2004) Absorption and metabolism of flavonoids. Free Radic Biol Med 36 829-837. [DOI] [PubMed] [Google Scholar]
- Wang SW, Chen J, Jia X, Tam VH, and Hu M (2006) Disposition of flavonoids via enteric recycling: structural effects and lack of correlations between in vitro and in situ metabolic properties. Drug Metab Dispos 34 1837-1848. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Furukawa T, Sharyo S, Ohashi Y, Yasuda M, Takaoka M, and Manabe S (2000) Effect of troglitazone on the liver of a Gunn rat model of genetic enzyme polymorphism. J Toxicol Sci 25 423-431. [DOI] [PubMed] [Google Scholar]