Abstract
Human immunodeficiency virus (HIV)-1 Tat protein plays a key role in the pathogenesis of both HIV-1-associated cognitive-motor disorder and drug abuse. Dopamine (DA) transporter (DAT) function is strikingly altered in patients with HIV-1-associated dementia and a history of chronic drug abuse. This study is the first in vitro evaluation of potential mechanisms underlying the effects of Tat protein on DAT function. Rat striatal synaptosomes were incubated with recombinant Tat1–86 protein, and [3H]DA uptake and the binding of [3H]2β-carbomethoxy-3-β-(4-fluorophenyl)tropane (WIN 35,428) and [3H]1-[2-(diphenylmethoxy)ethyl]-4-(3-phenylpropyl)-piperazine (GBR 12935) were determined. Tat decreased [3H]DA uptake, [3H]WIN 35,428 binding, and [3H]GBR 12935 binding in a time-dependent manner. The potency of Tat for inhibiting [3H]DA uptake (Ki = 1.2 μM) was the same as that for inhibiting [3H]GBR 12935 binding but 3-fold less than that for inhibiting [3H]WIN 35,428 binding. Mutant Tat proteins did not alter [3H]DA uptake. Kinetic analysis of [3H]DA uptake revealed that Tat (1 or 10 μM) decreased the Vmax value and increased the Km value in a dose-dependent manner. The Vmax value, decreased by Tat (1 μM), returned to the control level after washout of Tat, indicating that the inhibitory effect of Tat on DA uptake was reversible. Saturation studies revealed that Tat decreased the Bmax value and increased the Kd value of [3H]WIN 35,428 binding, whereas Tat decreased the Bmax value of [3H]GBR 12935 binding, without a change in the Kd value. These findings provide new insight into understanding the pharmacological mechanisms of Tat-induced dysfunction of the DAT in the dopaminergic system in HIV-infected patients.
Recent estimates indicate that there are 30 to 40 million people infected with HIV worldwide (for review, see Ferris et al., 2008). Injection drug use is a high-risk behavior associated with the transmission of HIV and accounts for approximately one third of the total number of acquired immunodeficiency syndrome cases in the United States (Ferris et al., 2008). HIV-1 transactivator of transcription (Tat) protein is essential for efficient viral replication and can be released from acutely infected cells as a biologically active protein (Dingwall et al., 1989; Ensoli et al., 1990). Tat protein is thought to play a crucial role in the pathogenesis of HIV-1-associated dementia (Hudson et al., 2000; Ferris et al., 2008). This Tat-associated neurotoxicity may, in part, reflect an apparent dysfunction of the dopaminergic system (Nath et al., 1987; Berger and Arendt, 2000).
The dopamine (DA) transporter (DAT) is a specific marker for DA terminals and plays a critical role in many neurological disorders, including drug abuse (Gainetdinov and Caron, 2003). The reuptake of DA through the DAT is the primary mechanism that regulates extracellular DA acting on DA receptors (Gainetdinov and Caron, 2003). DATs are high-affinity targets for cocaine and amphetamine, both of which are highly addictive and widely abused substances (Zhu and Reith, 2008). Clinical studies using positron emission tomography have found that HIV-1-infected patients with HIV-1-associated dementia display a significant reduction in DAT density in the putamen and ventral striatum (Wang et al., 2004; Chang et al., 2008).
HIV-1 Tat is a nonstructural viral protein of 101 amino acids, encoded from two separate exons. Peptides derived from the first exon of Tat, including Tat46–60, Tat37–72, and Tat31–61, have been found to cause neurotoxicity (Philippon et al., 1994; Nath et al., 1996). Intrastriatal injections of Tat damage both efferent and afferent projections of the striatum (Bansal et al., 2000), including nigrostriatal DA neurons (Zauli et al., 2000). An in vivo microdialysis study recently reported that intrastriatal infusion of Tat decreased K+-evoked DA levels in rat, suggesting that in vivo exposure to Tat causes alterations in DA transmission (Ferris et al., 2009). In addition, intrastriatal Tat decreases amphetamine-evoked DA overflow and a depletion of DA levels 7 days after local injections (Cass et al., 2003). The protein and mRNA levels of tyrosine hydroxylase are decreased in rat dopaminergic PC12 cells in the presence of Tat (Zauli et al., 2000). Collectively, these results indicate that Tat interferes with the biosynthetic pathway, production, and release of DA. Thus, Tat-induced neurotoxicity may reflect damage of the dopaminergic system with functional Parkinsonian features and pathology. In fact, several publications have reported clinical outcomes of Parkinsonian symptoms (Berger and Nath, 1997) and depletion of substantia nigra DA neurons in HIV-infected patients (Wang et al., 2004).
Tat protein decreases [3H]DA uptake into rat striatal synaptosomes (Wallace et al., 2006). Intra-accumbal Tat1–72 increases acute cocaine-induced locomotor activity and attenuates repeated cocaine-induced behavioral sensitization (Harrod et al., 2008). Rats injected with Tat and systemic methamphetamine show a reduction in [125I]RTI-121 binding to DAT in striatum (Maragos et al., 2002) and significantly decreased levels of tyrosine hydroxylase 24 h later (Theodore et al., 2006). In rat fetal midbrain primary culture cells, incubation with Tat protein causes reductions in [3H]WIN 35,428 binding, which was associated with Tat-induced neurotoxicity (Aksenova et al., 2006). Taken together, these results suggest that Tat protein-induced neurotoxicity, at least in part, is mediated through reduced DAT activity and that a combination of Tat protein and abused drugs enhances neurotoxicity in a synergistic manner (Ferris et al., 2008).
To date, the mechanisms underlying the effect of Tat protein on DAT function have not been subject to a detailed pharmacological assessment. In the present study, we determined the effects of Tat on function of DAT and its radioligand binding sites, including dose-response and time course studies. [3H]WIN 35,428 binding has been shown to share pharmacological identity with the DA uptake carrier and to be part of the cocaine binding domain (Pristupa et al., 1994; Reith and Coffey, 1994). We found that recombinant Tat1–86 protein differentially inhibited specific [3H]DA uptake and the binding of [3H]WIN 35,428 and [3H]GBR 12935 in rat striatal synaptosomes. In addition, the present study examined the effects of Tat protein on total DAT immunoreactivity in striatal synaptosomes by Western blotting and the potential interaction between Tat and DAT by surface plasmon resonance (SPR) measurement.
Materials and Methods
Materials. [3H]DA (3,4-ethyl-2[N-3H]dihydroxyphenylethylamine; specific activity, 31 Ci/mmol), [3H]WIN 35,428 (specific activity, 85 Ci/mmol), [3H]GBR 12935 (GBR 12935 [propylene-2,3-3H]; specific activity, 43.5 Ci/mmol), and [3H]l-leucine (specific activity, 140 Ci/mmol) were purchased from PerkinElmer Life and Analytical Sciences (Boston, MA). Recombinant HIV-1 transactivator of transcription (Tat1–86, REP0002a) protein and its mutant protein Tat Cys22 (cysteine 22 was substituted to glycine, REP0032) were purchased from Diatheva (Fano, Italy). The Tat1–86 (clade B) and its mutated Tat Cys22 are produced in Escherichia coli and have >90% purity as estimated by analysis of Coomassie Blue-stained SDS-polyacrylamide gel electrophoresis. A deletion mutant of Tat protein devoid of amino acids 31 to 61 (TatΔ31–61) was kindly provided by Dr. Avindra Nath (Department of Neurology, Johns Hopkins Hospital, Baltimore, MD). Antibodies recognizing rat DAT (C-20; goat polyclonal antibody) and actin (C-2; mouse monoclonal antibody) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Anti-goat IgG horseradish peroxidase was purchased from Jackson ImmunoResearch Laboratories Inc. (West Grove, PA). Goat anti-mouse IgG-horseradish peroxidase was purchased from Santa Cruz Biotechnology, Inc. Neuro2A cells were purchased from American Type Culture Collection (Manassas, VA). The vectors pEGFP-N3 and pIRES-neo were purchased from BD Biosciences (San Jose, CA). Fetal bovine serum, penicillin/streptomycin, and all culture media were purchased from Atlanta Biologicals (Norcross, GA). Lipofectamine 2000 and Geneticin were purchased from Invitrogen (G418; Carlsbad, CA). cDNA for hDAT in PCMV6-XL5 was purchased from Origene (Rockville, MD). d-Glucose, l-ascorbic acid, GBR 12909, WIN 35,428, l-leucine, l-lysine, bovine serum albumin, pyrocatechol, α-d-glucose, EDC, HEPES, isopropanol, nomifensine maleate, pargyline hydrochloride, polyethylene glycol, NHS, sucrose, and Tween 20 were purchased from Sigma-Aldrich (St. Louis, MO). All other chemicals were purchased from Thermo Fisher Scientific (Waltham, MA).
Animals. Adult male Sprague-Dawley rats (200–225 g body weight) were obtained from Harlan (Indianapolis, IN) and housed in standard polyurethane cages with free access to food and water. The colony room was maintained at 22 ± 2°C and 45 ± 10% humidity, with a 12/12-h light/dark cycle (lights on 7:00 AM Eastern Standard Time). The experimental procedures were approved by the University of South Carolina Institutional Animal Care and Use Committee and conformed to the 2003 National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Synaptosomal Preparation. Striata from individual rats were homogenized in 20 ml of ice-cold 0.32 M sucrose containing 5 mM NaHCO3, pH 7.4, with 16 up-and-down strokes using a Teflon pestle homogenizer (clearance, approximately 0.003 in.). The crude synaptosomal preparation was centrifuged at 2000g for 10 min at 4°C, and the resulting supernatants were then centrifuged at 20,000g for 15 min at 4°C. The resulting pellets were resuspended in 5.0 ml of ice-cold Krebs-Ringer-HEPES assay buffer for the [3H]DA uptake assay (125 mM NaCl, 5 mM KCl, 1.5 mM MgSO4, 1.25 mM CaCl2, 1.5 mM KH2PO4, 10 mM d-glucose, 25 mM HEPES, 0.1 mM EDTA, 0.1 mM pargyline, and 0.1 mM l-ascorbic acid, saturated with 95% O2, 5% CO2; pH 7.4). Protein concentration was determined by the Bradford protein assay (Bradford, 1976) using bovine serum albumin as the standard (Bio-Rad Laboratories, Hercules, CA).
Cell Culture. Neuro2A cells (mouse neuroblastoma; CCL-131) were cultured in minimal essential medium with Earle's balanced salt solution, supplemented with 10% heat inactivated fetal bovine serum and 1% penicillin/streptomycin, 10,000 IU/ml and 10,000 μg/ ml. Cells were maintained in vented culture flasks at 37°C in a 5% CO2-humidified atmosphere. Cultures were grown to 70% confluence and subcultured weekly.
Neuro2A (hDAT-GFP) Construct. A fluorescently tagged hDAT was constructed by fusing the N terminus of the enhanced green fluorescent protein (GFP) from pEGFP-N3 to the C terminus of the human synthetic DAT cDNA from pCMV6-XL5, thereby creating the fusion construct hDAT-GFP. The hDAT was derived from the human “solute carrier family 6” (neurotransmitter transporter, DA), member 3 (SLC6A3), and purchased as transfection-ready DNA (accession no. NM_001044.2). This construct was subcloned into a pIRES-Neo3 expression vector and modified to express the hDAT from a cytomegalovirus promoter and a neomycin resistance gene. N2A cells were transfected with the hDAT-GFP construct using Lipofectamine 2000 (Invitrogen). Correct insertion was confirmed by KpnI digestion and DNA sequencing. A stably transfected pool of N2A (hDAT-GFP) cells was selected in 500 μg/ml G-418. Cells were observed using fluorescent microscopy, and only colonies, or cultures, that displayed a high level of hDAT expression were chosen for future studies. Expression level was determined using GFP fluorescence observed at both the plasma membrane of the cell and as punctate formations within intracellular organelles.
[3H]DA Uptake Assay. [3H]DA uptake was determined using previously described methods (Zhu et al., 2004). Assays were performed in duplicate with a final volume of 250 μl. Aliquots of striatal synaptosomes (30 μl containing 20 μg of protein) were added to the tubes containing 170 μl of assay buffer and 25 μl of one of nine concentrations of Tat, TatΔ31–61, Tat Cys22, or GBR 12909 (final concentration, 0.1 nM–10 μM). GBR 12909 was used as a positive control for these experiments. TatΔ31–61 was chosen as a negative control based on a previous study showing that the region of the 31 to 61 amino acids plays an important role in the cellular uptake of Tat (Ma and Nath, 1997). Tat Cys22 was another negative control for these experiments; a single mutation in the cysteine 22 plays a critical role in the structural integrity of the activating domain of Tat protein (Koken et al., 1994). For the Tat dose-response study, samples were incubated at 34°C for 15 min in an oxygenated metabolic shaker. For the time course study, assay tubes were incubated at 34°C for a range of times (1, 5, 15, 30, 45, and 60 min). Subsequently, 25 μl of 0.1 μM[3H]DA (final concentration) was added to each tube, and the incubation was continued at 34°C for 10 min. The 10-min time period was chosen based on a preliminary study; the reuptake of [3H]DA into rat striatal synaptosomes was linear over 2 to 10 min (data not shown). The reactions were terminated by the addition of 3 ml of ice-cold assay buffer. Samples were filtered through Whatman GF/B glass fiber filters (Whatman, Maidstone, UK), presoaked with assay buffer containing 1 mM pyrocatechol. Filters were washed three times with 3 ml of ice-cold assay buffer containing 1 mM pyrocatechol using a Brandel cell harvester (model M-48; Biochemical Research and Development Laboratories Inc., Gaithersburg, MD). Pyrocatechol (catechol) is a catechol-O-methyltransferase inhibitor. Catechol-O-methyltransferase and monoamine oxidases A and B are the major mammalian enzymes involved in the metabolic degradation of DA (Napolitano et al., 1995). In the current study, pyrocatechol (1 mM) was included in the DA uptake assay buffer to prevent the degradation of [3H]DA during the processes of washes and harvesting (Zhu et al., 2004). Radioactivity was determined by liquid scintillation spectrometry (model Tri-Carb 2900TR; PerkinElmer Life and Analytical Sciences).
To determine whether Tat-induced inhibition of [3H]DA uptake was the result of an alteration in either the maximal velocity (Vmax) or Michaelis-Menten constant (Km) of [3H]DA uptake, kinetic analyses were conducted in the absence or presence of Tat. To generate saturation isotherms, duplicate assay tubes were prepared as described above for the inhibition assays except that the concentration of DA added to each tube was varied. A fixed concentration of [3H]DA (500,000 dpm/tube) was isotopically diluted with one of eight concentrations of unlabeled DA (final DA concentrations, 1.0 nM–1 μM). Synaptosomal samples were incubated with each concentration of [3H]DA in the absence or presence of the concentrations of Tat (1 or 10 μM). Nonspecific uptake was determined in duplicate at each [3H]DA concentration by including 10 μM nomifensine in the assay buffer. Kinetic parameters (Vmax and Km) were determined using Prism 4.0 (GraphPad Software Inc., San Diego, CA).
[3H]WIN 35,428 Binding Assay. The ability of Tat to inhibit [3H]WIN 35,428 binding in striatal synaptosomes was examined. [3H]WIN 35,428 binding was determined using a previously described method (Zhu et al., 2007). Synaptosomes were prepared as described above, and the pellets were resuspended in 5.0 ml of ice-cold sodium-phosphate buffer (2.1 mM NaH2PO4, 7.3 mM Na2HPO4·7H2O, and 320 mM sucrose, pH 7.4). The sucrose-phosphate buffer for [3H]WIN 35,428 binding assay was chosen based on previous studies (Reith and Coffey, 1994; Aloyo et al., 1995). There are two types of assay buffers for [3H]WIN 35,428 binding, i.e., sucrose-phosphate buffer and Tris-NaCl buffer (Madras et al., 1989; Coffey and Reith, 1994; Reith and Coffey, 1994). The sucrose-phosphate buffer contains a lower sodium ion concentration (∼30 mM) compared with that in Tris-NaCl buffer (100 mM). It has been reported that sucrose-phosphate buffer is more suitable to P2 membrane preparation (the current study) and that Tris-NaCl buffer is commonly used in crude membrane preparation (Reith and Coffey, 1994). Because WIN 35,428 is an analog of cocaine, the binding assay in the sucrose-phosphate buffer is similar to that used originally for cocaine binding (Coffey and Reith, 1994). The presence of sucrose in the binding assay also increases [3H]WIN 35,428 binding compared with a reduction in binding seen with hypotonic media (Coffey and Reith, 1994).
For the competitive inhibition experiment and time course study, assays were performed in duplicate in a final volume of 250 μl. Aliquots of the striatal synaptosomes (25 μl) were added to the assay tubes containing 25 μl of [3H]WIN 35,428 (final concentration, 5 nM) and assay buffer (170 μl), 25 μl containing buffer or one of nine concentrations (final concentration, 1 nM–10 μM) of Tat, WIN 35,428, or GBR 12909. To determine whether Tat-induced inhibition of [3H]WIN 35,428 was the result of alterations in the maximal number of binding sites (Bmax) or affinity (Kd) for this radioligand, kinetic analysis of [3H]WIN 35,428 binding was conducted. To generate saturation isotherms, striatal synaptosomes from two rats were pooled, and half of the pooled sample was used for the [3H]WIN 35,428 binding in the presence of Tat1–86 (1 μM); the other half of the pooled sample was used for control (in the absence of Tat1–86). This concentration of Tat was based on the results from the inhibition assays. Duplicate assay tubes were prepared as described above for the inhibition assays. Striatal synaptosomes were preincubated with Tat1–86 (0.2 or 1 μM) or the same amount of assay buffer (control) for 15 min; subsequently, they were incubated with one of the eight concentrations of [3H]WIN 35,428 (final concentration, 0.5–30 nM) on ice for 2 h. In parallel, nonspecific binding at each concentration of [3H]WIN 35,428 (in the presence of 30 μM cocaine, final concentration) was subtracted from total binding to calculate the specific binding.
For the time course experiments, assay tubes were incubated on ice for a range of time (1, 5, 15, 30, 45, and 60 min). Nonspecific binding was determined in the presence of 30 μM cocaine. Assays were terminated by rapid filtration onto Whatman GF/B glass fiber filters, presoaked for 2 h with assay buffer containing 0.5% polyethylenimine, using a Brandel cell harvester. Filters were rinsed three times with 3 ml of ice-cold assay buffer. Radioactivity remaining on the filters was determined by liquid scintillation spectrometry (model Tri-Carb 2900TR; PerkinElmer Life and Analytical Sciences).
[3H]GBR 12935 Binding Assay. The methods used to assay [3H]GBR 12935 binding have been described previously (Zhu et al., 2004). In brief, synaptosomes were prepared as described above, and resulting pellets after 20,000g for 15 min were resuspended in 5.0 ml of ice-cold assay buffer (118 mM NaCl, 4.8 mM KCl, 2.5 mM CaCl2, 1.2 mM MgCl2, 25 mM HEPES, and 10 mM d-glucose, pH 7.4). For the competitive inhibition experiment and time course study, assays were performed in duplicate in a final volume of 250 μl. Aliquots of striatal synaptosomes (25 μl) were added to assay tubes containing 25 μl of [3H]GBR 12935 (final concentration, 5 nM) and assay buffer (170 μl), 25 μl containing buffer or one of nine concentrations (final concentration, 1 nM–10 μM) of Tat, WIN 35,428, or GBR 12909. Saturation experiments of [3H]GBR 12935 binding were conducted in the absence or presence of Tat. Striatal synaptosomes were preincubated with Tat1–86 (0.2, 1, or 10 μM) or same amount of assay buffer (control) for 15 min; subsequently, they were incubated with one of the eight concentrations of [3H]GBR 12935 (final concentration, 0.5–30 nM) for 1 h. All procedures for the experiments were conducted at room temperature, except for the Tat solution, which was prepared with ice-cold assay buffer. An equal amount of ice-cold buffer was added into all assay tubes in the absence of Tat. For the time course experiments, assay tubes were incubated at room temperature for a range of time (1, 5, 15, 30, 45, and 60 min). Nonspecific binding was determined in the presence of 30 μM GBR 12909. Assays were terminated by rapid filtration onto Whatman GF/B glass fiber filters, presoaked for 2 h with assay buffer containing 0.5% polyethylenimine using a Brandel cell harvester. Filters were rinsed three times with 3 ml of ice-cold assay buffer.
[3H]Leucine Uptake Assay. The potential inhibitory effect of Tat on electrochemical gradients was assessed using [3H]leucine uptake assay. The uptake assay was performed in duplicate with a final volume of 250 μl. Aliquots of striatal synaptosomes (30 μl) containing 20 μg of protein were preincubated with Tat, TatΔ31–61, or leucine and assay buffer (125 mM NaCl, 5 mM KCl, 1.5 mM MgSO4, 1.25 mM CaCl2, 1.5 mM KH2PO4, 10 mM d-glucose, 25 mM HEPES, 0.1 mM EDTA, 0.1 mM pargyline, and 0.1 mM l-ascorbic acid, saturated with 95% O2,5%CO2; pH 7.4). TatΔ31–61 and leucine were chosen as negative and positive controls, respectively. Assay tubes were placed in an oxygenated metabolic shaker at 34°C for 15 min. Subsequently, 1 μM [3H]leucine (final concentration, 25 μl) was added to each tube, and incubation was continued for 10 min at 34°C in the metabolic shaker. The competitive inhibition experiments for [3H]leucine and [3H]DA uptake were performed in the absence or presence of Tat (final concentration, 0.1 nM–10 μM), with a preincubation limited to 5 min, followed by adding 1 μM[3H]leucine or 0.1 μM[3H]DA (final concentration) for 4 min. Nonspecific uptake for [3H]leucine and [3H]DA was determined in the presence of 10 mM l-lysine and 10 μM nomifensine, respectively. Incubation was terminated by the addition of 3 ml of ice-cold assay buffer, followed by immediate filtration through Whatman GF/B glass fiber filters. Filters were washed three times with 3 ml of ice-cold assay buffer using a Brandel cell harvester.
Western Blots. To determine whether Tat-induced inhibitions of [3H]DA uptake and the binding of [3H]WIN 35,428 and [3H]GBR 12935 were the result of changes in DAT protein levels, DAT immunoreactivity in striatal synaptosomes was assessed after exposure to 1 μM Tat under different incubation times and temperatures. Synaptosomes were prepared as described above, and aliquots of striatal synaptosomes (30 μl containing 20 μg of protein) were added to the tubes containing 195 μl of assay buffer as well as 25 μl of Tat (final concentration, 1 μM) or the same amount of assay buffer. Similar incubation conditions were used for [3H]DA uptake and the binding of [3H]WIN 35,428 and [3H]GBR 12935. Samples were incubated at 34°C, on ice or at room temperature, for 15 and 60 min and then mixed with Laemmli sample buffer and boiled for 5 min. To detect immunoreactive DAT protein in synaptosomes, samples were subjected to gel electrophoresis and Western blotting. Proteins were separated by 10% SDS-polyacrylamide gel electrophoresis for 90 min at 150 V and transferred to Immobilon-P transfer membranes (0.45-μm pore size; Millipore, Billerica, MA) in transfer buffer (50 mM Tris, 250 mM glycine, and 3.5 mM SDS) using a Mini Trans-Blot Electrophoretic Transfer Cell (Bio-Rad Laboratories) for 110 min at 72 V. Transfer membranes were incubated with blocking buffer (5% dry milk powder in phosphate-buffered saline containing 0.5% Tween 20) for 1 h at room temperature, followed by incubation with goat polyclonal DAT antibody (1 μg/ml in blocking buffer) overnight at 4°C. Transfer membranes were washed five times with wash buffer (phosphate-buffered saline containing 0.5% Tween 20) at room temperature and then incubated with rabbit anti-goat DAT antibody (1:2500 dilution in blocking buffer) for 1 h at 22°C. Blots on transfer membranes were detected using enhanced chemiluminescence and developed on Hyperfilm ECL-Plus (GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK). After detection and quantification of DAT protein, each blot was stripped in 10% Re-blot plus mild antibody stripping solution (Millipore Bioscience Research Reagents, Temecula, CA) for 20 min at room temperature and reprobed for detection of actin. Actin was used as an intracellular control protein to monitor protein loading between samples and determined using mouse monoclonal antibody (1:1000 dilution in blocking buffer). Multiple autoradiographs were obtained using different exposure times, and immunoreactive bands within the linear range of detection were quantified by densitometric scanning (Scion Image software; Scion Corporation, Frederick, MD). Band density measurements, expressed as relative optical density, were used to determine levels of DAT immunoreactivity in synaptosomes.
SPR Measurements. SPR is a relatively new technology that allows for real-time measurement of ligand-protein and protein-protein interactions using immobilized membranes that overexpress a desired protein. Recent studies examining ligand-protein interactions with a variety of sensor technologies have demonstrated the usefulness of SPR in determining interactions with G protein-coupled receptors (Silin et al., 2006; Kumbhat et al., 2007).
A standardized assay protocol, as developed by Icx-Nomadics (Oklahoma City, OK), was used for the immobilization of biological membranes onto the sensor chip. The Vesicle Capture (VesCap) chip sensor was chosen for its ability to immobilize cellular membranes. The system was the Sensi-Q SPR package also provided by Icx-Nomadics. The lipophilic hydrocarbon chains of the immobilized decylamine insert into the phospholipid bilayer of the biological membrane, thereby “capturing” them. The lipid bilayer and the native structure of membrane proteins are maintained throughout the duration of experimentation. Capture of vesicles or membranes onto the sensing surface is noncovalent, allowing free diffusion of membrane components in all directions. The polyethylene glycoldecylamine layer presents a simple two-dimensional interaction plane. VesCap chemistry is especially suitable for cell lines overexpressing a surface receptor.
Preparation of the sensor chip involved multiple steps before immobilization of the membranes. The sensor surface carboxyl group was first converted to an NHS ester by injection of 1:1 mix of EDC/NHS (0.4 M EDC and 0.1 M NHS). A primary amine group on the analyte displaced the NHS group, and an amide bond was formed, covalently linking the analyte to the surface. Unreacted NHS esters were blocked by injection of 1 M N-decylamine, pH 7.0. The surface was washed with 10 mM HCl for 3 min. These steps activated the carboxyl surface of the sensor and allowed the NHS ester to immobilize the biological membrane onto the sensor surface. Two cell membrane suspensions were prepared from both nontransfected N2A (control) and transfected N2A (hDAT-GFP) cells. Cells were trypsinized, centrifuged, lysed with water, centrifuged, and resuspended in HEPES-buffered saline with Tween 20 and EDTA buffer to 1 mg/ml, then 50 μl of N2A (control). Membranes were injected (10 μl/min over a 5-min period) over the sensor surface through channels 1 and 2. Channel 1 was then regenerated by removal of control membrane by injection of 50 μl of 50:50 (v/v) isopropanol/10 mM HCl at the rate of 50 μl/min followed by 50-μl injection of 10 mM HCl (50 μl/min) alone. The regeneration step was repeated twice. After the sample loop was purged and washed, 50 μl of N2A (hDAT-GFP) was injected into channel 1 (10 μl/min). The membranes were allowed to stabilize for 30 min before analysis. To determine the interaction of Tat1–86 and Tat Cys22 with hDAT-GFP, two concentrations of each protein (0.5 and 1.0 μM) were prepared in low-retention tubes with HEPES-buffered saline and injected sequentially. To determine specific interactions between Tat and hDAT-GFP, the following equation was used: response units (RU) channel 1 (N2A-hDAT) - RU channel 2 (control). The change in RU was measured in real time for 600 s. At the end of 600 s, the cells were washed with assay buffer and allowed to restabilize before the next injection.
Increases in mass associated with the binding event (protein-protein interaction) results in an increase in refractive index as measured by RU. A response of 1 RU is equivalent to 10-6 refractive index units, which represents approximately 1 pg of protein/mm2.An increase in refractive index of 1000 RU equates to approximately 1 ng/mm2. The high sensitivity and low signal-to-noise ratio obtained with the Sensi-Q SPR device is achieved in part through a stable design based on Kretschmann optics and Peltier temperature control. The area under each curve (AUC) was then calculated by subtracting the negative area from the positive to yield “net” area. The AUC was calculated by Prism (GraphPad Software Inc.) using the trapezoid rule without curve fitting or curve smoothing. The trapezoid equation was ΔX × (Y1 + Y2)/2, where X is discrete units of time (0.1-s intervals) between Y1 and Y2, Y1 was the response in RU at the first time point, and Y2 is the response RU at the second time point. Once the ligand-protein complex was formed, laser signal intensity increases proportionally with the degree of ligand-protein complex. Due to the nature of the response, net AUC was chosen to compare the response of Tat Cys22.
Data Analysis and Statistics. Data are presented as mean ± S.E.M., and n represents the number of independent experiments for each treatment group. For Tat-induced inhibition of [3H]DA uptake, [3H]WIN 35,428 binding, and [3H]GBR 12935 binding, data are expressed as a percentage of control, i.e., specific [3H]DA uptake, [3H]WIN 35,428 binding, or [3H]GBR 12935 binding in the absence of Tat. IC50 values for Tat-induced inhibition in specific [3H]DA uptake or in specific binding of [3H]WIN 35,428 and [3H]GBR 12935 were determined from inhibition curves by nonlinear regression analysis using a one-site model with variable slope. Inhibition curves were generated using four independent experiments with Tat. Ki values were calculated according to the Cheng-Prusoff equation Ki = IC50/(1 + L/Kd). The Km value for [H]DA uptake was defined as 30 nM (data not shown). The Kd value for [3H]WIN 35,428 and [3H]GBR 12935 binding was defined as 3 and 1.5 nM, respectively (data not shown). Kinetic parameters (Bmax, Vmax, Km,or Kd) of [3H]DA uptake, [3H]WIN 35,428 binding, and [3H]GBR 12935 binding were determined from saturation curves by nonlinear regression analysis using a one-site model with variable slope. Experiments involving exposure to a single concentration (1 or 10 μM) of Tat with multiple time points were analyzed by separate one-factor ANOVAs, followed by simple comparison tests. For experiments involving comparisons between two paired samples, paired Student's t tests were used to determine the ability of Tat to alter the kinetic parameters [Km and Vmax for [3H]DA uptake; Kd and Bmax for [3H]WIN 35,428 and [3H]GBR 12935 binding compared with control (the absence of Tat)]; log-transformed values of Km or Kd were used for these statistical comparisons. Separate paired Student's t tests were conducted on DAT immunoreactivity for comparisons between control and Tat-treated samples. To analyze data from SPR measurement, a two-way ANOVA was performed with two between-group factors (Tat protein type and concentration). Student-Newman-Keuls comparisons were made for post hoc analyses. All statistical analyses were performed using SPSS, standard version 16.0 (SPSS Inc., Chicago, IL), and differences were considered significant at p < 0.05.
Results
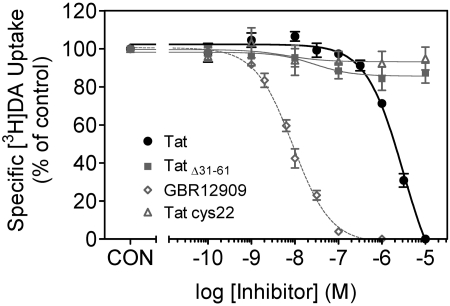
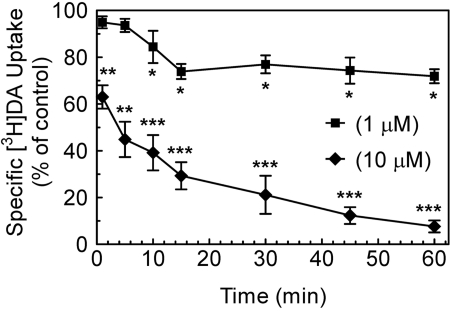
Tat Protein Inhibits [3H]DA Uptake into Rat Striatal Synaptosomes. To determine the concentration-dependent inhibitory effect of Tat on specific [3H]DA uptake into striatal synaptosomes, [3H]DA uptake was examined in the presence of various concentrations of Tat. Specific [3H]DA uptake was substantially inhibited by Tat (Ki = 1160 ± 100 nM). GBR 12909 was used as a positive control for the [3H]DA uptake assay and had a Ki value of 19 ± 1.5 nM (Table 1). TatΔ31–61 and Tat Cys22 were negative controls for the assay because neither inhibited [3H]DA uptake across the concentration range from 0.1 nM to 10 μM (Fig. 1). The time course of Tat-induced inhibitory effects on [3H]DA uptake was determined at 1 to 60 min by incubation with 1 or 10 μM Tat. As seen in Fig. 2, one-way ANOVAs revealed significant time-dependent inhibition in [3H]DA uptake for Tat at a concentration of 1 μM(F7,24 = 49.2; p < 0.001) and 10 μM(F7,24 = 51.1; p < 0.001). At 10 μM, Tat inhibited [3H]DA uptake by 37 ± 2.7% at 1 min (p < 0.01, paired Student's t test), and the inhibitory effects remained until 60 min.
TABLE 1.
Summary of inhibitory activities in [3H]DA uptake assay and [3H]WIN 35,428 and [3H]GBR 12935 binding assays in rat striatal synaptosomes in the presence of Tat, TatΔ31–61, Tat Cys22, GBR 12909, or WIN 35,428 Data are presented as mean ± S.E.M. of three to five independent experiments performed in duplicate.
|
[3H]DA Uptake
|
[3H]WIN 35,428 Binding
|
[3H]GBR 12935 Binding
|
||||
|---|---|---|---|---|---|---|
| Ki | Hill Coefficient | Ki | Hill Coefficient | Ki | Hill Coefficient | |
| nM | nM | nM | ||||
| Tat | 1160 ± 100 | 1.18 ± 0.1 | 400 ± 39 | 1.03 ± 0.05 | 1610 ± 150 | 0.93 ± 0.02 |
| TatΔ31–61 | >100,000 | >100,000 | >100,000 | |||
| Tat Cys22 | >100,000 | >100,000 | >100,000 | |||
| GBR 12909 | 19 ± 1.5 | 0.96 ± 0.02 | 30.0 ± 2.3 | 1.23 ± 0.10 | 1.20 ± 0.2 | 0.88 ± 0.01 |
| WIN 35,428 | 2.81 ± 0.14 | 0.85 ± 0.02 | 17.9 ± 4.43 | 1.23 ± 0.11 | ||
Fig. 1.
Pharmacological profiles of [3H]DA uptake in the presence of Tat1–86, TatΔ31–61, Tat Cys22, or GBR 12909. Striatal synaptosomes were preincubated with various concentrations of Tat1–86, TatΔ31–61, Tat Cys22, or GBR 12909 (0.1 nM–10 μM) at 34°C for 15 min followed by the addition of [3H]DA (final concentration, 0.1 μM) for 10 min. TatΔ31–61 and Tat Cys22 were used as negative controls. GBR 12909 was used as a positive control. Data are expressed as mean ± S.E.M. as percentage of control (CON) values (25,205 ± 2065 dpm) from five independent experiments performed in duplicate. Nonspecific [3H]DA uptake was determined in the presence of 10 μM nomifensine. All curves were best fit to a single class of binding site and generated by nonlinear regression.
Fig. 2.
Time course of Tat effects on [3H]DA uptake into rat striatal synaptosomes. Striatal synaptosomes pooled from two rats were used for all samples. For a single experiment, aliquots of synaptosomes were preincubated with Tat1–86 (1 or 10 μM) or the same volume of assay buffer (control) at 34°C for the indicated time (1–60 min), followed by the addition of [3H]DA (final concentration, 0.1 μM) at 34°C for 10 min. Four pairs of independent inhibition experiments were performed at each time, and the raw data were analyzed as matched pairs. Data are expressed as mean ± S.E.M. as percentage of the respective control values from three independent experiments performed in duplicate. *, p < 0.05; **, p < 0.01; ***, p < 0.001 compared with the respective controls.
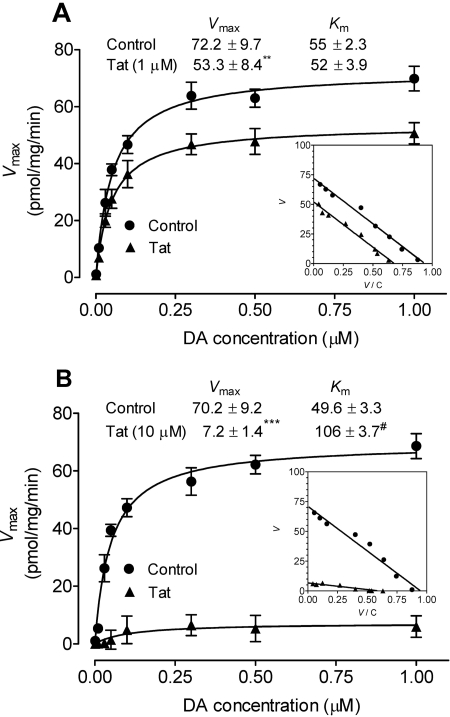
To determine the effect of Tat on Vmax and Km values for [3H]DA uptake into striatal synaptosomes, kinetic analysis of [3H]DA uptake was performed in the absence (control) or presence of Tat (1 and 10 μM). The Eadie-Hofstee analysis of the [3H]DA uptake showed that 15-min preincubation with Tat (1 μM) significantly decreased the Vmax value by 26 ± 1.9% (53.3 ± 8.4 pmol/mg/min) compared with control [72.2 ± 9.7 pmol/mg/min; t(3) = 13.5, p < 0.01, paired Student's t test] (Fig. 3A). There was no change in the Km value between Tat-treated and control samples (55.2 ± 2.3 and 52.2 ± 3.9 nM; Fig. 3A). Although this may be regarded as an example of a mixed inhibition mechanism of the noncompetitive variant-type (Tomlinson, 1988), the reduction in Vmax value could also reflect a trafficking phenomenon rather than a particular kinetic mechanism (see Discussion). A high concentration of Tat (10 μM) was used to determine whether the inhibitory effect of Tat on the parameters (Vmax and Km)of[3H]DA uptake was concentration-dependent. As shown in Fig. 3B, Tat (10 μM) caused a 10-fold decrease in the Vmax value (7.2 ± 1.4 pmol/mg/min) compared with control [70.2 ± 9.2 pmol/mg/min; paired t(3) = 7.8, p < 0.001, paired Student's t test]; the Km was increased from 49 ± 3.3 to 106 ± 3.7 nM [t(3) = 4.64, p < 0.05, paired Student's t test].
Fig. 3.
Effect of Tat on kinetic analysis of [3H]DA uptake into rat striatal synaptosomes. Kinetic analysis of the synaptosomal [3H]DA uptake was determined in the absence (control) or presence of Tat1–86 at concentration of 1 μM (A) or 10 μM (B). Striatal synaptosomes were preincubated with or without Tat at 34°C for 15 min followed by the addition of one of eight mixed concentrations of the [3H]DA. In parallel, nonspecific uptake at each concentration of [3H]DA (in the presence of 10 μM nomifensine, final concentration) was subtracted from total uptake to calculate DAT-mediated uptake. The Vmax and Km values were estimated by fitting the data to the Michaelis-Menten equation and represent the means from five independent experiments ± S.E.M. Inset, Eadie-Hofstee transformation of the same kinetic data. #, p < 0.05; **, p < 0.01; ***, p < 0.001 compared with the respective control values.
We then evaluated whether the Tat-induced decrease in Vmax was reversible. For this study, paired samples of striatal synaptosomes were preincubated with Tat (1 μM) or without Tat (control) for 15 min. As shown in Table 2, neither the Vmax nor Km value relative to the control value was altered followed by washout of Tat. These results suggest that the inhibitory effect of Tat on Vmax value is reversible.
TABLE 2.
The Vmax and Km values of [3H]DA uptake into rat striatal synaptosomes in the absence and presence of Tat protein Striatal synaptosomes from two rats were pooled, and half of the pooled sample was preincubated with and without (control) Tat1–86 (1 μ M) for 15 min, and the other half of the pooled sample was preincubated with and without (control) Tat1–86 (1 μ M) for 15 min followed by washing out of Tat protein with fresh assay buffer (washout). All samples were incubated with 0.1 μ M [3H]DA and increased concentrations of unlabeled DA. Data are presented as mean ± S.E.M. of five independent experiments performed in duplicate.
|
Vmax
|
Km
|
|||
|---|---|---|---|---|
| Control | Tat | Control | Tat | |
| fmol/min | nM | |||
| 15-min incubation | 72.2 ± 9.7 | 53.3 ± 8.4** | 52.2 ± 3.9 | 55.0 ± 2.3 |
| Washout | 70.3 ± 5.7 | 60.7 ± 3.1 | 51.1 ± 7.7 | 61.8 ± 7.4 |
p < 0.01 (paired Student's t test)
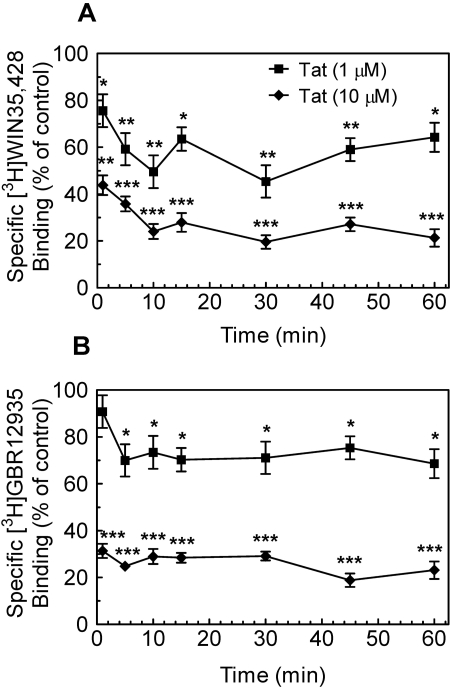
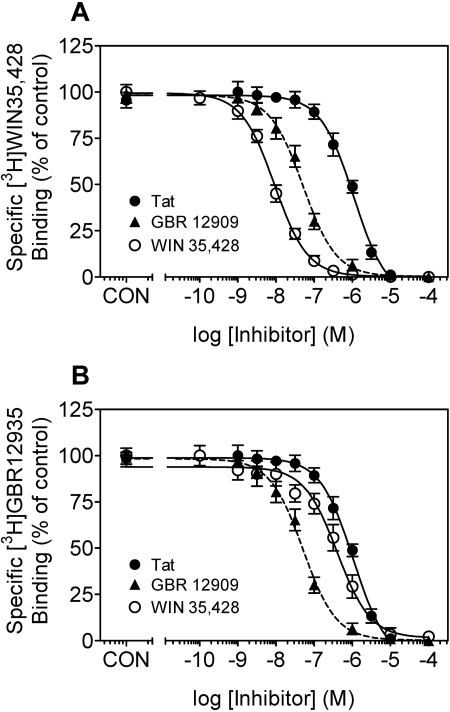
Tat Protein Inhibits [3H]WIN 35,428 Binding. To assess whether the inhibitory effect of Tat on DAT function also involved known cocaine-like radioligand binding sites on DAT, the ability of Tat protein to inhibit [3H]WIN 35,428 binding was determined with striatal synaptosomes. As shown in Fig. 4A, Tat at 1 or 10 μM concentration significantly inhibited [3H]WIN 35,428 binding during the 60-min period (1 μM Tat: F7,24 = 45.6, p < 0.001 or 10 μM Tat: F7,24 = 250, p < 0.001). Exposure to Tat for 1 min caused a 24 ± 1.8 and 56 ± 2.2% reduction in [3H]WIN 35,428 binding at 1 and 10 μM concentrations, respectively, and these significant inhibitory effects remained through 60 min. Concentration-response curves for Tat protein, GBR 12909, or WIN 35,428 in inhibiting [3H]WIN 35,428 binding in striatal synaptosomes were assessed with an exposure time of 2 h (Fig. 5A). GBR 12909 and WIN 35,429 were used to clarify different binding sites for [3H]WIN 35,428 binding on DAT protein, and they were found to have Ki values of 30 ± 2.3 and 2.81 ± 0.14 nM, respectively (Table 1). Tat (1 μM) inhibited the specific [3H]WIN 35,428 binding, with Ki values of 400 ± 39 nM (Table 1).
Fig. 4.
Tat protein inhibited [3H]WIN 35,428 and [3H]GBR 12935 binding in a time-dependent manner. A, aliquots of synaptosomes were incubated with Tat1–86 (1 or 10 μM) and 5 nM [3H]WIN 35,428 on ice for the indicated time (1–60 min). B, Tat1–86 (1 or 10 μM) was incubated with striatal synaptosomes and 5 nM [3H]GBR 12935 at room temperature for the indicated time. Four pairs of independent inhibition experiments were performed at each time, and the raw data were analyzed as matched pairs. Nonspecific binding for [3H]WIN 35,428 and [3H]GBR 12935 was determined in the presence of 30 μM cocaine and 30 μM GBR 12909, respectively. Data are expressed as mean percentage ± S.E.M. of the respective control (in the absence of Tat) values from three independent experiments performed in duplicate. *, p < 0.05; **, p < 0.01; ***, p < 0.001 compared with the respective controls.
Fig. 5.
Pharmacological profiles of Tat1–86, WIN 35,428, or GBR 12909 inhibition of [3H]WIN 35,428 and [3H]GBR 12935 binding in rat striatal synaptosomes. For a single experiment, striatal synaptosomes were incubated with the indicated concentrations of Tat1–86, WIN 35,428, or GBR 12909 in the presence of 5 nM [3H]WIN 35,428 on ice for 2 h or 5 nM [3H]GBR 12935 at room temperature for 60 min. A, specific [3H]WIN 35,428 competition curves expressed as mean ± S.E.M. percentage of control (CON) values (7926 ± 499 dpm) from five independent experiments performed in duplicate. B, specific [3H]GBR 12935 competition curves are expressed as mean ± S.E.M. percentage of CON values (8500 ± 340 dpm) from five independent experiments performed in duplicate. Nonspecific binding for [3H]WIN 35,428 and [3H]GBR 12935 was determined in the presence of 30 μM cocaine and 30 μM GBR 12909, respectively. All curves were best fit to a single class of binding site.
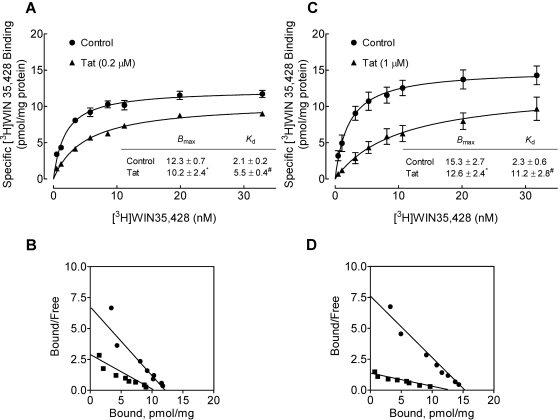
We also determined the effect of Tat on the Bmax and Kd of [3H]WIN 35,428 binding in striatal synaptosomes in the absence or presence of Tat (0.2 and 1 μM). As shown in Fig. 6A, a low concentration of Tat (0.2 μM) significantly decreased the Bmax value of [3H]WIN 35,428 binding by 17 ± 1.1% (10.2 ± 2.4 pmol/mg protein) compared with control [12.3 ± 0.7 pmol/mg protein; t(3) = 4.35, p < 0.05, paired Student's t test]. Tat (0.2 μM) also significantly increased the Kd value (5.5 ± 0.4 nM) compared with the control [2.1 ± 0.2 nM; t(3) = 7.94, p < 0.05, paired Student's t test]. Likewise, Tat at 1 μM caused a 18 ± 1.5% decrease in the Bmax value (12.6 ± 2.4 nM) compared with the control [15.3 ± 2.7 nM; t(3) = 2.83, p < 0.05, paired Student's t test] (Fig. 6C), with an increase in the Kd value [Tat: 11.2 ± 2.8 nM; control: 2.3 ± 0.6 nM; t(3) = 10.2, p < 0.05, paired Student's t test]. Although one interpretation of these results might be that Tat protein acts as an uncompetitive inhibitor on [3H]WIN 35,428 binding, it should be kept in mind that this is only valid as a descriptive narrative regarding the appearance of the Scatchard plot (binding) in comparison with an Eadie-Hofstee plot (uptake): application of the underlying uncompetitive mechanism to the binding situation (inhibitor binding preferentially to the transporter-ligand complex) leads to radically different kinetic outcomes compared with the substrate transport case (Tomlinson, 1988).
Fig. 6.
Characterization of [3H]WIN 35,428 binding in striatal synaptosomes in the absence and presence of Tat. For a single experiment, striatal synaptosomes from two rats were pooled, and half of the pooled sample was used for [3H]WIN 35,428 binding in the presence of Tat1–86, and the other half of the pooled sample was used for control. Saturation isotherms for [3H]WIN 35,428 binding to striatal synaptosomes was determined in the absence (control) or presence of Tat1–86 at the concentration of 0.2 μM (A) or 1 μM (C). Bmax (picomoles per milligram of protein) and Kd (nanomolar) values for control and Tat are presented. Scatchard transformations of same data are presented for control and Tat1–86 at the concentration of 0.2 μM (B) or 1 μM (D). Nonspecific binding for [3H]WIN 35,428 was determined in the presence of 30 μM cocaine. Data were best fit to a single class of binding site and are presented as means ± S.E.M. from four independent experiments. *,#, p < 0.05 compared with the respective control values.
Tat Protein Inhibits [3H]GBR 12935 Binding. To assess whether the inhibitory effect of Tat on DAT function also involved known noncocaine-like binding sites on DAT, the ability of Tat protein to inhibit [3H]GBR 12935 binding was evaluated with striatal synaptosomes. As illustrated in Fig. 4B, results from the time course study revealed a significant time-dependent inhibition of [3H]GBR 12935 binding for Tat at 1 μM(F7,24 = 10.3; p < 0.001) and 10 μM(F7,24 = 280; p < 0.001), respectively. At 1 μM, Tat-induced inhibition on [3H]GBR 12935 binding was 25 ± 2.1% from 5 to 60 min. At a high concentration (10 μM), Tat inhibited [3H]GBR 12935 binding by 77 ± 3.6% throughout the 60-min period (p < 0.001, paired Student's t test).
Concentration-response curves for Tat protein, GBR 12909, or WIN 35,428 in inhibiting [3H]GBR 12935 binding in striatal synaptosomes are illustrated in Fig. 5B. GBR 12909 and WIN 35,428 were used to distinguish different binding sites for [3H]GBR 12935 binding on DAT protein, and they showed Ki values of 1.2 ± 0.2 and 17.9 ± 4.4 nM, respectively (Table 1). Tat (1 μM) inhibited the specific [3H]GBR 12935 binding, with Ki values of 1610 ± 150 nM (Table 1).
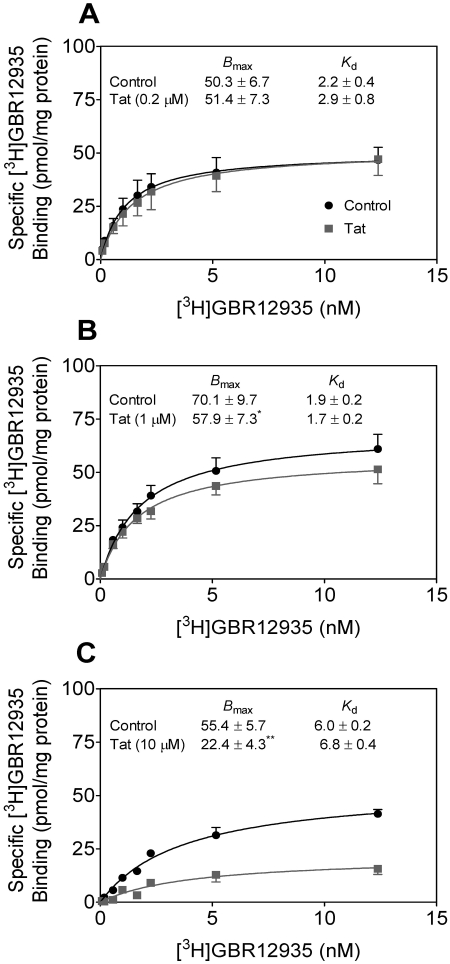
We also determined the effect of Tat on the Bmax and Kd of [3H]GBR 12935 binding in striatal synaptosomes in the absence or presence of Tat (0.2, 1, or 10 μM). As shown in Fig. 7A, a low concentration of Tat (0.2 μM) did not decrease either the Bmax or Kd values of [3H]GBR 12925 binding. Tat (1 μM) decreased the Bmax value by 17 ± 1.2% (57.9 ± 7.3 pmol/mg protein) compared with the control [70.1 ± 9.7 pmol/mg protein; t(4) = 2.94, p < 0.05, paired Student's t test], with no change in the Kd value (Fig. 7B). At a high concentration (10 μM), Tat caused a 60 ± 5.2% reduction in the Bmax value (22.4 ± 4.3 pmol/mg protein) compared with the control [55.4 ± 5.7 pmol/mg protein; t(4) = 12.5, p < 0.01, paired Student's t test], with no change in the Kd value (Fig. 7C). These results suggest that Tat protein inhibits [3H]GBR 12935 binding in a noncompetitive manner but that this is only true descriptively: actual noncompetitiveness in the binding situation (as opposed to the transport case) would not result in any change in binding as inhibitor binds equally well to the free or ligand-complexed transporter (Tomlinson, 1988). In addition, Bmax changes could reflect trafficking rather than kinetic effects (see Discussion).
Fig. 7.
Characterization of [3H]GBR 12935 binding in striatal synaptosomes in the absence and presence of Tat. Saturation isotherms for [3H]GBR 12935 binding to striatal synaptosomes were determined in the absence (control) or presence of Tat1–86. For a single experiment, striatal synaptosomes from two rats were pooled, and half of the pooled sample was used for [3H]GBR 12935 binding in the presence of Tat, and other half of the pooled sample was used for control. Bmax (picomoles per milligram of protein) and Kd (nanomolar) values are presented for control and Tat1–86 at the concentration of 0.2 μM (A), 1 μM (B), or 10 μM (C). Nonspecific binding for [3H]GBR 12935 was determined in the presence of 30 μM GBR 12909. Data were best fit to a single class of binding site and are presented as means ± S.E.M. from four independent experiments. *, p < 0.05; **, p < 0.01 compared with the respective control values.
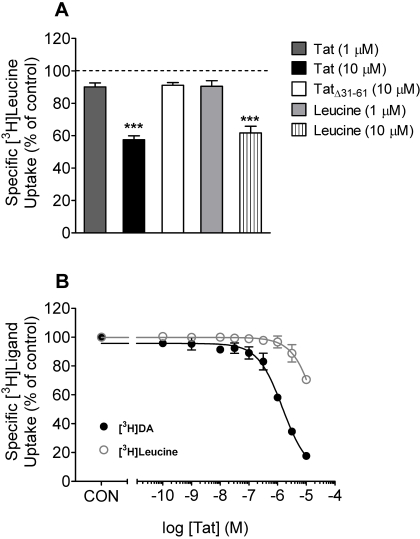
Effect of Tat Protein on [3H]Leucine Uptake into Rat Striatal Synaptosomes. The potential inhibitory effect of Tat on electrochemical gradients was determined by monitoring [3H]leucine uptake, which, as DAT function, depends on membrane potential and ion gradients. TatΔ31–61 and leucine were used as the negative and positive control, respectively. As illustrated in Fig. 8A, 10 μM TatΔ31–61,1 μM Tat, or 1 μM leucine resulted in a similar inhibition (∼10%) of [3H]leucine uptake. At a concentration of 10 μM, either Tat or leucine caused an approximate 40 ± 1.6% decrease in [3H]leucine uptake (p < 0.001, paired Student's t test). To reduce the Tat effect on electrochemical gradients, a separate experiment was performed with a reduced exposure time to Tat (Fig. 8B). Under these conditions, Tat inhibited [3H]DA uptake, with a Ki value of 0.51 ± 0.01 μM, which was 100-fold higher than that for [3H]leucine uptake (Ki = 56 ± 6.7 μM) (Fig. 8B). At 1 μM, Tat had no effect on the [3H]leucine uptake, whereas 10 μM Tat inhibited the [3H]leucine uptake by 30 ± 2.5%. A clear separation of inhibition curves for Tat was observed between the [3H]DA and [3H]leucine uptake assays (Fig. 8B). These results indicate that at a low concentration of 1 μM, the inhibition of [3H]DA uptake by Tat as shown in Fig. 1 was not attributable to alterations in electrochemical gradients.
Fig. 8.
Effects of Tat on [3H]leucine and [3H]DA uptake into striatal synaptosomes. A, striatal synaptosomes were preincubated with various concentrations of Tat1–86, TatΔ31–61, or leucine at the concentration as indicated at 34°C for 15 min followed by the addition of [3H]leucine (final concentration, 1 μM) for 10 min. Data are expressed as mean ± S.E.M. as percentage of control (CON) values (1480 ± 87 dpm) from five independent experiments performed in duplicate. ***, p < 0.001 compared with the control values. B, synaptosomes were preincubated with various concentrations of Tat1–86 (0.1 nM–10 μM) at 34°C for 5 min followed by the addition of [3H]leucine (final concentration, 1 μM) or [3H]DA (final concentration, 0.1 μM) for 4 min. Nonspecific uptake for [3H]leucine and [3H]DA was determined in the presence of 10 mM l-lysine and 10 μM nomifensine, respectively. Data are expressed as mean ± S.E.M. as percentage of CON values (1307 ± 97 dpm) and (26,000 ± 1300 dpm) for [3H]leucine and [3H]DA, respectively, from four independent experiments performed in duplicate. All curves were best fit to a single class of binding site and generated by nonlinear regression.
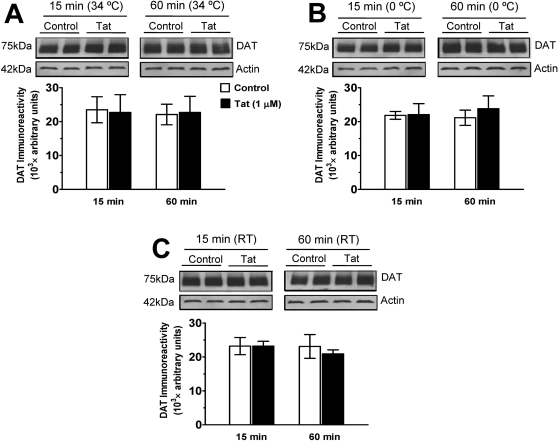
Effect of Tat Protein on DAT Immunoreactivity in Rat Striatal Synaptosomes. To determine whether Tat (1 μM)-induced inhibition in [3H]DA uptake, [3H]WIN 35,428 and [3H]GBR 12935 binding was associated with an alteration of total DAT immunoreactivity in synaptosomes, Western blot assays were performed. Figure 9 shows that exposure to a low concentration of Tat (1 μM) did not alter total synaptosomal DAT immunoreactivity at any time point and temperature compared with control samples. These results suggest that the Tat-induced decreases in [3H]DA uptake and DAT binding sites are not due to reduction of total DAT immunoreactivity in striatal synaptosomes.
Fig. 9.
Effects of Tat on DAT immunoreactivity in striatal synaptosomes. Striatal synaptosomes were incubated with 1 μM Tat1–86 at 34°C (A), on ice at 0°C (B), or at room temperature (RT; C) for 15 and 60 min. Top, representative immunoblots from synaptosomes incubated with Tat or same amount of assay buffer (control). Each condition was repeated in the adjacent lane on the gel. Actin was monitored as a control intracellular protein (see Materials and Methods). Bottom, DAT immunoreactivity is expressed as mean ± S.E.M. densitometry units from three independent experiments (n = 3).
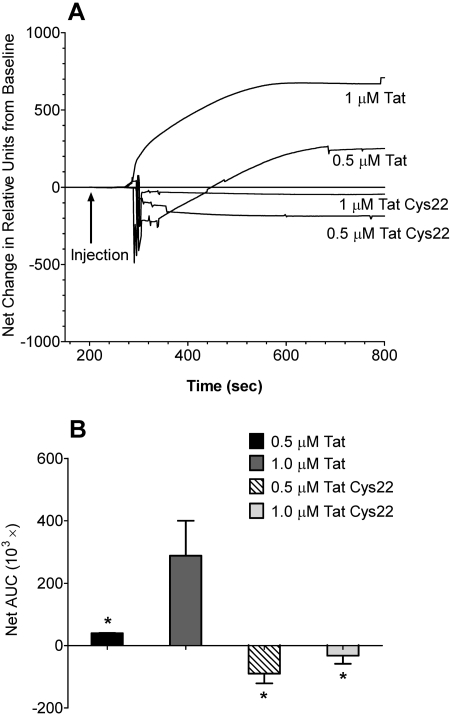
SPR Analysis of Tat and Mutant Tat Interactions with hDAT-GFP. Injection of Tat resulted in a robust change in signal intensity (Fig. 10A). Based on sensor technology, this represents an increase in SPR signal angle, and an interaction between Tat and hDAT-GFP. Nonselective Tat interactions are eliminated by the subtraction of channel 2 (control) from the response observed in channel 1 (hDAT-GFP). There were significant main effects of protein type (F1,8 = 14.21; p < 0.05) and concentration (F1,8 = 6.55; p < 0.05) on net changes in AUC (Fig. 10B). No significant interaction between protein type and concentration was found (F1,8 = 2.56; p > 0.05). The response to 1.0 μM Tat was greater than that observed in 0.5 μM Tat and Tat Cys22 at either 0.5 or 1.0 μM concentration (p < 0.05, Student-Newman-Keuls comparisons) (Fig. 10B). The curves for both Tat Cys22 groups were not different from baseline (S.D. curves not shown for clarity) (Fig. 10A). These data suggest that Tat was interacting with hDAT-GFP on the membranes immobilized in channel 1, whereas Tat Cys22 was not.
Fig. 10.
SPR analysis of interactions between Tat protein and hDAT-GFP. Tat1–86 or Tat Cys22 (0.5 or 1.0 μM) was injected sequentially across the VesCap sensor chip. An interaction between Tat and hDAT-GFP was indicated by a positive change from baseline values. Baseline was determined as the average response units for the 10 s before injection. A, raw data curves represent the change from baseline as measured in “relative units” measured at 0.1-s intervals over 600 s. Data are expressed as the mean curves from three independent experiments performed in duplicate. B, net AUC was determined by averaging all positive and negative responses during the 600-s period. Comparisons between the net changes in AUC showed that 1.0 μM Tat1–86 was significantly different from each of the other groups. Data are expressed as the mean ± S.E.M. of the net changes in baseline of three independent experiments performed in duplicate. *, p < 0.05 compared with 1 μM Tat1–86.
Discussion
The current study determined the impact of HIV-1 Tat protein on DAT function in rat striatum with [3H]DA uptake assays and [3H]WIN 35,428 and [3H]GBR 12935 binding assays. The results demonstrated time- and concentration-dependent effects of Tat in inhibiting [3H]DA uptake into striatal synaptosomes. It is important to note that the data presented here provide novel evidence that the Tat-induced decrease in DAT activity is accompanied by distinctly different changes in both the binding sites of [3H]WIN 35,428 and [3H]GBR 12935 and in the regulatory properties of DAT. These changes have profound implications for understanding dysfunctional DA regulation via DAT alteration, which may underlie the neurochemical basis of HIV-induced neuronal dysfunction and synergistic neurotoxicity of HIV with drugs of abuse.
The influence of Tat on [3H]DA uptake revealed itself in inhibitory effects at micromolar Tat concentrations, which corresponded to the potency for Tat inhibition of [3H]GBR 12935 binding. In contrast, the Ki value for Tat in inhibiting [3H]WIN 35,428 binding was 3-fold lower (i.e., increased potency) than that for [3H]DA uptake, suggesting that some functions of DAT are more sensitive to Tat than others, possibly related to slightly different domains for various ligands (Pristupa et al., 1994). In addition, the time course studies revealed that Tat inhibited [3H]DA uptake and the binding of [3H]WIN 35,428 and [3H]GBR 12935 in a time-dependent manner. Compared with the effects of Tat on the binding of the two radioligands, the effects of Tat on [3H]DA uptake were more delayed. At the 5-min time point, Tat-induced inhibition of the binding of [3H]WIN 35,428 and [3H]GBR 12935 reached a plateau, whereas the effects of Tat on [3H]DA uptake exhibited a maximal inhibition at 15 min, which remained through 60 min. The Tat protein used in the current study contains 86 amino acids encoded by two exons. The deletion mutant proteins TatΔ31–61 and Tat Cys22 exerted no inhibitory effect on [3H]DA uptake, suggesting that these amino acids in the first exon of Tat play a crucial role in the influence of Tat on DAT. Interestingly, SPR analysis of interactions between Tat protein and hDAT-GFP revealed that Tat interacted with hDAT-GFP in a concentration-dependent manner. In contrast, Tat Cys22 had little effect. Thus, these data suggest that the influence of Tat on DAT function and DAT ligand binding sites could involve a protein-protein interaction between Tat and DAT.
Our findings indicate that Tat protein influences selective binding sites on the DAT, with differential impact on [3H]WIN 35,428 and [3H]GBR 12935 binding. First, the Ki value for Tat inhibition in the [3H]GBR 12935 binding is 4-fold higher than that in the [3H]WIN 35,428 binding (Table 1). Second, Tat increases the Kd value for [3H]WIN 35,428 binding but not for [3H]GBR 12935 (Figs. 6 and 7). Third, WIN 35,428 has higher affinity in displacing [3H]WIN 35,428 binding than [3H]GBR 12935 binding (Fig. 5). WIN 35,428 and GBR 12935 are useful for labeling elements of the DAT; GBR 12935 labels the classic DA uptake site in rodent brain and binds to a piperazine acceptor site, whereas WIN 35,428 binds to cocaine binding sites (Andersen et al., 1987; Richfield, 1991). For example, the Bmax value for [3H]GBR 12935 in the control group is approximately 5 times higher than observed with the [3H]WIN 35,428 binding (Figs. 6 and 7), which is consistent with a previous report (Akunne et al., 1994). The [3H]WIN 35,428- and the [3H]GBR 12935-labeled DAT binding sites have some overlap (Pristupa et al., 1994). However, DAT sites labeled by these ligands may reflect different aspects of the functional DA uptake process, because [3H]GBR 12935 and [3H]WIN 35,428 do not seem to bind the same functional form/state of DAT in COS-7 expressed human DAT (Pristupa et al., 1994); in rodent brain, the two radioligands seem to have different binding characteristics as well (Andersen et al., 1987; Richfield, 1991). A caveat, however, is that the different temperature conditions used for measuring the binding of the two radioligands might also play a role in the divergent results. Nevertheless, these assays were always performed in the presence or absence of Tat, in which effects of Tat on [3H]GRB 12935 or [3H]WIN 35,428 binding were always compared with the respective controls (i.e., data present as percentage of control, in the absence of Tat).
The present findings show that 1 μM Tat decreased the Vmax value by 26%, whereas 10 μM Tat decreased the Vmax value by 90%, demonstrating a dose-dependent effect of Tat on DAT function. We also determined whether exposure to Tat in this concentration range produces a global effect on ionic gradients around plasma membranes required for uptake; the results showed that 1 μM Tat had no effect on [3H]leucine uptake but that 10 μM Tat decreased [3H]leucine uptake by 40% (Fig. 8A). Thus, a low level of Tat (1 μM) can be thought to functionally regulate DAT function, which the current results show is a reversible process upon washout (Table 2). In contrast, a high concentration of Tat (10 μM) exerts a more global effect on membrane proteins and electrochemical gradients. Although a detectable Tat concentration (140 pmol) in frontal cortical brain sample from HIV-1-infected patients has been reported (Hudson et al., 2000), it is unclear what the actual concentration of Tat (for producing effects on DAT activity) is at or around the synapse or how this effective concentration of Tat might be reflected in cerebrospinal fluid of HIV-infected patients. Tat (1 μM) caused an ∼18% decrease in the Bmax value of [3H]WIN 35,428 and [3H]GBR 12935 binding. However, both [3H]WIN 35,428 and [3H]GBR 12935 binding assays were conducted in the presence of Tat for at least 1 h, whereas the Vmax value was determined in the presence of Tat only for 15 min. Furthermore, results from Western blots show that the Tat (1 μM)-induced decreases in the Vmax and Bmax values were not accompanied by reduction of total DAT immunoreactivity in striatal synaptosomes after exposure to Tat. One interpretation is that the Tat (1 μM)-induced decreases observed in Bmax value (∼18%) of radioligand binding and in Vmax value (26%) reflect a trafficking effect on DAT away from the surface, whereas total synaptosomal DAT remains unchanged. A divergence between total DAT immunoreactivity and DAT function ([3H]DA uptake and [3H]WIN 35,428 binding) was observed in a previous study by Little et al. (2002), which reported an increase in the latter functional measures with exposure to cocaine but no change in total DAT immunoreactivity in human DAT-transfected N2A cells. The increase in functional activity was accompanied by an enhancement in cell surface DAT expression. Thus, the present results with low levels of Tat protein may represent a combination of trafficking and functional effects on DAT. It will be important in future studies to assess the potential redistribution of DAT protein in cytoplasmic pools and its cell surface expression induced by exposure to low levels of Tat.
As an alternative explanation to DAT trafficking away from the neuronal cell surface in combination with functional effects, the current results can be interpreted in terms of Tat-induced allosteric modulation of DAT function. Tat at 1 μM decreased the Vmax value (26%) without changing the Km value (Fig. 3A), but 10 μM Tat decreased the Vmax by 90% and increased the apparent Km value (Fig. 3B), consonant with Tat allosterically modulating DAT function in a dose-dependent manner. In addition, Tat dose-dependently decreased the Bmax value of [3H]WIN 35,428 binding and increased the apparent Kd value (Fig. 6), in deviation from a purely competitive mechanism of inhibition by Tat. Thus, Tat may act as an allosteric modulator of DAT rather than as either a reuptake inhibitor or a substrate-type releaser, such as cocaine and amphetamine. The different kinetics for inhibition of [3H]GBR 12935 binding by Tat are consonant with the different binding characteristics reported for the two radioligands in rodent brain (Andersen et al., 1987; Richfield, 1991). A recent study reported that the novel allosteric modulators of the DAT, SoRI-20040, SoRI-20041, and SoRI-2827, decreased the Vmax and Bmax values of [3H]DA uptake and [125I]RTI-55 binding and increased the apparent Km and Kd values in a dose-dependent manner (Pariser et al., 2008). In general, drugs interact with transporter proteins in two ways, either as a reuptake inhibitor or as a substrate. There is growing interest in an additional layer of complexity on how compounds can interact with transporters: allosteric modulation. Such allosterism could represent a novel therapeutic potential, or as we speculate for Tat in the present study, underlie a neurobiological mechanism of dysfunction of DAT reported in the patients with HIV infection (Wang et al., 2004; Chang et al., 2008), consonant with a potential susceptibility to those patients with drug intake. Understanding this mechanism has the potential to facilitate the development of therapeutic programs for HIV infection in humans.
Acknowledgments
We acknowledge Dr. Maarten E. A. Reith (New York University, New York, NY) for reading and commenting on the manuscript.
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grants DA013137, DA026721]; the National Institutes of Health National Institute of Child Health and Development [Grant HD043680]; and by the University of South Carolina, Research and Productive Scholarship Program.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.108.150144.
ABBREVIATIONS: Tat, transactivator of transcription; DA, dopamine; DAT, dopamine transporter; RTI-121, 3β-(4-iodophenyl) tropane-2β-carboxylic acid isopropyl ester; WIN 35,428, 2β-carbomethoxy-3-β-(4-fluorophenyl)tropane; GBR 12935, 1-[2-(diphenylmethoxy)ethyl]-4-(3-phenylpropyl)-piperazine; SPR, surface plasmon resonance; h, human; GBR 12909, 1-[2-(bis[4-fluorophenyl]methoxy)ethyl]-4-[3-phenylpropyl]piperazine; EDC, 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide; NHS, N-hydroxysuccinimide; GFP, green fluorescent protein; RU, response units; AUC, area under curve; ANOVA, analysis of variance; SoRI-20040, N-(2,2-diphenylethyl)-2-phenyl-4-quinazolinamine; SoRI-20041, N-(3,3-diphenylpropyl)-2-phenyl-4-quinazolinamine; SoRI-2827, [4-amino-6-[(diphenylmethyl)amino]-5-nitro-2-pyridinyl]carbamic acid ethyl ester; RTI-55, 2β-carbomethoxy-3β-(4-iodophenyl)tropane; VesCap, Vesicle Capture.
References
- Aksenova MV, Silvers JM, Aksenov MY, Nath A, Ray PD, Mactutus CF, and Booze RM (2006) HIV-1 Tat neurotoxicity in primary cultures of rat midbrain fetal neurons: changes in dopamine transporter binding and immunoreactivity. Neurosci Lett 395 235-239. [DOI] [PubMed] [Google Scholar]
- Akunne HC, Dersch CM, Cadet JL, Baumann MH, Char GU, Partilla JS, de Costa BR, Rice KC, Carroll FI, and Rothman RB (1994) Studies of the biogenic amine transporters. III. Demonstration of two binding sites for [3H]GBR12935 and [3H]BTCP in rat caudate membranes. J Pharmacol Exp Ther 268 1462-1475. [PubMed] [Google Scholar]
- Aloyo VJ, Ruffin JS, Pazdalski PS, Kirifides AL, and Harvey JA (1995) [3H]WIN 35,428 binding in the caudate nucleus of the rabbit: evidence for a single site on the dopamine transporter. J Pharmacol Exp Ther 273 435-444. [PubMed] [Google Scholar]
- Andersen PH, Jansen JA, and Nielsen EB (1987) [3H]GBR 12935 binding in vivo in mouse brain: labeling of a piperazine acceptor site. Eur J Pharmacol 144 1-6. [DOI] [PubMed] [Google Scholar]
- Bansal AK, Mactutus CF, Nath A, Maragos W, Hauser KF, and Booze RM (2000) Neurotoxicity of HIV-1 proteins gp120 and Tat in the rat striatum. Brain Res 879 42-49. [DOI] [PubMed] [Google Scholar]
- Berger JR and Arendt G (2000) HIV dementia the role of the basal ganglia and dopaminergic systems. J Psychopharmacol 14 214-221. [DOI] [PubMed] [Google Scholar]
- Berger JR and Nath A (1997) HIV dementia and the basal ganglia. Intervirology 40 122-131. [DOI] [PubMed] [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72 248-254. [DOI] [PubMed] [Google Scholar]
- Cass WA, Harned ME, Peters LE, Nath A, and Maragos WF (2003) H IV-1 protein Tat potentiation of methamphetamine-induced decreases in evoked overflow of dopamine in the striatum of the rat. Brain Res 984 133-142. [DOI] [PubMed] [Google Scholar]
- Chang L, Wang GJ, Volkow ND, Ernst T, Telang F, Logan J, and Fowler JS (2008) Decreased brain dopamine transporters are related to cognitive deficits in HIV patients with or without cocaine abuse. Neuroimage 42 869-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey LL and Reith MEA (1994) [3H]WIN 35,428 binding to the dopamine uptake carrier. I. Effect of tonicity and buffer composition. J Neurosci Methods 51 23-30. [DOI] [PubMed] [Google Scholar]
- Dingwall C, Ernberg I, Gait MJ, Green SM, Heaphy S, Karn J, Lowe AD, Singh M, Skinner MA, and Valerio R (1989) Human immunodeficiency virus 1 tat protein binds trans-activation-responsive region (TAR) RNA in vitro. Proc Natl Acad Sci USA 86 6925-6929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensoli B, Barillari G, Salahuddin SZ, Gallo RC, and Wong-Staal F (1990) Tat protein of HIV-1 stimulates growth of cells derived from Kaposi's sarcoma lesions of AIDS patients. Nature 345 84-86. [DOI] [PubMed] [Google Scholar]
- Ferris MJ, Frederick-Duus D, Fadel J, Mactutus CF, and Booze RM (2009) In vivo microdialysis in awake, freely moving rats demonstrates HIV-1 Tat-induced alterations in dopamine transmission. Synapse 63 181-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris MJ, Mactutus CF, and Booze RM (2008) Neurotoxic profiles of HIV, psychostimulant drugs of abuse, and their concerted effect on the brain: current status of dopamine system vulnerability in neuroADIS. Neurosci Biobehav Rev 32 883-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainetdinov RR and Caron MG (2003) Monoamine transporters: from genes to behavior. Annu Rev Pharmacol Toxicol 43 261-284. [DOI] [PubMed] [Google Scholar]
- Harrod SB, Mactutus CF, Fitting S, Hasselrot U, and Booze RM (2008) Intraaccumbal Tat (1–72) alters acute and sensitized responses to cocaine. Pharmacol Biochem Behav 90 723-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson L, Liu J, Nath A, Jones M, Raghavan R, Narayan O, Male D, and Everall I (2000) Detection of the human immunodeficiency virus regulatory protein tat in CNS tissues. J Neurovirol 6 145-155. [DOI] [PubMed] [Google Scholar]
- Koken SE, Greijer AE, Verhoef K, van Wamel J, Bukrinskaya AG, and Berkhout B (1994) Intracellular analysis of in vitro modified HIV Tat protein. J Biol Chem 269 8366-8375. [PubMed] [Google Scholar]
- Kumbhat S, Shankaran DR, Kim SJ, Gobi KV, Joshi V, and Miura N (2007) Surface plasmon resonance biosensor for dopamine using D3 dopamine receptor as a biorecognition molecule. Biosens Bioelectron 23 421-427. [DOI] [PubMed] [Google Scholar]
- Little KY, Elmer LW, Zhong H, Scheys JO, and Zhang L (2002) Cocaine induction of dopamine transporter trafficking to the plasma membrane. Mol Pharmacol 61 436-445. [DOI] [PubMed] [Google Scholar]
- Ma M and Nath A (1997) Molecular determinants for cellular uptake of Tat protein of human immunodeficiency virus type 1 in brain cells. J Virol 71 2495-2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madras BK, Fahey MA, Bergman J, Canfield DR, and Spealman RD (1989) Effects of cocaine and related drugs in nonhuman primates. I. [3H]Cocaine binding sites in caudate-putamen. J Pharmacol Exp Ther 251 131-141. [PubMed] [Google Scholar]
- Maragos WF, Young KL, Turchan JT, Guseva M, Pauly JR, Nath A, and Cass WA (2002) Human immunodeficiency virus-1 Tat protein and methamphetamine interact synergistically to impair striatal dopaminergic function. J Neurochem 83 955-963. [DOI] [PubMed] [Google Scholar]
- Napolitano A, Zürcher G, and Da Prada M (1995) Effects of tolcapone, a novel catechol-O-methyltransferase inhibitor, on striatal metabolism of L-DOPA and dopamine in rats. Eur J Pharmacol 273 215-221. [DOI] [PubMed] [Google Scholar]
- Nath A, Jankovic J, and Pettigrew LC (1987) Movement disorders and AIDS. Neurology 37 37-41. [DOI] [PubMed] [Google Scholar]
- Nath A, Psooy K, Martin C, Knudsen B, Magnuson DS, Haughey N, and Geiger JD (1996) Identification of a human immunodeficiency virus type 1 Tat epitope that is neuroexcitatory and neurotoxic. J Virol 70 1475-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariser JJ, Partilla JS, Dersch CM, Ananthan S, and Rothman RB (2008) Studies of the biogenic amine transporters. 12. Identification of novel partial inhibitors of amphetamine-induced dopamine release. J Pharmacol Exp Ther 326 286-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippon V, Vellutini C, Gambarelli D, Harkiss G, Arbuthnott G, Metzger D, Roubin R, and Filippi P (1994) The basic domain of the lentiviral Tat protein is responsible for damages in mouse brain: involvement of cytokines. Virology 205 519-529. [DOI] [PubMed] [Google Scholar]
- Pristupa ZB, Wilson JM, Hoffman BJ, Kish SJ, and Niznik HB (1994) Pharmacological heterogeneity of the cloned and native human dopamine transporter: disassociation of [3H]WIN 35,428 and [3H]GBR 12,935 binding. Mol Pharmacol 45 125-135. [PubMed] [Google Scholar]
- Reith ME and Coffey LL (1994) [3H]WIN 35,428 binding to the dopamine uptake carrier. II. Effect of membrane fractionation procedure and freezing. J Neurosci Methods 51 31-38. [DOI] [PubMed] [Google Scholar]
- Richfield EK (1991) Quantitative autoradiograph of the dopamine uptake complex in rat brain using [3H]GBR 12935: binding characteristics. Brain Res 540 1-13. [DOI] [PubMed] [Google Scholar]
- Silin VI, Karlik EA, Ridge KD, and Vanderah DJ (2006) Development of surface-based assays for transmembrane proteins: selective immobilization of functional CCR5, a G protein-coupled receptor. Anal Biochem 349 247-253. [DOI] [PubMed] [Google Scholar]
- Theodore S, Stolberg S, Cass WA, and Maragos WF (2006) Human immunodeficiency virus-1 protein Tat and methamphetamine interactions. Ann N Y Acad Sci 1074 178-190. [DOI] [PubMed] [Google Scholar]
- Tomlinson G (1988) Potential misconceptions arising from the application of enzyme kinetic equations to ligand-receptor system at equilibrium. Can J Physiol Pharmacol 66 342-349. [DOI] [PubMed] [Google Scholar]
- Wallace DR, Dodson S, Nath A, and Booze RM (2006) Estrogen attenuates gp120- and tat1–72-induced oxidative stress and prevents loss of dopamine transporter function. Synapse 59 51-60. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Chang L, Volkow ND, Telang F, Logan J, Ernst T, and Fowler JS (2004) Decreased brain dopaminergic transporters in HIV-associated dementia patients. Brain 127 2452-2458. [DOI] [PubMed] [Google Scholar]
- Zauli G, Secchiero P, Rodella L, Gibellini D, Mirandola P, Mazzoni M, Milani D, Dowd DR, Capitani S, and Vitale M (2000) HIV-1 Tat-mediated inhibition of the tyrosine hydroxylase gene expression in dopaminergic neuronal cells. J Biol Chem 275 4159-4165. [DOI] [PubMed] [Google Scholar]
- Zhu J and Reith MEA (2008) Role of dopamine transporter in the action of psychostimulants, nicotine, and other drugs of abuse. CNS Neurol Disord Drug Targets 7 393-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Bardo MT, Bruntz RC, Stairs DJ, and Dwoskin LP (2007) Individual differences in response to novelty predict prefrontal cortex dopamine transporter function and cell surface expression. Eur J Neurosci 26 717-728. [DOI] [PubMed] [Google Scholar]
- Zhu J, Green T, Bardo MT, and Dwoskin LP (2004) Environmental enrichment enhances sensitization to GBR 12935-induced activity and decreases dopamine transporter function in the medial prefrontal cortex. Behav Brain Res 148 107-117. [DOI] [PubMed] [Google Scholar]