Abstract
The cyclooxygenase (COX) pathway generates the reactive lipid electrophile 15-deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2), which forms covalent protein adducts that modulate cell signaling pathways. It has been shown that this regulates important biological responses, including protection against oxidative stress, and supports the proposal that 15d-PGJ2 has pharmacological potential. Protective pathways activated by 15d-PGJ2 include those controlling the synthesis of the intracellular antioxidants glutathione (GSH) and the enzyme heme oxygenase-1 (HO-1). The induction of the synthesis of these intracellular antioxidants is, in large part, regulated by covalent modification of Keap1 by the lipid and the subsequent activation of the electrophile response element (EpRE). For the first time, we show that the potency of 15d-PGJ2 as a signaling molecule in endothelial cells is significantly enhanced by the accumulation of the covalent adduct with 15d-PGJ2 and endogenous Keap1 over the time of exposure to the prostaglandin. The consequence of this finding is that signaling initiated by electrophilic lipids differs from agonists that do not form covalent adducts with proteins because the constant generation of very low concentrations of 15d-PGJ2 can lead to induction of GSH or HO-1. In the course of these studies we also found that a substantial amount (97-99%) of exogenously added 15d-PGJ2 is inactivated in the media and does not enter the cells to initiate cell signaling. In summary, we propose that the accumulation of covalent adduct formation with signaling proteins provides a mechanism through which endogenous intracellular formation of electrophilic lipids from cyclooxygenase can exert anti-inflammatory effect in vivo.
Keywords: Glutathione, heme oxygenase, Keap1, antioxidant response element
Introduction
It has long been recognized that enzymes such as cyclooxygenase (COX) generate signaling molecules through the controlled oxidation of lipids [1, 2]. A sub-class of these signaling molecules can act through mechanisms which involve the specific covalent modification of proteins [3-6]. For example, some of the cyclopentenone prostaglandins contain α,β-unsaturated carbonyl groups and an associated electrophilic center. This results in reactivity with nucleophilic amino acid residues such as cysteine [3, 7-9]. In this respect, the cyclopentenone 15-deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2) is particularly interesting since it is formed in vivo downstream of COX-2 from the dehydration of PGD2 [10, 11]. It is now clear that 15d-PGJ2 has distinct signaling properties which cannot be explained solely by its interaction with PPARγ or other established prostaglandin receptors [8, 10, 12-14]. The electrophilic carbons are critical for these biological actions of 15d-PGJ2, since closely related compounds lacking this group cannot activate the PPARγ-independent signaling pathways [10, 12]. These PPARγ-independent pathways are associated with the formation of covalent lipid-protein adducts with a sub-proteome that includes members such as Keap1, ras, thioredoxin, and NFκB in the cell [7, 8, 15-19]. We have termed this family of proteins the electrophile responsive proteome [3, 5]. Interestingly, the effects of covalent protein modification with a signaling molecule such as Keap1 have not been fully characterized, and this has led to an under appreciation of the potential role of 15d-PGJ2 in cytoprotective signaling.
An important protein belonging to the electrophile responsive proteome is Keap1, a cytosolic protein that binds the transcription factor Nrf-2 and controls the levels available for nuclear translocation [20]. Covalent modification of critical thiol residues of Keap1 decreases the proteasomal degradation of Nrf-2, and results in the translocation of Nrf-2 to the nucleus where it heterodimerizes with other transcription factors to increase the expression of proteins under the control of the electrophile response element (EpRE) [20]. Such proteins include heme oxygenase-1 (HO-1) and the enzyme responsible for glutathione (GSH) synthesis, glutamyl cysteine ligase. It has been shown that heme oxygenase-1 (HO-1) expression is induced by isolated cyclopentenone prostaglandins [21-24]. An important question in the redox signaling field is whether sufficient amounts of electrophilic lipids such as 15d-PGJ2 can be generated by COX-2 to activate signaling pathways. Indeed, free 15d-PGJ2 has been measured in vivo, is elevated during the late stages of inflammation and is associated with the COX-2-dependent induction of intracellular antioxidants [24-27]. For example, in a recent study, the levels of free 15d-PGJ2 were reported in the nM range, a level insufficient to activate PPARγ, although more recent reports suggest that PPARγ may be activated at biologically relevant concentrations [28-30]. This raises a critical question important for understanding how lipid electrophiles, such as 15d-PGJ2, mediate cell signaling; what is the “real” concentration to which cells are exposed and how does this relate to the formation of the covalent protein adducts which modulate the biological effects of the lipid? Other factors which are likely to affect the availability of electrophiles such as 15d-PGJ2 to initiate cell signaling are the competition with non-specific binding sites in the media or metabolism and conjugation with glutathione through the enzymatic action of glutathione S-transferases [17, 31-33]. To approach these questions, we investigated the relationship between exogenously added 15d-PGJ2 and the levels of covalent adduct formation with proteins in endothelial cells in culture.
In addition, we hypothesized that covalent modification of signaling proteins over time will increase the sensitivity of the pathways activated by the EpRE system through the accumulation of covalent adducts with Keap1. Accordingly, we have designed experiments to characterize the conditions of exposure required to activate the EpRE in endothelial cells by 15d-PGJ2, measured by increases in both cellular GSH and HO-1. In this study, we demonstrate for the first time that adduct formation with endogenous Keap1 and low amounts of 15d-PGJ2 can accumulate over time leading to increased levels of cellular GSH and HO-1. In addition, a substantial proportion of the 15d-PGJ2 is rapidly inactivated under cell culture conditions and not available for activation of cell signaling pathways. The implications of these data for understanding the mechanisms of reactive lipid signaling in vivo are profound since they suggest that the accumulation of a signal can occur over time at biologically relevant levels of 15d-PGJ2. These findings support the hypothesis that this and similar electrophiles can contribute to the regulation of the EpRE in vivo.
MATERIALS AND METHODS
All biochemicals were purchased from Sigma (St Louis, MO) except as noted.
Cell culture
Bovine aortic endothelial cells (BAEC) were prepared and used between passages 4-10 as described previously [34] and cultured in 6-well plates in 10% fetal bovine serum (FBS, Atlanta Biologicals, Atlanta, GA), 5.6 mM glucose, 2 mM glutamine (Gibco, Carlsbad, CA) and penicillin/streptomycin (Gibco) containing media (Dulbecco's modified Eagle's medium; DMEM, Cellgro, Herndon, VA). After reaching confluency media were replaced with 0.5% FBS containing media for 24h before exposure to the lipid under the conditions described for each experiment.
Determination of lipid concentration and cell treatment
The 15d-PGJ2 (Cayman, Ann Arbor, MI) and biotinylated 15d-PGJ2 (BT-15d-PGJ2) concentration were measured by absorbance at 306nm using the extinction coefficient 12,000 M−1 cm−1 in EtOH. The lipid concentration in aqueous media was recalibrated by absorbance at 315nm using the extinction coefficient 9,588 M−1 cm−1 and 9,014 M−1 cm−1 for unmodified and biotinylated lipids, respectively. Confluent cells were treated with lipid under the conditions described and all cellular responses were compared to a vehicle (ethanol) control. In the comparison of the bolus versus sequential addition of 15d-PGJ2 over a period of three days, the experiment was initiated at a confluency of approximately 80% at day 1.
Determination of lipid adduct formation in cells
To follow the fate of 15d-PGJ2, the biotinylated derivative (BT-15d-PGJ2) was prepared as described previously [8]. For the detection of protein adducts, BAEC were grown in 0.5% serum for 24h and then either treated with 10 μM BT-15d-PGJ2 (30 mins-16h) or with increasing concentrations of BT-15d-PGJ2 for 4h. Cells were washed with phosphate buffered saline (PBS) and harvested in 10 mM Tris, 1% Triton X-100, pH 7.4 containing protease inhibitor cocktail (PIC; Mini-Complete, Roche, Indianapolis, IN). Cell lysate proteins were separated by SDS-PAGE and transferred to nitrocellulose membrane at 100V for 2h. After blocking with 5% skimmed milk in TBS-T for 1h, the membranes were probed with streptavidin-HRP (Amersham, Piscataway, NJ) in TBS-T and developed using a chemiluminescence imager (AlphaInnotech, San Leandro, CA). 10 ng and 20 ng of BT-cyt c (biotin levels are 2.41 pmol and 4.82 pmol, respectively) were used as an internal standard to calibrate the adduct formation in cell lysates [35].
Determination of lipid adduct distribution between cellular and media proteins
To determine the distribution of protein adducts by BT-15d-PGJ2 between cells and media, BT-15d-PGJ2 (10 μM) was added to media containing 0% or 0.5% serum for 4h. The total amount of lipid added to the medium in these experiments was verified spectrophotometrically by using the characteristic absorbance of BT-15d-PGJ2 at 306 or 315nm and corrected for any background absorbance of the medium as described above. Media were then collected and cells were lysed in 10 mM Tris, 1% Triton X-100 buffer (pH 7.4) with PIC and the pellets were washed with PBS. After centrifugation, the cell pellets were sonicated in 10 mM Tris, 2% SDS (pH 6.8) with PIC for 20 min. Protein content was determined by Lowry assay and BT-15d-PGJ2 adduct on proteins measured as described previously [35]. Proteins were visualized using Sypro Ruby stain (Molecular Probes, Eugene, OR) with a CCD camera imager equipped with a SYPRO-500 filter (AlphaInnotech, San Leandro, CA).
To determine the extent of modification of Keap1 by 15d-PGJ2, confluent cells were treated with BT-15d-PGJ2 at a range of concentrations and for varying periods of time. After the treatment, cell lysates were prepared as described above. Biotinylated proteins were affinity precipitated using a 100 μL of a 50% slurry of Neutravidin beads (Pierce, Rockford, IL) washed with 20 mM Tris-HCl (pH 7.4, 6 times). Cell lysates (500-800 μg protein) were added to the beads containing PIC and incubated overnight at 4°C with rotation. Beads were then washed with 500 μl 0.1 M glycine (pH 2.8, 6 times) followed by washing with 1 ml 20 mM Tris-HCl (pH 7.4). Samples were then prepared for analysis by heating the beads to 80°C for 10 min in 80 μl of 2x sample buffer (0.1 M Tris, 4% SDS, 10% Glycerol, 0.2% Bromophenol blue, pH 6.8) containing β-mercaptoethanol to release the biotin labeled proteins. Samples were then centrifuged at 12,500 rpm for 10 min at 4°C, and supernatants used for analysis. Proteins were separated by SDS-PAGE, and transferred to nitrocellulose membrane at 100V for 2h and membranes blocked with 5% skimmed milk in TBS-T. Membranes were incubated with polyclonal primary antibody against Keap1 (Santa Cruz: E-20 1:1000 dilution in 5% skimmed milk in TBS-T), followed by a HRP (horseradish peroxidase)-conjugated donkey anti-goat secondary antibody (Santa Cruz; 1:1000 dilution in 5% skimmed milk in TBS-T). Membranes were developed by chemiluminescence using SuperSignal West Dura substrate (Pierce, Rockford, IL) and sequential images taken with quantitation only performed on bands which had not reached saturation as described in [36].
Determination of Glutathione and HO-1 levels
After incubation with 15d-PGJ2 or BT-15d-PGJ2, cells in each well were washed twice with 2 ml of 10 μM DTPA (diethylene triamine pentaacetic acid) in PBS and lysed in the presence PIC containing lysis buffer (10 μM DTPA, 0.1% Triton X-100 in PBS, pH 7.4). Protein content was assayed by the Bradford method (Bio-Rad, Hercules, CA). Total glutathione (GSH+GSSG) levels were determined as described previously and are expressed relative to cell lysate protein [12]. To determine HO-1 protein expression, cell lysate proteins were separated by SDS-PAGE and transferred to nitrocellulose at 100V for 2h before membranes were blocked with 5% skimmed milk and 1% bovine serum albumin in TBS-T. Membranes were incubated with polyclonal primary antibody against HO-1 (Stressgen: SPA-896 1:3000 dilution in blocking buffer), followed by a HRP (horseradish peroxidase)-conjugated donkey anti-rabbit IgG secondary antibody (Amersham: NA934V; 1:5000 dilution in 5% skimmed milk only in TBS-T). Membranes were developed by chemiluminescence using SuperSignal West Dura substrate and sequential images taken with quantitation only performed on bands which had not reached saturation.
Statistical analysis
Data are reported as mean ± S.E.M. for sample sizes of three to nine, as indicated in the legends. Statistical significance was evaluated by one-way or two-way ANOVA (analysis of variance) among the groups using SPSS window (version 8.0). The minimum level of significance was set at P <0.05. The least significant difference (LSD) test was used as a post hoc test for the significant difference between groups.
RESULTS
Quantitation of protein adducts upon addition of BT-15d-PGJ2 to endothelial cells in culture
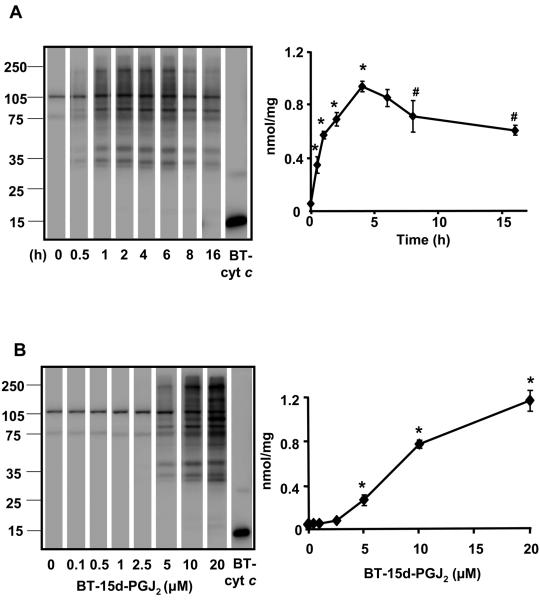
To establish the time course and concentration-dependence for protein adduct formation mediated by 15d-PGJ2 bovine aortic endothelial cells (BAEC) were treated for the times shown with BT-15d-PGJ2 (10 μM) or a range of concentrations for 4h (Figure 1) and the amount of protein adducts quantitated using a biotinylated internal standard protein [35]. Two bands were detected in either the untreated cells (result not shown) or at the zero time point which are due to the endogenous biotin-containing carboxylases. Protein adducts in the samples containing 10 μM BT-15d-PGJ2 increased over time reaching a maximum of approximately 1 nmol/mg protein at 4-5h (Figure 1A). The formation of protein adducts was also dependent on the concentration of BT-15d-PGJ2 (Figure 1B). No toxicity was detected in the presence of BT-15d-PGJ2 after 4h, but cell death occurred in the presence of 20 μM BT-15d-PGJ2 at 16h (data not shown) as reported previously [12].
Figure 1. Formation of protein adducts in BAEC on exposure of BT-15d-PGJ2 is time and concentration dependent.
Cells were treated with BT-15d-PGJ2 (0-20 μM) in medium containing 0.5% serum for 0-16h before preparation of a cell lysate and analysis for BT-15d-PGJ2 protein adducts by SDS-PAGE followed by western blotting. The position of the molecular weight markers is indicated on the left panel of each representative blots. Panel A: Cells were exposed to BT-15d-PGJ2 (10 μM) for 0-16h and BT-15d-PGJ2 adducts formation on protein determined. Panel B: Cells were exposed to increasing concentrations of BT-15d-PGJ2 for 4h and BT-15d-PGJ2 adduct formation determined. In both cases BT-cyt c was used as an internal standard 20 ng (4.82 pmoles biotin) for panel A. 10 ng (2.41 pmoles) for Panel B. Data are expressed as nmol BT/mg protein and values represent the mean ± S.E.M., n=3. Significance was analyzed by one-way ANOVA followed by the LSD as post hoc test. * represents a significant difference compared to control (t=0 or 0 μM) at p<0.001 and # represents the significant difference compared to 4h at <0.001 (Panel A).
Effect of serum on the distribution of protein adduct formation by BT-15d-PGJ2 and induction of HO-1 synthesis
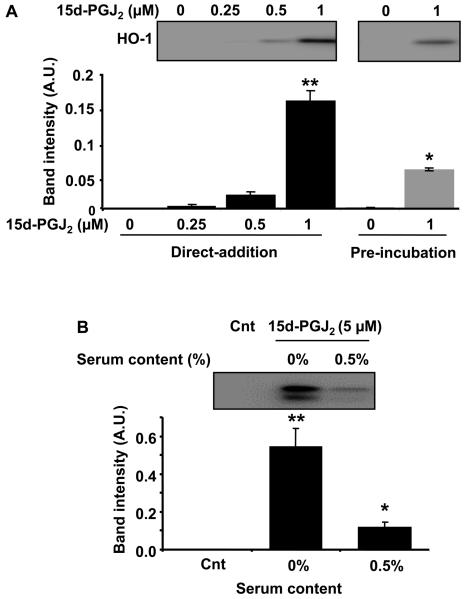
It has previously been shown that the presence of serum in the medium can decrease the signaling in epithelial cells through a competing reaction with albumin which appears to prevent access to the cells [37]. We confirmed this result by adding 15d-PGJ2 (0-1 μM) to the cells either directly or after pre-incubation for 5 min in media containing 0.5% FBS followed by incubation for 16h and measurement of HO-1 levels (Figure 2A). As a further test of the inhibitory effect of serum, 15d-PGJ2 (5 μM) was added directly to BAEC with and without 0.5% FBS and HO-1 levels measured after 16h. As shown in Figure 2B, the presence of FBS decreased HO-1 induction by approximately 90%.
Figure 2. The effect of FBS on HO-1 induction by 15d-PGJ2 in BAEC.
Panel A: Cells were treated with increasing concentrations of 15d-PGJ2 for 16h immediately after mixing with medium (black bar) or incubating in medium for 5 min before adding to cells (grey bars). Cells were lysed after 16h to determine HO-1 induction by western blotting as described in method. The statistical significance among the groups was evaluated by two-way ANOVA. The least significant difference (LSD) was used as post hoc tests for the difference between the groups and the interaction of the variables. ** significantly different to control (0 μM of 15d-PGJ2) and * between 0 min and 5 min incubation before adding 1 μM 15d-PGJ2, at p<0.0001. Panel B: Cells were incubated with 15d PGJ2 (5 μM) for 4h (0% or 0.5% FBS media) after which the medium was changed (0.5% FBS) and the cells incubated for a further 12h before lysis and analysis for HO-1 induction by western blotting. Statistical significance was evaluated by one-way ANOVA followed by the LSD as post hoc tests. ** represents a significant difference compared to control (0 μM of 15d-PGJ2) at p<0.0001 and * between 0% and 0.5% serum containing media with 5 μM 15d-PGJ2 at p<0.0001. The insets show representative blots and the bar charts the quantitation of replicated samples and data are represented as the mean ±S.E.M. n=3.
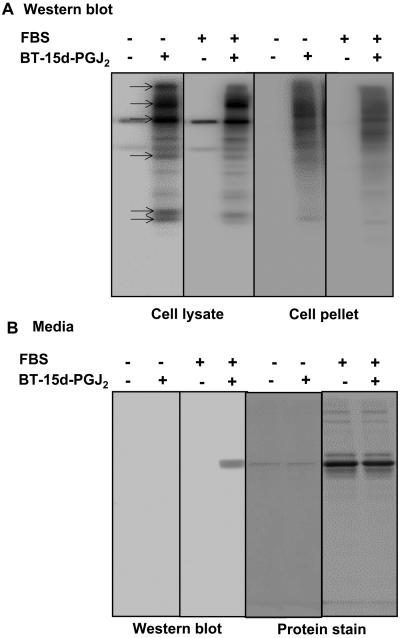
Using the conditions defined in Figure 2B adduct formation was determined in the medium, cell lysate, and SDS solubilized cell pellet. In this experiment, the amount of lipid added to the cells was 7.5 μM (15 nmol total) and the distribution of BT-lipid in the different fractions is shown in Figure 3 and Table 1. The levels of protein adduct formation on a number of the protein bands (indicated with arrows, Figure 3A) were decreased by the presence of FBS. Quantitation of these experiments indicated that 2.8% of the total BT-lipid added in the absence of FBS formed protein adducts versus 0.35% in its presence (Table 1). Protein adduct formation was detected in the medium on bovine serum albumin (Figure 3B), which accounted for another 0.2% of adducts in the presence of FBS. Taken together, these data demonstrate that the pool of 15d-PGJ2 capable of forming covalent adducts in the cell is decreased over a short time period by interaction with serum.
Figure 3. Distribution of BT-15d-PGJ2 between cellular and media protein adducts.
Cells were incubated with BT-15d-PGJ2 (10 μM) in 0% or 0.5% serum containing media for 4h. Media were then collected and Triton soluble cell lysates and SDS soluble cell pellets prepared for analysis of BT-15d-PGJ2 protein adducts in the cell by SDS-PAGE followed by western blotting. Panel A: BT-15d-PGJ2 adduct formation in cell fractions in the absence and presence of FBS determined by western blot and protein stained gel from media. Protein bands showing decreased adduct formation after incubation with 0.5% FBS are indicated with arrows. Panel B: BT-15d-PGJ2 adduct formation in media and protein staining in the absence and presence of 0.5% FBS. The quantitation of these data is reported in Table 1.
Table 1. Effect of serum on the distribution of protein adduct formation by BT-15d-PGJ2.
Values are Mean ± S.E.M. (n= 3). The BT-15d-PGJ2 protein adduct formation was determined in the medium, cell lysate, and SDS solubilized cell pellet according to the internal standard (BT-cyt c) from a western blot using streptavidin-conjugated HRP (for cell lysate and pellets) or spectrophotometric analysis (for media).
| BT-15d-PGJ2 protein adducts | 0% FBS | 0.5% FBS |
|---|---|---|
| Media (pmol) | N.D. | 30.2±4.6 |
| Soluble cell lysate (pmol) | 294.3±2.2 | 41.1±1.5 |
| SDS soluble cell pellets (pmol) | 125.8±12.3 | 11.3±1.5 |
| Total BT-15d-PGJ2 in cells (pmol) | 420±12 | 52.4±0.57 |
| BT-15d-PGJ2 in media (nmol) | 15.0 ± 0.4 | 14.9 ± 0.2 |
Determining the concentration of 15d-PGJ2 required to induce GSH and HO-1 in BAEC
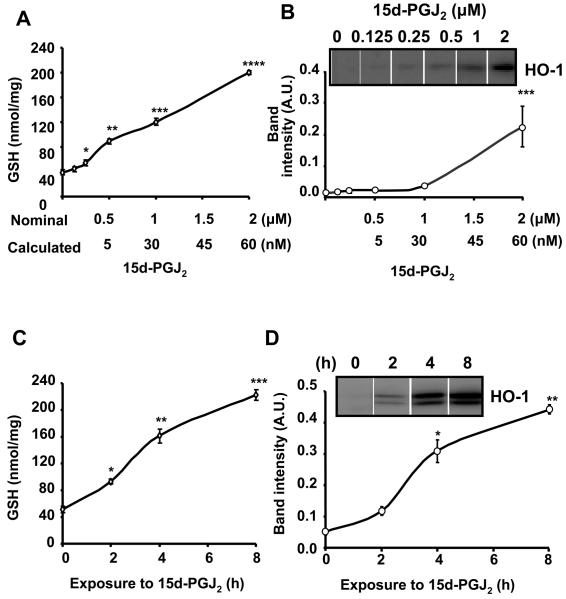
In the next series of experiments, we assessed cell signaling induced by 15d-PGJ2 using both GSH and HO-1 protein levels which are sensitive indicators of the covalent modification of Keap1 and subsequent activation of the EpRE. To establish a concentration dependence for 15d-PGJ2 in regulating these antioxidants, BAEC were exposed to increasing concentrations of 15d-PGJ2 in serum-free media. After 4h incubation, the lipid-containing medium was removed and replaced with fresh media containing 0.5% FBS and the cells incubated for a further 12h before lysis and measurement of total GSH or HO-1 protein. In Figure 4, we show the data for both GSH induction (Panel A) and HO-1 (Panel B) as a function of both the nominal concentration of 15d-PGJ2 added to the media and also adjusted for the loss to the cell culture medium (97%-Table 1). As has been reported previously, we found that the levels of GSH increase at least four fold on exposure to 15d-PGJ2 (Figure 4A). In the case of HO-1, a significant increase was only detected at nominal concentrations of 1 μM 15d-PGJ2 or above (Figure 4B).
Figure 4. Induction of GSH and HO-1 in BAEC in response to increasing concentration of 15d-PGJ2 and time of exposure to the lipid.
Cells were incubated with increasing concentration of 15d-PGJ2 for 4h (0% FBS) after which the medium was changed (0.5% FBS) and the cells incubated for a further 12h. The numbers below the nominal concentration (Panel A,B) represent the calculated concentration of 15d-PGJ2 when loss to the media is taken into account. Panel A: total GSH levels Panel B: HO-I protein expression from the same cell lysates. Panel C, D; Cells were incubated with 2 μM 15d-PGJ2 for the times shown (0% FBS) before the medium was changed (0.5% FBS) and the cell incubated for a total time of 16 h before preparation of cell lysates. Panel C: Total GSH. Panel D: HO-I protein levels. Values are the mean ± S.E.M., n=3 and statistical significance was evaluated by one-way ANOVA followed by the LSD as post hoc tests. *, **, ***, **** represent a significant difference compared to control (0 μM 15d-PGJ2) at p<0.05, p<0.01, p<0.001, p<0.0001 respectively.
The effect of time on the induction of GSH and HO-1 by 15d-PGJ2in BAEC
The data in Figure 1 suggests that increasing the time of exposure of 15d-PGJ2 to the cells should increase the final level of GSH or HO-1. To test this, cells were incubated with 2 μM 15d-PGJ2 in the absence of FBS for 2, 4 or 8h after which the lipid was removed by changing the media and cells incubated for a further 16h. In these experiments, the lower molecular weight band detected in the HO-1 western blot represents a recently described processed form of the enzyme and was included in the quantitation of the protein [38]. Measurement of GSH or HO-1 in these cells revealed that a longer time of exposure to 15d-PGJ2 resulted in a higher level of GSH or HO-1 (Figure 4C, D). This response was not linear, however, since the earliest time points of exposure showed disproportionately lower levels of both GSH and HO-1. This threshold effect was not due to saturation of the signal for either HO-1 or GSH at the longer time points since preliminary experiments indicated that these levels for both antioxidants were approximately 50-70% of the maximum levels that can be achieved (result not shown). Control experiments in which the vehicle was added under identical conditions indicated that the time of incubation under serum free conditions had no impact on the basal GSH or HO-1 levels (result not shown).
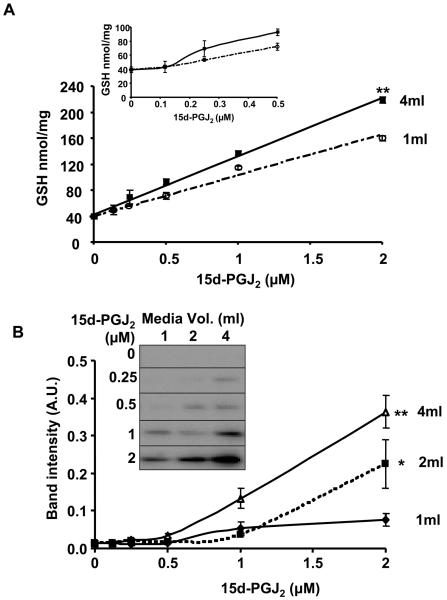
The effect of media volume on the induction of GSH and HO-1 by 15d-PGJ2
In the vasculature, endothelial cells are exposed to blood containing electrophiles at low concentrations. To model this effect, we varied both the volume of media (1-4ml) and concentration of 15d-PGJ2 exposed to the cells and determined the resulting levels of GSH and HO-1. The concentration dependence for the induction of GSH for two volumes (1ml and 4ml) are shown in Figure 5A. The inset shows the lower part of the dose-response curves for GSH and demonstrates that even at very low concentrations of the electrophile exposure of the cells to the same concentration at a higher volume results in a greater induction of antioxidant. When the same samples were also analyzed for HO-1 induction, a similar pattern was observed. A potentiation of the effects of 2μM 15d-PGJ2 occurs in higher volumes of media, which corresponds to increasing amounts (2, 4 and 8 nmol) of the lipid (Figure 5B). These data are consistent with a response to 15d-PGJ2 that is sensitive to both the concentration and absolute amount of lipid exposed to the cells. Thus GSH and HO-1 levels increase as the volume of a fixed concentration of 15d-PGJ2 is increased.
Figure 5. Induction of GSH and HO-1 in BAEC in response to increasing volumes of media containing 15d-PGJ2.
Panel A: Cells were incubated with increasing volumes of media (1, 2, 4ml) containing a range of concentrations of 15d-PGJ2 (0.125, 0.25, 0.5, 1 or 2 μM) for 4 h (0% FBS) after which the medium was changed (0.5% FBS, 2ml) and the cells incubated for further 12h. The curves for the 1ml and 4ml volume were shown. The extent of GSH induction below 0.5 μM 15d-PGJ2 is shown in the inset. Panel B: HO-1 protein expression as a function of volume and concentration of media containing 15d-PGJ2. Values are mean ± S.E.M., n=3 and statistical significance among the groups was evaluated as described in Figure 6 and *, ** represent a significant difference among the groups at p<0.01, p<0.001 respectively.
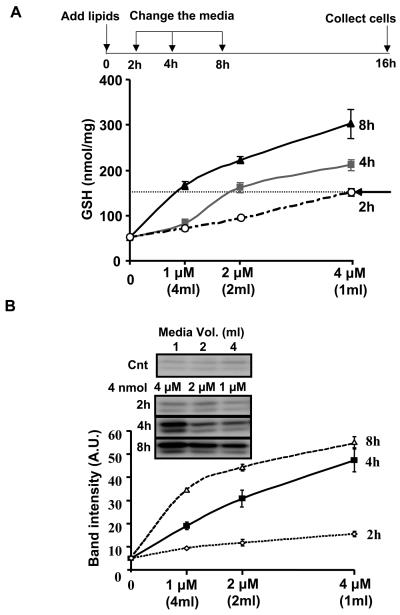
The importance of time of exposure and amount of electrophile in determining induction of HO-1 and GSH through covalent adduct formation with Keap1
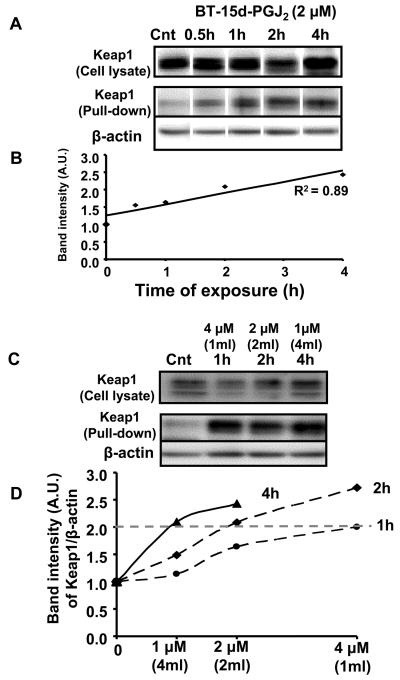
Based on the previous experiments, we predicted that lower concentrations of lipid will accumulate adducts with signaling proteins more slowly, thus requiring more time to obtain the same biological response. In other words, if the amount of the electrophile is kept constant and the volume of the media doubled (i.e. the lipid concentration is decreased by half) the time taken to achieve the same biological response will also double. This experiment was performed as described in the scheme shown in Figure 6A, where a constant amount (4 nmol) of 15d-PGJ2 was exposed to cells in different volumes (shown in parenthesis) for the times shown. As before, both GSH and HO-1 were measured and are reported as a function of 15d-PGJ2 concentration. It is evident from these data that increased time of lipid exposure to the cells leads to increased GSH levels at all concentrations tested. From Figure 6A it is also clear that the time taken to reach a fixed level of cellular GSH (e.g. 150 nmol-dotted line) increases as the concentration of 15d-PGJ2 exposed to the cells decreases. These samples were also assessed for changes in HO-1 levels in the cell (Figure 6B). While there was little or no response after only 2h, 2 μM 15d-PGJ2 exposed to the cells for 4h resulted in similar levels of HO-1 to 1 μM 15d-PGJ2 exposed to the cells for 8h. These data predict that similar responses in the formation of covalent protein adduct formation with Keap1 will also occur. To test this, cells were prepared as described in Figure 6. Levels of Keap1 (Figure 7A top panels) and actin (data not shown) were determined by western blot analysis and did not change in response to BT-15d-PGJ2. Samples were then subjected to affinity purification of biotinylated proteins. We found that a small amount of contaminating Keap1 was pulled down in the control samples together with actin and other cytoskeletal proteins. This is most likely due to the non-specific association of actin with the neutravidin resin used for the affinity purification. As can be seen in Figure 7A,B, on exposure to BT-15d-PGJ2 the amount of Keap1 retained on the neutravidin resin increased with time, a result consistent with increased lipid-adduct formation with Keap1. In contrast, the amount of actin retained on the resin remained relatively constant and was used as a normalizing control. These data strongly indicate for the first time that adduct formation with endogenous Keap1 is formed with low levels of lipid electrophiles and accumulate with time. Next, we used the same experimental design to determine the effect of time of lipid exposure and concentration on Keap1 adduct formation. We found that lipid adduct formation with Keap1 was the same at different concentrations (but the same amount) of 15d-PGJ2 (Figure 7C). Taken together, these data show that if sufficient time is allowed for covalent adducts to accumulate, the amount of Keap1 associated with the Neutravidin resin is essentially the same (Dotted line in Figure 7D).
Figure 6. The induction of GSH and HO-1 in response to a constant amount of 15d-PGJ2 with varying concentration.
Panel A: cells were incubated with increasing volume (shown in parenthesis) of media containing the same amount of 15d-PGJ2 at the concentrations shown for 2, 4, 8h (0% FBS) after which the medium was changed (0.5% FBS, 2ml) and the cells incubated for a further 14, 12, and 8h respectively after which cell lysates were prepared. Panel A: The dotted line allows comparison of the conditions needed to achieve the same level of GSH (150 nmol GSH/mg protein) in response to a constant amount (4 nmol) but varying concentrations of 15d-PGJ2. Panel B: HO-1 protein expression. Values are mean ±S.E.M., n=3. Values are mean ±S.E.M., n=3.
Figure 7. Adduct formation with Keap1 in response to changing amounts of BT-15d-PGJ2 and length of exposure.
Cells were incubated with BT-15d-PGJ2 under the same conditions as shown in Figure 6. Panel A: Upper panels of Western Blots represent the total levels of Keap1 in the cell lysates prior to pull down. The lower two panels represent the levels of both Keap1 and actin after affinity purification with Neutravidin resin. Panel B: Correlation of Keap1 retained on the Neutravidin resin with the time of exposure to BT-15d-PGJ2 (0-4h). Panel C: The upper panel of the Western Blots represents the total levels of Keap1 in the cell lysates. The lower panels show the amount of Keap1 and actin retained by the Neutravidin resin after exposure to different concentrations of BT-15d-PGJ2 and times of exposure to a fixed amount of lipid (4 nmol). Panel D: The amount of Keap1 retained on the Neutravidin resin was normalized to actin and is shown plotted as function of lipid concentration (volume of media shown in parenthesis). The dotted line allows comparison of the conditions needed to achieve the same level of Keap1 modification in response to a constant amount (4 nmol) but varying concentrations of 15d-PGJ2.
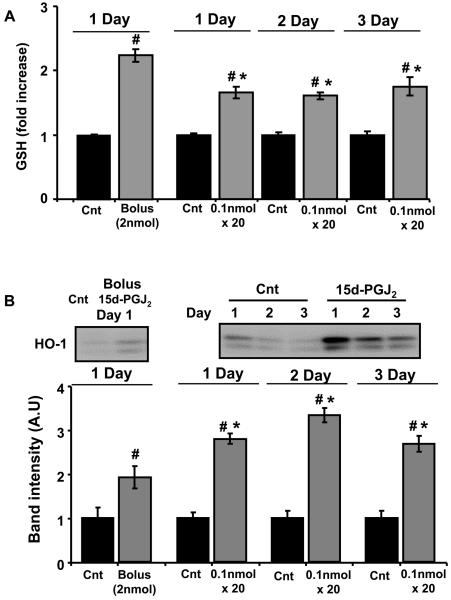
Comparison of bolus or sequential additions of low concentrations of 15d-PGJ2 on the induction of GSH and HO-1 in BAEC
As a further test of whether the signal for induction of GSH or HO-1 can accumulate over time, we selected one of the lowest amounts of 15d-PGJ2 which could result in detectable levels of GSH or HO-1 (2 nmol) and added to BAEC as either one bolus addition or 20 sequential additions of 100 pmol each per day for 1-3 days. This is equivalent to a concentration 25 nM for each addition which is within the range of 15d-PGJ2 levels that could be produced in cell culture by COX-2 [11]. The time between each addition was selected as 20 min on the basis of the data shown in Figure 2 which shows rapid inactivation of 15d-PGJ2 5 min after addition to the media. The basal levels of both GSH and HO-1 increase with lower cell density and for this reason the responses are expressed as a fold changes relative to a controls cultured under identical conditions. As shown in Figure 8, the levels of GSH were induced approximately 2 fold by the bolus addition of 15d-PGJ2 and a slightly lesser amount (approximately 1.7 fold) after addition of the same amount of the lipid in 100 pmol aliquots. Importantly, this response to 15d-PGJ2 was sustained over three days. In the case of HO-1, the bolus addition of 15d-PGJ2 resulted in an approximately 2 fold increase in the levels of HO-1 and this was increased with the sequential additions of 100 pmol to approximately 2.7 fold and was again responsive over the three days of the experiment.
Figure 8. Induction of GSH and HO-1 in BAEC by bolus and sequential treatment with the same amount of 15d-PGJ2.
Cells at 80% confluency in media (4ml) were incubated with a bolus addition of 2 nmol (0.5 μM) of 15d-PGJ2 (0.5% FBS) or sequentially treated with 100 pmol (25 nM) every 20 min (20 times over 7h). Cells were incubated with 0.5% serum containing media for a further 9h before measurement of HO-1 and GSH. This process was repeated with samples treated identically for Day 2 and Day 3. Thus the cells on Day 3 had been exposed to 25 nM 15d-PGJ2 over 3 days. Panel A: Total GSH normalized to vehicle treated controls. Panel B: HO-1 protein induction normalized to controls. Value are mean ± S.E.M., n=3 and statistical significance among the groups was evaluated by one-way ANOVA followed by the LSD as post hoc tests. *, # represents a significant difference compared to control within the groups (Bolus or Day 1-3 group) at p<0.0001 and between lipid treated groups or bolus to sequential treatment (Day1-3) for at p<0.0001 respectively.
Discussion
A number of lines of evidence support the concept that endogenous formation of COX-derived electrophilic lipid oxidation products may play an anti-inflammatory role [3, 6, 9, 24, 39-43]. These findings have also led to the suggestion that exogenous administration of these lipids has potential anti-inflammatory properties. It is then important to understand their biochemical properties and mechanisms of action [24, 44-47]. Several studies have defined potential mechanisms through which the anti-inflammatory effects may be elicited by 15d-PGJ2, and these include inhibition of the NFκB signaling pathway, induction of antioxidant defenses, and potentiation of apoptosis in macrophages [8, 10, 39, 48-50]. These findings have generated considerable interest since they explain how COX-2 activity may contribute to the resolution of inflammation [10]. In endothelial cells, one mechanism leading to induction of COX-2 and other cytoprotective proteins is the shear stress caused by laminar blood flow [51, 52]. Recently, it has been shown that laminar, but not turbulent, flow stimulates the activation of the Nrf-2/Keap1 pathway through a COX-2 dependent mechanism. Laminar flow has also been shown to increase both GSH and HO-1 [27, 51, 53]. Importantly, the Nrf-2/Keap1 pathway is also activated by the addition of exogenous electrophilic cyclopentenones such as 15d-PGJ2 [8, 12, 13, 23, 54]. Interestingly, the free concentrations of both 15d-PGJ2 and its precursor PGD2 were determined in the medium after flow and found to be approximately 0.5-1 nM [27]. A number of studies have reported similar concentrations of free 15d-PGJ2 in a wide range of tissue and cell culture conditions, as well as plasma levels in human patients [11, 40, 42, 43, 55, 56]. However, the levels of free 15d-PGJ2 that can be measured in vivo are far lower than these required in vitro to modulate the same signaling pathways, and this point has led to some controversy about the biological relevance of this lipid [27, 28, 56]. For example, it has been shown that the concentrations of 15d-PGJ2 found in adipocytes are lower than those predicted for the activation of PPARγ [28]. This is also the case for the activation of the Nrf-2/Keap1 pathway, which leads to the synthesis of antioxidants such as GSH or HO-1 [27]. We hypothesized that cell culture conditions used to recapitulate 15d-PGJ2 exposure in vivo result in a substantial loss of the electrophile, thereby preventing covalent protein adduct formation required for Nrf-2/Keap1 activation. We also reasoned that the formation of covalent protein adducts in the cell is a more accurate reflection of the amount of 15d-PGJ2 available to initiate cell signaling in cell culture experiments.
In the present study, we have tested this hypothesis using a number of approaches. First we used BT-15d-PGJ2 in combination with a biotinylated protein standard to determine the absolute amount of lipid-protein adducts formed in endothelial cells [35]. We have previously demonstrated that this tagged analog of 15d-PGJ2 forms a covalent adduct with Keap1 and this is responsible for the induction of GSH synthesis in endothelial cells [8]. Others have demonstrated induction of HO-1 by electrophiles through the EpRE, and we and others have demonstrated induction of HO-1 by 15d-PGJ2 [51, 57, 58]. Earlier studies with radiolabeled electrophilic cyclopentenones demonstrated that the lipids were irreversibly associated with cellular proteins and the label accumulated over time consistent with covalent protein adduct formation [59]. Here, we have shown that covalent protein adduct formation in general and with Keap1 specifically is dependent on both the concentration of 15d-PGJ2 and the time of exposure (Figures 1, 7). We also found that although μM concentrations (0-40 nmol) of 15d-PGJ2 were added to the cells, only pmol amounts of adduct were detected on cellular proteins (Figure 1, 3 and Table 1). Inactivation occurred upon addition to the cell culture media and was enhanced by the presence of FBS. In addition, inactivation of 15d-PGJ2 does not require cells, indicating that the failure of the 15d-PGJ2 to react with cellular proteins and upregulate HO-1 and GSH is not due to cellular metabolism of the lipid. These results are consistent with other reports using radioactively labeled 15d-PGJ2 which show that a small proportion (less than 5%) of the lipid is associated with cell proteins when added to fibroblasts in culture [28]. Previous studies have also suggested that albumin can interact with 15d-PGJ2 and here we demonstrate that 15d-PGJ2 does in fact covalently modify albumin [37].
Importantly, of the inactivation of 15d-PGJ2 decreased the amount of electrophile available for the induction of HO-1 (Figure 3, Table 1). For the first time, the extent of this decrease has been quantified and is substantial with approximately 97-99% of the amount of 15d-PGJ2 unavailable to either form protein adducts or initiate cell signaling. This result may explain the enormous variability in literature reports in the concentrations of 15d-PGJ2 required to elicit cell signaling [8, 28-30]. In addition these data, now allow us to reassess the amount of 15d-PGJ2 which is available to initiate the induction of GSH and HO-1 on addition of the pure lipid to cell culture media (Figure 4). For example, we can calculate that the addition of 1 nmol of BT-15d-PGJ2, which is sufficient to induce both GSH and HO-1 synthesis, results in only 30 pmol adducted to cellular protein. In the case of the human subjects, this would be the amount present in approximately 100 ml of plasma containing 0.33 nM 15d-PGJ2 [55].
In assessing the formation of protein adducts by BT-15d-PGJ2 we found that these adducts increase progressively with time up to a period of approximately 4h. We also found that the induction of both GSH and HO-1 also increased over a similar time range and this was associated with increasing modification of Keap1 by the electrophile (Figure 4, 7). This is important since lower concentrations of electrophilic lipid will form covalent adducts with proteins more slowly and explains why more time is required at lower concentrations of 15d-PGJ2 to activate the signaling pathways leading to HO-1 or GSH induction (Figure 6). Taken together, our experiments revealed that three key factors, which have not been previously considered, make a major contribution to the magnitude of the cell signaling in response to 15d-PGJ2 and the consequent synthesis of GSH or HO-1. These are
1) The importance of considering the total amount (i.e. number of molecules) of 15d-PGJ2 to which the cells are exposed in contributing to the final response of cells to electrophilic lipids.
2) Concentration also plays a role in determining the time of exposure to 15d-PGJ2 needed to result in increased levels of GSH or HO-1. Higher concentrations of 15d-PGJ2 resulted in more rapid responses, while low concentrations in higher volumes have the potential to achieve similar biological responses given sufficient time (Figure 6-8).
3) An equivalent biological response can be achieved by sequential additions of low concentrations of 15d-PGJ2 over time as a bolus addition of a single higher concentration of the same absolute amount of lipid (Figure 8).
In summary, we have demonstrated that the minimum concentrations of 15d-PGJ2 required to activate signaling pathways in cells are approximately 1-2 orders of magnitude lower than that assumed from the amounts added to the media. In addition, the formation of covalent adducts is a key element of the cell signaling pathway that enhances the sensitivity of these pathways to low concentrations of electrophiles over time. Although this was not addressed in the present study, the data also suggests that metabolism by GSH-dependent mechanisms, including the glutathione S-transferases, will decrease substantially under conditions below the Km for these enzymes where low levels of electrophile-protein adducts accumulate over time. These data also have important implications not only for the cell signaling mediated by electrophilic lipids but also other mechanisms of redox cell signaling which involve the post-translational and stable modification of cell signaling proteins. The accumulation of protein adduct formation over time allows the cell to sense the “redox tone” of the cellular milieu [5, 6]. In complex biological processes such as inflammation, this provides a mechanism through which cellular responses can be modulated over the hours or days over which these biological processes evolve.
Acknowledgements
This study was supported by NIH grants ES 10167 and HL 58013 (to V. D.-U.), and an American Heart Association Scientist Development Grant (to A.L.).
Abbreviations
- 15d-PGJ2
15-deoxy-Δ12,14-prostaglandin J2
- BT
Biotinylated
- BAEC
Bovine aortic endothelial cells
- EpRE
Electrophile response element
- ROS
Reactive oxygen species
- COX
Cyclooxygenase
- Keap1
Kelch-like erythroid cell-derived protein with Cap n' Collar homology-associated protein
- Nrf-2
Nuclear factor-erythroid 2-related factor
- HO-1
Heme oxygenase-1
- PPARγ
Peroxisome proliferator activated receptor gamma
- SDS-PAGE
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- NFkB
Nuclear factor kappa B
- PBS
Phosphate buffer saline
References
- 1.Warner TD, Mitchell JA. Cyclooxygenases: new forms, new inhibitors, and lessons from the clinic. Faseb. J. 2004;18:790–804. doi: 10.1096/fj.03-0645rev. [DOI] [PubMed] [Google Scholar]
- 2.Belton O, Fitzgerald D. Cyclooxygenase-2 inhibitors and atherosclerosis. J. Am. Coll. Cardiol. 2003;41:1820–1822. doi: 10.1016/s0735-1097(03)00305-x. [DOI] [PubMed] [Google Scholar]
- 3.Stamatakis K, Perez-Sala D. Prostanoids with cyclopentenone structure as tools for the characterization of electrophilic lipid-protein interactomes. Ann. N. Y. Acad. Sci. 2006;1091:548–570. doi: 10.1196/annals.1378.096. [DOI] [PubMed] [Google Scholar]
- 4.Landar A, Giles NM, Zmijewski JW, Watanabe N, Oh JY, Darley-Usmar VM. Modification of lipids by reactive oxygen and nitrogen species: the oxy-nitroxy-lipidome and its role in redox cell signaling. Future Lipidology. 2006;1:203–211. [Google Scholar]
- 5.Dickinson DA, Darley-Usmar VM, Landar A. The covalent advantage: a new paradigm for cell signaling by thiol reactive lipid oxidation products. In: Dalle-Donne I, Scalone A, Butterfield DA, editors. Redox Proteomics: from Protein Modifications to Cellular Dysfunction and Diseases. John Wiley & Sons, Inc.; Indianapolis: 2006. pp. 345–367. [Google Scholar]
- 6.Perez-Sala D, Cernuda-Morollon E, Pineda-Molina E, Canada FJ. Contribution of covalent protein modification to the antiinflammatory effects of cyclopentenone prostaglandins. Ann. N. Y. Acad. Sci. 2002;973:533–536. doi: 10.1111/j.1749-6632.2002.tb04695.x. [DOI] [PubMed] [Google Scholar]
- 7.Renedo M, Gayarre J, Garcia-Dominguez CA, Perez-Rodriguez A, Prieto A, Canada FJ, Rojas JM, Perez-Sala D. Modification and activation of Ras proteins by electrophilic prostanoids with different structure are site-selective. Biochemistry. 2007;46:6607–6616. doi: 10.1021/bi602389p. [DOI] [PubMed] [Google Scholar]
- 8.Levonen AL, Landar A, Ramachandran A, Ceaser EK, Dickinson DA, Zanoni G, Morrow JD, Darley-Usmar VM. Cellular mechanisms of redox cell signalling: role of cysteine modification in controlling antioxidant defences in response to electrophilic lipid oxidation products. Biochem. J. 2004;378:373–382. doi: 10.1042/BJ20031049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanchez-Gomez FJ, Cernuda-Morollon E, Stamatakis K, Perez-Sala D. Protein thiol modification by 15-deoxy-Delta12,14-prostaglandin J2 addition in mesangial cells: role in the inhibition of pro-inflammatory genes. Mol. Pharmacol. 2004;66:1349–1358. doi: 10.1124/mol.104.002824. [DOI] [PubMed] [Google Scholar]
- 10.Straus DS, Glass CK. Cyclopentenone prostaglandins: new insights on biological activities and cellular targets. Med. Res. Rev. 2001;21:185–210. doi: 10.1002/med.1006. [DOI] [PubMed] [Google Scholar]
- 11.Shibata T, Kondo M, Osawa T, Shibata N, Kobayashi M, Uchida K. 15-deoxy-delta 12,14-prostaglandin J2. A prostaglandin D2 metabolite generated during inflammatory processes. J. Biol. Chem. 2002;277:10459–10466. doi: 10.1074/jbc.M110314200. [DOI] [PubMed] [Google Scholar]
- 12.Levonen AL, Dickinson DA, Moellering DR, Mulcahy RT, Forman HJ, Darley-Usmar VM. Biphasic effects of 15-deoxy-delta(12,14)-prostaglandin J(2) on glutathione induction and apoptosis in human endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2001;21:1846–1851. doi: 10.1161/hq1101.098488. [DOI] [PubMed] [Google Scholar]
- 13.Ceaser EK, Ramachandran A, Levonen AL, Darley-Usmar VM. Oxidized low-density lipoprotein and 15-deoxy-delta 12,14-PGJ2 increase mitochondrial complex I activity in endothelial cells. Am. J. Physiol. Heart. Circ. Physiol. 2003;285:H2298–2308. doi: 10.1152/ajpheart.00508.2003. [DOI] [PubMed] [Google Scholar]
- 14.Clay CE, Monjazeb A, Thorburn J, Chilton FH, High KP. 15-Deoxy-delta12,14-prostaglandin J2-induced apoptosis does not require PPARgamma in breast cancer cells. J. Lipid Res. 2002;43:1818–1828. doi: 10.1194/jlr.m200224-jlr200. [DOI] [PubMed] [Google Scholar]
- 15.Ceaser EK, Moellering DR, Shiva S, Ramachandran A, Landar A, Venkartraman A, Crawford J, Patel R, Dickinson DA, Ulasova E, Ji S, Darley-Usmar VM. Mechanisms of signal transduction mediated by oxidized lipids: the role of the electrophile-responsive proteome. Biochem. Soc. Trans. 2004;32:151–155. doi: 10.1042/bst0320151. [DOI] [PubMed] [Google Scholar]
- 16.Dickinson DA, Levonen AL, Moellering DR, Arnold EK, Zhang H, Darley-Usmar VM, Forman HJ. Human glutamate cysteine ligase gene regulation through the electrophile response element. Free Radic. Biol. Med. 2004;37:1152–1159. doi: 10.1016/j.freeradbiomed.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 17.Sanchez-Gomez FJ, Gayarre J, Avellano MI, Perez-Sala D. Direct evidence for the covalent modification of glutathione-S-transferase P1-1 by electrophilic prostaglandins: implications for enzyme inactivation and cell survival. Arch. Biochem. Biophys. 2007;457:150–159. doi: 10.1016/j.abb.2006.10.032. [DOI] [PubMed] [Google Scholar]
- 18.Shibata T, Yamada T, Ishii T, Kumazawa S, Nakamura H, Masutani H, Yodoi J, Uchida K. Thioredoxin as a molecular target of cyclopentenone prostaglandins. J. Biol. Chem. 2003;278:26046–26054. doi: 10.1074/jbc.M303690200. [DOI] [PubMed] [Google Scholar]
- 19.Oliva JL, Perez-Sala D, Castrillo A, Martinez N, Canada FJ, Bosca L, Rojas JM. The cyclopentenone 15-deoxy-delta 12,14-prostaglandin J2 binds to and activates H-Ras. Proc. Natl. Acad. Sci. U S A. 2003;100:4772–4777. doi: 10.1073/pnas.0735842100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Itoh K, Tong KI, Yamamoto M. Molecular mechanism activating Nrf2-Keap1 pathway in regulation of adaptive response to electrophiles. Free Radic. Biol. Med. 2004;36:1208–1213. doi: 10.1016/j.freeradbiomed.2004.02.075. [DOI] [PubMed] [Google Scholar]
- 21.Andoh Y, Suzuki H, Araki M, Mizutani A, Ohashi T, Okumura T, Adachi Y, Ikehara S, Taketani S. Low- and high-level expressions of heme oxygenase-1 in cultured cells under uninduced conditions. Biochem. Biophys. Res. Commun. 2004;320:722–729. doi: 10.1016/j.bbrc.2004.05.212. [DOI] [PubMed] [Google Scholar]
- 22.Zhang X, Lu L, Dixon C, Wilmer W, Song H, Chen X, Rovin BH. Stress protein activation by the cyclopentenone prostaglandin 15-deoxy-Delta12,14-prostaglandin J2 in human mesangial cells. Kidney Int. 2004;65:798–810. doi: 10.1111/j.1523-1755.2004.00454.x. [DOI] [PubMed] [Google Scholar]
- 23.Lee TS, Tsai HL, Chau LY. Induction of heme oxygenase-1 expression in murine macrophages is essential for the anti-inflammatory effect of low dose 15-deoxy-Delta 12,14-prostaglandin J2. J. Biol. Chem. 2003;278:19325–19330. doi: 10.1074/jbc.M300498200. [DOI] [PubMed] [Google Scholar]
- 24.Itoh K, Mochizuki M, Ishii Y, Ishii T, Shibata T, Kawamoto Y, Kelly V, Sekizawa K, Uchida K, Yamamoto M. Transcription Factor Nrf2 Regulates Inflammation by Mediating the Effect of 15-Deoxy-Delta(12,14)-Prostaglandin J(2) Mol. Cell. Biol. 2004;24:36–45. doi: 10.1128/MCB.24.1.36-45.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Y, Morrow JD, Roberts LJ., 2nd Formation of reactive cyclopentenone compounds in vivo as products of the isoprostane pathway. J. Biol. Chem. 1999;274:10863–10868. doi: 10.1074/jbc.274.16.10863. [DOI] [PubMed] [Google Scholar]
- 26.Shibata T, Yamada T, Kondo M, Tanahashi N, Tanaka K, Nakamura H, Masutani H, Yodoi J, Uchida K. An endogenous electrophile that modulates the regulatory mechanism of protein turnover: inhibitory effects of 15-deoxy-delta(12,14)-prostaglandin J2 on proteasome. Biochemistry. 2003;42:13960–13968. doi: 10.1021/bi035215a. [DOI] [PubMed] [Google Scholar]
- 27.Hosoya T, Maruyama A, Kang MI, Kawatani Y, Shibata T, Uchida K, Warabi E, Noguchi N, Itoh K, Yamamoto M. Differential responses of the Nrf2-Keap1 system to laminar and oscillatory shear stresses in endothelial cells. J. Biol. Chem. 2005;280:27244–27250. doi: 10.1074/jbc.M502551200. [DOI] [PubMed] [Google Scholar]
- 28.Bell-Parikh LC, Ide T, Lawson JA, McNamara P, Reilly M, FitzGerald GA. Biosynthesis of 15-deoxy-delta12,14-PGJ2 and the ligation of PPARgamma. J. Clin. Invest. 2003;112:945–955. doi: 10.1172/JCI18012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berry EB, Keelan JA, Helliwell RJ, Gilmour RS, Mitchell MD. Nanomolar and micromolar effects of 15-deoxy-delta 12,14-prostaglandin J2 on amnion-derived WISH epithelial cells: differential roles of peroxisome proliferator-activated receptors gamma and delta and nuclear factor kappa B. Mol. Pharmacol. 2005;68:169–178. doi: 10.1124/mol.104.009449. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi Y, Ueki S, Mahemuti G, Chiba T, Oyamada H, Saito N, Kanda A, Kayaba H, Chihara J. Physiological levels of 15-deoxy-Delta12,14-prostaglandin J2 prime eotaxin-induced chemotaxis on human eosinophils through peroxisome proliferator-activated receptor-gamma ligation. J. Immunol. 2005;175:5744–5750. doi: 10.4049/jimmunol.175.9.5744. [DOI] [PubMed] [Google Scholar]
- 31.Gayarre J, Avellano MI, Sanchez-Gomez FJ, Carrasco MJ, Canada FJ, Perez-Sala D. Modification of proteins by cyclopentenone prostaglandins is differentially modulated by GSH in vitro. Ann. N. Y. Acad. Sci. 2007;1096:78–85. doi: 10.1196/annals.1397.072. [DOI] [PubMed] [Google Scholar]
- 32.Paumi CM, Smitherman PK, Townsend AJ, Morrow CS. Glutathione S-transferases (GSTs) inhibit transcriptional activation by the peroxisomal proliferator-activated receptor gamma (PPAR gamma) ligand, 15-deoxy-delta 12,14prostaglandin J2 (15-d-PGJ2) Biochemistry. 2004;43:2345–2352. doi: 10.1021/bi035936+. [DOI] [PubMed] [Google Scholar]
- 33.Brunoldi EM, Zanoni G, Vidari G, Sasi S, Freeman ML, Milne GL, Morrow JD. Cyclopentenone prostaglandin, 15-deoxy-Delta12,14-PGJ2, is metabolized by HepG2 cells via conjugation with glutathione. Chem. Res. Toxicol. 2007;20:1528–1535. doi: 10.1021/tx700231a. [DOI] [PubMed] [Google Scholar]
- 34.Go YM, Levonen AL, Moellering D, Ramachandran A, Patel RP, Jo H, Darley-Usmar VM. Endothelial NOS-dependent activation of c-Jun NH(2)- terminal kinase by oxidized low-density lipoprotein. Am. J. Physiol. Heart Circ. Physiol. 2001;281:H2705–2713. doi: 10.1152/ajpheart.2001.281.6.H2705. [DOI] [PubMed] [Google Scholar]
- 35.Landar A, Oh JY, Giles NM, Isom A, Kirk M, Barnes S, Darley-Usmar VM. A sensitive method for the quantitative measurement of protein thiol modification in response to oxidative stress. Free Radic. Biol. Med. 2006;40:459–468. doi: 10.1016/j.freeradbiomed.2005.08.046. [DOI] [PubMed] [Google Scholar]
- 36.Oh J, Johnson MS, Landar A. Methods for determining the modification of protein thiols by reactive lipids. Methods Cell. Biol. 2007;80:417–434. doi: 10.1016/S0091-679X(06)80021-X. [DOI] [PubMed] [Google Scholar]
- 37.Berry EB, Sato TA, Mitchell MD, Stewart Gilmour R, Helliwell RJ. Differential effects of serum constituents on apoptosis induced by the cyclopentenone prostaglandin 15-deoxy-delta12,14-prostaglandin J2 in WISH epithelial cells. Prostaglandins Leukot. Essent. Fatty Acids. 2004;71:191–197. doi: 10.1016/j.plefa.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 38.Lin Q, Weis S, Yang G, Weng YH, Helston R, Rish K, Smith A, Bordner J, Polte T, Gaunitz F, Dennery PA. Heme oxygenase-1 protein localizes to the nucleus and activates transcription factors important in oxidative stress. J. Biol. Chem. 2007;282:20621–20633. doi: 10.1074/jbc.M607954200. [DOI] [PubMed] [Google Scholar]
- 39.Cernuda-Morollon E, Pineda-Molina E, Canada FJ, Perez-Sala D. 15-Deoxy-Delta 12,14-prostaglandin J2 inhibition of NF-kappaB-DNA binding through covalent modification of the p50 subunit. J. Biol. Chem. 2001;276:35530–35536. doi: 10.1074/jbc.M104518200. [DOI] [PubMed] [Google Scholar]
- 40.Gilroy DW, Colville-Nash PR, McMaster S, Sawatzky DA, Willoughby DA, Lawrence T. Inducible cyclooxygenase-derived 15-deoxy(Delta)12-14PGJ2 brings about acute inflammatory resolution in rat pleurisy by inducing neutrophil and macrophage apoptosis. Faseb. J. 2003;17:2269–2271. doi: 10.1096/fj.02-1162fje. [DOI] [PubMed] [Google Scholar]
- 41.Scher JU, Pillinger MH. 15d-PGJ2: the anti-inflammatory prostaglandin? Clin. Immunol. 2005;114:100–109. doi: 10.1016/j.clim.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 42.Gilroy DW, Colville-Nash PR, Willis D, Chivers J, Paul-Clark MJ, Willoughby DA. Inducible cyclooxygenase may have anti-inflammatory properties. Nat. Med. 1999;5:698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- 43.Mouihate A, Boisse L, Pittman QJ. A novel antipyretic action of 15-deoxy-Delta12,14-prostaglandin J2 in the rat brain. J. Neurosci. 2004;24:1312–1318. doi: 10.1523/JNEUROSCI.3145-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rossi A, Kapahi P, Natoli G, Takahashi T, Chen Y, Karin M, Santoro MG. Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IkappaB kinase. Nature. 2000;403:103–108. doi: 10.1038/47520. [DOI] [PubMed] [Google Scholar]
- 45.Maggi LB, Jr., Sadeghi H, Weigand C, Scarim AL, Heitmeier MR, Corbett JA. Anti-inflammatory actions of 15-deoxy-delta 12,14-prostaglandin J2 and troglitazone: evidence for heat shock-dependent and -independent inhibition of cytokine-induced inducible nitric oxide synthase expression. Diabetes. 2000;49:346–355. doi: 10.2337/diabetes.49.3.346. [DOI] [PubMed] [Google Scholar]
- 46.Cuzzocrea S, Wayman NS, Mazzon E, Dugo L, Di Paola R, Serraino I, Britti D, Chatterjee PK, Caputi AP, Thiemermann C. The cyclopentenone prostaglandin 15-deoxy-Delta(12,14)-prostaglandin J(2) attenuates the development of acute and chronic inflammation. Mol. Pharmacol. 2002;61:997–1007. doi: 10.1124/mol.61.5.997. [DOI] [PubMed] [Google Scholar]
- 47.Ianaro A, Ialenti A, Maffia P, Pisano B, Di Rosa M. Role of cyclopentenone prostaglandins in rat carrageenin pleurisy. FEBS. Lett. 2001;508:61–66. doi: 10.1016/s0014-5793(01)03035-6. [DOI] [PubMed] [Google Scholar]
- 48.Musiek ES, Gao L, Milne GL, Han W, Everhart MB, Wang D, Backlund MG, Dubois RN, Zanoni G, Vidari G, Blackwell TS, Morrow JD. Cyclopentenone isoprostanes inhibit the inflammatory response in macrophages. J. Biol. Chem. 2005;280:35562–35570. doi: 10.1074/jbc.M504785200. [DOI] [PubMed] [Google Scholar]
- 49.Alvarez-Maqueda M, El Bekay R, Alba G, Monteseirin J, Chacon P, Vega A, Martin-Nieto J, Bedoya FJ, Pintado E, Sobrino F. 15-deoxy-delta 12,14-prostaglandin J2 induces heme oxygenase-1 gene expression in a reactive oxygen species-dependent manner in human lymphocytes. J. Biol. Chem. 2004;279:21929–21937. doi: 10.1074/jbc.M400492200. [DOI] [PubMed] [Google Scholar]
- 50.Hortelano S, Castrillo A, Alvarez AM, Bosca L. Contribution of cyclopentenone prostaglandins to the resolution of inflammation through the potentiation of apoptosis in activated macrophages. J. Immunol. 2000;165:6525–6531. doi: 10.4049/jimmunol.165.11.6525. [DOI] [PubMed] [Google Scholar]
- 51.Chen XL, Varner SE, Rao AS, Grey JY, Thomas S, Cook CK, Wasserman MA, Medford RM, Jaiswal AK, Kunsch C. Laminar flow induction of antioxidant response element-mediated genes in endothelial cells. A novel anti-inflammatory mechanism. J. Biol. Chem. 2003;278:703–711. doi: 10.1074/jbc.M203161200. [DOI] [PubMed] [Google Scholar]
- 52.Topper JN, Cai J, Falb D, Gimbrone MA., Jr. Identification of vascular endothelial genes differentially responsive to fluid mechanical stimuli: cyclooxygenase-2, manganese superoxide dismutase, and endothelial cell nitric oxide synthase are selectively up-regulated by steady laminar shear stress. Proc. Natl. Acad. Sci. U. S. A. 1996;93:10417–10422. doi: 10.1073/pnas.93.19.10417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Levonen AL, Patel RP, Brookes P, Go YM, Jo H, Parthasarathy S, Anderson PG, Darley-Usmar VM. Mechanisms of cell signaling by nitric oxide and peroxynitrite: from mitochondria to MAP kinases. Antioxid. Redox Signal. 2001;3:215–229. doi: 10.1089/152308601300185188. [DOI] [PubMed] [Google Scholar]
- 54.Koppal T, Petrova TV, Van Eldik LJ. Cyclopentenone prostaglandin 15-deoxy-Delta(12,14)-prostaglandin J(2) acts as a general inhibitor of inflammatory responses in activated BV-2 microglial cells. Brain Res. 2000;867:115–121. doi: 10.1016/s0006-8993(00)02270-8. [DOI] [PubMed] [Google Scholar]
- 55.Blanco M, Moro MA, Davalos A, Leira R, Castellanos M, Serena J, Vivancos J, Rodriguez-Yanez M, Lizasoain I, Castillo J. Increased plasma levels of 15-deoxyDelta prostaglandin J2 are associated with good outcome in acute atherothrombotic ischemic stroke. Stroke. 2005;36:1189–1194. doi: 10.1161/01.STR.0000166054.55993.e5. [DOI] [PubMed] [Google Scholar]
- 56.Shan ZZ, Masuko-Hongo K, Dai SM, Nakamura H, Kato T, Nishioka K. A potential role of 15-deoxy-delta(12,14)-prostaglandin J2 for induction of human articular chondrocyte apoptosis in arthritis. J. Biol. Chem. 2004;279:37939–37950. doi: 10.1074/jbc.M402424200. [DOI] [PubMed] [Google Scholar]
- 57.Ishii T, Itoh K, Takahashi S, Sato H, Yanagawa T, Katoh Y, Bannai S, Yamamoto M. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J. Biol. Chem. 2000;275:16023–16029. doi: 10.1074/jbc.275.21.16023. [DOI] [PubMed] [Google Scholar]
- 58.Landar A, Zmijewski JW, Dickinson DA, Le Goffe C, Johnson MS, Milne GL, Zanoni G, Vidari G, Morrow JD, Darley-Usmar VM. Interaction of electrophilic lipid oxidation products with mitochondria in endothelial cells and formation of reactive oxygen species. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H1777–1787. doi: 10.1152/ajpheart.01087.2005. [DOI] [PubMed] [Google Scholar]
- 59.Narumiya S, Ohno K, Fukushima M, Fujiwara M. Site and mechanism of growth inhibition by prostaglandins. III. Distribution and binding of prostaglandin A2 and delta 12-prostaglandin J2 in nuclei. J. Pharmacol. Exp. Ther. 1987;242:306–311. [PubMed] [Google Scholar]