Abstract
Background
Third-stage infective larvae (L3) of hookworms are in an obligatory state of developmental arrest that ends upon entering the definitive host, where they receive a signal that re-activates development. Recovery from the developmentally arrested dauer stage of Caenorhabditis elegans is analogous to the resumption of development during hookworm infection. Insulin-like signaling (ILS) mediates recovery from arrest in C. elegans and activation of hookworm dauer L3. In C. elegans, phosphorylation of the forkhead transcription factor DAF-16 in response to ILS creates binding cites for the 14-3-3 protein Ce-FTT-2, which translocates DAF-16 out of the nucleus, resulting in resumption of reproductive development.
Results
To determine if hookworm 14-3-3 proteins play a similar role in L3 activation, hookworm FTT-2 was identified and tested for its ability to interact with A. caninum DAF-16 in vitro. The Ac-FTT-2 amino acid sequence was 91% identical to the Ce-FTT-2, and was most closely related to FTT-2 from other nematodes. Ac-FTT-2 was expressed in HEK 293T cells, and was recognized by an antibody against human 14-3-3β isoform. Reciprocal co-immunoprecipitations using anti-epitope tag antibodies indicated that Ac-FTT-2 interacts with Ac-DAF-16 when co-expressed in serum-stimulated HEK 293T cells. This interaction requires intact Akt consensus phosphorylation sites at serine107 and threonine312, but not serine381. Ac-FTT-2 was undetectable by Western blot in excretory/secretory products from serum-stimulated (activated) L3 or adult A. caninum.
Conclusion
The results indicate that Ac-FTT-2 interacts with DAF-16 in a phosphorylation-site dependent manner, and suggests that Ac-FTT-2 mediates activation of L3 by binding Ac-DAF-16 during hookworm infection.
Background
Hookworm infection is one of the most common chronic illnesses in the world, with an estimated 740 million infections [1]. The developmentally arrested third-stage infective larvae (L3) of hookworms resume development in response to a host-specific signal encountered during invasion. This cue initiates a signaling pathway that results in expression of genes required for development and molting to the L4 and subsequent adult stage. This signaling pathway represents a potential target for intervention in the hookworm life cycle as a means of preventing infection.
When non-feeding arrested L3 of the canine hookworm Ancylostoma caninum are incubated with serum components in vitro, they resume feeding within 12 hrs [2,3]. While they do not molt in vitro, "activation" by serum induces the release of molecules associated with infection, including proteases and activation-associated secretory proteins (ASPs) [4-7], and differential gene expression [8]. Hookworm L3 activation is mediated by an insulin-like signaling (ILS) pathway, similar to recovery from the analogous dauer stage of the free-living nematode Caenorhabditis elegans [9,10]. This dauer stage is morphologically, behaviorally, and functionally analogous to the hookworm L3, and recovery from it has been proposed as a model for the hookworm infective process [11,12]. The molecular biology of dauer recovery is well-defined, and has provided a useful framework for investigation of the molecular biology of hookworm infection.
Recovery from dauer in C. elegans is mediated by an insulin-like signaling (ILS) pathway [13]. Replete environmental conditions initiate a signaling cascade through an insulin growth factor 1 (IGF-1)-like receptor (encoded by the daf-2 gene), a phosphatidylinositol 3-kinase (PI3-K, age-1), and protein kinase B/Akt [14-20]. One of the key downstream targets of ILS is the forkhead/FOXO transcription factor DAF-16. Under dauer-inducing conditions, DAF-16 is located primarily in the nucleus, where it binds to target genes and mediates entry into and maintenance of dauer [15,21]. ILS during recovery causes the phosphorylation of DAF-16 by the serine/threonine kinase Akt [18], thereby creating binding sites for 14-3-3 proteins [22]. Binding of 14-3-3 to phosphorylated DAF-16 results in translocation of DAF-16 from the nucleus to the cytoplasm, and expression of genes associated with growth and development [23,24].
The 14-3-3 proteins are highly conserved ~30 kDa acidic dimeric proteins found in all eukaryotes. They function in cell signaling, cell cycle regulation, intracellular trafficking, and other process by modulating protein-protein interactions [22]. Typically organisms contain several isoforms, and homo- or heterodimers bind to phosphoserine- or phosphothreonine-containing motifs [25]. All forms of 14-3-3 share a similar tertiary structure, the dimer contact residues are highly conserved, and each monomer is capable of binding a phosphopeptide independently [25]. There are two isoforms of 14-3-3 in C. elegans, ftt-2 and par-5, but only ftt-2 is involved in regulation of DAF-16. Specific knockdown by RNAi of ftt-2, but not par-5, caused increased transcription of DAF-16 target genes [26].
Activation of hookworm L3 requires ILS [13,27], and recently the ortholog of Ce-DAF-16 was identified from the hookworms A. caninum and A. ceylanicum and shown to bind to a conserved DNA binding element [28]. Given the conservation of this pathway and the similarity between L3 activation and dauer recovery, we asked whether Ac-DAF-16 was regulated by a 14-3-3 protein as well. We report here that the ortholog of Ce-FTT-2 from A. caninum interacts with Ac-DAF-16 in vivo, and that this interaction requires intact Akt phosphorylation sites on DAF-16. This provides further support for the use of dauer recovery as a model for larval activation during the hookworm infective process.
Results
Cloning and characterization of Ac-ftt-2
A consensus sequence of a cluster of eight A. caninum expressed sequence tag (EST) sequences homologous to 14-3-3 proteins was used to design oligonucleotide primers to isolate the complete Ac-ftt-2 cDNA from A. caninum. Using a hemi-nested strategy, the 5' end was isolated using the conserved nematode spliced leader sequence [29] as the forward primer, coupled with two nested gene-specific reverse primers in successive PCR reactions. The second reaction produced an amplicon of approximately 600 bp that contained the SL sequence and overlapped the 5' end of the A. caninum EST sequence. A similar strategy was used to isolate the 3' end from an A. caninum cDNA library. A reverse primer complementary to the flanking T7 sequence in the vector was used with two nested forward primers to generate an amplicon of approximately 700 bp that contained a poly d(A) tail, and overlapped the EST and the 5' end sequences. New primers were designed to amplify the entire coding sequence of A. caninum 14-3-3 cDNA, which was cloned into pET28 vector and confirmed by DNA sequencing of both strands. The full-length cDNA sequence was deposited in GenBank (accession number FJ842376).
The full-length 14-3-3 cDNA contained the conserved 22 nucleotide nematode SL at the 5' end [30], which was followed by 33 untranslated nucleotides and an ATG codon encoding the starting methionine at nucleotide 56. The cDNA is predicted to encode an open reading frame of 249 amino acids, ending with a TAA termination codon at nucleotide 803, and followed by a 327 bp 3'-untranslated region. A canonical AATAAT polyadenylation signal [31] was located 11 bp upstream of the poly (dA) tail (nucleotides 1116 to 1121). The deduced amino acid sequence has a predicted mass of 28174 Da and a calculated pI of 4.87. The hookworm 14-3-3 lacks a secretory signal peptide [32], and contains a nuclear export signal (LxxxLxL) [33] at amino acids 223 to 229, consistent with a role in nuclear transport [34].
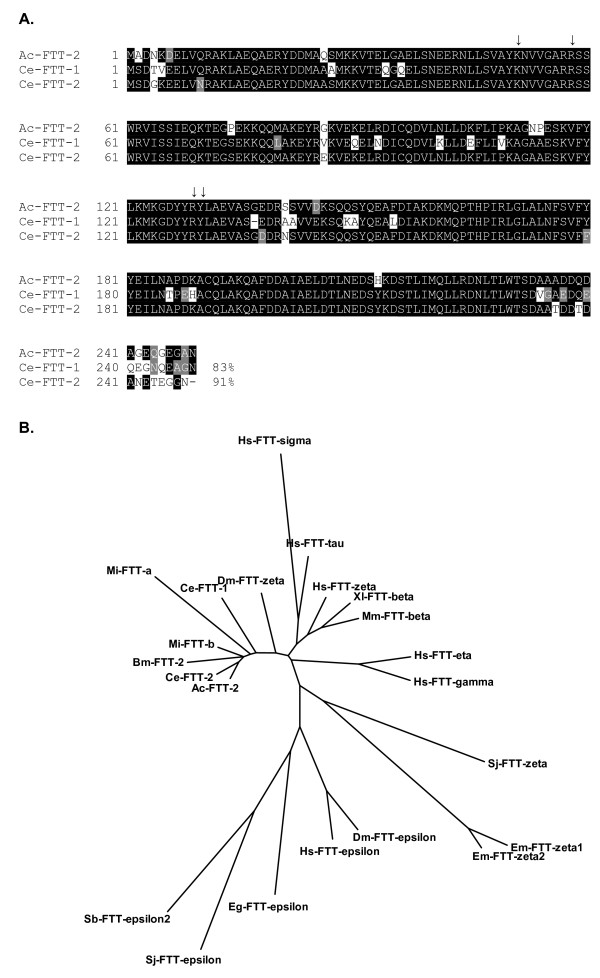
A BLASTP search [35] of the non-redundant GenBank database using the deduced amino acid sequence confirmed that the hookworm protein was a member of the 14-3-3 protein family (Pfam PF00244, Interpro IPR000308). The best matches were to nematode 14-3-3 proteins, including Caenorhabditis briggsae (score 464, 2e-129), C. elegans (score 464, 4e-129). C. brenneri (score 462, 8e-129), and Meloidogyne incognita (score 461, 3e-128). While mammals have at least seven closely related isoforms [22], C. elegans has only two [26]. Alignment with Ce-FTT-1 (also known as PAR-5) and Ce-FTT-2 amino acid sequences shows that the hookworm 14-3-3 is more closely related to Ce-FTT-2 (91% identity) than Ce-FTT-1 (83% identity) (Fig. 1A). Furthermore, phylogenetic analysis indicates the hookworm 14-3-3 clusters most closely with FTT-2 isoforms from several other nematodes, including M. incognita and Brugia malayi (Fig. 1B). Therefore, the hookworm 14-3-3 protein most likely represents the Ce-FTT-2 ortholog, and will be referred to as Ac-FTT-2.
Figure 1.
The phylogenetic relationship between Ac-FTT-2 and selected 14-3-3 protein family members. A. Alignment of Ac-FTT-2 with C. elegans 14-3-3 proteins. Shading indicates residues that are identical (black) or similar (gray) to Ac-FTT-2. Amino acid residues that directly contact phosphate groups of targets are marked with an arrow. Protein sequences were aligned using CLUSTAL W software and displayed using BOXSHADE software located on the Swiss EMBnet server [74]. B. Neighbor joining tree of representative 14-3-3 proteins. Proteins were aligned using CLUSTAL W software on the Swiss EMBnet server [74]. Amino acid distances were calculated using the Poisson correction model in the MEGA program version 3.1 [75]. Major bootstrap values (1000 replications) are shown at each node. Ac, A. caninum; Bm. Brugia malayi; Ce, Caenorhabditis elegans; Dm, Drosophila melanogaster; Eg, Echinococcus granulosus; Em, Echinococcus multilocularis; Hs, Homo sapiens; Mi, Meloidogyne incognita; Sb, Schistosoma bovis; Sj, Schistosoma japonicum, Xl, Xenopus laevis. Accession numbers: Ce-FTT-1, [Genbank:CAA98138]; Ce-FTT-2, [Genbank:CAA91474]; Bm-FTT-2, [Genbank:XP_001895095]; Dm-FTT-ε, [Genbank:P92177]; Dm-FTT-ζ, [Genbank:P29310]; Eg-FTT-ε, [Genbank:AAX73175]; Em-FTT-ζ1, [Genbank:AAC48315]; Em-FTT-ζ2, [Genbank:AAM94864]; Hs-FTT-γ, [Genbank:P61981]; Hs-FTT-ε, [Genbank:P62258];Hs-FTT-ζ, [Genbank:P63104];Hs-FTT-η, [Genbank:Q04917];Hs-FTT-σ, [Genbank:P31947];Hs-FTT-τ, [Genbank:P27348]; Mi-FTT-a, [Genbank:AAL40719]; Mi-FTT-b, [Genbank:AAR85527]; Mm-FTT-β, [Genbank:Q9CQV8]; Sb-FTT-ε2, [Genbank:AAT39381]; Sj-FTT-ζ, [Genbank:AAD56715]; Sj-FTT-ε, [Genbank:AAC62003]; Xl-FTT-β, [Genbank:Q5XGC8].
The 14-3-3 proteins are a family of dimeric proteins that modulate protein-protein interactions [22]. Phosphorylation of the interacting protein at sequence-specific sites mediates its interaction with the 14-3-3. Each subunit of the 14-3-3 dimer can bind to a phosphoserine or phosphothreonine ligand independently [34]. The most highly conserved region, the peptide-binding pocket, contains the residues that contact the phosphorylated amino acids. These residues (Lys51, Arg58, Arg129 and Tyr130) are completely conserved in Ac-FTT-2 (Fig. 1A). The N-terminal portion of 14-3-3 proteins is required for dimerization, and contains two conserved phosphorylation sites (Ser59 and Ser65) that are substrates for several kinases in mammals, including Akt and protein kinase C (PKC) [22]. Phosphorylation of Ser59 converted 14-3-3 dimers to monomers [36], suggesting a possible regulatory mechanism. However, a c-Jun N-terminal kinase (JNK) site at amino acid 186 [22] is absent from both Ac-FTT-2 and Ce-FTT-2, but is conserved in PAR-5. This further supports the conclusion that Ac-FTT-2 represents the ortholog of Ce-FTT-2.
Transfection and immunoprecipitation of Ac-FTT-2 from HEK 293 cells
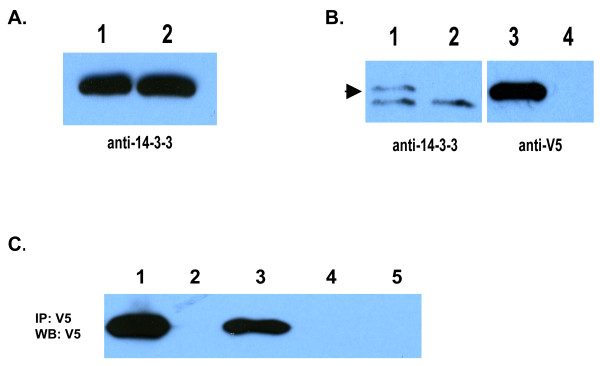
Given the high level of conservation in 14-3-3 molecules, we tested whether an antibody against the human β isoform of 14-3-3 could detect Ac-FTT-2. As shown in Figure 2A, the 14-3-3β antibody recognized a single band in lysates of both untreated and serum-stimulated (activated) A. caninum L3 by Western blot. Next, we expressed recombinant Ac-FTT containing a V5 epitope tag in HEK293 cells. Western blotting of cell lysates indicated that Ac-FTT-2 was expressed in mammalian cells (Fig. 2B). An anti-V5 body recognized a single band of the appropriate size in lysates of cells transfected with pcDNA3.1V5/Ac-ftt-2 plasmid, but not in lysates from cells transfected with empty vector. The 14-3-3β antibody recognized two bands in the transfected cells, and a single band in the mock transfected cells. The lower molecular weight band, present in both transfected and mock cells, is endogenous 14-3-3, while the higher molecular weight band is the expressed FTT-2/V5 fusion protein, which migrates more slowly in the gel because of the attached epitope tag. This indicates that recombinant Ac-FTT-2 can be expressed in mammalian cells, and recognized by an antibody against the epitope tag and an anti-human 14-3-3 antibody.
Figure 2.
Expression of Ac-FTT-2 and detection with anti-human 14-3-3β antibody. A. Western blot of A. caninum L3 worm lysates. Soluble protein (5 μg) from 65,000 activated and non-activated L3 lysate was separated by 4–20% gradient SDS-PAGE, followed by Western blotting with anti-human 14-3-3β antibody. Lane 1, non-activated L3 extract; lane 2, activated L3 extract. B. Expression of A. caninum V5-tagged recombinant FTT-2 in human embryonic kidney 293 cells. A cDNA encoding full length Ac-ftt-2 was cloned into vector pcDNA3.1/V5-His and transfected into HEK293 cells for expression. After 48 h, 16 μg of total protein was separated by SDS-PAGE, and proteins detected by Western blotting with anti-human 14-3-3β antibody (left panel) or anti-V5 antibody (right panel). Lanes 1 and 3, Ac-FTT-2 transfected; lanes 2 and 4, mock transfected (empty vector).C. Immunoprecipitation of A. caninum V5-tagged recombinant FTT-2 expressed in human embryonic kidney 293 cells. Lysates were prepared 48 h after transfection with pcDNA3.1/V5-His/Ac-ftt-2. Mock lysates were made from cells transfected with the empty vector alone. Recombinant Ac-FTT-2 was precipitated by the addition of anti-V5 antibody. Bound sample refers to precipitated beads, and unbound refers to supernatant removed from precipitated beads. Lane 1, Ac-ftt-2 whole lysate only (input); lane 2, Ac-ftt-2 lysate unbound sample; Lane 3, Ac-ftt-2 lysate bound sample; Lane 4, mock lysate unbound sample; Lane 5, mock lysate bound sample.
Dauer recovery in C. elegans is mediated by 14-3-3 through regulation of DAF-16 subcellular localization and transcriptional activities [26]. This regulation is dependent on insulin-like signalling [23]. Because the resumption of development associated with infection in hookworms is analogous to dauer recovery [11,37], we hypothesized that DAF-16 is regulated by 14-3-3 in response to ILS as in dauer recovery in C. elegans. Previously, we demonstrated that Ac-DAF-16 binds to and drives transcription from a conserved hookworm DAF-16 DNA binding element in mammalian cells [38]. As hookworms have yet to be transfected with foreign DNA, we again used mammalian cells to examine interactions between Ac-FTT-2 and Ac-DAF-16.
We first tested whether we could immunoprecipitate recombinant V5-tagged Ac-FTT-2 from HEK293 cell lysates. As shown in Figure 2C, anti-V5 antibody successfully pulled down recombinant A. caninum FTT-2, as detected by Western blot with HRP-conjugated anti-V5 antibody (lane 3) and with anti-14-3-3β (not shown). Ac-FTT was not detected in unbound supernatant from the immunoprecipitate (Fig. 2C, lane 2), nor in control (empty vector) transfected cell lysate supernatant or immunoprecipitate (lanes 4 and 5, respectively), indicating that the ant-V5 antibody specifically precipitated the recombinant Ac-FTT-2.
After confirming that we could express and immunoprecipitate recombinant Ac-FTT, we next tested if Ac-FTT-2 and Ac-DAF-16 interact in vivo. In C. elegans, FTT-2 interacts with and regulates Ce-DAF-16 by excluding it from the nucleus [26]. HEK 293 cells were co-transfected with equal amounts of plasmid pcDNA3.1V5/Ac-ftt-2, encoding V5-tagged Ac-FTT-2, and plasmid pCMV4FLAG/Ac-daf-16 encoding FLAG-tagged A. caninum forkhead transcription factor DAF-16 [38]. After serum treatment for 24 hrs, reciprocal co-IP was performed on cell lysates using anti-V5 and anti-FLAG antibody resin.
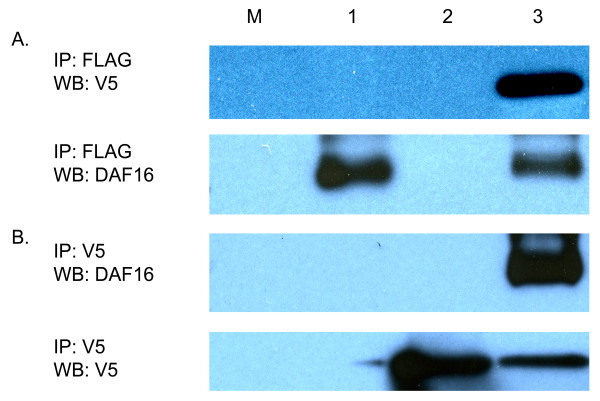
As shown in Figure 3A, anti-FLAG agarose pulled down both a 64 kDa FLAG-tagged DAF-16 and the V5-tagged recombinant Ac-FTT-2 from co-transfected cells (lanes 3), but only recombinant DAF-16 from cells singly transfected with Ac-daf-16 (lane 1). As expected, anti-FLAG agarose did not pull down any protein from the Ac-ftt-2 singly transfected cells (lane 2). Conversely, anti-V5 agarose precipitated both proteins from co-transfected cells, but only recombinant 14-3-3 from cells singly transfected with the Ac-ftt-2 construct, and nothing from cells expressing recombinant Ac-DAF-16 only (Fig. 3B). This indicates that Ac-FTT-2 interacts with Ac-DAF-16 in mammalian cell culture, and suggests that a similar interaction occurs in worms.
Figure 3.
Co-immunoprecipitation of FTT-2 and DAF-16 expressed in human embryonic kidney 293 cells. Cells were transfected singly (4 μg) or co-transfected (2 μg each) with pcDNA3.1V5/Ac-ftt-2 and pCMV4FLAG/Ac-daf-16. At 16 hrs, the cells were incubated with 20% serum, and lysates prepared 24 hrs later. Lane M, cells transfected with empty pcDNA3.1/V5-His vector (mock); lane 1, cells transfected with Ac-daf-16 alone; lane 2, cells transfected with Ac-ftt-2 alone; lane 3, serum treated co-transfected cells. A. Immunoprecipitation with anti-FLAG (M2) agarose. Top panel, Western blot with anti-V5 antibody; bottom panel, the same blot stripped and probed with DAF-16 antiserum. B. Immunoprecipitation with anti-V5 agarose. Top panel, Western blot with DAF-16 antiserum; bottom panel, the same blot stripped and probed with V5 antibody.
Intact phosphorylation sites are required for the FTT-2 – DAF-16 interaction
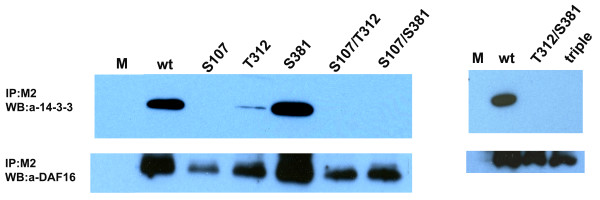
Interaction of 14-3-3 with Ce-DAF-16 requires phosphorylation of specific serine or threonine residues by the protein kinase Akt (Cahill et al., 2001). To determine if the predicted Akt phosphorylation sites are important for the interaction of hookworm 14-3-3 and DAF-16, recombinant Ac-DAF-16 proteins with the phosphorylation sites mutated to alanine were tested for their ability to co-IP recombinant FTT-2 when co-expressed in HEK293 cells. Anti-FLAG M2 resin co-immunoprecipitated recombinant Ac-FTT-2 and wild type FLAG-tagged DAF-16 from cells expressing both proteins (Figure 4). However, mutation of serine107 to alanine (S107A) completely abolished the interaction with Ac-FTT-2, either singly or in combination with mutations at the other Akt phosphorylation sites (S107A:T312A, S107A:S381A, or triple). Mutation of threonine 312 (T312A) alone or in combination with mutations at the other sites also severely diminished the interaction with FTT-2 However, mutation of serine 381 (S381A) had no effect on the interaction with 14-3-3 (Figure 4). These data indicate that serine107 and threonine312 are essential for the interaction of Ac-DAF-16 with Ac-FTT-2.
Figure 4.
Effect of phosphorylation site mutation on the interaction of Ac-FTT-2 with Ac-DAF-16. HEK293 cells were co-transfected with 2 μg of pcDNA3.1V5/Ac-ftt-2 and 2 μg of wild-type or mutant pCMV4FLAG/Ac-daf-16 plasmids as above. Mock cells received 4 μg of empty pcDNA3.1/V5-His vector. Cell lysates were prepared 48 h after transfection, and then incubated with anti-FLAG (M2) agarose resin. Immunoprecipations were separated by 4–20% gradient SDS-PAGE and transferred to PVDF membrane. Top panels, Western blot with anti-human 14-3-3β antibody; Bottom panels, the blot was stripped and re-probed with Ac-DAF-16 antiserum. See text for description of the Ac-DAF-16 mutants.
Secretion of Ac-FTT-2
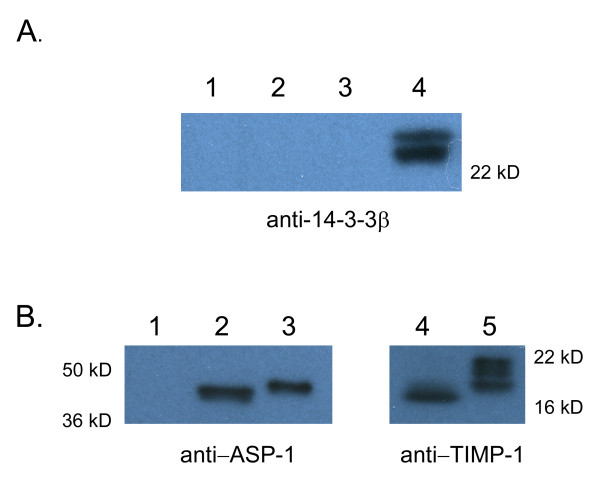
Hookworm L3 activated in vitro by incubation with serum components under host-like conditions release multiple proteins, including the activation associated secretory proteins 1 and 2 (ASP-1 and ASP-2) [5,6] and a metalloprotease [4]. Adult worms release molecules associated with feeding and survival in the host intestine, including anticoagulants [39], ASPs [40] and protease inhibitors [41]. To determine if Ac-FTT-2 was secreted, we took advantage of the cross-reactivity with the anti-human 14-3-3β antibody to examine ESP from L3 and adult stages by Western blot. As seen in Figure 5A, 14-3-3β antibody failed to detect any bands in non-activated, activated L3, or adult ESP (lanes 1–3), but recognized recombinant Ac-FTT-2 expressed in HEK293 cells (lane 4). As a control, L3 ESP were probed with ASP-1 antiserum, which detects a band in activated but not non-activated ESP (Figure 5B, lanes 1–3) [5]. Furthermore, antiserum against Ac-TMP-1, known to be released in adult ESP [42], detected Ac-TMP in the adult ESP (Figure 5B, lanes 4 and 5), indicating that the ESP contained secreted proteins. These data indicate that Ac-FTT-2 is not released in detectable amounts by either activated L3 or adult A. caninum.
Figure 5.
Absence of Ac-FTT-2 in excretory/secretory products of adult and L3 Ancylostoma caninum. ESP from 6000 non-activated or activated L3, and 10 μg of adult ESP were separated by 4–20% gradient SDS-PAGE and transferred to PVDF membrane for Western blotting. A. Lane 1, non-activated L3 ESP; lane 2, activated L3 ESP; lane 3, adult ESP; lane 4, lysate (20 μg) of HEK293 expressing Ac-FTT-2. The blot was probed with anti-14-3-3β antibody. B. Control gels. Lane 1, non-activated L3 ESP; lane 2, activated L3 ESP; lane 3, recombinant ASP-1 (80 ng); lane 4, adult ESP; recombinant TIMP-1 (130 ng). Blots were probed with anti-ASP-1 antiserum (left) or anti-TMP-1 antiserum (right).
Discussion
When hookworm L3 infective stage larvae encounter and invade a permissive host, developmental pathways are initiated by host-specific signals and lead to the maturation of larvae into adult parasites. The molecular details of this process are unknown, but clues to hookworm developmental processes have been revealed by studies of the related free-living nematode C. elegans. Specifically, dauer recovery in C. elegans has been compared to the transition to parasitism in hookworms [11,37], and they share several conserved pathways and molecules [27,38,43,44]. Despite these similarities, detailed mechanistic knowledge of the molecular biology of the transition to parasitism is difficult to obtain due to the inability of hookworms to complete their life cycle in vitro. For this reason, the regulation and function of hookworm molecules must be studied indirectly, using heterologous systems. This and a previous study [38] represent the first applications of a mammalian cell culture system to investigate hookworm molecular biology. This system will allow us to investigate molecular mechanisms of hookworm infection for the first time.
Dauer recovery in C. elegans is mediated by ILS signaling in response to improving environmental conditions [13]. ILS activates the serine/threonine kinase Akt/PKB, which in turn phosphorylates the forkhead transcription factor DAF-16 on multiple serine/threonine residues [18]. Phosphorylation of DAF-16 creates binding sites for the 14-3-3 family protein FTT-2 and translocates DAF-16 to the cytoplasm [23,26], thereby allowing a pattern of gene expression associated with reproductive development. Previously, we isolated an ortholog of DAF-16 from the hookworm A. caninum, and demonstrated that it bound to and drove transcription from a conserved DAF-16 binding element when expressed in mammalian cells [38]. The similarity between A. caninum and C. elegans ILS and recovery from dauer arrest led us to hypothesize that ILS plays a similar role in hookworms. To investigate the regulation of Ac-DAF-16 by 14-3-3, we isolated the FTT-2 ortholog from A. caninum, and co-expressed it with Ac-DAF-16 in HEK293 cells
Most organisms contain multiple isoforms of 14-3-3, including seven in mammals, two in Drosophila and yeast, and 15 in plants [45]. Several isoforms have been identified in platyhelminths of the genera Echinococcus and Schistosoma [46,47]. To date, no nematodes have been shown to encode more than two FTT-2 isoforms. Sequence comparison and phylogenetic analysis indicated that the hookworm 14-3-3 was most closely related to FTT-2 molecules from C. elegans and other nematodes. Despite 86% sequence identity between 14-3-3 isoforms FTT-2 and PAR-5 in C. elegans, only FTT-2 affects dauer recovery. RNAi knockdown of Ce-ftt-2 enhanced dauer formation at permissive temperatures in dauer constitutive daf-2 mutants, increased nuclear localization of DAF-16, and promoted transcription of several DAF-16 target genes [26]. The high level of sequence conservation of Ac-FTT-2 with Ce-FTT-2 suggested that this isoform was most likely to interact with Ac-DAF-16 in hookworms. While FTT-2 affects dauer formation in C. elegans, PAR-5 also weakly interacts with Ce-DAF-16, suggesting a possible role in aspects of DAF-16 function other than those listed above [26]. Indeed, over-expression of Ce-FTT-2 and PAR-5 extend lifespan in a DAF-16-dependent manner, and both interact with SIR-2.1 and DAF-16 in C. elegans [48]. While our data suggest that Ac-FTT-2 shares similar functions with Ce-FTT-2, we can not rule out a function in infection for another isoform of 14-3-3 in hookworms.
Once we identified the appropriate 14-3-3 ortholog from A. caninum, we introduced Ac-ftt-2 cDNA into mammalian expression vectors and expressed epitope tagged fusion protein in HEK293 cells. The native and recombinant hookworm FTT-2 protein expressed in HEK293 cells was recognized by an antibody against the β isoform of human 14-3-3. The antibody also detected an endogenous 14-3-3 protein in 293 cells, as indicated by the lower molecular weight band that is also present in cells transfected with empty vector. This antibody will permit further investigation of Ac-FTT-2 function in vitro and with native worm protein.
Ce-DAF-16 has been demonstrated to interact with 14-3-3 previously [26]. To determine if Ac-FTT-2 interacted with Ac-DAF-16, we co-expressed both proteins in HEK293 cells and performed reciprocal co-IPs using antibodies against the epitope tags on the recombinant proteins. We were able to successfully IP both proteins using both antibodies from serum stimulated cells, indicating that FTT-2 interacts with DAF-16.
14-3-3 proteins bind to the specific amino acid sequence motifs RSXpSXP and RXXXpSXP, where pS represents phosphoserine and X represents any amino acid [25,26]. RXRXXS is the consensus Akt phosphorylation site [23] and corresponds closely with the 14-3-3 binding sequence. Mammalian Akt phosphorylates FOXO proteins, the mammalian orthologs of DAF-16, at predicted 14-3-3 binding sites [49]. Using mutant DAF-16 molecules with altered phosphorylation sites, we demonstrated that serine 107 and threonine 312 are essential for the FTT-2 interaction with DAF-16. Both serine 107 and threonine 312 are in predicted 14-3-3 binding motifs. Typically, FOXO family proteins have three Akt phosphorylation sites, with sites P1 and P2 flanking the DNA binding domain in the N-terminal half of the molecule, and site P3 in the C-terminal half. In FOXO-4, as in Ac-DAF-16, the P1 and P2 sites are required for interaction with the 14-3-3 protein [49]. Binding of the 14-3-3 to these sites is believed to mask the DNA binding domain, thereby interrupting the FOXO/DAF-16 function. Interestingly, this differs from the FTT-2/DAF-16 interaction in C. elegans, where the analogous N-terminal P1 site and the C-terminal P3 site are required for interaction [23]. Our results demonstrate the requirement of Akt phosphorylation sites for 14-3-3/DAF-16 association, and suggest that Ac-14-3-3 binds to phosphorylated Ac-DAF-16 to mediate ILS, as occurs in C. elegans. Further investigations are underway to confirm this hypothesis.
14-3-3 proteins have been reported from a growing number of helminth parasites. In nematodes, aside from C. elegans and now A. caninum, the only other well characterized 14-3-3 proteins are from the root-knot nematode Meloidogyne incognita [50]. Partial sequences from EST databases have been reported from Strongyloides stercoralis, Haemonchus contortus [46], and the hookworm of humans Necator americanus [51]. A 29 kDa protein in ESP from the adult stage of the ovine stomach worm Teladorsagia circumcincta was identified as a 14-3-3 protein [52]. The complete descriptive and functional characterization of these proteins remains to be performed.
More is known about the 14-3-3 proteins of platyhelminths. Multiple isoforms and sub-isoforms have been identified and characterized in Schistosoma and Echinococcus species [46,47]. In S. mansoni, 14-3-3ε-1 interacts with the TGF-β Type 1 receptor and enhances TGF-β signaling [53]. TGF-β signaling is required for dauer formation (although not recovery) in C. elegans [13,54], and the TGF-β receptor ligand DAF-7 has been identified from A. caninum [44]. Also, TGF-β signaling has been implicated in the reactivation of tissue-arrested A. caninum L3 [55]. Demonstration of a functional role for Ac-FTT-2 in TGF-β signaling awaits further characterization of this pathway in hookworms.
The 14-3-3 proteins of platyhelminths are associated with the tegument, or are actively secreted, making them available as targets of the host immune system and therefore potential vaccine antigens [47,56]. In S. mansoni, all three isoforms of 14-3-3 are expressed during infection and induce antibody production by the host [57]. The 14-3-3ζ isoform has shown some protection against schistosome challenge infections, eliciting worm reductions ranging from 25 to 65% [58-61]. The greatest success of a 14-3-3 as an anti-parasite vaccine antigen was in Echinococcus multilocularis, where vaccination with the 14-3-3ζ isoform resulted in a 97% reduction in parasite load following challenge in a mouse [47,62]. Recombinant 14-3-3ζ reduced nitric oxide production from activated macrophages in vitro [63], and may contribute to the immunosuppressive effect associated with alveolar cyst infection [47,64].
The FTT-2 ortholog in the root-knot nematode M. incognita is synthesized in the dorsal esophageal gland cell and present in stylet secretions [50,65]. In this parasite, stylet secretions play a role in the induction and maintenance of the feeding site within the plant host [66]. 14-3-3 proteins in ESP suggest an extra-corporeal function, including potentially influencing host immune responses [47,64]. Hookworms are known to release abundant ESP from both activated L3 [4-6] and adult stages [41,42]. Minimally, release of 14-3-3 by a life history stage would be a prerequisite for a vaccine antigen. However, we could not demonstrate Ac-FTT-2 in either larval or adult ESP, indicating that unlike some other helminth 14-3-3 proteins, Ac-FTT-2 is not secreted. Therefore it is unlikely that this protein interacts with the host immune system. It is more likely that it is part of endogenous signaling pathways, including ILS downstream of Akt.
Conclusion
In conclusion, we report the identification and cloning of a 14-3-3 protein family member, Ac-FTT-2, from the canine hookworm A. caninum. Ac-FTT-2 is most closely related to Ce-FTT-2, a protein that mediates the effects of ILS in C. elegans. We demonstrated that Ac-FTT-2 interacts with the forkhead transcription factor Ac-DAF-16 when co-expressed in mammalian cells, and that this interaction requires intact Akt phosphorylation sites at serine 107 and threonine 312. Our data suggest that, as in C. elegans dauer recovery, the interaction of Ac-FTT-2 with phosphorylated Ac-DAF-16 mediates the effects of ILS on development, and therefore might play an important role in the hookworm infective process. Further investigations into the role of FTT-2 and DAF-16 in hookworm recovery will provide insights into the early, critical events of hookworm infection, and possibly novel intervention strategies for the prevention of hookworm disease.
Methods
Parasites
The Baltimore strain of A. caninum (U.S. National Parasite Collection accession 100655.00) was maintained in beagles as described previously [67]. The beagles were housed and treated according to a protocol approved by the George Washington University Institutional Care and Use Committee. Infective L3 were recovered from coproculture by a modified Baermann technique and stored at room temperature in buffer BU (50 mM Na2HPO4/22 mM KH2PO4/70 mM NaCL, pH 6.8) for up to one month [68], or snap-frozen in liquid N2 and stored at -80 C until lysates were prepared.
In vitro larval activation and collection of excretory/secretory products
Ancylostoma caninum L3 were activated under host-like conditions as described previously [6]. Briefly, approximately 5000 decontaminated L3 were incubated at 37C, 5% CO2 for 24 hr in 0.5 ml RPMI1640 tissue culture medium supplemented with 25 mM HEPES (pH 7.0) and antibiotics (RPMI-c) in individual wells of 24-well tissue culture plates. L3 were activated by the addition of 10% (v/v) of a less than 10 kDa ultrafiltrate of canine serum and 15 mM S-methyl-glutathione (GSM, Sigma Chemical, St. Louis, MO) in RPMI-c. Non-activated L3 were incubated with RPMI-c medium alone. The percentage of L3 feeding (or "activated") was determined as described elsewhere [2]
Following incubation, medium containing L3 was transferred to microcentifuge tubes and centrifuged at 13,000 rpm for 5 min. The supernatant containing the excretory/secretory products (ESP) was collected and concentrated by ultrafiltration using Centricon YM-10 cartridge (Millipore). The retentate was washed with 0.5 mL PBS, and concentrated 10-fold for electrophoresis.
Ac-FTT-2 amplification and cloning
A consensus sequence derived from a cluster of eight expressed sequence tags encoding an A. caninum 14-3-3 (AC01065) [69] was used to design specific forward and reverse oligonucleotide primers for PCR. The Ac-ftt-2 cDNA ends were amplified in two separate PCR reactions. For the 5' end, the reverse primer R2 (5'-AAATTGAGAGCGAGGCCAAGGCG-3'), the forward nematode spliced leader primer SL (5'-GGTTTAATTACCCAAG TTTGAG-3') and first strand cDNA [70] were incubated in a PCR reaction for 35 cycles of 1 min at 94 C, 1 min at 57 C, and 2 min at 72, followed by a final extension for 4 min at 72 C.
The 3' end was isolated using a nested strategy from an A. caninum L3 directional cDNA library constructed in Lambda ZAP II [71]. The first reaction employed the forward primer FTT-F1 (5'-GAACTCGTGTTGAGGGCCAAGC-3') together with a primer complementary to the T7 promoter site in the vector flanking the 3' end of the cDNA insert site (5'-TAATACGACTCACTATAG-3'). The primers and 1 μl of cDNA library were incubated in a PCR reaction of 1 min at 94 C, 1 min at 55 C, and 2 min at 72 C, and a final extension for 5 min at 72 C. The first round reaction was diluted 1:10 and used as template in a second reaction containing the nested forward primer FTT-F2 (5'-CGAGCTGGAGAGTTATTT CGTCG-3') and reverse primer T7-NEST (5'-ACTCACTATAGGGCGAATTG-3'), using identical cycling conditions. Amplicons of approximately 550 bp (5' end) and 900 bp (3'end) were gel purified, cloned using the pGEM-T Easy TA cloning kit (Promega, Madison, WI), and the DNA sequence of both strands determined. The DNA sequences of the 5' and 3' end clones were aligned and combined to give the 1165-bp full-length composite sequence of Ac-ftt-2, including a 32-bp poly-d(A) tail.
The composite sequence was used to design primers to amplify and clone the full-length coding sequence. Forward primer FTT-FX (5'-TTAGGATCCATG GCCGAT AACAAGGATGAACTCG-3') containing a 5'BamHI restriction site (underlined) and FTT-RX (5'-GATCTCGAGGAATCAGATCATA TGGGTTTAATTGGC-3') with a 5' XhoI restriction site were incubated in a PCR with A. caninum L3 first strand cDNA for 35 cycles of 95 C for 1 min, 55 C for 1 min, and 72 C for 2 min, followed by a final 5 min extension at 72 C. The amplicon was digested for 18 h at 37 C with 20 units each of XhoI and BamHI restriction enzymes (New England Biolabs, Beverley, MA) in BamHI buffer, gel purified and ligated into pET28a (Novagen, Gibbstown, NJ) which had been digested with the same enzymes. The ligation products were transformed into E. coli DH5α competent cells by standard methods, and the constructs confirmed by DNA sequencing.
The full length coding sequence of A. caninum 14-3-3 cDNA (Ac-ftt-2) in pET28a was used as a template to amplify the insert for subcloning into a mammalian expression vector. Forward primer FTT-FX and reverse primer FTT-RX2 (5'-ATAACTCGAGAT TGGCAC CCTCTCCTTGC-3') containing a XhoI site were incubated in a PCR reaction with 12 ng of plasmid DNA for 35 cycles of 95 C for 1 min, 55 C for 1 min, and 72 C for 2 min, followed by a 5 min extension at 72 C. An amplicon of approximately 700 bp was purified by Nucleospin column (ClonTech, Palo Alto, CA), and 2.5 μg of the product digested with XhoI and BamHI restriction enzymes as above. The gel purified insert was ligated into the mammalian expression vector pcDNA3.1-V5/His (Invitrogen, Carlsbad, CA) cut with the same enzymes, and the ligation products were transformed as above. Positive colonies were confirmed by colony PCR using FX and RX2 primers, and the DNA sequence of both strands determined. All DNA concentrations were determined spectrophotometrically (Nanodrop, Thermo Fisher Scientific, Waltham, MA).
Transfection of human embryonic kidney 293 cells
Growing human embryonic kidney 293 (HEK293) cells were re-suspended in fresh, pre-warmed RPMI1640 containing 10% FBS (Biowhittaker, Walkersville, MD), 2 mM L-glutamine (Mediatech, Herndon, VA), 100 IU/mL penicillin (Mediatech, Herndon, VA), and 100 μg/ml streptomycin (Mediatech, Herndon, VA), and evenly distributed into individual wells of a 6-well tissue culture plate. Medium was added to a final volume of 2 mL in each well and the plate was incubated overnight at 37 C, 5% CO2. The medium from each well was then replaced with fresh pre-warmed RPMI without disturbing attached cells and incubated for 2 h at 37 C. The cells were transfected with 4 μg of pcDNA3.1V5/Ac-ftt-2 plasmid DNA or empty pcDNA3.1V5 vector (mock) using Metafectene (Bionex, Munich) and incubated at 37°C for 48 h. Following incubation, media were removed and 2 mL of lysis buffer (20 mM Tris-HCl pH 7.5, 50 mM NaCl, 5 mM CHAPS, 1% Triton X-100, and 0.1% SDS) were added to each well to lyse the cells. Cell lysates were vortexed for 1 minute in microcentrifuge tubes, then frozen overnight at -80 C. Samples were thawed, vortexed again and centrifuged at room temperature for 5 min at 2300 × g. The supernatants were collected and stored at -20 C. Total protein concentrations were determined by bicinchoninic acid method (Micro BCA, Thermo Fisher) according to the manufacturer's protocol.
Co-transfections were performed as above with 2 μg each of pcDNA3.1V5/Ac-ftt-2 and pCMV4FLAG/Ac-daf-16 encoding the A. caninum forkhead transcription factor DAF-16 [38], or 2 μg each of the expression constructs and the corresponding empty vector. Cells were incubated overnight, followed by 20% serum treatment for 24 h, and lysates prepared.
Western Blot of HEK293 cell extracts
Western blotting with the specific anti-V5 antibody was used to determine if recombinant Ac-FTT-2 (rFTT-2) was expressed in HEK293 cells. Samples (16 μg of total protein) of mock and pcDNA3.1-V5-ftt-2 transfected cell lysates were separated in a 4–20% gradient pre-cast Novex Tris-glycine SDS polyacrylamide gel (Invitrogen). Separated proteins were transferred to a polyvinylidene fluoride (PVDF) membrane for 110 min at 22 V by standard methods [41]. Following transfer, the membrane was blocked in 15 mL of 5% non-fat dry milk (NFDM) in PBS-T (0.05% Tween-20) overnight at 4 C. Membranes were incubated with mouse anti-human 14-3-3β/HRP antibody (Santa Cruz Bioechnology, Santa Cruz, CA) (1:20,000) or anti-V5/HRP mouse antibody (Invitrogen) (1:5,000) in 1% NFDM in PBS-T for 2 h at 22 C. After 3 × 10 min washes in PBS-T and 2 × 10 min washes in distilled water, the membrane was incubated in Enhanced Chemoluminescence (ECL) solution (Thermo Fisher), and chemoluminescent immunoreactive bands were detected by exposure to radiographic film.
Immunoprecipitation of recombinant Ac-FTT from HEK 293 cell lysate
To determine if we could immunoprecipitate hookworm recombinant Ac-FTT-2, 5 μL of anti-V5 antibody (Invitrogen) were incubated for 16 h at 4 C with lysates of mock or Ac-ftt-2 transfected HEK 293 cells in a total volume of 500 μL of PBS-T (0.1% Tween-20). Protein A-agarose (Invitrogen) was pre-cleared with PBS-T and incubated with the antibody-antigen complexes for 1.5 h at 22 C with rotation. Following centrifugation for 30 s at 2300 × g, the supernatant was removed and stored frozen as the unbound sample, and the agarose complexes washed by rotation 3 × 5 min with 1 mL PBS-T. The agarose-antibody-antigen complexes were separated by SDS-PAGE and visualized by Western blot with HRP conjugated α-human 14-3-3β antibody or α-V5/HRP as described above.
Reciprocal co-immunoprecipitation with α-V5 and α-FLAG using HEK293 cell lysates
HEK293 cells co-transfected with Ac-daf-16 and Ac-ftt-2 were lysed with 200 μL of cold buffer containing 50 mM NaCl, 50 mM Tris-HCl pH7.5, 0.1% NP-40, 5 mM EDTA, 1× Phosphatase Inhibitor Cocktail II (Calbiochem), and 1× Halt Protease Inhibitor cocktail (Pierce). Lysates were centrifuged at 4 C for 10 min at 10,000 × g, and the protein concentration of the supernatants determined by Micro BCA. Forty μg of anti-FLAG M2 resin (Sigma, St. Louis, MO) was prepared according to manufacturer's directions, added to ~1 mg of cell lysates, and mixed by rotation overnight at 4 C. Tubes were centrifuged for 30 s at 8200 × g and washed 3 times in TBS (50 mM Tris HCl pH7.4, 150 mM NaCl). Co-immunoprecipitation (co-IP) complexes were separated by 4–20% gradient SDS-PAGE and transferred to PVDF membrane for 7.5 min in an iBlot apparatus (Invitrogen). Following blocking, the membrane was probed with anti-V5/HRP (1:5000 dilution) at 22 C for 2 h. The membrane was washed 3 times in PBS-T and 2 times in distilled deionized water (ddH2O), dried, and incubated with ECL solution as above. Following visualization, the membrane was stripped with Restore buffer (Pierce) and blocked overnight in 5% NFDM in PBS-T at 4 C. The stripped membrane was re-probed with a specific Ac-DAF-16 polyclonal antiserum (1:20,000 dilution) [38], and visualized as above. The reciprocal co-IP was performed as above, using anti-V5 resin (Sigma) to pull-down protein complexes, and anti-DAF-16/anti-rabbit IgG-HRP and anti-V5/HRP to probe the membranes.
Effect of phosphorylation on Ac-FTT-2 interaction with Ac-DAF-16
To determine the importance of phosphorylation at the predicted Akt phosphorylation sites for the interaction between 14-3-3 and Ac-DAF-16, the serine or threonine residue at each site was changed to alanine by site-directed mutagenesis. Using a Quickchange Site-Directed mutagenesis kit (Stratagene, La Jolla, CA), mutant Ac-DAF-16 plasmids were constructed in which each site was mutated separately (S107A, T312A, S381A), in pairs (S107A/T312A; S107A/S381A; T312A/S381A), or at all three phosphorylation sites (3A). The mutated plasmid constructs were confirmed by DNA sequencing.
HEK293 cells were co-transfected with 2 μg of pcDNA3.1V5/Ac-ftt-2 and 2 μg of wild-type or mutant pCMV4FLAG/Ac-daf-16 as above. Mock cells received 4 μg of empty pcDNA3.1/V5-His vector. After 24 hrs incubation, the cells were fed with 20% serum and grown overnight. Following cell lysis, co-IPs were performed using anti-FLAG agarose, separated by electrophoresis, and visualized by Western blot with anti-V5/HRP antibody as above. After stripping, the gel was re-probed with DAF-16 antiserum to visualize Ac-DAF-16.
Preparation of lysates from frozen A. caninum L3 larvae
Ancylostoma caninum L3 were activated by incubating with canine serum fractions and S-methylglutathione as described above [6]. Approximately 100,000 frozen non-activated and activated L3 A. caninum larvae were ground to fine powder with a sterile mortar and pestle pre-chilled with liquid N2. The powder was added to 500 μL of cold PBS plus 1% EDTA, 1% Halt Protease Inhibitor Cocktail, and 1% Phosphatase Inhibitor Cocktail II and mixed by inversion. The lysates were centrifuged at 16,100 × g for 6 min at 4 C. Aliquots of the supernatants were removed to determine protein concentration by Micro BCA, and the remaining supernatants were stored at -20 C until needed.
Secretion of Ac-FTT-2
To determine if Ac-FTT-2 was secreted during L3 activation, excretory/secretory products (ESP) from 6000 non-activated and activated L3 were collected as described previously [6]. Adult A. caninum ESP, recombinant tissue inhibitor of metalloproteases (Ac-TMP-1), and anti-TMP-1 antisera were gifts of Dr. Bin Zhan. Adult ESP were collected following overnight incubation of adult worms in vitro. Lysates (20 μg) of HEK293 cells expressing recombinant Ac-FTT-2, 80 ng of recombinant ASP-1 [5], and 130 ng of recombinant TMP-1 [42] were included as positive controls. Samples were separated at 140 V, 40 mA for 1.5 h in a 4–20% gradient pre-cast Novex Tris-glycine SDS polyacrylamide gel (Invitrogen), and transferred to PVDF membrane for 7.5 min in an iBlot apparatus (Invitrogen). Blots were incubated with ASP-1 antiserum (1:4000), TMP-1 antiserum (1:5000), or 14-3-3β antibody (1:20,000), respectively, and visualized using ECL Plus as described above.
Sequence Analysis
Dideoxy DNA sequencing using the ABI BigDye Terminator Cycle Sequencing Ready Reaction Kit v3.1 was performed by the Nevada Genomics Center [72], and the reactions run on an ABI3730 DNA Analyzer. Sequence analysis was performed using BioEdit Sequence Alignment Editor version 5.0.9 [73], and multiple alignments performed using CLUSTAL W at Swiss EMBnet [74]. Neighbor-joining trees were constructed using MEGA version 3.1 [75]. Homology searches were done using BLASTP [35,76] at the National Center for Biotechnology Information [77] and WU-BLAST Parasite Genome Database Query at the European Bioinformatics Institute [78]. Percentage identity and similarity was determined using the BLASTP algorithm with the BLOSUM62 matrix. Conserved motifs were identified by searching Scansite at MIT [79] using MotifScan software [80], and nuclear export signals identified by searching the NetNES 1.1 server [33,81] at the Center for Biological Sequence Analysis, Technical University of Denmark.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
JEK participated in the study design, made the expression constructs, conducted the cell culture experiments, and drafted the manuscript. XG constructed the DAF-16 mutants, conducted the secretion experiments, and helped coordinate the study. JMK isolated and cloned Ac-FTT-2. JMH conceived of, designed, and coordinated the study, and polished the manuscript. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
The authors thank Dr. Bin Zhan for the gift of adult hookworm ESP. This project was supported by NIH grants R21AI062857 and R01AI069293.
Contributor Information
Joshua E Kiss, Email: cepo85@gmail.com.
Xin Gao, Email: mtmxxg@gwumc.edu.
Joseph M Krepp, Email: joekrepp@gmail.com.
John M Hawdon, Email: mtmjmh@gwumc.edu.
References
- de Silva NR, Brooker S, Hotez PJ, Montresor A, Engels D, Savioli L. Soil-transmitted helminth infections: updating the global picture. Trends Parasitol. 2003;12:547–551. doi: 10.1016/j.pt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Hawdon JM, Schad GA. Serum stimulated feeding in vitro by third stage infective larvae of the canine hookworm Ancylostoma caninum. J Parasitol. 1990;76:394–398. [PubMed] [Google Scholar]
- Hawdon JM, Hotez PJ. Hookworm: developmental biology of the infectious process. Curr Opin Genet Dev. 1996;6:618–623. doi: 10.1016/s0959-437x(96)80092-x. [DOI] [PubMed] [Google Scholar]
- Hawdon JM, Jones BF, Perregaux MA, Hotez PJ. Ancylostoma caninum: metalloprotease release coincides with activation of infective larvae in vitro. Exp Parasitol. 1995;80:205–211. doi: 10.1006/expr.1995.1025. [DOI] [PubMed] [Google Scholar]
- Hawdon JM, Jones BF, Hoffman DR, Hotez PJ. Cloning and characterization of Ancylostoma-secreted protein. A novel protein associated with the transition to parasitism by infective hookworm larvae. J Biol Chem. 1996;271:6672–6678. doi: 10.1074/jbc.271.12.6672. [DOI] [PubMed] [Google Scholar]
- Hawdon JM, Narasimhan S, Hotez PJ. Ancylostoma secreted protein 2: cloning and characterization of a second member of a family of nematode secreted proteins from Ancylostoma caninum. Mol Biochem Parasitol. 1999;99:149–165. doi: 10.1016/s0166-6851(99)00011-0. [DOI] [PubMed] [Google Scholar]
- Zhan B, Hotez PJ, Wang Y, Hawdon JM. A developmentally regulated metalloprotease secreted by host-stimulated Ancylostoma caninum third-stage infective larvae is a member of the astacin family of proteases. Mol Biochem Parasitol. 2002;120:291–296. doi: 10.1016/s0166-6851(01)00453-4. [DOI] [PubMed] [Google Scholar]
- Datu BJ, Gasser RB, Nagaraj SH, Ong EK, O'Donoghue P, McInnes R, Ranganathan S, Loukas A. Transcriptional Changes in the Hookworm, Ancylostoma caninum, during the Transition from a Free-Living to a Parasitic Larva. PLoS Negl Trop Dis. 2008;2:e130. doi: 10.1371/journal.pntd.0000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassada RC, Russell RL. The dauer larva, a post-embryonic developmental variant of the nematode Caenorhabditis elegans. Dev Biol. 1975;46:326–342. doi: 10.1016/0012-1606(75)90109-8. [DOI] [PubMed] [Google Scholar]
- Riddle DL, Albert PS. Genetic and Environmental Regulation of Dauer Larva Development. In: Riddle DL, Blumenthal T, Meyer BJ, Priess JR, editor. C elegans II. Plainview, N.Y.: Cold Springs Harbor Laboratory Press; 1997. p. 1222. [PubMed] [Google Scholar]
- Rogers WP, Sommerville RI. The infective stage of nematode parasites and its significance in parasitism. Adv Parasitol. 1963;1:109–177. doi: 10.1016/s0065-308x(08)60503-5. [DOI] [PubMed] [Google Scholar]
- Hawdon JM. Hookworm infectious process: host signals and parasite responses. Ph.D. University of Pennsylvania, Parasitology; 1991. [Google Scholar]
- Tissenbaum HA, Hawdon J, Perregaux M, Hotez P, Guarente L, Ruvkun G. A common muscarinic pathway for diapause recovery in the distantly related nematode species Caenorhabditis elegans and Ancylostoma caninum. Proc Natl Acad Sci USA. 2000;97:460–465. doi: 10.1073/pnas.97.1.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogg S, Ruvkun G. The C. elegans PTEN homolog, DAF-18, acts in the insulin receptor-like metabolic signaling pathway. Mol Cell. 1998;2:887–893. doi: 10.1016/s1097-2765(00)80303-2. [DOI] [PubMed] [Google Scholar]
- Ogg S, Paradis S, Gottlieb S, Patterson GI, Lee L, Tissenbaum HA, Ruvkun G. The fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature. 1997;389:994–999. doi: 10.1038/40194. [DOI] [PubMed] [Google Scholar]
- Morris JZ, Tissenbaum HA, Ruvkun G. A phosphatidylinositol-3-OH kinase family member regulating longevity and diapause in Caenorhabditis elegans. Nature. 1996;382:536–539. doi: 10.1038/382536a0. [DOI] [PubMed] [Google Scholar]
- Kimura KD, Tissenbaum HA, Liu Y, Ruvkun G. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science. 1997;277:942–946. doi: 10.1126/science.277.5328.942. [DOI] [PubMed] [Google Scholar]
- Paradis S, Ruvkun G. Caenorhabditis elegans Akt/PKB transduces insulin receptor-like signals from AGE-1 PI3 kinase to the DAF-16 transcription factor. Genes Dev. 1998;12:2488–2498. doi: 10.1101/gad.12.16.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradis S, Ailion M, Toker A, Thomas JH, Ruvkun G. A PDK1 homolog is necessary and sufficient to transduce AGE-1 PI3 kinase signals that regulate diapause in Caenorhabditis elegans. Genes Dev. 1999;13:1438–1452. doi: 10.1101/gad.13.11.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb S, Ruvkun G. daf-2, daf-16 and daf-23: genetically interacting genes controlling dauer formation in Caenorhabditis elegans. Genetics. 1994;137:107–120. doi: 10.1093/genetics/137.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin K, Dorman JB, Rodan A, Kenyon C. daf-16: An HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science. 1997;278:1319–1322. doi: 10.1126/science.278.5341.1319. [DOI] [PubMed] [Google Scholar]
- Aitken A. 14-3-3 proteins: A historic overview. Semin Cancer Biol. 2006;16:162–172. doi: 10.1016/j.semcancer.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Cahill CM, Tzivion G, Nasrin N, Ogg S, Dore J, Ruvkun G, Alexander-Bridges M. Phosphatidylinositol 3-kinase signaling inhibits DAF-16 DNA binding and function via 14-3-3-dependent and 14-3-3-independent pathways. J Biol Chem. 2001;276:13402–13410. doi: 10.1074/jbc.M010042200. [DOI] [PubMed] [Google Scholar]
- Brownawell AM, Kops GJ, Macara IG, Burgering BM. Inhibition of nuclear import by protein kinase B (Akt) regulates the subcellular distribution and activity of the forkhead transcription factor AFX. Mol Cell Biol. 2001;21:3534–3546. doi: 10.1128/MCB.21.10.3534-3546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzivion G, Avruch J. 14-3-3 proteins: active cofactors in cellular regulation by serine/threonine phosphorylation. J Biol Chem. 2002;277:3061–3064. doi: 10.1074/jbc.R100059200. [DOI] [PubMed] [Google Scholar]
- Li J, Tewari M, Vidal M, Lee SS. The 14-3-3 protein FTT-2 regulates DAF-16 in Caenorhabditis elegans. Dev Biol. 2007;301:82–91. doi: 10.1016/j.ydbio.2006.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand A, Hawdon JM. Phosphoinositide-3-OH-kinase inhibitor LY294002 prevents activation of Ancylostoma caninum and Ancylostoma ceylanicum third-stage infective larvae. Int J Parasitol. 2004;34:909–914. doi: 10.1016/j.ijpara.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Gao X, Frank D, Hawdon JM. Molecular cloning and DNA binding characterization of DAF-16 orthologs from Ancylostoma hookworms. Int J Parasitol. 2009;39:407–415. doi: 10.1016/j.ijpara.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bektesh S, Van Doren K, Hirsh D. Presence of the Caenorhabditis elegans spliced leader on different mRNAs and in different genera of nematodes. Genes Devel. 1988;2:1277–1283. doi: 10.1101/gad.2.10.1277. [DOI] [PubMed] [Google Scholar]
- Bektesh SL, Hirsh DI. C. elegans mRNAs acquire a spliced leader through a trans-splicing mechanism. Nucleic Acids Res. 1988;16:5692. doi: 10.1093/nar/16.12.5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajarnavis A, Korf I, Durbin R. A probabilistic model of 3' end formation in Caenorhabditis elegans. Nucleic Acids Res. 2004;32:3392–3399. doi: 10.1093/nar/gkh656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendtsen JD, Nielsen H, von Heijne G, Brunak S. Improved prediction of signal peptides: SignalP 3.0. J Mol Biol. 2004;340:783–795. doi: 10.1016/j.jmb.2004.05.028. [DOI] [PubMed] [Google Scholar]
- la Cour T, Kiemer L, Molgaard A, Gupta R, Skriver K, Brunak S. Analysis and prediction of leucine-rich nuclear export signals. Protein Eng Des Sel. 2004;17:527–536. doi: 10.1093/protein/gzh062. [DOI] [PubMed] [Google Scholar]
- Bridges D, Moorhead GB. 14-3-3 proteins: a number of functions for a numbered protein. Sci STKE. 2005;2005:re10. doi: 10.1126/stke.2962005re10. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schaeffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucl Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock JM, Murphy J, Stomski FC, Berndt MC, Lopez AF. The dimeric versus monomeric status of 14-3-3zeta is controlled by phosphorylation of Ser58 at the dimer interface. J Biol Chem. 2003;278:36323–36327. doi: 10.1074/jbc.M304689200. [DOI] [PubMed] [Google Scholar]
- Hawdon JM, Schad GA. Developmental Adaptations in Nematodes. In: Toft CA, Aeschlimann A, Bolis L, editor. Parasitism: Coexistence or Conflict? Oxford: Oxford University Press; 1991. pp. 274–298. [Google Scholar]
- Gao M, Li R, FDai S, Wu Y, Yi D. Diversity of Bacillus thuringiensis strains from soil in China and their pesticidal activities. Biol Control. 2008;44:380–388. [Google Scholar]
- Cappello M, Hawdon JM, Jones BF, Kennedy WP, Hotez PJ. Ancylostoma caninum anticoagulant peptide: cloning by PCR and expression of soluble, active protein in E. coli. Mol Biochem Parasitol. 1996;80:113–117. doi: 10.1016/0166-6851(96)02658-8. [DOI] [PubMed] [Google Scholar]
- Zhan B, Liu Y, Badamchian M, Williamson A, Feng J, Loukas A, Hawdon JM, Hotez PJ. Molecular characterisation of the Ancylostoma-secreted protein family from the adult stage of Ancylostoma caninum. Int J Parasitol. 2003;33:897–907. doi: 10.1016/s0020-7519(03)00111-5. [DOI] [PubMed] [Google Scholar]
- Delaney A, Williamson A, Brand A, Ashcom J, Varghese G, Goud GN, Hawdon JM. Cloning and characterisation of an aspartyl protease inhibitor (API-1) from Ancylostoma hookworms. Int J Parasitol. 2005;35:303–313. doi: 10.1016/j.ijpara.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Zhan B, Badamchian M, Meihua B, Ashcom J, Feng J, Hawdon J, Shuhua X, Hotez PJ. Molecular cloning and purification of Ac-TMP, a developmentally regulated putative tissue inhibitor of metalloprotease released in relative abundance by adult Ancylostoma hookworms. Am J Trop Med Hyg. 2002;66:238–244. doi: 10.4269/ajtmh.2002.66.238. [DOI] [PubMed] [Google Scholar]
- Hawdon JM, Datu B. The second messenger cyclic GMP mediates activation in Ancylostoma caninum infective larvae. Int J Parasitol. 2003;33:787–793. doi: 10.1016/s0020-7519(03)00088-2. [DOI] [PubMed] [Google Scholar]
- Brand AM, Varghese G, Majewski W, Hawdon JM. Identification of a DAF-7 ortholog from the hookworm Ancylostoma caninum. Int J Parasitol. 2005;35:1489–1498. doi: 10.1016/j.ijpara.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Mackintosh C. Dynamic interactions between 14-3-3 proteins and phosphoproteins regulate diverse cellular processes. Biochem J. 2004;381:329–342. doi: 10.1042/BJ20031332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siles-Lucas Mdel M, Gottstein B. The 14-3-3 protein: a key molecule in parasites as in other organisms. Trends Parasitol. 2003;19:575–581. doi: 10.1016/j.pt.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Siles-Lucas M, Merli M, Gottstein B. 14-3-3 proteins in Echinococcus: their role and potential as protective antigens. Exp Parasitol. 2008;119:516–523. doi: 10.1016/j.exppara.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Wang Y, Oh SW, Deplancke B, Luo J, Walhout AJM, Tissenbaum HA. C. elegans 14-3-3 proteins regulate life span and interact with SIR-2.1 and DAF-16/FOXO. Mech Ageing Dev. 2006;127:741–747. doi: 10.1016/j.mad.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Obsil T, Ghirlando R, Anderson DE, Hickman AB, Dyda F. Two 14-3-3 Binding Motifs Are Required for Stable Association of Forkhead Transcription Factor FOXO4 with 14-3-3 Proteins and Inhibition of DNA Binding. Biochemistry. 2003;42:15264–15272. doi: 10.1021/bi0352724. [DOI] [PubMed] [Google Scholar]
- Jaubert S, Laffaire JB, Ledger TN, Escoubas P, Amri EZ, Abad P, Rosso MN. Comparative analysis of two 14-3-3 homologues and their expression pattern in the root-knot nematode Meloidogyne incognita. Int J Parasitol. 2004;34:873–880. doi: 10.1016/j.ijpara.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Rabelo EM, Hall RS, Loukas A, Cooper L, Hu M, Ranganathan S, Gasser RB. Improved insights into the transcriptomes of the human hookworm Necator americanus – fundamental and biotechnological implications. Biotechnol Adv. 2009;27:122–132. doi: 10.1016/j.biotechadv.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Craig H, Wastling JM, Knox DP. A preliminary proteomic survey of the in vitro excretory/secretory products of fourth-stage larval and adult Teladorsagia circumcincta. Parasitology. 2006;132:535–543. doi: 10.1017/S0031182005009510. [DOI] [PubMed] [Google Scholar]
- McGonigle S, Beall MJ, Feeney EL, Pearce EJ. Conserved role for 14-3-3ε downstream of type I TGF-β receptors. FEBS Lett. 2001;490:65–69. doi: 10.1016/s0014-5793(01)02133-0. [DOI] [PubMed] [Google Scholar]
- Patterson GI, Padgett RW. TGF beta-related pathways. Roles in Caenorhabditis elegans development. Trends Genet. 2000;16:27–33. doi: 10.1016/s0168-9525(99)01916-2. [DOI] [PubMed] [Google Scholar]
- Arasu P. In vitro reactivation of Ancylostoma caninum tissue-arrested third-stage larvae by transforming growth factor-beta. J Parasitol. 2001;87:733–738. doi: 10.1645/0022-3395(2001)087[0733:IVROAC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Siles-Lucas M, Nunes CP, Zaha A, Breijo M. The 14-3-3 protein is secreted by the adult worm of Echinococcus granulosus. Parasite Immunol. 2000;22:521–528. doi: 10.1046/j.1365-3024.2000.00334.x. [DOI] [PubMed] [Google Scholar]
- McGonigle S, Loschiavo M, Pearce EJ. 14-3-3 proteins in Schistosoma mansoni; identification of a second epsilon isoform. Int J Parasitol. 2002;32:685–693. doi: 10.1016/s0020-7519(01)00323-x. [DOI] [PubMed] [Google Scholar]
- Uribe N, Siles-Lucas M, Lopez-Aban J, Esteban A, Suarez L, Martinez-Fernandez A, del Olmo E, Muro A. The Sb14-3-3ζ recombinant protein protects against Schistosoma bovis in BALB/c mice. Vaccine. 2007;25:4533–4539. doi: 10.1016/j.vaccine.2007.04.024. [DOI] [PubMed] [Google Scholar]
- Siles-Lucas M, Uribe N, Lopez-Aban J, Vicente B, Orfao A, Nogal-Ruiz JJ, Feliciano AS, Muro A. The Schistosoma bovis Sb14-3-3ζ recombinant protein cross-protects against Schistosoma mansoni in BALB/c mice. Vaccine. 2007;25:7217–7223. doi: 10.1016/j.vaccine.2007.07.021. [DOI] [PubMed] [Google Scholar]
- Schechtman D, Tarrab-Hazdai R, Arnon R. The 14-3-3 protein as a vaccine candidate against schistosomiasis. Parasite Immunol. 2001;23:213–217. doi: 10.1046/j.1365-3024.2001.00378.x. [DOI] [PubMed] [Google Scholar]
- Liu CH, Zhang XR, Qiu DC, Xiao SH, Hotez PJ, Zhen DF, Zhen HL, Li MD, Ren HN, Zhan B, et al. Epidemiology of human hookworm infections among adult villagers in Hejiang and Santai Counties, Sichuan Province, China. Acta Trop. 1999;73:243–249. doi: 10.1016/s0001-706x(99)00035-2. [DOI] [PubMed] [Google Scholar]
- Siles-Lucas M, Merli M, Mackenstedt U, Gottstein B. The Echinococcus multilocularis 14-3-3 protein protects mice against primary but not secondary alveolar echinococcosis. Vaccine. 2003;21:431–439. doi: 10.1016/s0264-410x(02)00517-0. [DOI] [PubMed] [Google Scholar]
- Andrade MA, Siles-Lucas M, Espinoza E, Perez Arellano JL, Gottstein B, Muro A. Echinococcus multilocularis laminated-layer components and the E14t 14-3-3 recombinant protein decrease NO production by activated rat macrophages in vitro. Nitric Oxide. 2004;10:150–155. doi: 10.1016/j.niox.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Gottstein B, Hemphill A. Echinococcus multilocularis: the parasite-host interplay. Exp Parasitol. 2008;119:447–452. doi: 10.1016/j.exppara.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Jaubert S, Ledger TN, Laffaire JB, Piotte C, Abad P, Rosso MN. Direct identification of stylet secreted proteins from root-knot nematodes by a proteomic approach. Mol Biochem Parasitol. 2002;121:205–211. doi: 10.1016/s0166-6851(02)00034-8. [DOI] [PubMed] [Google Scholar]
- Bird DM, Kaloshian I. Are roots special? Nematodes have their say. Physiol Mol Plant P. 2003;62:115–123. [Google Scholar]
- Schad GA. Arrested development of Ancylostoma caninum in dogs: influence of photoperiod and temperature on induction of a potential to arrest. In: Meerovitch E, editor. Aspects of Parasitology: a festschrift dedicated to the fiftieth anniversary of the Institute of Parasitology of McGill University. Montreal: McGill University; 1982. pp. 361–391. [Google Scholar]
- Hawdon JM, Schad GA. Long-term storage of hookworm infective larvae in buffered saline solution maintains larval responsiveness to host signals. J Helm Soc Wash. 1991;58:140–142. [Google Scholar]
- Wylie T, Martin JC, Dante M, Mitreva MD, Clifton SW, Chinwalla A, Waterston RH, Wilson RK, McCarter JP. Nematode.net: a tool for navigating sequences from parasitic and free-living nematodes. Nucl Acids Res. 2004;32:D423–426. doi: 10.1093/nar/gkh010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawdon JM, Datu B, Crowell M. Molecular cloning of a novel multidomain Kunitz-type proteinase inhibitor from the hookworm Ancylostoma caninum. J Parasitol. 2003;89:402–407. doi: 10.1645/0022-3395(2003)089[0402:MCOANM]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Hawdon JM, Jones BF, Hotez PJ. Cloning and characterization of a cDNA encoding the catalytic subunit of a cAMP-dependent protein kinase from Ancylostoma caninum third-stage infective larvae. Mol Biochem Parasitol. 1995;69:127–130. doi: 10.1016/0166-6851(94)00203-y. [DOI] [PubMed] [Google Scholar]
- Nevada Genomics Center http://www.ag.unr.edu/genomics/
- Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser. 1999;41:95–98. [Google Scholar]
- Swiss EMBnet http://www.ch.embnet.org
- Kumar S, Tamura K, Nei M. MEGA3: Integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief Bioinform. 2004;5:150–163. doi: 10.1093/bib/5.2.150. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic Local Alignment Search Tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- National Center for Biotechnology Information BLAST http://blast.ncbi.nlm.nih.gov/Blast.cgi
- European Bioinformatics Institute http://www.ebi.ac.uk/
- Scansite http://scansite.mit.edu/
- Obenauer JC, Cantley LC, Yaffe MB. Scansite 2.0: Proteome-wide prediction of cell signaling interactions using short sequence motifs. Nucleic Acids Res. 2003;31:3635–3641. doi: 10.1093/nar/gkg584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NetNES http://www.cbs.dtu.dk/services/NetNES/