Abstract
The authors assessed CSF and plasma HIV-1 RNA and neuropsychological test performance (composite neuropsychological test Z score [NPZ-4]) in 25 HIV-1–infected subjects 4 and 8 weeks after beginning potent antiretroviral therapy that included a protease inhibitor. In the 14 subjects who entered the study on no antiretroviral treatment, NPZ-4 improvement was associated with decline in CSF HIV-1 RNA at both visits (p = 0.001 and p = 0.02), and those treated with zidovudine or indinavir had greater improvement in NPZ-4 at both visits compared to those treated with other drugs (p = 0.003 and p = 0.01).
Plasma HIV-1 RNA concentrations guide decisions on antiretroviral therapy and help the clinician assess response to therapy.1 In untreated patients, plasma HIV-1 RNA concentration correlates with risk of progression to AIDS.2 The clinical utility of measuring CSF HIV-1 RNA is not known. We examined changes in CSF and plasma HIV-1 RNA and in neuropsychological test performance in individuals beginning a potent antiretroviral regimen that included a protease inhibitor.
Methods
Eligibility criteria included the following: HIV-1 infection, never having received a protease inhibitor, initiating therapy including a protease inhibitor or adding a protease inhibitor to a regimen that had not changed for ≥4 weeks, nonfocal neurologic examination, platelet count >50,000 cells/uL, normal prothrombin time and partial thromboplastin time, no active infection, medically stable, and no other contraindication to lumbar puncture (LP). Written informed consent was obtained from all participants. The study was approved by the University of Washington Institutional Review Board.
At each visit, subjects underwent a standardized neurologic history and examination; timed gait, grooved pegboard with the dominant hand, finger tapping with the nondominant hand, and digit symbol tests; venipuncture; and LP.
HIV-1 RNA in centrifuged CSF and plasma was measured by the Amplicor HIV-1 Monitor test with Ultrasensitive Specimen Preparation (Roche Molecular Systems, Pleasanton, CA). CSF or plasma samples with undetectable HIV-1 RNA were assigned the detection limit of 50 copies/mL.
Plasma and CSF indinavir levels were performed in two batches using different methods that are cross-validated (personal communication, Julie A. Stone, 2002). For the first batch, a published method was used.3 For the second batch, indinavir and the internal standard were isolated from plasma and CSF and detected by liquid chromatography tandem mass spectroscopy. Limit of quantitation was 50 ng/mL in plasma and 5 ng/mL in CSF.
Z scores were calculated for each neuropsychological test based on age-adjusted norms4 and a composite neuropsychological test Z score (NPZ-4) was calculated for each subject at each visit. Plasma and CSF HIV-1 RNA were analyzed as log10 copies/mL. Differences in means were assessed by t-test, and associations between continuous variables by Spearman rank correlation. Associations between change in NPZ-4 and plasma or CSF HIV-1 RNA were described by linear regression adjusted for the baseline values of the outcome variables. All regressions were computed using Huber-White robust variance estimators.5 p Values < 0.05 were considered significant. Analyses were not adjusted for multiple comparisons.
Results
The baseline characteristics of the 25 study subjects are shown in table 1. All subjects began taking at least three antiretroviral agents. The regimens included neither zidovudine (AZT) nor indinavir in 6 subjects, AZT and indinavir in 13 subjects, indinavir without AZT in 5 subjects, and AZT without indinavir in 1 subject. One subject stopped taking AZT between weeks 4 and 8; all subjects who began taking indinavir continued to take it throughout the 8 weeks of observation. Seventeen subjects were seen at 4 weeks and 22 were seen at 8 weeks.
Table 1.
Characteristics of the study subjects at the baseline visit
| Characteristics | All 25 subjects | Eleven subjects who entered on ARV | Fourteen Subjects who entered on no ARV | p Value* |
|---|---|---|---|---|
| Men | 24 (96) | 11 (100) | 13 (92.9) | 0.36 |
| Age, y | 37 (25–56) | 39 (25–56) | 34.5 (27–46) | 0.07 |
| CD4+ T cells/uL | 259 (3–665) | 267 (3–665) | 207 (5–543) | 0.48 |
| Years of education | 13 (2–19) | 14 (10–19) | 13 (2–18) | 0.07 |
| NPZ-4 | −0.36 (−2.28–1.01) | −0.39 (−2.28–0.37) | −0.31 (−0.83–1.01) | 0.48 |
| Detectable CSF HIV-1 RNA† | 19 (75) | 5 (45) | 14 (100) | 0.002 |
| CSF HIV-1 RNA log10 copies/mL | 2.58 (1.70–4.84) | 1.70 (1.70–3.52) | 2.95 (1.91–4.84) | 0.004 |
| Detectable plasma HIV-1 RNA† | 24 (96) | 10 (91) | 14 (100) | 0.25 |
| Plasma HIV-1 RNA log10 copies/mL | 4.73 (1.70–6.21) | 4.71 (1.70–5.88) | 4.73 (3.31–6.21) | 0.51 |
| CSF WBC/uL | 3.5 (0–18) | 2.0 (0–6) | 4.5 (0–18) | 0.12 |
| CSF protein mg/dL | 39 (22–58) | 43 (25–58) | 38 (22–50) | 0.46 |
Values are median (range) or n (%).
p Value for differences between those who entered on and off ARV.
≥1.70 log10 copies/mL.
ARV = antiretroviral drugs; NPZ-4 = composite neuropsychological test Z score; WBC = white blood cells.
NPZ-4 improved at 8 weeks (p = 0.01) but not at 4 weeks. NPZ-4 improvement was associated with decline in CSF but not plasma HIV-1 RNA at 4 weeks (table 2). Improvement in NPZ-4 was not associated with change in CSF or plasma HIV-1 RNA at 8 weeks. Compared to treatment with other agents, treatment with AZT or indinavir was not associated with change in CSF or plasma HIV-1 RNA concentrations at either visit. However, compared to treatment with other agents, and taking into account baseline NPZ-4, treatment with a regimen that included AZT or indinavir was associated with improvement in NPZ-4 at 4 weeks (p = 0.01), but not at 8 weeks. Baseline CSF HIV-1 RNA was comparable in subjects who did and did not begin taking AZT or indinavir, and the proportion of subjects with detectable CSF HIV-1 RNA did not differ between the two groups.
Table 2.
Change in NPZ-4 relative to change in CSF and plasma HIV-1 RNA at each follow-up visit taking into account baseline NPZ-4
| Change in NPZ-4 per log10 decrease in HIV-1 RNA concentration |
||||
|---|---|---|---|---|
| Compartment | 4 weeks | p Value | 8 weeks | p Value |
| In all 25 subjects | ||||
| CSF | 0.41 ± 0.10 | 0.001 | 0.06 ± 0.18 | 0.73 |
| Plasma | 0.24 ± 0.11 | 0.06 | 0.21 ± 0.13 | 0.13 |
| In 14 subjects who entered on no antiretrovial therapy | ||||
| CSF | 0.59 ± 0.13 | 0.001 | 0.28 ± 0.10 | 0.02 |
| Plasma | 0.26 ± 0.14 | 0.11 | 0.25 ± 0.10 | 0.04 |
Values are mean ± SD.
Indinavir levels were determined in eight CSF and nine plasma samples at 4 weeks and in 11 CSF and 10 plasma samples at 8 weeks. Median (range) indinavir concentrations at 4 weeks were CSF 77.4 ng/mL (5.0 to 205.0) and plasma 215.5 ng/mL (38.9 to 4,437.0). At 8 weeks they were 167.1 ng/mL (34.8 to 405.0) and 1,814.2 ng/mL (41.6 to 13,260.6).
There was an interaction between antiretroviral therapy at the baseline visit and decline in CSF and plasma HIV-1 RNA. Among subjects who entered on no antiretroviral agents, NPZ-4 improvement was associated with decline in CSF HIV-1 RNA at both visits (see table 2). NPZ-4 improvement was associated with decline in plasma HIV-1 RNA at 8 weeks but not at 4 weeks (see table 2). Compared to subjects taking antiretroviral drugs at entry, more subjects not taking them at entry had detectable CSF HIV-1 RNA and CSF HIV-1 RNA was higher (see table 1). The two groups did not differ in baseline plasma HIV-1 RNA or NPZ-4. Subjects treated with AZT or indinavir had greater improvement in NPZ-4 at both visits compared to those treated with other drugs (figure).
Figure.
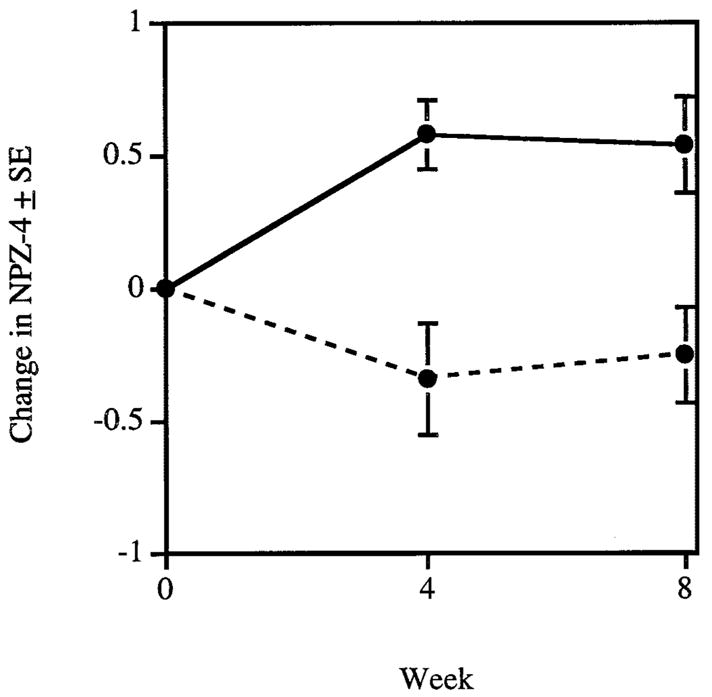
Mean change in composite neuropsychological test Z score (NPZ-4) (±SE) at each visit adjusted for baseline NPZ-4 among the 14 subjects who entered the study on no antiretroviral agents. The solid line depicts the change in NPZ-4 for subjects who began taking a regimen that included zidovudine (AZT) or indinavir and the dashed line depicts the change in NPZ-4 for subjects who began taking other antiretroviral agents. Change in NPZ-4 is greater at both visits in those subjects who began a regimen that included AZT or indinavir (p = 0.003 at 4 weeks and p = 0.01 at 8 weeks).
Discussion
HIV-1 enters the CSF from blood, particularly via the choroid plexus, by trafficking of infected peripheral blood lymphocytes or monocytes, and by infection of meninges or brain. Price et al.6 propose that transitory CSF infection is seen in individuals with CD4+ T cells >200/uL (early infection) and is mediated by trafficking of infected lymphocytes. Autonomous infection is seen in individuals with CD4+ cells <200/uL (late infection), particularly in those with dementia, and is mediated by infection of brain monocytes and macrophages. In transitory infection, treatment of peripheral HIV-1 is sufficient to treat CSF HIV-1. In autonomous infection, drug penetration into the CNS is required to decrease CSF virus.
The subjects in our study had moderately advanced HIV-1 infection with median CD46+ T cells of 259/uL. The association between CSF HIV-1 RNA and improvement in NPZ-4 was most robust in subjects who were not receiving antiretroviral therapy at entry. This association was seen for CSF HIV-1 RNA at both 4 and 8 weeks. A weaker association with plasma HIV-1 RNA was only seen at 8 weeks. Compared to subjects taking antiretroviral agents at entry, more subjects who were not taking them at entry had detectable CSF HIV-1 RNA at baseline and CSF HIV-1 RNA was higher. The lack of association between CSF HIV-1 RNA decline and improvement in NPZ-4 in subjects taking antiretroviral drugs at baseline may reflect a floor effect: they had less chance to demonstrate CSF HIV-1 RNA decline because their concentration of CSF virus was lower. The association between decline in CSF HIV-1 RNA and improvement in NPZ-4 may support the contention that CSF HIV-1 RNA in our subjects reflects brain HIV-1 infection. Because improvement in NPZ-4 was less associated with decline in plasma HIV-1 RNA, improvement in NPZ-4 is not due to improved overall health due to suppression of systemic HIV-1. Among subjects not receiving antiretroviral agents at baseline, those treated with AZT or indinavir, agents with good CSF penetration, had significantly greater improvement in NPZ-4 at both visits compared to subjects treated with other drugs.
Our study had limitations. The number of subjects was small, and therefore we did not perform linear regressions with more than two covariates. We suggest that the associations between decline in CSF HIV-1 RNA and improved NPZ-4 in subjects not receiving antiretroviral therapy at baseline is mostly explained by their higher CSF HIV-1 RNA. By taking into account the baseline value of the outcome variable in linear regressions we minimized differences that might reflect stage of disease or duration of infection.
Our results suggest that monitoring CSF viral load after beginning potent antiretroviral therapy may be useful for HIV-1–infected individuals with cognitive impairment. The goal of therapy in these patients is to suppress HIV-1 replication in the plasma and CSF. Better responses may be seen when agents with good CSF penetration are used.
Acknowledgments
Supported by NIH/AI 30731, NIH/AI 27664, NIH/MH 63647, and University of Washington Center for AIDS Research NIH/AI 30731.
The authors thank Julie A. Stone, PhD, and Ling Zhong, PhD, at Merck for CSF and plasma indinavir levels; and Joan Dragavon, Reggie Sampoleo, Corey Scherrer, and Stacy Smith for technical assistance.
Footnotes
Publisher's Disclaimer: Disclaimer. Dr. Collier has received research contracts in excess of $10,000 from Merck.
References
- 1.Carpenter CC, Cooper DA, Fischl MA, et al. Antiretroviral therapy in adults: updated recommendations of the International AIDS Society-USA Panel. JAMA. 2000;283:381–390. doi: 10.1001/jama.283.3.381. [DOI] [PubMed] [Google Scholar]
- 2.Mellors JW, Munoz A, Giorgi JV, et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med. 1997;126:946 –954. doi: 10.7326/0003-4819-126-12-199706150-00003. [DOI] [PubMed] [Google Scholar]
- 3.Zhong L, Yeh KC. Determination of indinavir in human cerebrospinal fluid and plasma by solid-phase extraction and high-performance liquid chromatography with column switching. J Chromatogr B Biomed Sci Appl. 1999;734:63–71. doi: 10.1016/s0378-4347(99)00342-4. [DOI] [PubMed] [Google Scholar]
- 4.Navia BA, Dafni U, Simpson D, et al. A phase I/II trial of nimodipine for HIV-related neurologic complications. Neurology. 1998;51:221–228. doi: 10.1212/wnl.51.1.221. [DOI] [PubMed] [Google Scholar]
- 5.White H. Maximum likelihood estimation of misspecified models. Econometrica. 1982;50:1–26. [Google Scholar]
- 6.Staprans S, Marlowe N, Glidden D, et al. Time course of cerebrospinal fluid responses to antiretroviral therapy: evidence for variable compartmentalization of infection. AIDS. 1999;13:1051–1061. doi: 10.1097/00002030-199906180-00008. [DOI] [PubMed] [Google Scholar]


