Abstract
Parasitic infection of the nervous system can produce a variety of symptoms and signs. Because symptoms of infection are often mild or nonspecific, diagnosis can be difficult. Familiarity with basic epidemiological characteristics and distinguishing radiographic findings can increase the likelihood of detection and proper treatment of parasitic infection of the nervous system. This article discusses the clinical presentation, diagnosis, and treatment for some of the more common infections of the nervous system caused by cestodes, trematodes and protozoans: Echinococcus spp., Spirometra spp. (sparganosis), Paragonimus spp., Schistosoma spp., Trypanosoma spp., Naegleria fowlerii, Acanthamoeba histolytica, and Balamuthia mandrillaris.
Keywords: Parasite, nervous system, cestodes, trematode, protozoan
Cestodes, trematodes, and protozoans can infect the central or peripheral nervous system, producing a variety of clinical symptoms and signs. Cestodes and trematodes are platyhelminthes, a phylum characterized (with one exception) by an inability to live outside of a host. Nervous system infections by platyhelminthes occur throughout the world. Cestodes, often referred to as “tapeworms,” may exist in either adult or larval forms. Structurally, cestodes are ribbon-shaped, segmented worms with a scolex in the anterior portion used to attach to a host. In general, larval forms are more pathogenic to the human nervous system because adult forms rarely spread beyond the gastrointestinal tract. Neurocysticercosis, caused by the cestode Taenia solium, is perhaps the most common cause of epilepsy in the world and is discussed in an article in this issue of Seminars in Neurology.
Trematodes, also referred to as “flukes,” can invade the nervous system secondarily after blood, liver, intestinal, or pulmonary infection. Most species, except Schistosoma, are hermaphroditic and capable of reproduction and survival within the host. Trematodes possess two anterior suckers for firm attachment to their host.
Protozoans are classified by mode of locomotion and can be either free-living or obligate parasites. The spectrum of clinical symptoms and signs of protozoal infection are diverse and nervous system infection can occur without involvement of other systems. Due to their large size, protozoans are often easier to identify by simple light microscopy than other parasites.
The typical clinical and radiological findings of nervous system infection by selected cestodes, trematodes, and protozoans will be reviewed (Table 1). Treatment of these infections varies greatly, from symptomatic management to surgical extraction. Choice of appropriate therapy can be difficult and when possible should be guided by expert opinion. Electronic resources are available to assist with diagnostic testing and treatment options and are provided at the end of the article.
Table 1.
Imaging Findings of Selected Cestode, Trematode, and Protozoan Infections of the CNS
| Parasite | Type of Lesion | Location | Other Features |
|---|---|---|---|
| Alveolar hydatid disease (Echinococcus multilocularis) | Contrast-enhancing round, solid lesions with contrast enhancement and surrounding edema evident on T2-weighted images | Any location | Lesions are calcified with definite margins |
| Chagas’ disease (Trypanosoma cruzi) | T1-weighted images may show hypointense lesions that enhance, with hyperintense edematous areas on T2-weighted images, producing mass effect | Parietal and frontal lobes only | Similar imaging to toxoplasmosis |
| Granulomatous amoebic encephalitis (Balamuthia spp., Acanthamoeba spp.) | Single or multiple ring-enhancing lesions with surrounding vasogenic edema and regional mass effect; evidence of large and small arterial occlusion with subsequent infarction | Gray and white matter | Lack of granuloma formation in the brain lesions can occur secondary to immunosuppression |
| Human African trypanosomiasis (Trypanosoma bruceii) | Diffuse hyperintensity in the basal ganglia bilaterally on T1-weighted images | Deep gray matter, internal capsule | CT may reveal diffuse atrophy |
| Multiple intracranial hydatidosis (Echinococcus granulosis) | Either multiple thin-walled, large, spherical or egg-shaped cysts are seen or a large cyst with smaller ones around it | Most common in parietal region | Nonenhancing cysts without perilesion edema or calcification |
| Paragonimus | T2-weighted axial images show inflammatory changes in tissue surrounding clusters of lesions which are contrast-enhancing on T1 | Frontal and temporal gray and white matter most commonly affected | Early active stage of infection is a conglomerate of ringlike enhancing lesions (grape-cluster appearance) with surrounding edema usually confined to a single hemisphere |
| Primary amoebic meningoencephalitis (Naegleria spp.) | T2-weighted imaging may show global edema and stroke secondary to severe intracranial pressure | Base of brain most severely affected but may involve any region | Nonspecific brain edema on CT |
| Schistosomiasis | T1-weighted images reveal central linear enhancement, clustered in a masslike structure with an arborized appearance; T2-weighted images show hyperintense lesions with associated surrounding vasogenic edema and mass effect | S. hematobium preferentially occurs in the spinal cord, other spp. may occur throughout CNS | CT shows single or multiple hyperdense lesions with variable enhancement surrounded by low-density edema with an associated mass effect |
| Sparganosis | T2-weighted images show increased signal intensity suggestive of edema; T1 can sometimes show an enhancing mass; MRI sometimes shows linear or curvilinear contrast-enhancing lesions conforming to the shape of the worm | Frequently involves the frontal and parietal lobes, and rarely occurs in the cerebellum, brain stem, or spinal canal | Often diagnosed as vasculitis or ischemic stroke; CT shows multiple small punctate foci of calcification, which represent calcospherules in the tegmentum of the organism |
For additional information and references, please refer to text.
CESTODES
Echinococcus (Hydatid Disease)
EPIDEMIOLOGY
The most common Echinococcus species causing human infection are E. granulosis and E. multilocularis.1 E. granulosis causes cystic echinococcosis (cystic hydatid disease) and is endemic in the Mediterranean, the Middle East, and Latin America.2 E. multilocularis is about half the size of E. granulosis, causes alveolar echinococcosis (alveolar hydatid disease), and is endemic in Alaska, central Europe, Turkey, and China.1 Artic and red foxes are the definitive hosts, but domestic dogs and cats can also become infected. Epidemiological data support transmission of E. multilocularis between dogs or foxes and rodents.3 In countries where infection is endemic, females and children are disproportionately affected because of more frequent contact with infected animals.4
PATHOPHYSIOLOGY
E. granulosis resides in the intestinal tract of dogs and other canids.5 After ingestion by a canid, the parasite quickly transits from the small intestine to the liver and then travels through blood or lymph vessels to lung, brain, vertebrae, pericardium, kidney, or periorbital tissue. Man and sheep are intermediate hosts and acquire infection by ingesting eggs eliminated by infected animals. Human infection results in the formation of hydatid cysts that contain a serous fluid rich in scolices.6 Most infections result in solitary hydatid cysts in the liver. E. multilocularis also travels through blood or lymph vessels to other organs but unlike E. granulosis rarely involves organs other than the liver.2
CLINICAL FINDINGS
Hydatid infection often remains undetected until cyst enlargement produces symptoms. The cyst can cause more severe symptoms if it ruptures or becomes super-infected. Central nervous system (CNS) involvement complicates 2 and 5% of infections with E. granulosis and E. multilocularis, respectively.1,3 The clinical symptoms of neuroechinococcosis are produced by cyst formation rather than direct tissue invasion; symptoms result from compression of brain tissue or intracerebral blood vessels. As intracranial pressure increases, initial subtle clinical symptoms, such as headache, are accompanied by nausea, vomiting, and seizures. Some Echinococcus spp. can secondarily infect the CNS from either the liver or lungs.7,8
DIAGNOSIS
Elevated erythrocyte sedimentation rate (ESR) and serum eosinophilia are usually present in patients with echinococcosis. However, because echinococcal infections are encapsulated, cerebrospinal fluid (CSF) eosinophilia is typically absent. When human echinococcal infection is diagnosed, a veterinarian should also be consulted, as household pets, especially dogs, or farm animals are often the source of infection. Diagnosis of E. granulosus infection can be confirmed by serum indirect hemagglutination (IHA), indirect fluorescent antibody (IFA), or enzyme-linked immunosorbent assay (ELISA), with assay sensitivity rates ranging from 50 to 60% in patients with pulmonary cysts to 98% in patients with hepatic cysts.9 Serum assays to detect E. multilocularis are more sensitive than assays for E. granulosis and are not cross-reactive.10 False-positive results can occur with other Taenia spp. infections (such as Taenia solium infection).11 Some patients who are cyst carriers do not produce detectable antibody levels, so a negative antibody detection test does not exclude the diagnosis of echinococcosis. Serological testing does not predict CNS involvement and is not recommended as a measure of treatment response.12
NEUROIMAGING
Contrast-enhanced computerized tomography (CT) of the brain is usually sufficient for evaluation, but magnetic resonance imaging (MRI) is warranted if surgical intervention is planned. CT demonstrates cysts of various sizes, sometimes in grapelike clusters.13 Chronic disease may develop a granulomatous appearance (Fig. 1).
Figure 1.
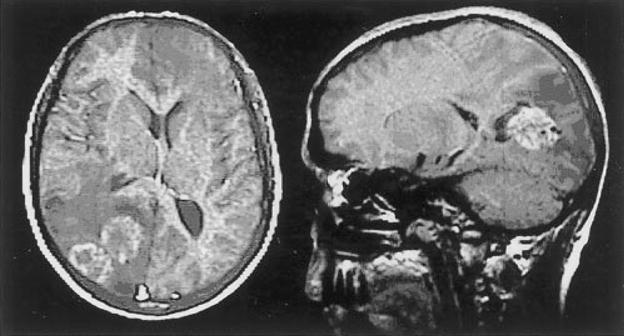
A 29-year-old male with E. multilocularis. Axial T1 MRI with contrast (left) shows several adjacent ring-enhancing lesions, with hypodense periphery and midline shift to the right. Contrast-enhanced sagittal T1 MRI (right) demonstrates cystic appearing parieto-occipital lesions with surrounding vasogenic edema. (Reprinted with permission from Oktar N, Karabiyikoğlu M, Demirtaş E, Altintaş N, Korkmaz M, Özdamar N. Cerebral alveolar Echinococcosis: review of the literature and report of a case. Norol Bil D 1999;16:#8.)
TREATMENT
Surgery is the most effective treatment for echinococcal infection, whether localized to the internal organs or to the CNS, and is often curative.14 Cyst removal can be complicated, and intraoperative rupture can provoke an intense anaphylactic reaction. In addition, medical treatment should be initiated at the time of diagnosis and must be continued postoperatively for months to prevent cyst recurrence.15 Anticonvulsants may also be required.
Sparganosis
EPIDEMIOLOGY
Sparganosis is an infection caused by the larval form of tapeworms from the genus Spirometra or Diphyllobothrium. Spirometra are parasites of fish-eating cats, dogs, and birds, and human infection usually results from eating undercooked fish. S. mansoni is most commonly encountered in Asia, and S. mansonoides is most commonly encountered in the Gulf States of North America.16,17 Recently, S. proliferum was reported as a cause of a disseminated and proliferative infection with CNS involvement.18 The highest endemicity of Spirometra spp. is in Korea and Japan, primarily because of dietary customs. In Korea, seroprevalence is as high as 8% in some regions, with males affected 10 times more often than females.16 Cases of sparganosis have been reported in Asia, Africa, Australia, South America, and the United States.17,19–25
Human infection most often occurs after ingestion of infected raw snake, frog, or pig.25–27 Water infected with larva in the tiny crustacean Cyclops may also be infectious.28 Contact with infected flesh of any of the intermediate hosts can cause infection and may spread beyond the initial site of infection.24 Raw snake consumption, practiced during some Asian masculinity rituals, is one of the most common modes of transmission.26 Use of poultice containing raw frog can also transmit infection, sometimes causing orbital sparganosis.29 In the Western hemisphere, water contamination is the most common cause of infection given the infrequency of raw snake consumption or poultice use.30,31
PATHOPHYSIOLOGY
The life cycle of Spirometra spp. includes several different hosts; definitive hosts include dogs, cats, and wild carnivores, and humans are accidental hosts.25,32 Immature Spirometra reside in the host intestine, and eggs shed with feces later hatch in water and are consumed by small aquatic crustaceans.21 When the crustaceans are consumed by an intermediate host, the larvae penetrate the gastrointestinal tract, become plerocercoids, and spread throughout the body tissues and muscles.31 The life cycle is completed within the intestine of the final host, which can be a human. Adult worms can survive up to 30 years.33 Many case reports describe patients who deny consumption or contact with potentially infectious vectors for more than 20 years.34
CLINICAL FINDINGS
Infection with S. mansonoides or S. mansoni cause similar symptoms. Patients initially develop a discrete subcutaneous nodule that migrates over days to months.35 Sparganosis, in addition to gnathostomiasis, paragonimiasis, and the filarial infection Loa Loa, are the most common parasitic infections causing migratory subcutaneous masses. CNS involvement is common, more often with proliferative disease, but the mechanism of dissemination is unknown. As with many other parasitic infections, the clinical presentation depends upon the location and size of the lesion. Seizures, hemiparesis, and headache are present in most patients.36 A recent retrospective review of European cases reported seizure as the most common presenting symptom, but also described a slowly progressive hemiparesis that developed over years.35 Ocular involvement with sparganosis can cause considerable pain and irritation of the conjunctival tissue and can also invade the periorbital tissues, resulting in proptosis and blindness.37 There is no evidence to suggest that immunosuppressed hosts are at higher risk of developing CNS involvement.
DIAGNOSIS
Peripheral eosinophilia can be pronounced, even with superficial infection.38 CNS involvement is usually associated with serum eosinophilia.39 CSF eosinophilia has not been reported. Identification of the parasite in a tissue specimen provides the definitive diagnosis but is not always possible.40 If biopsy or removal of the organism is not feasible, diagnosis can be made by antisparganum ELISA, which is both sensitive and specific.41
NEUROIMAGING
CT imaging is more sensitive than MRI for detecting punctate calcified “calcospherules” within the parasite.42 Most experts recommend repeat imaging to assess for change in size or location of enhancing nodules, which would indicate the parasite is still alive and surgical extrication is indicated.36 MRI can demonstrate hypodense white matter lesions with variable enhancement; findings suggestive of mass lesion or vasculopathy may also be present (Fig. 2).24,43 Vasculitis with stroke caused by sparganosis has also been reported.44
Figure 2.
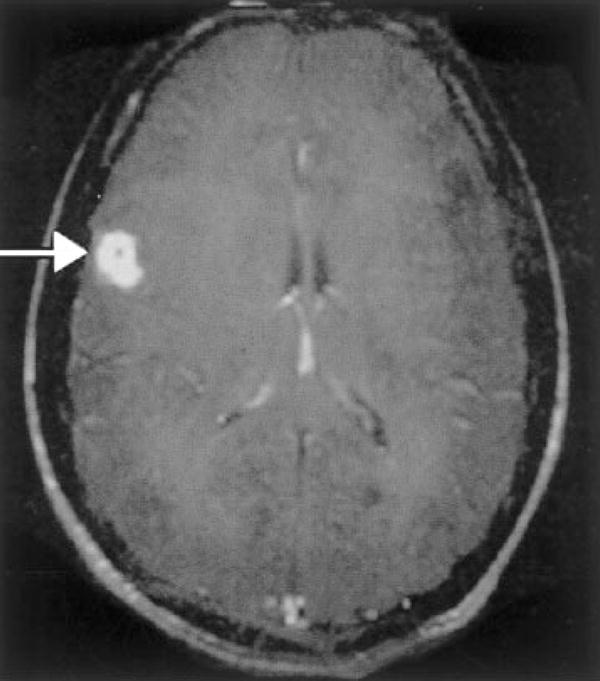
Patient with cerebral sparganosis confirmed by biopsy. MRI shows a ring-enhancing lesion in the parietal cortex (arrow). Change of shape and position of the lesions corresponding to movement of the organism has been described. (Reprinted with permission from Hughes AJ, Biggs BA. Parasitic worms of the central nervous system: an Australian perspective. Intern Med J 2002;32:541–553.)
TREATMENT
Treatment of peripheral infection with praziquantel has produced limited success.45 Cerebral infection responds best to surgical excision of the parasite, as praziquantel has no effect on adult worms in the CNS.46 Most Spirometra spp. respond to medical or surgical therapy, but attempts at surgical removal of S. proliferum are usually unsuccessful due to the widespread dissemination of this species.18 Although various treatments have been used, none has been effective for proliferative disease.47
TREMATODES
Paragonimus
EPIDEMIOLOGY
Paragonimus spp. is the only mammalian lung fluke capable of infecting humans. An estimated 20 million people are infected worldwide, 10 million in China alone.48 The “Oriental Lung Fluke,” P. westermani, is endemic throughout Asia and Western Africa and is the subspecies most often responsible for human infection. Prevalence of infection is slightly higher in females than males, with a peak in young adulthood. Human Paragonimus spp. infection is most often caused by consumption of freshwater crab or crayfish.49 Fully cooked crabs and crayfish do not transmit infection, but many regional dishes use pickling or marinating rather than cooking and are potentially infectious. Domesticated cats, dogs, wild boars, and pigs can also harbor the fluke and transmit disease to humans.48,50
PATHOPHYSIOLOGY
Once the organism has been ingested, fluke metacercariae are released into the small bowel.51 Over 2 to 8 weeks, the larvae migrate through the intestinal wall and peritoneum to invade lung parenchyma. The larvae remain in the lungs until maturity, at which time migration recurs. Adult worms may live up to 20 years. Migration to the brain is complicated and the mechanism not well understood; some studies suggest larvae migrate through loose connective tissue around the jugular vein and enter the posterior circulation via the skull base foramina.52 The predilection of infection for the occipital and temporal lobes supports this theory.
CLINICAL FINDINGS
Initial infection produces gastrointestinal symptoms that can be very mild.53 Most people recover from the initial infection but death infrequently occurs. During any phase of the disease, migratory subcutaneous masses are often present.54 Migratory swellings contain immature worms and are most often located over the lower abdominal region but can occur over any skin surface. Within 4 to 6 months of initial infection, pulmonary symptoms develop, ranging from mild cough to fulminant dyspnea or hemoptysis.55 Active pulmonary disease, either acute or chronic, in combination with neurological symptoms, especially in the absence of tuberculosis, should prompt consideration of paragonimiasis. CNS complications occur in ~1% of patients with pulmonary paragonimiasis.53 Meningoencephalitis is the most common CNS complication and may persist for 6 to 8 weeks. Symptoms during chronic CNS infection are often vague and may include headache, weakness, or nausea. Other CNS complications include transverse myelitis and myelopathy.52 Seizures are usually present in patients with more extensive CNS involvement. Untreated CNS disease carries a mortality rate of almost 5%.56
DIAGNOSIS
Serum eosinophilia and CSF eosinophilia are often pronounced, regardless of the severity of neurological symptoms.57 Definitive diagnosis of CNS involvement requires demonstration of eggs in CSF or brain biopsy material. Although eggs are often present in the stool, sputum, and peritoneal fluid, serum antibody detection tests are more likely to yield the diagnosis.58 Diagnosis is confirmed by detection of antibodies to Paragonimus spp. in the CSF by complement fixation testing.14 Because neurological symptoms occur during the chronic phase of disease, CSF examination may not be as helpful as neuroimaging or other diagnostic testing.
NEUROIMAGING
Plain skull films are often dramatic, demonstrating a characteristic “soap bubble” appearance.59 As noted earlier, Paragonimus spp. infection has a predilection for the temporal and occipital lobe, and contrast CT usually demonstrates clusters of ring-enhancing lesions in these areas (Fig. 3).60,61 Brain MRI may reveal additional lesions.
Figure 3.
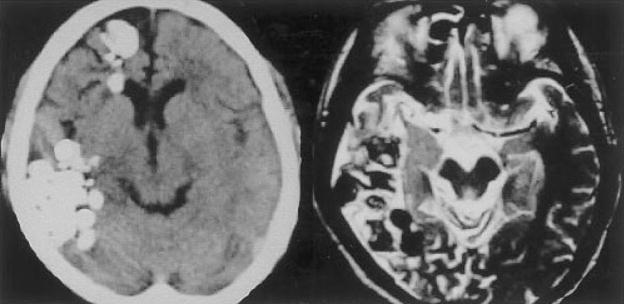
Patient with chronic active cerebral paragonimiasis. Chronic active disease: noncontrast head CT on left shows multiple clusters of calcified densities in the right frontal and temporal regions. T2 axial brain MRI demonstrates edema in the surrounding tissue. (Reprinted with permission from Kang SY, Kim TK, Kim TY, Ha YI, Choi SW, Hong SJ. A case of chronic cerebral Paragonimiasis Westermani. Korean J Parasitol 2000;38:167–171.)
TREATMENT
Praziquantel is the treatment of choice.14 Bithional and triclabendazole are also effective but may require repeat or prolonged therapy. Steroids are synergistic with praziquantel but not recommended for use with the other medications.
Schistosomiasis (Bilharzia)
EPIDEMIOLOGY
Schistosomiasis occurs in up to 300 million people worldwide each year and is caused by five species of blood flukes (digenetic trematodes): Schistosoma mansoni, S. haematobium, S. japonicum, S. intercalculatum, and S. mekongi.62 CNS involvement has been reported with three of the five species: S. mansoni, S. haematobium, S. japonicum.63 Humans are definitive hosts, but at least 30 other mammals are also susceptible to infection. Transmission of schistosomiasis requires contact with water, prompting some experts to call it a “man-made disease.” Endemicity requires an intermediate mollusk host (aquatic or amphibious snails), but additional factors are also important.64 In general, countries with poor sanitary facilities and scarce potable water have higher rates of endemicity. Unfortunately, improvements intended to ameliorate poor sanitary conditions and water supplies in developing countries often increase endemicity by damming up or irrigation with polluted infected water.64,65 In addition, tourists who are instructed to avoid tap water in endemic countries often miss more subtle forms of contact with the schisotosome, such as bathing, washing clothes, and walking barefoot.
PATHOPHYSIOLOGY
Initial infection occurs when the forked tail of the larvae penetrates human skin. The tail is then shed and the larva migrates into the venous system, favoring venules and venous plexi. Clinical symptoms of schistosomiasis depend upon the infecting species and can occur at several stages during the parasitic life cycle. Each species has a predilection for different sites within the human body: the inferior mesenteric vein (S. mansoni), peri-bladder veins (S. haematobium), or superior mesenteric veins (S. japonicum).66–68 Eggs of S. japonicum are smaller than eggs of other schistosomal species and cause 60% of all schistosomal brain infections; the larger egg size of S. mansoni usually limits infection to the spinal cord.69 S. haematobium may cause either brain or spinal cord infection.70 Eggs within the CNS do not develop into worms, and adult worms are not believed to migrate into the CNS. Entry into the CNS is theorized to occur via Batson’s plexus. Once entry has occurred, eggs induce a granulomatous response as tissues attempt to wall off the invading parasite. During chronic infection, granulomas become exudative and necrotic. Necrosis can be extensive and involve vascular walls as well as local tissue.71
CLINICAL FINDINGS
Initial infection usually produces an acute febrile infection called Katayama fever with urticarial swellings, myalgias, eosinophilia, and bloody diarrhea.72 These symptoms may last for several weeks, but are uncommon in populations where infection is endemic. Symptoms may not occur until weeks after the initial infection, especially when caused by S. mansoni or S. japonicum.73 Neurological involvement usually appears weeks or months after initial infection when eggs migrate through the vascular system to the brain or spinal cord; symptoms may result from mass effect of the egg itself or from granuloma formation around the egg.63 Because the parasite likely enters the CNS via Batson’s plexus, the spinal cord and posterior fossa are the most common sites of involvement. Mass effect produced by granuloma formation can cause increased intracranial pressure, and erosion of vascular walls can produce intracranial hemorrhage. Spinal cord involvement may cause symptoms due to granuloma formation or necrotic myelitis, sometimes resulting in a cauda equina or conus syndrome.66 Children with schistosomal infection often develop cognitive impairment.74
DIAGNOSIS
Definitive diagnosis of CNS schistosomiasis is obtained by identification of an egg in biopsy tissue. Detection of schistosomal eggs in stool or urine confirms the diagnosis of schistosomiasis.14 Stool examination is more sensitive for S. mansoni and S. japonicum, and examination of urine is best for S. hematobium.75 Infection can also be confirmed by antibody detection, but antibodies often persist after anthelminthic treatment and should therefore not be used as an indication of the response to treatment. Examination of three stool specimens is more sensitive than immunodiagnosis. For patients with spinal cord infection, CSF ELISA for immunoglobulin G against egg antigens is recommended.76 When clinical suspicion is high and stool and urine examinations are negative, tissue biopsy should be considered.
NEUROIMAGING
Although Schistosoma infect the CNS, granuloma formation around the eggs is usually inflammatory and may be mistaken for neoplasm.77 CT imaging of the brain typically reveals single or multiple hyperdense lesions surrounded by edema with variable contrast enhancement.78 MRI of the brain may reveal a characteristic “arborized” appearance, with linear enhancement surrounded by punctate enhancing nodules (Fig. 4).79,80
Figure 4.
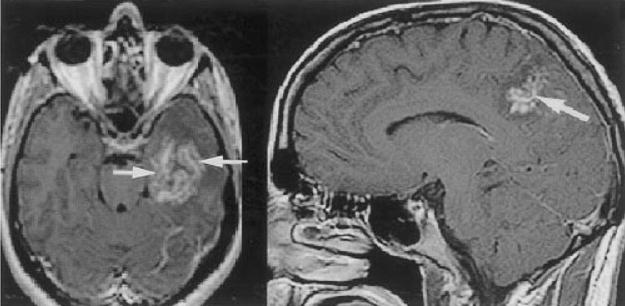
Two different patients with cerebral schistosomal infection. Contrast-enhanced T1-weighted axial and sagittal images demonstrate central linear enhancement surrounded by multiple enhancing punctate nodules, forming an “arborized” appearance (arrows). Pathologically, this pattern correlated with a host granulomatous response to Schistosoma spp. ova. (Reprinted with permission from the American Journal of Roentgenology: Sanelli PC, Lev MH, Gonzalez RG, Schaefer PW. Unique linear and nodular MRI enhancement pattern in schistosomiasis of the central nervous system: report of three patients. AJR Am J Roentgenol 2001; 177:1471–1474.)
TREATMENT
Praziquantel is effective against all Schistosoma spp. and is curative for 60 to 90% of patients.81 Patients who continue to shed eggs in feces should be retreated.82 When praziquantel is ineffective, oxamniquine may be used.83 Artemether, unlike praziquantel, kills immature migrating larvae (schistosomula) and is synergistic with praziquantel.84 Steroids are recommended for patients with edematous lesions or rapidly progressive neurological deficits.78 In the absence of edema, addition of steroids to anthelminthic treatment probably does not improve prognosis of either cerebral or spinal cord infection. When large granulomas are present, surgical extirpation is usually warranted.
PROTOZOANS
American Trypanosomiasis (Chagas’ Disease)
EPIDEMIOLOGY
Trypanosoma cruzi is endemic to most South and Central American countries.85 Infection is most often acquired from the bite of the reduviid bug but can also occur transplacentally, by ingestion of infected guinea pig or by blood transfusion or organ transplantation.86 With increasing urbanization and emigration, infection has spread beyond rural Latin America to the United States and other parts of the world.87 Endemicity is highest where the reduviid bug (Triatoma infestan) or other Triatoma spp. reside. The reduviid bug typically inhabits dank areas but has adapted to survival in urban settings.88 Transmission via transfusion occurs more often in urban areas, when migrants from highly endemic rural areas contribute infected blood to blood banks.89 Increased blood product screening has reduced the prevalence of infected blood products in some cities, but trypanosome-infected transfusions are still common in many South American countries.86 In the United States, tourists and immigrants were previously at highest risk for acquiring infection but have been supplanted by immunosuppressed patients.90
PATHOPHYSIOLOGY
While taking a blood meal from a potential host, the vector leaves fecal waste containing T. cruzi eggs on the skin or mucous membranes.91 Eggs are introduced into the human host through broken skin produced by itching around the site of the insect bite. Once inside the host, the larvae mature and divide via binary fission. These cells are shed into the bloodstream and travel to distant sites where they become intracellular organisms and mature into adults. Unlike African trypanosomes, T. cruzi do not replicate in the bloodstream, but divide only after infecting a new cell or after ingestion by an accidental host. Rupture of infected cells releases infectious parasites as well as potent inflammatory parasitic molecules that induce a strong host response.92
CLINICAL FINDINGS
Acute manifestations of infection include malaise, myalgia, headache, asthenia, and anorexia.93 In the majority of patients, these symptoms recede after several weeks. Romaña’s sign (unilateral or bilateral palpebral edema) is pathognomonic for Chagas’ disease and represents a sensitization response to the bite of an infected bug.94,95 Cardiac failure and intestinal involvement are major causes of morbidity and mortality.96 In Brazil, Chagas’ disease is the primary cause of cardiac failure in men under 40.97 Progression from an acute generalized febrile illness to a symptomatic (chronic) infection occurs in less than 5% of infected people.96 The most common CNS manifestation of chronic infection is meningoencephalitis. CNS symptoms depend largely on the severity of the host immune response and the number, size, and location of focal lesion(s). As infection can be partially suppressed by the host immune system, chronic disease may be asymptomatic.
DIAGNOSIS
Definitive diagnosis is based upon demonstration of the trypanosome in serum or CSF.14 Microscopic examination of blood can identify intracellular motile organisms. During chronic infection, direct visualization of the parasite is uncommon. Serum antibody detection tests are sensitive and specific for both acute and chronic forms of infection.98 Clinical history, rather than diagnostic testing, is more useful for determining chronicity of infection and is important when considering treatment options. Cross-reactivity with leishmaniasis can occur.99
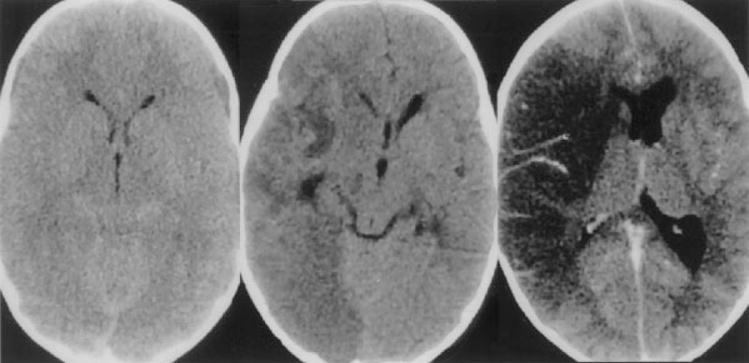
NEUROIMAGING
Imaging findings are variable and depend primarily upon the patient’s immune status. The most common abnormality encountered is one or more ring-enhancing lesions involving both gray and white matter (Fig. 5).100
Figure 5.
Patient with HIV and Chagas’ disease. Contrast-enhanced CT demonstrating edema surrounding a left frontal mass. (Image courtesy of Dr. Henry Masur.)
TREATMENT
Only acute Chagas’ disease can be eradicated by treatment.101 Treatment of chronic infection is symptomatic. When clinical suspicion is high, treatment of acute infection should be initiated early, even if preliminary testing is negative.101 Currently, benzonidazole is the treatment of choice for acute Chagas’ disease.102 Up to 30% of adults treated with benzonidazole experience adverse side effects; allergic dermatitis is common and usually occurs within 2 weeks of starting treatment.103,104 If dermatitis occurs, corticosteroids should be administered and may permit continued treatment when no alternate therapy is available. Nifurtimox is available as an investigational drug when primary therapy fails or is not readily available.
African Trypanosomiasis (Sleeping Sickness)
EPIDEMIOLOGY
Human African Trypanosomiasis (HAT) is endemic to sub-Saharan Africa.105 An estimated 300,000 new cases are diagnosed each year, most in the Democratic Republic of Congo and Uganda.106 Reporting is not standardized and widespread political unrest likely diminishes the actual number of cases reported. Two subspecies of Trypanosoma brucei cause human disease: T. brucei gambiense (West African or Gambian) and T. brucei rhodesiense (East African or Rhodesian, also known as sleeping sickness).107 The tsetse fly is the vector for both Trypanosoma spp. and is unique to Africa. Incidence of disease is proportional to contact between humans and the tsetse fly.105 Thus, expanding human encroachment into areas with higher fly density can produce marked increases in incidence of infection. Only a few dozen cases of sleeping sickness in United States citizens have been reported over the past half-century, the majority occurring after travel through an endemic region.105
PATHOPHYSIOLOGY
A superficial chancre usually develops at the site of the tsetse fly bite.108 Larvae within the chancre migrate through blood and lymphatic vessels, maturing and reproducing during migration. The final destination of both parasites is the CNS. Parasites reaching the CNS encounter a variable immune response.109 The parasite can periodically modify surface glycoproteins to evade detection by the host immune system. Host immune response by monocytes, macrophages, and plasma cells often causes vascular permeability, resulting in adverse patient outcomes. Most often, vascular infiltration produces a meningoencephalitis, with prominent hemorrhage and edema.
CLINICAL FINDINGS
Although Trypanosoma subspecies are morphologically identical, they produce markedly different clinical syndromes. During acute Eastern and Western African trypanosomiasis, rash, intermittent fever, and lymphadenopathy are common.110 Whereas Eastern trypanosomiasis tends to be more aggressive with symptoms developing soon after the tsetse fly bite, Western trypanosomiasis is usually more indolent and can take years to fully manifest. Behavioral abnormalities can be subtle but are often present during the acute febrile illness. Severe biorhythm dysfunction eventually develops and includes daytime somnolence and nighttime insomnia.111 Additional neurological abnormalities encountered include hypo- and hyperthermia, ataxia, rigidity, and akinesia. Although brain and meninges are involved, meningismus is uncommon. Death often results from coma or secondary infection caused by the severe neurological damage (e.g., aspiration during seizures).112 Fatal arrhythmias produced by trypanosomal invasion of the heart are also a common cause of death.
DIAGNOSIS
Definitive diagnosis is achieved by identifying trypanosomes in wet prep of stained blood, centrifuged CSF, or biopsied tissue.14 Acute infection can be diagnosed by demonstrating the parasite in a chancre or site of tsetse fly bite. Treatment depends upon the stage of infection (local, systemic, CNS); thus, diagnostic biopsy should be directed toward the tissue that is most likely to be infected.14 Identification of the organism by microscopy is not sensitive during late-stage disease. Antibody and polymerase chain reaction (PCR) assays are not as sensitive or specific as direct identification of the parasite. If clinical suspicion for HAT is high, CSF should be examined using PCR assay and light microscopy.113
NEUROIMAGING
No specific CT findings have been reported. Brain MRI may demonstrate focal high signal abnormalities in the white matter, specifically with T2 sequences (Fig. 6). These findings can disappear after treatment.114
Figure 6.
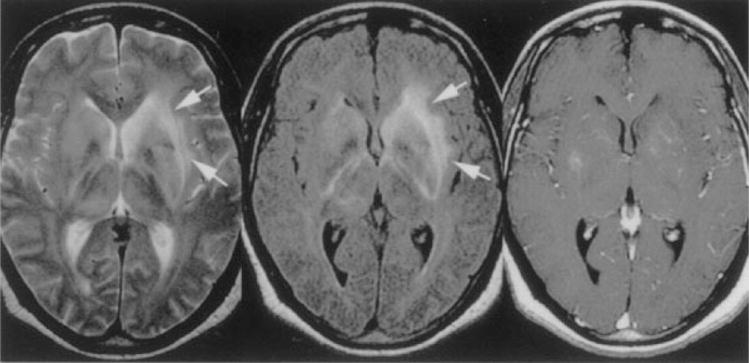
Patient with African trypanosomiasis. Axial T2-weighted image (left) shows diffuse hyperintensity in both basal ganglia and along the internal, external, and extreme capsules (arrows). Axial FLAIR sequence (center, arrows) shows bilateral increased signal intensity involving the internal and external capsules bilaterally. Axial T1-weighted gadolinium-enhanced image (right) shows minimal enhancement in the basal ganglia bilaterally. (Reprinted with permission from Gill DS, Chatha DS, del Cardio-O’Donovan R. MR imaging findings in African trypansomiasis. AJNR Am J Neuroradiol 2003;24:1383–1385. Copyright # by American Society of Neuroradiology, www.ajnr.org.)
TREATMENT
Recommended treatment is dependent upon whether CSF examination demonstrates CNS involvement. Suramin and pentamidine isethionate are the most frequently recommended therapies for systemic trypanosomiasis but are not effective for CNS infection.115 When CNS involvement is documented or suspected, melarsoprol should be initiated immediately. Melarsoprol is effective against both Eastern and Western variants, but drug-resistance is emerging.116 Eflornithine is not effective against T. rhodesiense but can be used to treat CNS infection by T. gambiense if melarsoprol fails.117
Naegleria fowlerii/Primary Amoebic Meningoencephalitis
EPIDEMIOLOGY
Naegleria fowlerii, the cause of primary amoebic meningoencephalitis (PAM), is present throughout the world.118 Most infections occur in children and young adults who play or dive in bodies of stagnant freshwater during warm summer months.119 Less than 100 infections have been reported worldwide. Infection is almost invariably fatal, with mortality approaching 95% and usually occurring within 72 hours of onset of symptoms.120 Early recognition of infection has permitted effective treatment for a few patients.121
PATHOPHYSIOLOGY
N. fowlerii initially enters the human via the nose and erodes through the cribiform plate to gain access into the CNS.122 Once the meninges have been penetrated, infection localizes initially to the olfactory bulbs. Invasion of the subarachnoid space and intracerebral dissemination usually occurs within hours. Both adult and immature parasites cluster near blood vessels in the base of the brain, brain stem, and posterior fossa.123 Cytotoxic enzymes released by the organism induce purulence and necrosis.
CLINICAL FINDINGS
Early symptoms include severe, throbbing headache, fever, nausea, and vomiting.124 Most patients have a history of swimming or bathing in stagnant water. Meningismus is common, and some patients present with seizures or coma. Differentiation between PAM and bacterial meningitis can be difficult but is crucial given the rapid progression of N. fowlerii infection.
DIAGNOSIS
Most patients develop a neutrophilic leukocytosis. CSF findings typically resemble bacterial meningitis, with low glucose and a neutrophilic pleocytosis.14 Organisms are not visualized with Gram’s stain because amoebas are killed during the fixation process. CSF wet mount should be performed to look for trophozoites.14 Trichrome and Giemsa staining of CSF may also be useful. In the past, PCR assays and antibody probes were not clinically useful as patients were usually dead before test results were available, but when treatment is initiated early, PCR may be useful for providing definitive diagnosis.125
NEUROIMAGING
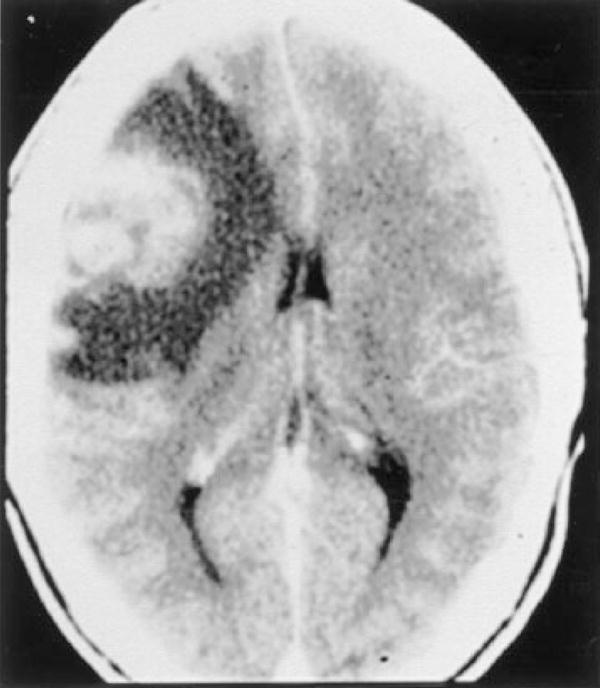
Neuroimaging is usually normal initially, but severe edema may be present (Fig. 7).126 Findings at autopsy are localized to the posterior fossa and brain stem.127
Figure 7.
Patient with Naegleria fowlerii infection identified postmortem. Noncontrast CT (left) at the level of the superior colliculus showing effacement of the quadrigeminal plate cistern. Noncontrast CT in middle shows low attenuation in the right hemisphere with mass effect. Contrast infusion 1 week later (right) shows mild vascular enhancement and persistent low attenuation consistent with infarction of both middle cerebral and posterior cerebral artery territories. (Reprinted with permission from Schumacher DJ, Tien RD, Lane K. Neuroimaging findings in rare amebic infections of the central nervous system. AJNR Am J Neuroradiol 1995;16(suppl):930–935. Copyright # by American Society of Neuroradiology, www.ajnr.org.)
TREATMENT
Very few people have survived PAM, and no standardized treatment exists. Only three successful treatments have been reported; all infections were diagnosed early, and most included high-dose systemic and intrathecal amphotericin B.128 Adjunct hyperbaric therapy has been used, with limited success. Miconazole, rifampin, and sulfisoxazole may also be effective.129
Acanthamoeba histolytica and Balamuthia mandrillaris/Granulomatous Amoebic Encephalitis
EPIDEMIOLOGY
Both Acanthamoeba histolytica and Balamuthia mandrillaris are free-living amoebas that cause granulomatous amoebic encephalitis (GAM).130,131 Both are present throughout the world in soil and sometimes in freshwater. Although both organisms are capable of living in water, they are not encountered as frequently as Naegleria spp., and stagnated water is not a requirement.132 A. histolytica has been isolated from water fountains and contact lens and is a common cause of self-limited and mild keratitis.133
PATHOPHYSIOLOGY
CNS infection by A. histolytica is uncommon in immunocompetent hosts.134 In contrast to A. histolytica, B. mandrillaris causes infection in immunocompetent and immunosuppressed hosts with equal frequency.135
CLINICAL FINDINGS
Until 1997, all human cases of B. mandrillaris infection had been diagnosed at autopsy.132 Clinical presentation is similar to A. histolytica infection. No systemic findings are specific for either infection. Encephalitis develops more slowly with B. mandrillaris infection and often takes months until clinical symptoms develop. Immunosuppressed hosts are more likely to develop a virulent hematogenous or cutaneous infection or granulomatous meningitis.136
DIAGNOSIS
Definitive diagnosis can be obtained by demonstration of trophozoites or cysts of A. histolytica on stained smears of biopsy specimens or corneal scrapings.14 Direct IFA tests can be useful. Differentiation between B. mandrillaris and A. histolytica infection requires immunofluorescence studies.14 Examination of contact lenses from patients with keratitis can reveal A. histolytica.
NEUROIMAGING
Contrast-enhanced head CT of patients with B. mandrillaris CNS infection usually demonstrates ring-enhancing lesions. MRI shows diffusion-restriction within the abscess cavity and prominent edema on T2 that can resolve after treatment. Calcifications are seen in patients with chronic infection (Fig. 8).132
Figure 8.
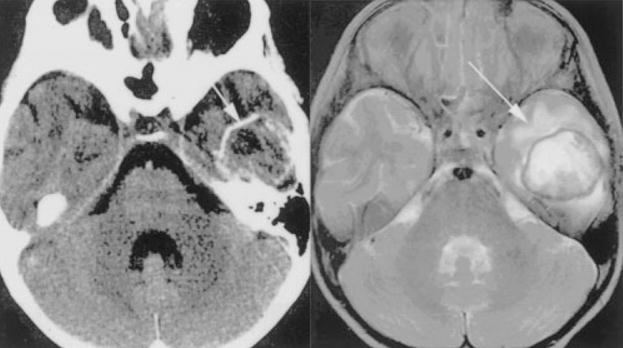
Balamuthia mandrillaris infection in a 5-year-old immunocompetent girl. Head CT with contrast (left) demonstrates a ring-enhancing lesion in the left temporal lobe and T2 MRI (right) confirms the presence of edema surrounding the well-demarcated solitary abscess (arrows). (Reprinted with permission from Healy JF. Balamuthia amebic encephalitis: radiographic and pathologic findings. AJNR Am J Neuroradiol 2002;23:486–489. Copyright # by American Society of Neuroradiology, www.ajnr.org.)
TREATMENT
There are no standardized treatments for CNS infection with either B. mandrillaris or A. histolytica. In children, successful treatment has included trimethoprim-sulfamethoxazole, rifampin, and ketoconazole.137 Eye and skin infections are treatable, but if the CNS is infected, death usually occurs within weeks to months.136
CONCLUSION
Parasitic infections of the CNS, previously restricted mainly to people living in developing countries, are becoming increasingly more prevalent throughout the world. With the advent of increasing global travel, more potent immunosuppression, and HIV infection, parasitic infections will likely become even more commonplace. Basic familiarity with common pathogens can make diagnosis more expeditious and efficient. For the clinician confronted with a patient with suspected parasitic infection, additional assistance with diagnostic evaluation and therapy can be obtained at the following web sites (see also Table 2):
Table 2.
Treatment of Selected Cestode, Trematode, and Protozoan Infections of the CNS
| Parasite | Medication | Dosage | Precautions | Potential Side Effects |
|---|---|---|---|---|
| Trypansomiasis (Chagas’ disease) | Benznidazole; steroids may be helpful | 5–7 mg/kg/d in adults and 10 mg/kg/d bidtid for 60 d | Up to 30% of adults experience severe side effects | Peripheral neuropathy; neutropenia, nausea, vomiting, liver failure |
| Echinococcosis | Albendazole | 400 mg bid for 1–6 months, pediatrics 15 mg/kg/d for 1–6 months | Concurrent use of dexamethasone or praziquantel may cause toxicity | Abdominal pain, jaundice, and alopecia |
| Human African trypanosomiasis | Pentamidine isethionate; steroids only if using melarsorpol as treatment | 4 mg/kg/d IM for 10 d | Hypotension, pancreatitis, and cardiac arrhythmias can occur with IV or IM administration | Nausea, leucopenia, and elevated creatinine |
| Naegleria fowlerii | Amphotericin B | 1.5 mg/kg/d IV or intrathecally | Steroids may induce hypokalemia | Seizures, hearing loss, cardiac arrhythmia, rash |
| Paragonimiasis | Praziquantel | 75 mg/kg TID for 3 d | Breast-feeding should be delayed for 3 days posttreatment; seizures can occur if disease burden is high | Nausea, vomiting, abdominal pain, dizziness, headache, rash |
| Schistosomiasis | Praziquantel; treatment of coexisting infections | 60 mg/kg/d bid or tid for 1 d | Breast-feeding should be delayed for 3 days posttreatment | Mild; generally well tolerated |
| Sparganosis | Praziquantel | Not yet determined | Breast-feeding should be delayed for 3 days post-treatment; seizures can occur if disease burden is high | Nausea, vomiting, abdominal pain, dizziness, headache, rash |
For treatment options and further information, please consult The Medical Letter or contact the CDC.
The World Health Organization Fact Sheet, available at http://www.who.int/inffs/en/index.html
Centers for Disease Control, Division of Parasitic Diseases, available at http://www.dpd.cdc.gov/dpdx/
The Medical Letter, available at http://www.medletter.com/index.html
Acknowledgments
Supported by NIH Grants K23-AI01600 and University of Washington Center for AIDS Research (CFAR) Grant AI27757.
Footnotes
Objectives: On completion of this article, the reader should be able to summarize the clinical presentation, diagnosis, and treatment of parasitic infections of the central nervous system.
Accreditation: The Indiana University School of Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Credit: The Indiana University School of Medicine designates this educational activity for a maximum of 1 Category 1 credit toward the AMA Physicians Recognition Award. Each physician should claim only those credits that he/she actually spent in the educational activity.
Disclosure: Statements of disclosure have been obtained regarding the authors’ relevant financial relationships. The authors have nothing to disclose.
References
- 1.Algros MP, Majo F, Bresson-Hadni S, et al. Intracerebral alveolar echinococcosis. Infection. 2003;31:63–65. doi: 10.1007/s15010-002-2178-y. [DOI] [PubMed] [Google Scholar]
- 2.Al Zain TJ, Al-Witry SH, Khalili HM, et al. Multiple intracranial hydatidosis. Acta Neurochir (Wien) 2002;144:1179–1185. doi: 10.1007/s00701-002-0987-5. [DOI] [PubMed] [Google Scholar]
- 3.Lewall DB. Hydatid disease: biology, pathology, imaging and classification. Clin Radiol. 1998;53:863–874. doi: 10.1016/s0009-9260(98)80212-2. [DOI] [PubMed] [Google Scholar]
- 4.Kern P, Bardonnet K, Renner E, et al. European echinococcosis registry: human alveolar echinococcosis, Europe, 1982–2000. Emerg Infect Dis. 2003;9:343–349. doi: 10.3201/eid0903.020341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouree P. Hydatidosis: dynamics of transmission. World J Surg. 2001;25:4–9. doi: 10.1007/s002680020001. [DOI] [PubMed] [Google Scholar]
- 6.Garret M, Herbsman H, Fierst S. Cytologic diagnosis of echinococcosis. Acta Cytol. 1977;21:553–554. [PubMed] [Google Scholar]
- 7.Farmer PM, Chatterley S, Spier N. Echinococcal cyst of the liver: diagnosis and surgical management. Ann Clin Lab Sci. 1990;20:385–391. [PubMed] [Google Scholar]
- 8.Gottstein B, Reichen J. Hydatid lung disease (echinococcosis/hydatidosis) Clin Chest Med. 2002;23:397–408. doi: 10.1016/s0272-5231(02)00007-2. [DOI] [PubMed] [Google Scholar]
- 9.Ramadan NI, Abel Aaty HE, Mahmoud MS, El Nori A. An enzyme-linked immunoelectrotransfer blot assay for diagnosis of human cystic echinococcosis. J Egypt Soc Parasitol. 1999;29:849–857. [PubMed] [Google Scholar]
- 10.Jiang L, Wen H, Ito A. Immunodiagnostic differentiation of alveolar and cystic echinococcosis using ELISA test with 18-kDa antigen extracted from Echinococcus protoscoleces. Trans R Soc Trop Med Hyg. 2001;95:285–288. doi: 10.1016/s0035-9203(01)90235-4. [DOI] [PubMed] [Google Scholar]
- 11.Kanwar JR, Kaushik SP, Sawhney IM, et al. Specific antibodies in serum of patients with hydatidosis recognised by immunoblotting. J Med Microbiol. 1992;36:46–51. doi: 10.1099/00222615-36-1-46. [DOI] [PubMed] [Google Scholar]
- 12.Gottstein B. Molecular and immunological diagnosis of echinococcosis. Clin Microbiol Rev. 1992;5:248–261. doi: 10.1128/cmr.5.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tuzun M, Altinors N, Arda IS, Hekimoglu B. Cerebral hydatid disease CT and MR findings. Clin Imaging. 2002;26:353–357. doi: 10.1016/s0899-7071(02)00449-7. [DOI] [PubMed] [Google Scholar]
- 14.CDC. DPDx Laboratory diagnosis of parasites of public health concern. Vol. 2003. Centers for Disease Control and Prevention; 2003. [Google Scholar]
- 15.el-Mufti M, Kamag A, Ibrahim H, et al. Albendazole therapy of hydatid disease: 2-year follow-up of 40 cases. Ann Trop Med Parasitol. 1993;87:241–246. doi: 10.1080/00034983.1993.11812762. [DOI] [PubMed] [Google Scholar]
- 16.Lee KJ, Bae YT, Kim DH, et al. A seroepidemiologic survey for human sparganosis in Gangweon-do. Korean J Parasitol. 2002;40:177–180. doi: 10.3347/kjp.2002.40.4.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Landero A, Hernandez F, Abasolo MA, et al. Cerebral sparganosis caused by Spirometra mansonoides. Case report J Neurosurg. 1991;75:472–474. doi: 10.3171/jns.1991.75.3.0472. [DOI] [PubMed] [Google Scholar]
- 18.Noya O, Alarcon de Noya B, Arrechedera H, et al. Sparganum proliferum: an overview of its structure and ultrastructure. Int J Parasitol. 1992;22:631–640. doi: 10.1016/0020-7519(92)90012-a. [DOI] [PubMed] [Google Scholar]
- 19.Fontan R, Beauchamp F, Beaver PC. New helminthiases in Laos. II. Plathelminths. Bull Soc Pathol Exot Filiales. 1975;68:566–573. [PubMed] [Google Scholar]
- 20.Li X. Food-borne parasitic zoonoses in the People’s Republic of China. Southeast Asian J Trop Med Public Health. 1991;22(suppl):31–35. [PubMed] [Google Scholar]
- 21.Hughes AJ, Biggs BA. Parasitic worms of the central nervous system: an Australian perspective. Intern Med J. 2002;32:541–553. doi: 10.1046/j.1445-5994.2002.00265.x. [DOI] [PubMed] [Google Scholar]
- 22.Schmid H, Watschinger H. Sparganosis in the Masailand. Acta Trop. 1972;29:218–230. [PubMed] [Google Scholar]
- 23.Moulinier R, Martinez E, Torres J, et al. Human proliferative sparganosis in Venezuela: report of a case. Am J Trop Med Hyg. 1982;31:358–363. doi: 10.4269/ajtmh.1982.31.358. [DOI] [PubMed] [Google Scholar]
- 24.Cummings TJ, Madden JF, Gray L, et al. Parasitic lesion of the insula suggesting cerebral sparganosis: case report. Neuroradiology. 2000;42:206–208. doi: 10.1007/s002340050047. [DOI] [PubMed] [Google Scholar]
- 25.Gray ML, Rogers F, Little S, et al. Sparganosis in feral hogs (Sus scrofa) from Florida. J Am Vet Med Assoc. 1999;215:204–208. [PubMed] [Google Scholar]
- 26.Park HY, Lee SU, Kim SH, et al. Epidemiological significance of sero-positive inhabitants against sparganum in Kangwon-do, Korea. Yonsei Med J. 2001;42:371–374. doi: 10.3349/ymj.2001.42.4.371. [DOI] [PubMed] [Google Scholar]
- 27.Mastura AB, Ambu S, Hasnah O, Rosli R. Spargana infection of frogs in Malaysia. Southeast Asian J Trop Med Public Health. 1996;27:51–52. [PubMed] [Google Scholar]
- 28.Tsou MH, Huang TW. Pathology of subcutaneous sparganosis: report of two cases. J Formos Med Assoc. 1993;92:649–653. [PubMed] [Google Scholar]
- 29.Kron MA, Guderian R, Guevara A, Hidalgo A. Abdominal sparganosis in Ecuador: a case report. Am J Trop Med Hyg. 1991;44:146–150. doi: 10.4269/ajtmh.1991.44.146. [DOI] [PubMed] [Google Scholar]
- 30.Rywlin AM, Beck JW, Snyder GB. Sparganosis in Florida. Arch Dermatol. 1968;97:425–427. [PubMed] [Google Scholar]
- 31.Norman SH, Kreutner A., Jr Sparganosis: clinical and pathologic observations in ten cases. South Med J. 1980;73:297–300. [PubMed] [Google Scholar]
- 32.Taylor RL. Sparganosis in the United States. Report of a case Am J Clin Pathol. 1976;66:560–564. doi: 10.1093/ajcp/66.3.560. [DOI] [PubMed] [Google Scholar]
- 33.Mueller JF. Potential longevity of life history stages of Spirometra spp. J Parasitol. 1974;60:376–377. [PubMed] [Google Scholar]
- 34.Chang JH, Lin OS, Yeh KT. Subcutaneous sparganosis—a case report and a review of human sparganosis in Taiwan. Kaohsiung J Med Sci. 1999;15:567–571. [PubMed] [Google Scholar]
- 35.Pampiglione S, Fioravanti ML, Rivasi F. Human sparganosis in Italy. Case report and review of the European cases. APMIS. 2003;111:349–354. doi: 10.1034/j.1600-0463.2003.1110208.x. [DOI] [PubMed] [Google Scholar]
- 36.Chang KH, Chi JG, Cho SY, et al. Cerebral sparganosis: analysis of 34 cases with emphasis on CT features. Neuroradiology. 1992;34:1–8. doi: 10.1007/BF00588423. [DOI] [PubMed] [Google Scholar]
- 37.Botterel F, Bouree P. Ocular sparganosis: a case report. J Travel Med. 2003;10:245–246. doi: 10.2310/7060.2003.40468. [DOI] [PubMed] [Google Scholar]
- 38.Campbell EW, Beals C. Striking eosinophilia in sparganosis. Postgrad Med. 1977;62:138–140. doi: 10.1080/00325481.1977.11714709. [DOI] [PubMed] [Google Scholar]
- 39.Fan KJ, Pezeshkpour GH. Cerebral sparganosis. Neurology. 1986;36:1249–1251. doi: 10.1212/wnl.36.9.1249. [DOI] [PubMed] [Google Scholar]
- 40.Sarma DP, Weilbaecher TG. Human sparganosis. J Am Acad Dermatol. 1986;15:1145–1148. doi: 10.1016/s0190-9622(86)70284-3. [DOI] [PubMed] [Google Scholar]
- 41.Nishiyama T, Ide T, Himes SR, Jr, et al. Immunodiagnosis of human sparganosis mansoni by micro-chemiluminescence enzyme-linked immunosorbent assay. Trans R Soc Trop Med Hyg. 1994;88:663–665. doi: 10.1016/0035-9203(94)90218-6. [DOI] [PubMed] [Google Scholar]
- 42.Moon WK, Chang KH, Cho SY, et al. Cerebral sparganosis: MR imaging versus CT features. Radiology. 1993;188:751–757. doi: 10.1148/radiology.188.3.8351344. [DOI] [PubMed] [Google Scholar]
- 43.Jeong SC, Bae JC, Hwang SH, et al. Cerebral sparganosis with intracerebral hemorrhage: a case report. Neurology. 1998;50:503–506. doi: 10.1212/wnl.50.2.503. [DOI] [PubMed] [Google Scholar]
- 44.Han SR, Park JK, Kim YI, Son BC. Posterior cerebral artery infarction caused by cerebral sparganosis-induced vasculitis. Eur Neurol. 2001;46:105–107. doi: 10.1159/000050776. [DOI] [PubMed] [Google Scholar]
- 45.Ishii H, Mukae H, Inoue Y, et al. A rare case of eosinophilic pleuritis due to sparganosis. Intern Med. 2001;40:783–785. doi: 10.2169/internalmedicine.40.783. [DOI] [PubMed] [Google Scholar]
- 46.Kim DG, Paek SH, Chang KH, et al. Cerebral sparganosis: clinical manifestations, treatment, and outcome. J Neurosurg. 1996;85:1066–1071. doi: 10.3171/jns.1996.85.6.1066. [DOI] [PubMed] [Google Scholar]
- 47.Torres JR, Noya OO, Noya BA, et al. Treatment of proliferative sparganosis with mebendazole and praziquantel. Trans R Soc Trop Med Hyg. 1981;75:846–847. doi: 10.1016/0035-9203(81)90428-4. [DOI] [PubMed] [Google Scholar]
- 48.Blair D, Xu ZB, Agatsuma T. Paragonimiasis and the genus Paragonimus. Adv Parasitol. 1999;42:113–222. doi: 10.1016/s0065-308x(08)60149-9. [DOI] [PubMed] [Google Scholar]
- 49.Kim DC. Paragonimus westermani: life cycle, intermediate hosts, transmission to man and geographical distribution in Korea. Arzneimittelforschung. 1984;34:1180–1183. [PubMed] [Google Scholar]
- 50.Miyazaki I, Hirose H. Immature lung flukes first found in the muscle of the wild boar in Japan. J Parasitol. 1976;62:836–837. [PubMed] [Google Scholar]
- 51.Choi WY. Paragonimus westermani: pathogenesis and clinical features of infection. Arzneimittelforschung. 1984;34:1184–1185. [PubMed] [Google Scholar]
- 52.Oh SJ. Cerebral and spinal paragonimiasis. A histopathological study. J Neurol Sci. 1969;9:205–236. doi: 10.1016/0022-510x(69)90072-0. [DOI] [PubMed] [Google Scholar]
- 53.Kusner DJ, King CH. Cerebral paragonimiasis. Semin Neurol. 1993;13:201–208. doi: 10.1055/s-2008-1041126. [DOI] [PubMed] [Google Scholar]
- 54.Ashitani J, Kumamoto K, Matsukura S. Paragonimiasis westermani with multifocal lesions in lungs and skin. Intern Med. 2000;39:433–436. doi: 10.2169/internalmedicine.39.433. [DOI] [PubMed] [Google Scholar]
- 55.Kagawa FT. Pulmonary paragonimiasis. Semin Respir Infect. 1997;12:149–158. [PubMed] [Google Scholar]
- 56.Kang SY, Kim TK, Kim TY, et al. A case of chronic cerebral paragonimiasis westermani. Korean J Parasitol. 2000;38:167–171. doi: 10.3347/kjp.2000.38.3.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kan H, Ogata T, Taniyama A, et al. Extraordinarily high eosinophilia and elevated serum interleukin-5 level observed in a patient infected with Paragonimus westermani. Pediatrics. 1995;96:351–354. [PubMed] [Google Scholar]
- 58.Kim TY, Joo IJ, Kang SY, et al. Recombinant Paragonimus westermani yolk ferritin is a useful serodiagnostic antigen. J Infect Dis. 2002;185:1373–1375. doi: 10.1086/339880. [DOI] [PubMed] [Google Scholar]
- 59.Oh SJ. Paragonimus meningitis. J Neurol Sci. 1968;6:419–433. doi: 10.1016/0022-510x(68)90028-2. [DOI] [PubMed] [Google Scholar]
- 60.Yoshida M, Moritaka K, Kuga S, Anegawa S. CT findings of cerebral paragonimiasis in the chronic state. J Comput Assist Tomogr. 1982;6:195–196. doi: 10.1097/00004728-198202000-00039. [DOI] [PubMed] [Google Scholar]
- 61.Udaka F, Okuda B, Okada M, et al. CT findings of cerebral paragonimiasis in the chronic state. Neuroradiology. 1988;30:31–34. doi: 10.1007/BF00341939. [DOI] [PubMed] [Google Scholar]
- 62.El-Garem AA. Schistosomiasis. Digestion. 1998;59:589–605. doi: 10.1159/000007534. [DOI] [PubMed] [Google Scholar]
- 63.Pittella JE. Neuroschistosomiasis. Brain Pathol. 1997;7:649–662. doi: 10.1111/j.1750-3639.1997.tb01080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zheng J, Gu XG, Xu YL, et al. Relationship between the transmission of schistosomiasis japonica and the construction of the Three Gorge Reservoir. Acta Trop. 2002;82:147–156. doi: 10.1016/s0001-706x(02)00046-3. [DOI] [PubMed] [Google Scholar]
- 65.Babiker SM, Blankespoor HD, Wassila M, et al. Transmission of Schistosoma haematobium in North Gezira, Sudan. J Trop Med Hyg. 1985;88:65–73. [PubMed] [Google Scholar]
- 66.Liu LX. Spinal and cerebral schistosomiasis. Semin Neurol. 1993;13:189–200. doi: 10.1055/s-2008-1041125. [DOI] [PubMed] [Google Scholar]
- 67.Pollner JH, Schwartz A, Kobrine A, Parenti DM. Cerebral schistosomiasis caused by Schistosoma haematobium: case report. Clin Infect Dis. 1994;18:354–357. doi: 10.1093/clinids/18.3.354. [DOI] [PubMed] [Google Scholar]
- 68.Haedicke TA. Cerebral schistosomiasis. A report of 10 cases caused by Schistosomiasis japonica. Minn Med. 1972;55:1105–1114. [PubMed] [Google Scholar]
- 69.Pittella JE. The relation between involvement of the central nervous system in schistosomiasis mansoni and the clinical forms of the parasitosis. A review. J Trop Med Hyg. 1991;94:15–21. [PubMed] [Google Scholar]
- 70.Scrimgeour EM, Gajdusek DC. Involvement of the central nervous system in Schistosoma mansoni and S. haematobium infection. A review. Brain. 1985;108:1023–1038. doi: 10.1093/brain/108.4.1023. [DOI] [PubMed] [Google Scholar]
- 71.File S. Interaction of schistosome eggs with vascular endothelium. J Parasitol. 1995;81:234–238. [PubMed] [Google Scholar]
- 72.Stuiver PC. Acute schistosomiasis (Katayama fever) Br Med J (Clin Res Ed) 1984;288:221–222. doi: 10.1136/bmj.288.6412.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.King CH, Mahmoud AA. Drugs five years later: praziquantel. Ann Intern Med. 1989;110:290–296. doi: 10.7326/0003-4819-110-4-290. [DOI] [PubMed] [Google Scholar]
- 74.Jukes MC, Nokes CA, Alcock KJ, et al. Heavy schistosomiasis associated with poor short-term memory and slower reaction times in Tanzanian schoolchildren. Trop Med Int Health. 2002;7:104–117. doi: 10.1046/j.1365-3156.2002.00843.x. [DOI] [PubMed] [Google Scholar]
- 75.Brinkmann UK, Powollik W, Werler C, Traore M. An evaluation of sampling methods within communities and the validity of parasitological examination techniques in the field. Trop Med Parasitol. 1988;39:162–166. [PubMed] [Google Scholar]
- 76.Ferrari TC, Moreira PR, Oliveira RC, et al. The value of an enzyme-linked immunosorbent assay for the diagnosis of schistosomiasis mansoni myeloradiculopathy. Trans R Soc Trop Med Hyg. 1995;89:496–500. doi: 10.1016/0035-9203(95)90083-7. [DOI] [PubMed] [Google Scholar]
- 77.Gjerde IO, Mork S, Larsen JL, et al. Cerebral schistosomiasis presenting as a brain tumor. Eur Neurol. 1984;23:229–236. doi: 10.1159/000115735. [DOI] [PubMed] [Google Scholar]
- 78.Fowler R, Lee C, Keystone JS. The role of corticosteroids in the treatment of cerebral schistosomiasis caused by Schistosoma mansoni: case report and discussion. Am J Trop Med Hyg. 1999;61:47–50. doi: 10.4269/ajtmh.1999.61.47. [DOI] [PubMed] [Google Scholar]
- 79.Ching HT, Clark AE, Hendrix VJ, et al. MR imaging appearance of intracerebral schistosomiasis. AJR Am J Roentgenol. 1994;162:693–694. doi: 10.2214/ajr.162.3.8109523. [DOI] [PubMed] [Google Scholar]
- 80.Preidler KW, Riepl T, Szolar D, Ranner G. Cerebral schistosomiasis: MR and CT appearance. AJNR Am J Neuroradiol. 1996;17:1598–1600. [PMC free article] [PubMed] [Google Scholar]
- 81.Kardaman MW, Amin MA, Fenwick A, et al. A field trial using praziquantel (BiltricideR) to treat Schistosoma mansoni and Schistosoma haematobium infection in Gezira, Sudan. Ann Trop Med Parasitol. 1983;77:297–304. doi: 10.1080/00034983.1983.11811711. [DOI] [PubMed] [Google Scholar]
- 82.Watt G, Adapon B, Long GW, et al. Praziquantel in treatment of cerebral schistosomiasis. Lancet. 1986;2:529–532. doi: 10.1016/s0140-6736(86)90110-8. [DOI] [PubMed] [Google Scholar]
- 83.Ferrari ML, Coelho PM, Antunes CM, et al. Efficacy of oxamniquine and praziquantel in the treatment of Schistosoma mansoni infection: a controlled trial. Bull World Health Organ. 2003;81:190–196. [PMC free article] [PubMed] [Google Scholar]
- 84.Xiao S, Tanner M, N’Goran EK, et al. Recent investigations of artemether, a novel agent for the prevention of schistosomiasis japonica, mansoni and haematobia. Acta Trop. 2002;82:175–181. doi: 10.1016/s0001-706x(02)00009-8. [DOI] [PubMed] [Google Scholar]
- 85.Moncayo A. Chagas disease: current epidemiological trends after the interruption of vectorial and transfusional transmission in the Southern Cone countries. Mem Inst Oswaldo Cruz. 2003;98:577–591. doi: 10.1590/s0074-02762003000500001. [DOI] [PubMed] [Google Scholar]
- 86.Busch MP, Kleinman SH, Nemo GJ. Current and emerging infectious risks of blood transfusions. JAMA. 2003;289:959–962. doi: 10.1001/jama.289.8.959. [DOI] [PubMed] [Google Scholar]
- 87.Dias JC, Silveira AC, Schofield CJ. The impact of Chagas disease control in Latin America: a review. Mem Inst Oswaldo Cruz. 2002;97:603–612. doi: 10.1590/s0074-02762002000500002. [DOI] [PubMed] [Google Scholar]
- 88.Leiby DA, Herron RM, Jr, Read EJ, et al. Trypanosoma cruzi in Los Angeles and Miami blood donors: impact of evolving donor demographics on seroprevalence and implications for transfusion transmission. Transfusion. 2002;42:549–555. doi: 10.1046/j.1537-2995.2002.00077.x. [DOI] [PubMed] [Google Scholar]
- 89.Sanchez-Guillen MC, Barnabe C, Guegan JF, et al. High prevalence anti-Trypanosoma cruzi antibodies, among blood donors in the State of Puebla, a non-endemic area of Mexico. Mem Inst Oswaldo Cruz. 2002;97:947–952. doi: 10.1590/s0074-02762002000700004. [DOI] [PubMed] [Google Scholar]
- 90.Leiguarda R, Roncoroni A, Taratuto AL, et al. Acute CNS infection by Trypanosoma cruzi (Chagas’ disease) in immunosuppressed patients. Neurology. 1990;40:850–851. doi: 10.1212/wnl.40.5.850. [DOI] [PubMed] [Google Scholar]
- 91.Kirk ML, Schofield CJ. Density-dependent timing of defaecation by Rhodnius prolixus, and its implications for the transmission of Trypanosoma cruzi. Trans R Soc Trop Med Hyg. 1987;81:348–349. doi: 10.1016/0035-9203(87)90262-8. [DOI] [PubMed] [Google Scholar]
- 92.Hall BF, Joiner KA. Developmentally-regulated virulence factors of Trypanosoma cruzi and their relationship to evasion of host defences. J Eukaryot Microbiol. 1993;40:207–213. doi: 10.1111/j.1550-7408.1993.tb04905.x. [DOI] [PubMed] [Google Scholar]
- 93.Anez N, Carrasco H, Parada H, et al. Acute Chagas’ disease in western Venezuela: a clinical, seroparasitologic, and epidemiologic study. Am J Trop Med Hyg. 1999;60:215–222. doi: 10.4269/ajtmh.1999.60.215. [DOI] [PubMed] [Google Scholar]
- 94.Delaporte F. Romana’s sign. J Hist Biol. 1997;30:357–366. doi: 10.1023/a:1004221722554. [DOI] [PubMed] [Google Scholar]
- 95.Dias JC. [Cecilio Romana, Romana’s sign and Chagas’ disease] Rev Soc Bras Med Trop. 1997;30:407–413. doi: 10.1590/s0037-86821997000500012. [DOI] [PubMed] [Google Scholar]
- 96.Prata A. Clinical and epidemiological aspects of Chagas disease. Lancet Infect Dis. 2001;1:92–100. doi: 10.1016/S1473-3099(01)00065-2. [DOI] [PubMed] [Google Scholar]
- 97.Bestetti RB, Muccillo G. Clinical course of Chagas’ heart disease: a comparison with dilated cardiomyopathy. Int J Cardiol. 1997;60:187–193. doi: 10.1016/s0167-5273(97)00083-1. [DOI] [PubMed] [Google Scholar]
- 98.Matsumoto TK, Hoshino-Shimizu S, Nakamura PM, et al. High resolution of Trypanosoma cruzi amastigote antigen in serodiagnosis of different clinical forms of Chagas’ disease. J Clin Microbiol. 1993;31:1486–1492. doi: 10.1128/jcm.31.6.1486-1492.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Umezawa ES, Nascimento MS, Stolf AM. Enzyme-linked immunosorbent assay with Trypanosoma cruzi excreted-secreted antigens (TESA-ELISA) for serodiagnosis of acute and chronic Chagas’ disease. Diagn Microbiol Infect Dis. 2001;39:169–176. doi: 10.1016/s0732-8893(01)00216-4. [DOI] [PubMed] [Google Scholar]
- 100.Di Lorenzo GA, Pagano MA, Taratuto AL, et al. Chagasic granulomatous encephalitis in immunosuppressed patients. Computed tomography and magnetic resonance imaging findings. J Neuroimaging. 1996;6:94–97. doi: 10.1111/jon19966294. [DOI] [PubMed] [Google Scholar]
- 101.Urbina JA. Specific treatment of Chagas disease: current status and new developments. Curr Opin Infect Dis. 2001;14:733–741. doi: 10.1097/00001432-200112000-00012. [DOI] [PubMed] [Google Scholar]
- 102.Rodriques Coura J, de Castro SL. A critical review on Chagas disease chemotherapy. Mem Inst Oswaldo Cruz. 2002;97:3–24. doi: 10.1590/s0074-02762002000100001. [DOI] [PubMed] [Google Scholar]
- 103.Viotti R, Vigliano C, Armenti H, Segura E. Treatment of chronic Chagas’ disease with benznidazole: clinical and serologic evolution of patients with long-term follow-up. Am Heart J. 1994;127:151–162. doi: 10.1016/0002-8703(94)90521-5. [DOI] [PubMed] [Google Scholar]
- 104.Cerisola JA, Neves da Silva N, Prata A, et al. [Evaluation of the efficacy of nifurtimox in chronic human chagasic infection by using xenodiagnosis (author’s transl)] Bol Chil Parasitol. 1977;32:51–62. [PubMed] [Google Scholar]
- 105.Smith DH, Pepin J, Stich AH. Human African trypanosomiasis: an emerging public health crisis. Br Med Bull. 1998;54:341–355. doi: 10.1093/oxfordjournals.bmb.a011692. [DOI] [PubMed] [Google Scholar]
- 106.Van Nieuwenhove S, Betu-Ku-Mesu VK, Diabakana PM, et al. Sleeping sickness resurgence in the DRC: the past decade. Trop Med Int Health. 2001;6:335–341. doi: 10.1046/j.1365-3156.2001.00731.x. [DOI] [PubMed] [Google Scholar]
- 107.Welburn SC, Fevre EM, Coleman PG, et al. Sleeping sickness: a tale of two diseases. Trends Parasitol. 2001;17:19–24. doi: 10.1016/s1471-4922(00)01839-0. [DOI] [PubMed] [Google Scholar]
- 108.McGovern TW, Williams W, Fitzpatrick JE, et al. Cutaneous manifestations of African trypanosomiasis. Arch Dermatol. 1995;131:1178–1182. [PubMed] [Google Scholar]
- 109.Kristensson K, Mhlanga JD, Bentivoglio M. Parasites and the brain: neuroinvasion, immunopathogenesis and neuronal dysfunctions. Curr Top Microbiol Immunol. 2002;265:227–257. doi: 10.1007/978-3-662-09525-6_12. [DOI] [PubMed] [Google Scholar]
- 110.Villanueva MS. Trypanosomiasis of the central nervous system. Semin Neurol. 1993;13:209–218. doi: 10.1055/s-2008-1041127. [DOI] [PubMed] [Google Scholar]
- 111.Enanga B, Burchmore RJ, Stewart ML, Barrett MP. Sleeping sickness and the brain. Cell Mol Life Sci. 2002;59:845–858. doi: 10.1007/s00018-002-8472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mhlanga JD, Bentivoglio M, Kristensson K. Neurobiology of cerebral malaria and African sleeping sickness. Brain Res Bull. 1997;44:579–589. doi: 10.1016/s0361-9230(97)00309-2. [DOI] [PubMed] [Google Scholar]
- 113.Lejon V, Buscher P. Stage determination and follow-up in sleeping sickness. Med Trop (Mars) 2001;61:355–360. [PubMed] [Google Scholar]
- 114.Gill DS, Chatha DS, del O’Donovan-O’Donovan R. MR imaging findings in African trypansomiasis. AJNR Am J Neuroradiol. 2003;24:1383–1385. [PMC free article] [PubMed] [Google Scholar]
- 115.Nok AJ. Arsenicals (melarsoprol), pentamidine and suramin in the treatment of human African trypanosomiasis. Parasitol Res. 2003;90:71–79. doi: 10.1007/s00436-002-0799-9. [DOI] [PubMed] [Google Scholar]
- 116.Matovu E, Enyaru JC, Legros D, et al. Melarsoprol refractory T. b. gambiense from Omugo, north-western Uganda. Trop Med Int Health. 2001;6:407–411. doi: 10.1046/j.1365-3156.2001.00712.x. [DOI] [PubMed] [Google Scholar]
- 117.Docampo R, Moreno SN. Current chemotherapy of human African trypanosomiasis. Parasitol Res. 2003;90(suppl 1):S10–S13. doi: 10.1007/s00436-002-0752-y. [DOI] [PubMed] [Google Scholar]
- 118.Parija SC, Jayakeerthee SR. Naegleria fowleri: a free living amoeba of emerging medical importance. J Commun Dis. 1999;31:153–159. [PubMed] [Google Scholar]
- 119.Gyori E. December 2002: 19-year old male with febrile illness after jet ski accident. Brain Pathol. 2003;13:237–239. [PubMed] [Google Scholar]
- 120.Jain R, Prabhakar S, Modi M, et al. Naegleria meningitis: a rare survival. Neurol India. 2002;50:470–472. [PubMed] [Google Scholar]
- 121.Barnett ND, Kaplan AM, Hopkin RJ, et al. Primary amoebic meningoencephalitis with Naegleria fowleri: clinical review. Pediatr Neurol. 1996;15:230–234. doi: 10.1016/s0887-8994(96)00173-7. [DOI] [PubMed] [Google Scholar]
- 122.Martinez AJ. Free-living amebic meningoencephalitides: comparative study. Neurol Neurocir Psiquiatr. 1977;18:391–401. [PubMed] [Google Scholar]
- 123.Carter RF. Primary amoebic meningo-encephalitis: clinical, pathological and epidemiological features of six fatal cases. J Pathol Bacteriol. 1968;96:1–25. doi: 10.1002/path.1700960102. [DOI] [PubMed] [Google Scholar]
- 124.Viriyavejakul P, Rochanawutanon M, Sirinavin S. Naegleria meningomyeloencephalitis. Southeast Asian J Trop Med Public Health. 1997;28:237–240. [PubMed] [Google Scholar]
- 125.Reveiller FL, Cabanes PA, Marciano-Cabral F. Development of a nested PCR assay to detect the pathogenic free-living amoeba Naegleria fowleri. Parasitol Res. 2002;88:443–450. doi: 10.1007/s00436-002-0591-x. [DOI] [PubMed] [Google Scholar]
- 126.Schumacher DJ, Tien RD, Lane K. Neuroimaging findings in rare amebic infections of the central nervous system. AJNR Am J Neuroradiol. 1995;16:930–935. [PMC free article] [PubMed] [Google Scholar]
- 127.Ferrante A. Free-living amoebae: pathogenicity and immunity. Parasite Immunol. 1991;13:31–47. doi: 10.1111/j.1365-3024.1991.tb00261.x. [DOI] [PubMed] [Google Scholar]
- 128.Loschiavo F, Ventura-Spagnolo T, Sessa E, Bramanti P. Acute primary meningoencephalitis from entamoeba Naegleria Fowleri. Report of a clinical case with a favourable outcome. Acta Neurol (Napoli) 1993;15:333–340. [PubMed] [Google Scholar]
- 129.Seidel JS, Harmatz P, Visvesvara GS, et al. Successful treatment of primary amebic meningoencephalitis. N Engl J Med. 1982;306:346–348. doi: 10.1056/NEJM198202113060607. [DOI] [PubMed] [Google Scholar]
- 130.Sell JJ, Rupp FW, Orrison WW., Jr Granulomatous amebic encephalitis caused by acanthamoeba. Neuroradiology. 1997;39:434–436. doi: 10.1007/s002340050440. [DOI] [PubMed] [Google Scholar]
- 131.Deol I, Robledo L, Meza A, et al. Encephalitis due to a free-living amoeba (Balamuthia mandrillaris): case report with literature review. Surg Neurol. 2000;53:611–616. doi: 10.1016/s0090-3019(00)00232-9. [DOI] [PubMed] [Google Scholar]
- 132.Healy JF. Balamuthia amebic encephalitis: radiographic and pathologic findings. AJNR Am J Neuroradiol. 2002;23:486–489. [PMC free article] [PubMed] [Google Scholar]
- 133.McCulley JP, Alizadeh H, Niederkorn JY. The diagnosis and management of Acanthamoeba keratitis. CLAO J. 2000;26:47–51. [PubMed] [Google Scholar]
- 134.Marciano-Cabral F, Cabral G. Acanthamoeba spp. as agents of disease in humans. Clin Microbiol Rev. 2003;16:273–307. doi: 10.1128/CMR.16.2.273-307.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Denney CF, Iragui VJ, Uber-Zak LD, et al. Amebic meningoencephalitis caused by Balamuthia mandrillaris: case report and review. Clin Infect Dis. 1997;25:1354–1358. doi: 10.1086/516141. [DOI] [PubMed] [Google Scholar]
- 136.Martinez AJ, Visvesvara GS. Free-living, amphizoic and opportunistic amebas. Brain Pathol. 1997;7:583–598. doi: 10.1111/j.1750-3639.1997.tb01076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Singhal T, Bajpai A, Kalra V, et al. Successful treatment of Acanthamoeba meningitis with combination oral antimicrobials. Pediatr Infect Dis J. 2001;20:623–627. doi: 10.1097/00006454-200106000-00016. [DOI] [PubMed] [Google Scholar]


