Abstract
Background
It can be seen from the available mollusk mitogenomes that the family Pectinidae exhibits the most variation in genome organization. In this study, comparative mitogenomic analyses were performed for three scallops from the subfamily Chlamydinae (Pectinidae), with the goal of characterizing the degree of variability of mitogenome organization and other characteristics among species from the same subfamily and exploring their possible evolution route.
Findings
The complete or nearly complete mtDNA sequences of scallop Mimachlamys nobilis (17 935 bp), Mizuhopecten yessoensis (20 964 bp) and Chlamys farreri (17 035 bp) were determined using long PCR amplification and primer walking sequencing strategy. Highly variable size difference of the three genomes resulted primarily from length and number variations of non-coding regions, and the major difference in gene content of the three scallop species are due to varying tRNA gene sets. Only 21, 16, and 17 tRNA genes were detected in the mitogenomes of M. nobilis, M. yessoensis and C. farreri, respectively. Remarkably, no trnS gene could be identified in any of the three scallops. A newly-detected trnA-like sequence within the mitogenome of M. yessoensis seems to exemplify the functional loss of a tRNA gene, and the duplication of trnD in M. yessoensis raises a fundamental question of whether the retention of the tRNA gene copy of 2-tRNAs is easier than that of 4-tRNAs. Analysis of putative evolutionary pathways of gene rearrangement indicates that transposition of neighboring gene blocks may play an important role in the evolution of mitogenomes in scallops. Parsimonious analysis of the genomic variations implies that the mitogenomes of M. yessoensis and C. farreri are likely to derive independently from a common ancestor that was closely related to M. nobilis.
Conclusion
Comparative mitogenomic analyses among three species from the subfamily Chlamydinae show that the three genomes exhibit a high level of genomic variation and a diversity of tRNA gene sets, characterized by extensive translocation of genes. These features provide useful clues and information for evolutionary analysis of scallop mitogenomes.
Findings
It can be seen from the available mollusk mitogenomes that the Pectinidae exhibits the most variation in genome organization. When we were initiating the current study, three complete or nearly complete mitogenomes, representing three subfamilies, were available from this family, i.e., Argopecten irradians (Aequipectini group, GenBank: EU023915), Mizuhopecten yessoensis (Chlamydinae, GenBank: AB271769) and Placopecten magellanicus (Palliolinae, GenBank: DQ088274). Obvious differences in mitogenome organization of three scallops were observed: 1) the sizes of three mitogenomes are distinct from each other, i.e. 16 221 bp for A. irradians, 20 414 bp for M. yessoensis and 32 115 bp for P. magellanicus [1]; 2) allegedly, the three mitogenomes have significantly different tRNA gene sets, with the numbers of 22, 32 and 9 for A. irradians, P. magellanicus and M. yessoensis, respectively; 3) the genomes show distinct gene arrangement patterns, namely unique rearrangements involving nearly every gene.
The degree of gene arrangements of mitogenomes from different species in the same subfamily, Chlamydinae, is one of our concerns. Therefore, in this study the complete mtDNA sequences of Mimachlamys nobilis and M. yessoensis, and nearly complete mtDNA sequence of Chlamys farreri are determined using long PCR amplification and primer walking sequencing strategy (see Additional file 1), and used for comparative analyses. Another reason for inclusion of M. yessoensis is that its first mitogenome data deposited in GenBank (AB271769) seems to bear significant omissions and mis-annotations of tRNA genes and protein-coding gene. Apparently, these mis-annotations need to be amended for further studies of gene order variation, evolution and phylogenetic analysis.
Genome organization and nucleotide composition
The size of the mitogenome is 17 935 bp for M. nobilis (GenBank: FJ595958). Due to technical difficulties in sequencing, a small part (up to a couple of hundreds base pairs) of the mitogenome of C. farreri was not obtained and the nearly complete genome is 17 035 bp in length (GenBank: FJ595957). Genome assembly indicated that the unfinished section is the start part of the major non-coding region (MNR). The complete mtDNA sequence of M. yessoensis obtained in this study is 20 964 bp in length (GenBank: FJ595959), which is 550 bp longer than the nearly complete genome. Genome assembly indicated that the previously unfinished section is part of the MNR. Annotation to the mitogenome obtained in the current study and a re-annotation to AB271769 revealed the following findings: 1) the "absent" cox2 gene in previous annotation is actually present, corresponding to nucleotides 14 638–15 325, with "CTG" as initiation codon and "T" as termination codon; and 2) the genome has a total of 16 tRNA genes, instead of nine identified in that nearly complete genome. Additionally, 56 transitions were detected from a comparison of mitogenomes FJ595959 and AB271769.
The three mitogenomes contain 12 protein-coding genes (PCGs), lacking the atp8 gene as in most bivalves, two ribosomal RNA genes and varying numbers of tRNA gene set (see Additional file 2; Additional file 3; Additional file 4; Additional file 5; Figure 1). No obvious difference in gene length was observed among three scallops (Additional file 2); thus, what contributes to the differences in genome sizes is primarily the number and length of non-coding regions (NCRs). An interesting finding of this study is the existence of repeat units within the NCR of M. yessoensis mtDNA (see Additional file 6). The nucleotide compositions and usage frequencies of entire mitogenome for three scallops have a similar pattern (Additional file 2). The nucleotide compositions are all strongly skewed away from C in favor of G (the GC-skews are 0.312, 0.248 and 0.319 separately) and from A in favor of T (the AT-skews are -0.252, -0.197 and -0.240 respectively) (Additional file 2). The AT content of the three genomes are 59.6%, 55.3% and 57.9% in M. nobilis, M. yessoensis, and C. farreri respectively, lower than the average AT content of 16 available bivalves (61.6%), but comparable to that of other two pectinids (57% for A. irradians; 55.7% for P. magellanicus). Up to date, the five species within the Pectinidae represent the lowest average AT content (57.1%) compared to other molluscan lineages (66% for Gastropoda; 69.4 for Polyplacophora; 71.2% for Scaphopoda; 71.7% for Cephalopoda). The AT contents are slightly higher in rrnL (61.0%, 57.8% and 58% separately) and lower in rrnS (56.7%, 50.6% and 52.5%) than that of each full mitogenome.
Figure 1.
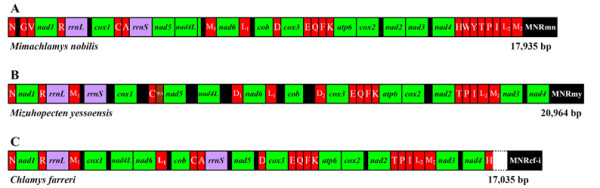
Organization of the mitochondrial genome of Mimachlamys nobilis (A), Mizuhopecten yessoensis (B) and Chlamys farreri (C). Protein and rRNA coding genes are abbreviated as in the text, and transfer RNA genes are depicted by their corresponding one-letter amino acid code. Non-coding regions (>50 bp in length) are labeled by black box and the major non-coding region is designated as "MNRmn", "MNRmy" and "MNRcf-i", respectively.
Protein-coding genes
Amino acid identity in proteins for pairs of three scallops ranged from as low as 54.3% in nad2 between M. nobilis and C. farreri to as high as 93.3% in cox1 between M. yessoensis and C. farreri (Table 1). M. yessoensis and C. farreri share a higher degree of amino acid similarity in all genes than do between other pairs. According to our data, it appears that the most conserved PCGs are cox1, nad1, and nad3 (identity > 80%), and the least conserved is nad2 (identity = 61.1%).
Table 1.
Protein-coding gene assignments and identity of Mimachlamys nobilis (Mnob), Mizuhopecten yessoensis (Myes) and Chlamys farreri (Cfar)
| Protein | % identity | ||
| Mnob-Myes | Mnob-Cfar | Myes-Cfar | |
| atp6 | 65.8 | 65.8 | 75.3 |
| cob | 64.2 | 67 | 80.5 |
| cox1 | 87 | 87.4 | 93.3 |
| cox2 | 64.9 | 65.3 | 75.7 |
| cox3 | 72.7 | 74.8 | 86.2 |
| nad1 | 82.2 | 81.2 | 85.4 |
| nad2 | 57.4 | 54.3 | 71.6 |
| nad3 | 75.8 | 77.8 | 89.9 |
| nad4 | 77.2 | 75.8 | 86 |
| nad4L | 72.1 | 71.2 | 85.6 |
| nad5 | 71.9 | 68.6 | 77.3 |
| nad6 | 64.8 | 67.9 | 75.9 |
In this study, most of PCGs in three mitogenomes use conventional initiation codons (20 for ATG, 10 for ATA and 1 for ATT), but two genes use the alternative ones (M. nobilis: nad1-TTG; M. yessoensis: cox2-CTG, nad1-GTG; C. farreri: cox2-GTG, nad1-GTG). Notably, one of the alternative initiation codons, GTG was also frequently used in two previously described scallop mitogenomes (5 for A. irradians and 2 for P. magellanicus). Only two genes use CTG as an initiation codon in the 45 reported molluscan mitogenomes (M. yessoensis: cox2; Lottia digitalis: cox1). Interestingly, six of 12 PCGs in M. yessoensis and C. farreri show a derived characteristic in their use of initiation codons when compared with those of M. nobilis, i.e. atp6 (ATA [M. nobilis]→ATG [M. yessoensis and C. farreri]), cox1 (ATA→ATG), cob (ATA→ATG), nad1 (TTG→GTG), nad2 (ATG→ATA) and nad4 (ATG→ATA). In general, the usage of initiation codons among three scallops is flexible, but not random.
Three genes stop with identical termination codon in all three scallops, i.e. nad1 (TAG), nad4 (TAG) and nad4L (TAA). Five genes (atp6, cox2, cox3, cob and nad2) share the same termination codon in the mitogenomes of C. farreri and M. nobili and three genes (cox1, nad3 and nad5) use the same termination codon in the genomes of M. yessoensis and M. nobilis. The nad6 in M. yessoensis and C. farreri display the derivative feature of termination codon usage when compared with that of M. nobilis (TAA [M. nobilis]→TAG [M. yessoensis and C. farreri]). The cox2 gene in both M. yessoensis and C. farreri contains a truncated termination codon, ending with a single thymine.
Transfer RNA genes
Despite an extensive search with the tRNAscan-SE [2] and by eye inspection, only 21, 16, and 17 tRNA genes were detected in the mitogenomes of M. nobilis, M. yessoensis and C. farreri, respectively (Additional file 7; Additional file 8; Additional file 9). Particularly, no trnS gene could be identified in any of the three scallops, and four tRNAs (trnG, trnV, trnW and trnY) were absent in the mitogenome of both M. yessoensis and C. farreri. Nevertheless, we presume that the loss of trnW and trnY in the mitogenome of C. farreri may be an artifact, due to unfinished sequencing of the region downstream of trnH. The loss of trnS may present the ancestral state for all three scallops of the subfamily Chlamydinae. The loss of the tRNA cluster "GV" in the mitogenome of M. yessoensis and C. farreri may present the first step of tRNA gene loss from the common ancestor of these two species belonging to the same tribe (Chlamydini). Additional derived features of tRNA gene loss and translocations were observed separately for both M. yessoensis and C. farreri, e.g. the deletions of trnH, trnW, trnY and trnA, and the duplication of trnD in the mitogenome of M. yessoensis.
Another interesting finding of this study is the identification of a trnA-like sequence within the mitogenome of M. yessoensis. For confirmation of this finding, at least three individuals of M. yessoensis were used separately for amplification and sequencing of this fragment. This trnA-like structure is just located following the trnC, where the tRNA gene cluster "CA" has been observed in the mitogenome of both M. nobilis and C. farreri. Alignment of this trnA-like sequence of M. yessoensis with trnA gene sequences of other four available scallops (A. irradians, P. magellanicus, M. nobilis and C. farreri) shows that it shares similar nucleotide sequences in amino acid arm, DHU arm, and anticodon arm with those of trnA genes, with a distinct sequence in the anticodon loop (Figure 2A). We therefore define this trnA-like genomic region as a pseudo-tRNA gene (PsA). The existence of PsA may provide evidence of intermediate status of tRNA gene loss. Several mechanisms have been proposed for tRNA gene loss [3-5]. In this case, trnA gene loss seems fit the model that tRNA genes are gradually lost functionally, and then physically, over long evolutionary periods of time. Gene functional loss via mutation of the anticodon loop may play the most important role in this case.
Figure 2.
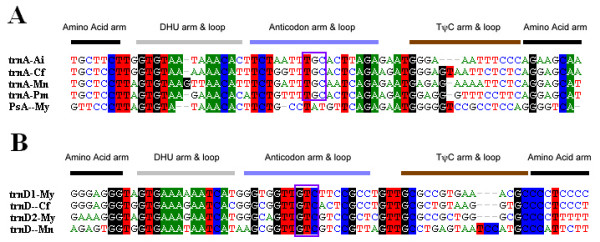
Alignment of the trnA and pseudogene (PsA) in mitochondrial genomes of five scallops (A), and alignment of trnD in mitochondrial genomes of the three scallops (B). tRNA secondary structure is designed above the alignment, and the position of the anticodon is highlighted with rectangular frame. Ai: Argopecten irradians Cf: Chlamys farreri Mn: Mimachlamys nobilis My: Mizuhopecten yessoensis Pm: Placopecten magellanicus.
In the mitogenomes of metazoan, almost all amino acids codons but leucine and serine are decoded by only one tRNA each [6]. The presence of two trnM genes was reported in the mitogenomes of tunicates [7]. However, it is a common phenomenon that mitogenomes of most bivalves contain two trnM genes. In this study, the trnM2 gene was found clustered with other tRNA genes, but the locations of trnM1 were variable over species. Another case of tRNA gene duplication is the trnD in M. yessoensis, which is found in single copy in the mitogenomes of both M. nobilis and C. farreri. Both trnD1 and trnD2 are considered true tRNA genes, based on sequence comparison (Figure 2B) as well as their putative secondary structures (Additional file 8). In order to probe into the mechanism of the retention of two copies of non-trnL/trnS tRNA genes, we examined the mitogenomes of all available mollusks for their tRNA gene usage, and found that a total of eight tRNA genes (trnD, trnE, trnF, trnK, trnM, trnN, trnQ, trnV) appear in at least two copies for each (see Additional file 10). Notably, all but trnV have anticodons corresponding to the 2-fold degenerate codons. This finding raises a fundamental question of whether the retention of the tRNA gene copy of 2-tRNAs is easier than that of 4-tRNAs (also see [6]). Perhaps there are differences in the gene loss and import mechanisms from the cytoplasm to the mitochondria of 4-tRNAs and 2-tRNAs. It is desirable to investigate the mechanisms of tRNA gene loss in further studies; more mitogenomes with variable tRNA gene sets should be included in future studies to draw a solid conclusion.
Putative evolutionary pathway of gene rearrangements
It is often difficult to trace the evolutionary pathway of metazoan mitogenomes due either to the generally low number of reorganization events (e.g., mammals) or drastic reorganizations in some animal lineages (e.g., nematodes, snails and brachiopods etc; see [8,9]), particularly in bivalves [e.g., [10,11]]. Phylogentic analyses for five scallops, using the concatenated amino acid sequences of 12 PCGs, resulted a high supported relationship which can be depicted as (A. irradians, (P. magellanicus, (M. nobilis, (M. yessoensis, C. farreri)))) (see Additional file 11). Based on the inferred phylogeny, a putative evolutionary pathway of gene rearrangement in the three species is assumed, and our preferred scenario of mitogenome evolution is further elaborated in Figure 3. In detail, there are at least three permutations between the mitogenomes of M. nobilis and that of a putative common ancestor of M. yessoensis and C. farreri: transposition of trnM1; transposition of the tRNA gene cluster "TPIL2M2", and loss of trnG and trnV. The tRNA gene cluster "HWY" is assumed to have been present in the putative common ancestor, based on the fact that trnH is just downstream of nad4 in C. farreri. Additionally, the absence of trnW and trnY may be artificial due to the incompletely sequenced downstream region. At least four independent events occurred in the mt gene rearrangement of M. yessoensis in the process of derivation from the putative common ancestor: deletion of the tRNA gene cluster "HWY", transposition of gene block "cox1-trnC-trnA" with rrnS, duplication of trnD and the generation of pseudogene PsA. Only one step is then necessary for the rearrangement of C. farreri from the common ancestor, i.e. the transposition of the large fragment "trnC-trnA-rrnS-nad5" with its neighbor block "nad4L-nad6-trnL1-cob".
Figure 3.
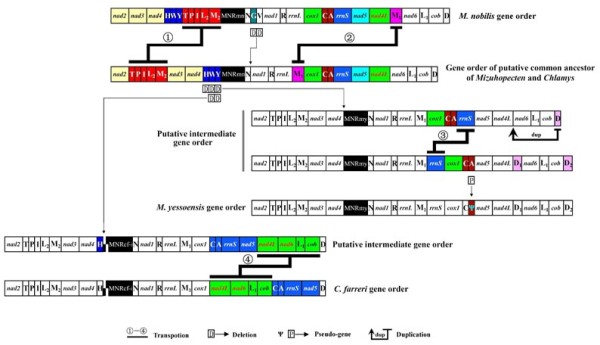
The putative evolutionary pathway of mitochondrial genomes for three scallops. The hypothetical pathway with the minimum number of gene duplication, deletion and transposition is illustrated. A putative common ancestor of M. yessoensis and C. farreri is shown as it can be most easily and parsimoniously explained. Putative intermediate gene order between "common ancestral gene order" and "M. yessoensis/C. farreri gene order" is also shown to explain the most parsimoniously possible evolutionary pathway of these two species. Symbols are explained under the figure and gene abbreviations are designated as in the text. Genes with identical order in the lineage are put in black/white boxes.
It has been commonly recognized that variations in gene order are relatively rare in mitogenomes of most metazoan lineages [8]. Dowton et al. [12] estimated a probability of 1/2664 for a single event of gene translocation occurring independently in two mitogenomes (starting from the same gene order in both). However, this probability could be an underestimate according to yet unidentified constraints on modes of gene rearrangements, and it should be applied cautiously. Pectinid bivalves seem to represent another example, as five sequenced mitogenomes exhibit significant genomic rearrangements, suggesting that gene rearrangements occurred frequently among lineages in this family. On the other hand, high amino acid sequence identity between M. yessoensis and C. farreri indicate that these species may have diverged only recently. As illustrated in Figure 3, transposition of neighbor gene blocks (transposition 1, 3 and 4) may play an important role in the evolution of scallop mitogenomes. There is no doubt that mt genomic rearrangements are in most cases appropriate markers to resolve both ancient and recent divergence processes [6], but the result of this study implies that a careful estimation of the rearrangement pathway is especially required in analyzing the highly variable organizations of mitogenomes in the Pectinidae. However, the scallop mitogenomes with such diversity in their organization seem to be a good model to elucidate molecular evolutionary and phylogenetic issues of mitogenomes in future studies.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
XW designed the research, carried out most of the experiments, performed the data analyses and drafted the manuscript; XX and XK carried out part of the experiments and participated in data analyses; ZY initiated, led the research, supervised all laboratory work and finalized the manuscript. All authors have read and approved the final manuscript.
Supplementary Material
Methods of PCR, sequencing and sequence analyses. Detailed methods for molecular experiments and data analyses are described in this file.
Basic information of mitochondrial genomes of three scallops. This table presents the positions and nucleotide sequence lengths of mitochondrial genomes of three scallops, and initiation and termination codons for protein-coding genes as well as tRNA gene anticodons (starting from trnN).
Organization of the mitochondrial genome of Mimachlamys nobilis. Protein and rRNA coding genes are abbreviated as in the text, and transfer RNA genes are depicted by their corresponding one-letter amino acid code. Non-coding regions (>50 bp in length) are labeled and the major non-coding region is designated as "MNRmn".
Organization of the mitochondrial genome of Mizuhopecten yessoensis. Protein and rRNA coding genes are abbreviated as in the text, and transfer RNA genes are depicted by their corresponding one-letter amino acid code. Non-coding regions (>50 bp in length) are labeled and the major non-coding region is designated as "MNRmy".
Organization of the mitochondrial genome of Chlamys farreri. Protein and rRNA coding genes are abbreviated as in the text, and transfer RNA genes are depicted by their corresponding one-letter amino acid code. Non-coding regions (>50 bp in length) are labeled and the incomplete sequenced major non-coding region is designated as "MNRcf-i".
Analyses of non-coding regions and repeat units in mitogenomes of three scallops. This section describes the unique character of non-coding regions and repeat units in mitogenomes of three scallops, including figure and references.
Putative secondary structures for the 21 transfer RNA genes of the Mimachlamys nobilis mitogenome. This figure show the putative secondary structures of tRNA generated by tRNAscan-SE 1.21.
Putative secondary structures for the 16 transfer RNA genes of the Mizuhopecten yessoensis mitogenome. This figure show the putative secondary structures of tRNA generated by tRNAscan-SE 1.21.
Putative secondary structures for the 17 transfer RNA genes of the Chlamys farreri mitogenome. This figure show the putative secondary structures of tRNA generated by tRNAscan-SE 1.21.
List of tRNA usage frequency in 45 available mollusk mitogenomes. This table shows the tRNA usage frequency in 45 available mollusk mitogenomes.
Phylogentic analyses for five scallops, using the concatenated amino acid sequences of 12 PCGs. Phylogenetic inferring were carried out using MEGA 4.1 for NJ (neighbor-joining), PAUP* 4b10 for MP (maximum parsimony) and PhyML for ML (maximum likelihood).
Acknowledgments
Acknowledgements
This work was financially supported by the CAS/SAFEA International Partnership Program for Creative Research Teams, Guangdong Science Foundation (No. 8451030101001660) and National Science Foundation of China (No. 40776088).
Contributor Information
Xiangyun Wu, Email: xywu2007@scsio.ac.cn.
Xiaodong Xu, Email: right2468@163.com.
Ziniu Yu, Email: carlzyu@scsio.ac.cn.
Xiaoyu Kong, Email: xykong@scsio.ac.cn.
References
- Smith DR, Snyder M. Complete mitochondrial DNA sequence of the scallop Placopecten magellanicus: Evidence of transposition leading to an uncharacteristically large mitochondrial genome. J Mol Evol. 2007;65:380–391. doi: 10.1007/s00239-007-9016-x. [DOI] [PubMed] [Google Scholar]
- Lowe TM, Eddy SR. A program for improved detection of transfer RNA genes in genomic sequence. Nucl Acids Res. 1997;25:955–964. doi: 10.1093/nar/25.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams KL, Palmer JD. Evolution of mitochondrial gene content: gene loss and transfer to the nucleus. Mol Phylogenet Evol. 2003;29:380–395. doi: 10.1016/S1055-7903(03)00194-5. [DOI] [PubMed] [Google Scholar]
- Borner GV, Morl M, Janke A, Paabo S. RNA editing changes the identity of a mitochondrial tRNA in marsupials. EMBO J. 1996;15:5949–5957. [PMC free article] [PubMed] [Google Scholar]
- Small I, Akashi K, Chapron A, Dietrich A, Duchene AM, Lancelin D, Marechal-Drouard L, Menand B, Mireau H, Moudden Y, Ovesna J, Peeters N, Sakamoto W, Souciet G, Wintz H. The strange evolutionary history of plant mitochondrial tRNAs and their aminoacyl-tRNA synthetases. J Hered. 1999;90:333–337. doi: 10.1093/jhered/90.3.333. [DOI] [Google Scholar]
- Podsiadlowski L, Braband A, Mayer G. The Complete mitochondrial genome of the onychophoran Epiperipatus biolleyi reveals a unique transfer RNA set and provides further support for the ecdysozoa hypothesis. Mol Biol Evol. 2008;25:42–51. doi: 10.1093/molbev/msm223. [DOI] [PubMed] [Google Scholar]
- Gissi C, Iannelli F, Pesole G. Complete mtDNA of Ciona intestinalis reveals extensive gene rearrangement and the presence of an atp8 and an extra trnM gene in ascidians. J Mol Evol. 2004;58:376–389. doi: 10.1007/s00239-003-2559-6. [DOI] [PubMed] [Google Scholar]
- Boore JL. Animal mitochondrial genomes. Nucl Acids Res. 1999;27:1767–1780. doi: 10.1093/nar/27.8.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi Y, Endo K, Tajima F, Ueshima R. The mitochondrial genome of the brachiopod Laqueus rubellus. Genetics. 2000;155:245–259. doi: 10.1093/genetics/155.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serb JM, Lydeard C. Complete mtDNA sequence of the North American freshwater mussel, Lampsilis ornata (Unionidae): An examination of the evolution and phylogenetic utility of mitochondrial genome organization in Bivalvia (Mollusca) Mol Biol Evol. 2003;20:1854–1866. doi: 10.1093/molbev/msg218. [DOI] [PubMed] [Google Scholar]
- Boore JL, Medina M, Rosenberg LA. Complete sequences of the highly rearranged molluscan mitochondrial genomes of the scaphopod Graptacme eborea and the bivalve Mytilus edulis. Mol Biol Evol. 2004;21:1492–1503. doi: 10.1093/molbev/msh090. [DOI] [PubMed] [Google Scholar]
- Dowton M, Castro LR, Austin AD. Mitochondrial gene rearrangements as phylogenetic characters in the invertebrates: The examination of genome "morphology". Invertebr Syst. 2002;16:345–356. doi: 10.1071/IS02003. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Methods of PCR, sequencing and sequence analyses. Detailed methods for molecular experiments and data analyses are described in this file.
Basic information of mitochondrial genomes of three scallops. This table presents the positions and nucleotide sequence lengths of mitochondrial genomes of three scallops, and initiation and termination codons for protein-coding genes as well as tRNA gene anticodons (starting from trnN).
Organization of the mitochondrial genome of Mimachlamys nobilis. Protein and rRNA coding genes are abbreviated as in the text, and transfer RNA genes are depicted by their corresponding one-letter amino acid code. Non-coding regions (>50 bp in length) are labeled and the major non-coding region is designated as "MNRmn".
Organization of the mitochondrial genome of Mizuhopecten yessoensis. Protein and rRNA coding genes are abbreviated as in the text, and transfer RNA genes are depicted by their corresponding one-letter amino acid code. Non-coding regions (>50 bp in length) are labeled and the major non-coding region is designated as "MNRmy".
Organization of the mitochondrial genome of Chlamys farreri. Protein and rRNA coding genes are abbreviated as in the text, and transfer RNA genes are depicted by their corresponding one-letter amino acid code. Non-coding regions (>50 bp in length) are labeled and the incomplete sequenced major non-coding region is designated as "MNRcf-i".
Analyses of non-coding regions and repeat units in mitogenomes of three scallops. This section describes the unique character of non-coding regions and repeat units in mitogenomes of three scallops, including figure and references.
Putative secondary structures for the 21 transfer RNA genes of the Mimachlamys nobilis mitogenome. This figure show the putative secondary structures of tRNA generated by tRNAscan-SE 1.21.
Putative secondary structures for the 16 transfer RNA genes of the Mizuhopecten yessoensis mitogenome. This figure show the putative secondary structures of tRNA generated by tRNAscan-SE 1.21.
Putative secondary structures for the 17 transfer RNA genes of the Chlamys farreri mitogenome. This figure show the putative secondary structures of tRNA generated by tRNAscan-SE 1.21.
List of tRNA usage frequency in 45 available mollusk mitogenomes. This table shows the tRNA usage frequency in 45 available mollusk mitogenomes.
Phylogentic analyses for five scallops, using the concatenated amino acid sequences of 12 PCGs. Phylogenetic inferring were carried out using MEGA 4.1 for NJ (neighbor-joining), PAUP* 4b10 for MP (maximum parsimony) and PhyML for ML (maximum likelihood).


