Abstract
This study evaluates the effect of Medicare Part D among seniors who previously lacked drug coverage, using time-trend analyses of patient-level dispensing data from three pharmacy chains. Of 114,766 seniors without drug benefits, 55 percent initiated drug insurance under Part D. After the penalty-free Part D enrollment period, use of statins, clopidogrel, and proton pump inhibitors stabilized at levels ranging from 11 percent to 37 percent above the trend that would have been expected if Part D had not been implemented. Patients reaching the Part D coverage gap (12 percent) experienced a decrease in essential medication use ranging from 5.7 percentage points per month for warfarin to 6.3 percentage points for statins.
Before implementation of Medicare’s Part D prescription drug benefit, one-third of Medicare’s forty-three million elderly beneficiaries had no prescription drug coverage.1 Surveys of seniors without such coverage indicated that they often restricted their medication use because of high costs.2 The Medicare prescription drug benefit was established to assist seniors by covering prescription drugs for all who voluntarily enroll. As of June 2006, 22.5 million seniors had enrolled in a Medicare Part D plan, 15.8 million had another source of drug coverage, and approximately 4.4 million (10 percent) still had no coverage.3
To control costs, legislators relied on cost-sharing requirements and formulary management by private prescription drug plans. Beneficiaries enrolling in Part D were required to pay monthly premiums, a deductible, and differing copayments depending on their absolute drug spending during the year, with exact amounts varying by drug benefit plan and subsidy level.4 One controversial component of the standard Part D benefit is a period of no coverage (the “doughnut hole”). Once total spending on a beneficiary’s prescription drugs reached $2,250 in 2006, he or she was required to pay the entire cost of medications until reaching $3,600 in out-of-pocket spending, at which point coverage resumed.5 In the first months of Part D, 1,429 stand-alone drug plans, regulated and overseen by the Centers for Medicare and Medicaid Services (CMS), were offered to seniors.6 These plans offered a wide variety of formularies and patient cost-sharing requirements, all of which used the defined standard Part D benefit or a design shown to be “actuarially equivalent.”7
Studies on the overall effect of Medicare Part D on seniors’ drug use and out-of-pocket spending have suggested that the policy resulted in a 5.9–12.8 percent increase in prescription drug use and a 13.1–15.6 percent decrease in out-of-pocket spending.8 However, these studies did not examine the experiences of seniors who lacked drug coverage before Part D, nor did they evaluate the effect of the coverage gap. This study sought to assess the effect of Medicare Part D on the use of selected essential drugs among seniors who previously lacked drug coverage, using data from three large pharmacy chains.
Study Data And Methods
Using time-trend analyses of pharmacy dispensing data from before and after the implementation of Medicare Part D, we assessed how drug use and out-of-pocket spending were affected, focusing on the consequences for those patients with sufficient prescription drug use to have reached the coverage gap.
Data sources and study population
To study drug-use patterns among patients without drug coverage, it was by definition necessary to identify our study population without using insurance claims databases. We therefore chose computerized pharmacy dispensing information recorded by pharmacy chains. We used records of all prescription drugs dispensed to people over age sixty-five at three pharmacy chains operating in multiple states from 1 January 2005 through 31 December 2006. Data included randomly assigned study identification numbers that were linkable over time within a chain but not between chains; patients’ ZIP codes; and, for each dispensed drug, the out-of-pocket payment amount, the quantity and strength dispensed, and the National Drug Code (NDC).
Insurance status
Detailed drug plan (third-party payment) information was not available. We therefore assigned patients’ insurance status (uninsured, Part D, or Part D coverage gap) with algorithms that considered medication costs and patients’ out-of-pocket spending. Drug costs were calculated as 80 percent of the average wholesale price for each NDC as recommended by the U.S. Department of Health and Human Services (HHS).9 Our algorithm to identify seniors who were uninsured in 2005 examined all drugs costing more than $20 per fill that patients filled during 2005. Patients paying 60 percent or more of the drug price for 80 percent or more of relevant prescription fills were considered uninsured. We ignored drugs costing $20 or less in the algorithm to determine insurance status because copays for such drugs might easily exceed 60 percent of the drug’s cost, artificially making patients appear uninsured. Drugs costing $20 or less were included in our subsequent analyses of the effects of Part D. This algorithm aimed to provide high specificity at the cost of lower sensitivity to ensure that seniors categorized as having no drug insurance truly had no coverage.
Newly insured
Previously uninsured patients were assigned to the group of newly insured under Part D based on their out-of-pocket payments in 2006; payments suggesting coverage were either even dollar amounts or less than 40 percent of the costs of obtained drugs that cost more than $20. To be considered insured, patients were required to meet this definition for at least half of all relevant prescriptions filled between 1 January 2006 and the date when the cumulative price of the patient’s prescriptions reached $2,000. The Part D insurance date was defined as the date of the first of two consecutive prescriptions meeting these criteria. Although we were unable to validate this algorithm, sensitivity analyses using more and less stringent algorithms had little influence on the number of patients identified and on Part D effect estimates, leading to the conclusion that cutoff points were chosen in a range where there was little ambiguity about Part D coverage status.10
Coverage gap
Insured subjects were assigned to the coverage gap group when cumulative total spending on all prescription fills in 2006 reached $2,250. When cumulative patient out-of-pocket payments reached $3,600, patients reached catastrophic coverage; at that point, their data were censored from the coverage gap group.
Same-chain requirement
Because data would be lost if patients used an out-of-chain pharmacy, we analyzed the records only of patients who were continuous users—having at least one fill of any prescription drug with the same chain in the last quarter of 2004 and in the first quarter of 2007.
Study drugs
We selected four essential medication classes representing expensive drugs (clopidogrel), less expensive drugs (warfarin), drugs used to treat symptomatic conditions (clopidogrel, proton-pump inhibitors [PPIs]), and drugs used to treat asymptomatic conditions (statins). Also, although there is overuse and underuse of all study drugs, the cardiovascular drugs included have been shown to be underused in elderly patients, while some believe that PPIs are overused.11 For all four classes, at least one form of generic medication was available or became available during the study period.
Analysis
To estimate the effect of the introduction of Part D, we calculated drug use in defined daily doses (DDDs) and out-of-pocket payments in U.S. dollars per thirty DDDs for each calendar month from 1 January 2005 through 31 December 2006.12 We separately conducted the analysis for all previously uninsured patients and for those who actually enrolled in a Part D plan in 2006. The former analysis assesses the net Part D policy effect among the previously uninsured; the latter, the effect on seniors who actually took advantage of Part D.13
We also evaluated the effects of reaching the coverage gap on usage and out-of-pocket spending among subjects who became insured under Part D in 2006 and had at least one fill for the class of interest before reaching the coverage gap in 2006 (indicating the need for the drug). For this analysis, the proportion of patients filling a prescription was defined for each thirty-day interval before and after the date each patient reached the coverage gap. For each subject, interval 0 was defined as the first such interval beginning on the date when the coverage gap was reached. Interval −1 was the thirty-day interval preceding interval 0.
Patients’ characteristics
We used dispensing information from 1 July through 31 December 2005 to characterize our study populations, including age; sex; region of residence; median income level and population density of residential ZIP code; Chronic Disease Score; the number of different drugs used; and, specifically, use of anticoagulants, loop diuretics, nitrates, or antidiabetic drugs as proxies for the presence of atrial fibrillation, congestive heart failure, angina, and diabetes, respectively.14
Statistical analysis
We used segmented linear regression models that estimate sudden changes in trends of monthly outcome rates (monthly drug use and out-of-pocket spending) from one segment of time to the next.15 We defined three time segments: a twelve-month baseline period (January–December 2005), a four-month transition period during which Medicare recipients were allowed to enroll in Part D without penalty (January–April 2006), and an eight-month stable Part D period (May–December 2006).
Changes in trends of monthly outcome rates attributable to the introduction of Medicare Part D were estimated as changes in levels and slopes of linear trend estimates in linear regression models adjusted for autoregression by repeated observations (one-month lag time). Models included a constant term, a linear baseline time trend, a binary indicator and linear time trend for the transition period to compare the transition period versus baseline, and a binary indicator and a linear time trend for the stable Part D period versus baseline. Normally distributed errors were assumed for total monthly use and out-of-pocket spending per DDD, based on the large number of observations and the central limit theorem.16
To calculate cumulative changes in use and out-of-pocket spending attributable to Part D during January–December 2006, we summed the differences between the predicted values from the model above and those obtained by extrapolating the 2005 baseline trend.17
For the analysis of the effects of the coverage gap on drug usage, the regression model included a linear time trend for the eight thirty-day intervals before reaching the gap, and a binary indicator and a linear time trend for the eight thirty-day intervals afterward. We excluded the intervals in which the coverage gap was reached (interval 0) because in that interval, by definition, every patient had one or more fills to push the cumulative spending above the Medicare threshold. Since by design we will find particularly high usage in interval 0, we would expect to find unusually low usage in the interval immediately preceding (interval −1), because the average time between refills was about thirty days. This interval was excluded from the analysis as well.
Study Results
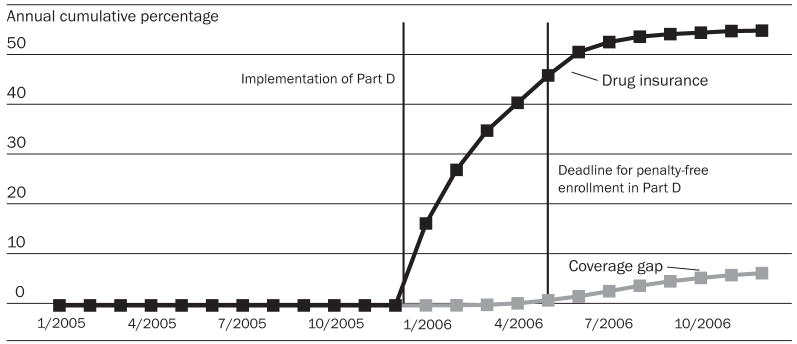
Out of 1.5 million patients age sixty-five and older identified in three pharmacy chains, 202,548 (13.7 percent) had no drug coverage from any source throughout 2005. Of those, 114,766 were continuous users of one chain and constituted the primary study cohort.18 In total, 62,495 patients (55 percent) without prior drug coverage received such coverage after the introduction of Medicare Part D in 2006. Most of this coverage was initiated in the first half-year of 2006 ( Exhibit 1). Among those patients, 7,325 (11.7 percent) met our criteria for reaching the coverage gap by December 2006, and, of these, 196 reached a catastrophic coverage level.
EXHIBIT 1. Prescription Drug Coverage Among Elderly Medicare Beneficiaries Who Did Not Have Drug Coverage Before The Implementation Of Medicare Part D, By Month, January 2005–December 2006.
SOURCE: Authors’ analysis of computerized pharmacy dispensing information from three pharmacy chains.
NOTE: Subjects without drug coverage were identified as those who paid 60 percent or more of a drug’s price for 80 percent or more of drugs dispensed in 2005.
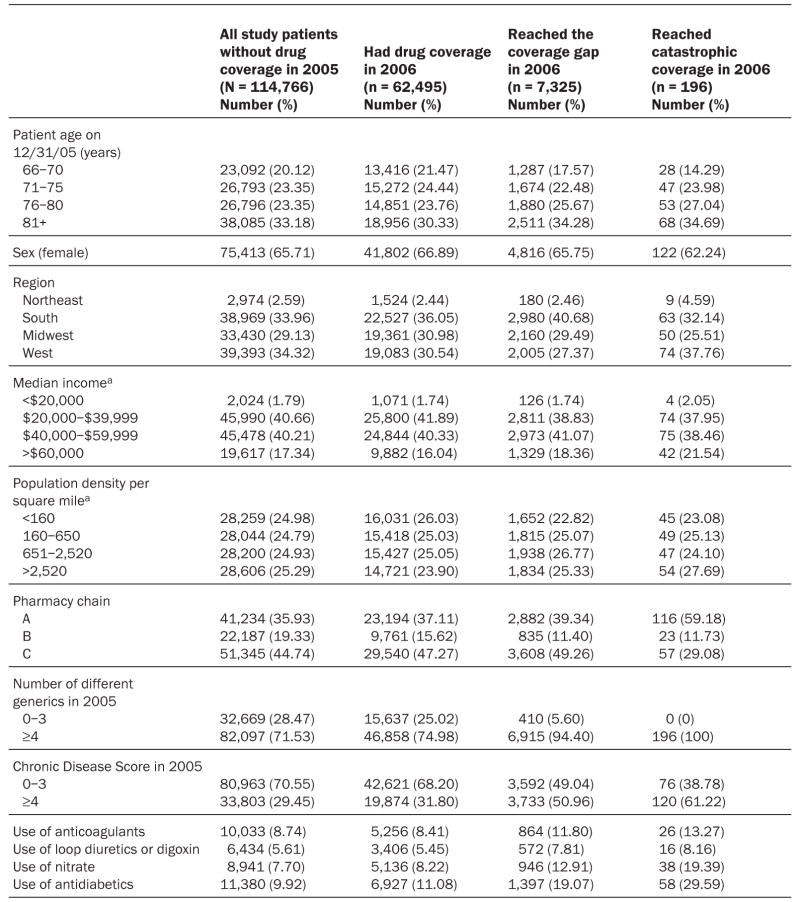
The mean age of the primary study population was 77.4 years (±7.1), and two-thirds were women ( Exhibit 2). Patients lived in forty-nine states and were equally likely to live in densely populated areas as in rural areas. Only 2.6 percent of the study sample resided in the Northeast.
EXHIBIT 2. Characteristics Of Elderly Medicare Beneficiaries Who Continuously Filled Prescriptions In One Of Three Pharmacy Chains Over The Study Period, January 2005–December 2006.
SOURCE: Authors’ analysis of computerized pharmacy dispensing information from three pharmacy chains.
a According to patient ZIP code and national survey data.
Medication use
The majority of patients (71 percent) had used four or more different medications in the six months before Part D, and sizable fractions had a Chronic Disease Score of 4 or higher (30 percent) or used antidiabetic drugs (10 percent) or nitrates (8 percent). The subgroup of patients who received drug coverage under Part D was, on average, slightly sicker and used more essential medications at baseline than those without drug coverage. The subgroup of patients who then reached the coverage gap was substantially sicker than those without coverage (51 percent had a Chronic Disease Score of 4 or higher), and 19 percent used antidiabetic drugs.
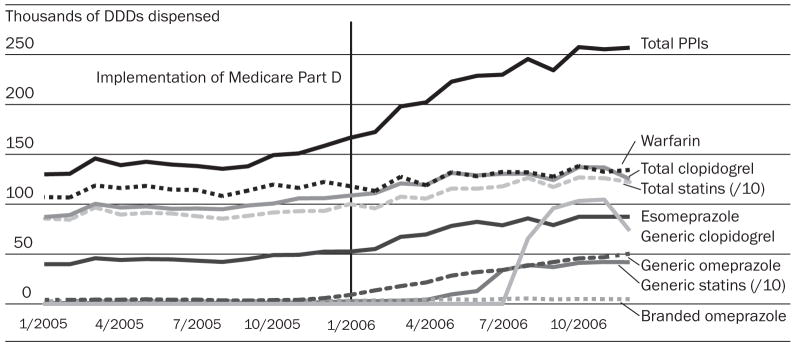
For all seniors without prior drug benefits, usage trends for almost all drugs began to increase in January 2006 with the start of Part D (Exhibit 3). During the stable Part D period, we found large and statistically significant increases in the use of all study drugs except warfarin, compared with the 2005 baseline trends (Exhibit 4). This resulted in a total increase of 22 percent for statin use (more than 2.5 million daily doses), 11 percent for clopidogrel use (more than 154,000), and 37 percent for use of PPIs (more than 723,000).
EXHIBIT 3. Use Of Selected Essential Drugs Among Elderly Medicare Beneficiaries Who Did Not Have Drug Coverage Before The Implementation Of Medicare Part D, January 2005–December 2006.
SOURCE: Authors’ analysis of computerized pharmacy dispensing information from three pharmacy chains.
NOTES: Generally we were interested in studying the effects of Part D on the entire drug classes selected for this study. For statins as well as clopidogrel, generic formulations became available during the study period, which enabled us to assess the amount of generic substitution under Part D for those drug classes. Esomeprazole was broken out because it is therapeutically equivalent to omeprazole but priced higher; this allowed us to study potentially wasteful prescribing under Part D. DDDs are defined daily doses. PPIs are proton pump inhibitors.
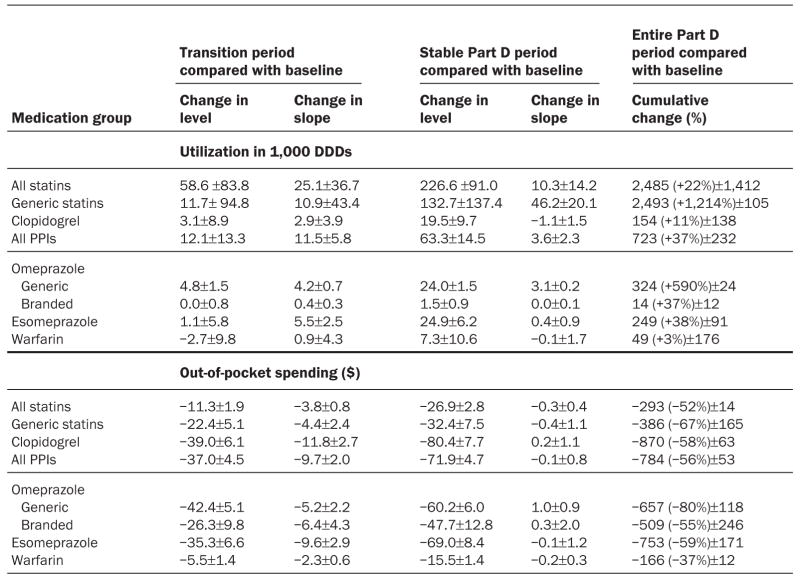
EXHIBIT 4. Changes In Utilization And Copayment Trends Under Medicare Part D For Selected Medication Groups Among Elderly Medicare Beneficiaries With No Prior Drug Coverage, Relative To 2005 Baseline Trends.
SOURCE: Authors’ analysis of computerized pharmacy dispensing information from three pharmacy chains.
NOTES: Transition period was January–April 2006. Stable Part D period was May–December 2006. Baseline period was January–December 2005. The R2 value was 0.89 or greater for all models. DDD is defined daily doses.
Generic pravastatin and simvastatin made up about 29 percent of all statins dispensed shortly after their market entry in April and June 2006, respectively. Use of these less costly generics immediately increased by 132,700 daily doses per month in the Part D period (Exhibit 4). Generic clopidogrel entered the market in August 2006; almost immediately, its market share rose to 68 percent. The substantial uptake of generic clopidogrel resulted in an additional 26 percent reduction in out-of-pocket spending for this drug (Exhibit 4).
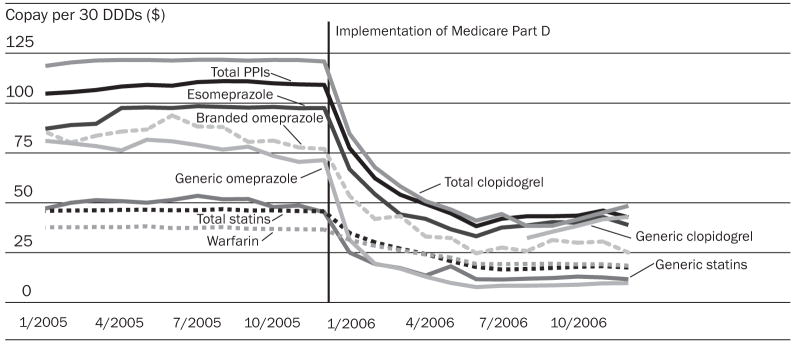
Range of copayments
During the transition period, copays for all drugs, with the exception of brand-name omeprazole, declined significantly (Exhibit 5). As a result, monthly copays per thirty DDDs were $15–$80 lower during the stable Part D period as compared to the pre-Part D period (Exhibit 4).
EXHIBIT 5. Out-Of-Pocket Spending For Selected Essential Drugs Among Elderly Medicare Beneficiaries Who Did Not Have Drug Coverage Before The Implementation Of Medicare Part D, January 2005–December 2006.
SOURCE: Authors’ analysis of computerized pharmacy dispensing information from three pharmacy chains.
NOTES: See Exhibit 3 Notes. DDDs are defined daily doses. PPIs are proton pump inhibitors.
Compared to the entire population of seniors who were uninsured in 2005, the 55 percent of patients who actually enrolled in a drug insurance plan under Part D experienced proportionally larger changes in usage and out-of-pocket spending, including a statistically significant increase in warfarin use.19
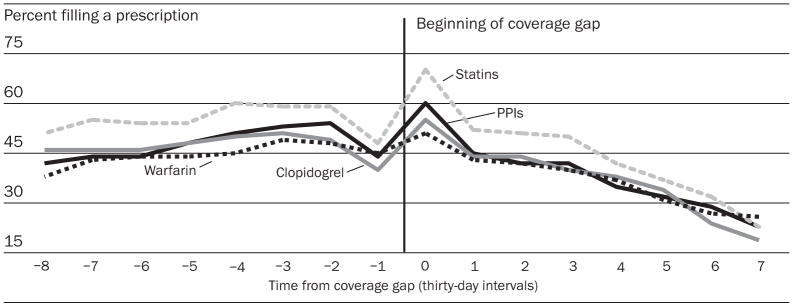
Among patients who reached the coverage gap, we observed a decline in the proportion of patients filling the study drugs that they used in previous months under Part D. Use of clopidogrel, which had been stable prior to the coverage gap, decreased at a rate of 5.0 percentage points per month (95 percent confidence interval [CI]: 3.2–6.8). Warfarin and statin use, which had been increasing at a rate of one percentage point per month prior to the coverage gap, decreased at 4.8 (95 percent CI: 3.8–5.7) and 6.3 (95 percent CI: 4.8–7.8) percentage points relative to the baseline trend. These decreases in use (Exhibit 6) corresponded temporally to increased copayment requirements during the coverage gap. Out-of-pocket payment increases from the months before the coverage gap to the months after it ranged from $12 per thirty DDDs for warfarin (95 percent CI: $11–$14) to $65 for clopidogrel (95 percent CI: $59–$70).
EXHIBIT 6. Use Of Four Selected Essential Drugs Among Elderly Medicare Beneficiaries Who Did Not Have Drug Coverage Before The Implementation Of Medicare Part D, Who Reached The Part D Coverage Gap Before 1 September 2006.
SOURCE: Authors’ analysis of computerized pharmacy dispensing information from three pharmacy chains.
NOTES: Two data points were omitted from the analysis. We excluded the thirty-day interval in which the coverage gap was reached (interval 0), because in that interval, by definition every patient had one or more prescription fills to push the cumulative spending above the Medicare Part D threshold. Because, by design, we will find particularly high usage in interval 0, we would expect to find unusually low use in the interval immediately preceding (interval −1), because the average time between refills is about thirty days. PPIs are proton pump inhibitors.
Discussion
Our analysis of the effects of Medicare Part D, a large-scale natural experiment among uninsured seniors, showed that the implementation of the benefit was associated with a sizable initial reduction in out-of-pocket drug spending and a meaningful increase in the use of selected essential medications.
The extent to which Medicare Part D stimulated increased dispensing of overused versus underused medications requires further study. Warfarin use was the least affected by the introduction of Medicare Part D, likely because of its relatively low cost and narrow therapeutic range requiring closer physician monitoring. In contrast, PPIs, a medication class that is frequently overused, experienced the steepest increase in use.
The benefit of Medicare Part D was not evenly distributed throughout the year. A sizable proportion of patients reached the coverage gap before the end of the year, which resulted in meaningfully reduced dispensing of previously used essential medications. Consequently, overall use of statins and clopidogrel, drugs with proven effectiveness, reached a plateau and started to decline in late 2006. Although the coverage gap may have been a necessary component of the Part D legislation to allow for passage of the bill, our results suggest that it mitigated gains in access to prescription drugs in our study setting.
Consistency with previous studies
Our results are consistent with those of two previous studies, which estimated 5.9 percent and 12.8 percent increases in prescription drug use and 13.1 percent and 15.6 percent decreases in out-of-pocket spending, respectively, among all seniors, regardless of pre- and post-Part D insurance status.20 We found that among previously uninsured seniors, drug use increased 3–37 percent and out-of-pocket spending decreased 37–58 percent, with the results varying by drug class. Assuming that the effects of Medicare Part D were limited largely to the one-third of seniors who were previously uninsured, our estimates should be approximately three times those previously reported. The demand elasticities implied by our results are 0.35 for warfarin, 0.44 for statins and clopidogrel, and 0.76 for PPIs. Previous estimates used by Mark Pauly in forecasting the effect of Medicare Part D (0.4) and calculated by Frank Lichtenberg and Sean Sun from an analysis of Walgreens data (0.72) are within the range observed in our study.21 One advantage to our class-based analysis is that it allowed for a detailed assessment of the effect of the policy change on medication use for patients with specific diseases; Part D evaluations that lump all drug classes together risk generalizing findings that are in fact quite heterogeneous.
Our findings were not entirely unexpected. A preponderance of evidence indicates that increased patient cost sharing has a strong effect on overall medication use and adherence to specific therapies.22 Reduced drug usage as a consequence of reaching a coverage gap has been associated with worse health outcomes.23 Our study found that increasing and limiting coverage similarly affected the use of under-used and overused medications, consistent with results from the RAND Health Insurance Experiment, which showed patients’ difficulty discriminating between more and less essential treatments under increased cost sharing.24
Impact of Part D on generic drug use
There was a rapid uptake of newly marketed generic statins and esomeprazole under Medicare Part D. Advocates of the use of private drug plans to deliver prescription drugs to seniors have argued that such plans could reduce costs for prescribed medications by steering patients toward more cost-effective medications such as generic versions of their current medications.25 We did not directly test the effect of Part D on generic medication use, but our findings are consistent with this contention.
Strengths of our analysis
Implementation of Medicare Part D on 1 January 2006 constituted a large-scale natural experiment. Interrupted time-trend analyses of drug coverage changes are considered among the most valid study designs, short of randomization.26 By establishing baseline time trends of drug usage in 2005 that were then followed into 2006, we can be confident that a comparison of post– to pre–Part D utilization trends provides a valid estimate of the Part D effect, assuming no other major interventions in early 2006 that would have affected drug use. Because the patients we studied were continuous users of the same pharmacy chain before and after the policy change, we believe that changes in the composition of our population over time do not account for the substantial utilization changes that occurred immediately after the policy change in this aggregate-level analysis.27 Although individual subjects’ characteristics could have changed over time as a result of aging, and we did not have diagnostic information to fully characterize patients’ health states, only an implausibly massive and sudden change in health or a substantial, simultaneous health system intervention could explain our findings.28 Our study is based on drug-use data that include patients with and without drug benefits, since they were collected independent of coverage status.
Limitations of our analysis
Despite these strengths, several limitations must be considered. Generalizability to all Part D–eligible patients without prior drug coverage may be limited because the patients in our study were continuous users of the same pharmacy chain before and after the study period. Although this does not rule out that patients received drugs in other pharmacies concurrently, our findings are generalizable to patients who tend to prefer a particular pharmacy or live near only one pharmacy. The U.S. Northeast was underrepresented in our study, but it is unlikely that the uninsured in the Northeast would respond differently to Part D than uninsured patients elsewhere in the United States. We could not account for over-the-counter (OTC) medication use, which may have complicated the PPI drug class analysis, because enrollment in Part D may have led some patients to switch from OTC to prescription medications.
We also did not have information about enrollment in specific prescription drug plans, which are likely to vary greatly in their particular formularies and cost-sharing requirements. We generated specific but not necessarily sensitive algorithms to assign insurance status, including the coverage gap. Testing variations in the assignment algorithm of insurance status in 2006 confirmed the robustness of our definitions. The high specificity ensured high internal validity of the policy-effect estimates because we avoided contamination of our study populations. However, it resulted in an underestimation of the proportion of seniors without drug coverage and possibly underestimated the proportion of patients who registered for drug benefits in 2006 or those reaching the coverage gap.29 Only detailed insurance data would allow a precise assessment of drug benefits, but these data would not include drug usage data for previously uninsured patients, rendering the data useless for our study question. Additional studies linking pharmacy-level data to health plan data would allow researchers to better assess the population of patients who use multiple pharmacies and to better identify the presence or absence of drug coverage.
We also did not include a concurrent control group in this analysis to more completely account for any underlying temporal trends in medication use. However, an analysis of patients dually eligible for Medicare and Medicaid, who had coverage through Medicaid during 2005 and were automatically enrolled in Medicare Part D in 2006, was conducted with this data set and indicated no significant change in the use of medication classes in our study after the implementation of Part D.30 This finding diminishes but does not completely eliminate concerns regarding the absence of controls. Also, we did not consider the cost of Medicare Part D to the government.
As seniors participated in Part D plans, it is possible that some received their medication via mail, which would not be recorded in our data. This would lead to an underestimation of the increased use of medications under Part D and could possibly overstate the reduced use during the coverage gap. Although regression to the mean of the coverage gap effect cannot be fully ruled out in an uncontrolled time-series analysis, a cohort study approach showed similar results.31 We also cannot determine precisely which patients entered the coverage gap. Only 16 percent of plans provided coverage in the coverage gap in 2006, but numerous cost-sharing strategies were used.32 Our estimates of total drug spending per patient were based on cost estimates using 80 percent of the average wholesale price, leading to imprecision of cost estimates for individual drugs.33 Additionally, we may have randomly misclassified the exact date on which patients entered the coverage gap, which would lead to an underestimation of the observed reduction in usage. Despite the measurement error, there does seem to be a strong relationship between patients’ out-of-pocket spending and medication use that closely corresponds to the time that we would expect patients to be entering the coverage gap.
The introduction of Medicare Part D was a mixed blessing for seniors without prior drug benefits. To the credit of the benefit, patients who enrolled were more likely to use essential medications, including clopidogrel, statins, and to a lesser extent warfarin, that are likely to result in better health outcomes. However, a sizable proportion of sicker patients reached the coverage gap in the first year and experienced a drop in the use of drugs they had used before reaching the coverage gap, which may result in worse health outcomes.
Additionally, while private drug plans may promote the use of generic drugs, there is also evidence that coverage within these plans encourages greater use of drugs that tend to be overused as well as those that are underused, and it may not adequately distinguish between the two. Increased implementation of benefit designs that require evidence of clinical appropriateness before authorizing medication use, or implementation of value-based benefit designs that reduce cost-sharing requirements for the most effective medications, may help encourage more appropriate and cost-effective medication use in Part D. Moreover, efforts to provide additional coverage in the coverage gap for certain essential medications may assist in optimizing coverage and the health of seniors.
Acknowledgments
An earlier version of this paper was presented at the Twenty-third International Conference on Pharmacoepidemiology and Therapeutic Risk Management, 19–22 August 2007, in Québec City, Canada. The study was funded by grants from the Robert Wood Johnson Foundation’s Changes in Health Care Financing and Organization (HCFO) Initiative and from the National Institute of Mental Health (Grant no. R01-MH 079175). William Shrank is supported by a career development award from the National Heart, Lung, and Blood Institute (Grant no. K23HL090505-01). The funding agencies were not involved in the design, conduct, and analysis of this study. The human subjects review board of Brigham and Women’s Hospital approved the study.
NOTES
- 1.Safran DG, et al. Prescription Drug Coverage and Seniors: Findings from a 2003 National Survey. Health Affairs. 2005;24:w152–w166. doi: 10.1377/hlthaff.w5.152. published online 19 April 2005. [DOI] [PubMed] [Google Scholar]; Soumerai SB, et al. Cost-Related Medication Nonadherence among Elderly and Disabled Medicare Beneficiaries: A National Survey One Year before the Medicare Drug Benefit. Archives of Internal Medicine. 2006;166(17):1829–1835. doi: 10.1001/archinte.166.17.1829. [DOI] [PubMed] [Google Scholar]
- 2.Safran, et al. Prescription Drug Coverage and Seniors”; and M.A. Steinman, L.P. Sands, and K.E. Covinsky, “Self-Restriction of Medications Due to Cost in Seniors without Prescription Coverage. Journal of General Internal Medicine. 2001;16(12):793–799. doi: 10.1111/j.1525-1497.2001.10412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.U.S. Department of Health and Human Services. Over Thirty-eight Million People with Medicare Now Receiving Drug Coverage. [(accessed 9 December 2008)];Press Release. 2006 June 14; http://www.hhs.gov/news/press/2006pres/20060614.html.
- 4.Hoadley J, et al. An In-Depth Examination of Formularies and Other Features of Medicare Drug Plans” (Menlo Park, Calif.: Henry J. Kaiser Family Foundation, April 2006); and Social Security Administration, “Medicare Part D Subsidies: Final Rule 20 CFR Part 418. Federal Register. 2005;70(250):77664–77685. [Google Scholar]
- 5.Centers for Medicare and Medicaid Services. Medicare Program; Medicare Prescription Drug Benefit; Final Rule, 42 CFR. Federal Register. 2005;70(18):4460. [PubMed] [Google Scholar]
- 6.Henry J. Kaiser Family Foundation. The Medicare Prescription Drug Benefit. [(accessed 27 January 2009)];Fact Sheet. 2006 June; http://www.kff.org/medicare/upload/7044-04.pdf.
- 7.Hoadley, et al. An In-Depth Examination [Google Scholar]
- 8.Yin W, et al. The Effect of the Medicare Part D Prescription Benefit on Drug Utilization and Expenditures. Annals of Internal Medicine. 2008;148(3):169–177. doi: 10.7326/0003-4819-148-3-200802050-00200. [DOI] [PubMed] [Google Scholar]; Lichtenberg FR, Sun SX. The Impact of Medicare Part D on Prescription Drug Use by the Elderly. Health Affairs. 2007;26(6):1735–1744. doi: 10.1377/hlthaff.26.6.1735. [DOI] [PubMed] [Google Scholar]
- 9.Thomson Corporation. Drug Topics Red Book. Montvale, N.J.: Thompson Corporation; 2006. and HHS Office of Inspector General. [accessed 9 December 2008];Medicaid Pharmacy—Additional Analyses of the Actual Acquisition Cost of Prescription Drug Products. Pub. no. A-06-02-00041, September 2002, http://oig.hhs.gov/oas/reports/region6/60200041.pdf.
- 10.See Appendix 1 online at http://content.healthaffairs.org/cgi/content/full/hlthaff.28.2.w305.DC2.
- 11.Choudhry NK, et al. Warfarin Prescribing in Atrial Fibrillation: The Impact of Physician, Patient, and Hospital Characteristics. American Journal of Medicine. 2006;119(7):607–615. doi: 10.1016/j.amjmed.2005.09.052. [DOI] [PubMed] [Google Scholar]; Spertus JA, et al. Prevalence, Predictors, and Outcomes of Premature Discontinuation of Thienopyridine Therapy after Drug-Eluting Stent Placement: Results from the PREMIER Registry. Circulation. 2006;113(24):2803–2809. doi: 10.1161/CIRCULATIONAHA.106.618066. [DOI] [PubMed] [Google Scholar]; Lee TJ, Fennerty MB, Howden CW. Systematic Review: Is There Excessive Use of Proton Pump Inhibitors in Gastro-Oesophageal Reflux Disease? Alimentary Pharmacology and Therapeutics. 2004;20(11–12):1241–1251. doi: 10.1111/j.1365-2036.2004.02289.x. [DOI] [PubMed] [Google Scholar]
- 12.For more on defined daily doses, see the World Health Organization Collaborating Centre for Drug Statistics Methodology Web site, “The ATC/DDD System,” http://www.whocc.no/atcddd/ (accessed 9 December 2008).
- 13.Schneeweiss S, et al. On the Evaluation of Drug Benefits Policy Changes with Longitudinal Claims Data: The Policy Maker’s versus the Clinician’s Perspective. Health Policy. 2001;55(2):97–109. doi: 10.1016/s0168-8510(00)00120-2. [DOI] [PubMed] [Google Scholar]
- 14.On the Chronic Disease Score, see Von Korff M, Wagner EH, Saunders K. A Chronic Disease Score from Automated Pharmacy Data. Journal of Clinical Epidemiology. 1992;45(2):197–203. doi: 10.1016/0895-4356(92)90016-g.Schneeweiss S, et al. Performance of Comorbidity Scores to Control for Confounding in Epidemiologic Studies Using Claims Data. American Journal of Epidemiology. 2001;154(9):854–864. doi: 10.1093/aje/154.9.854.
- 15.Wagner AK, et al. Segmented Regression Analysis of Interrupted Time Series Studies in Medication Use Research. Journal of Clinical Pharmacy and Therapeutics. 2002;27(4):299–309. doi: 10.1046/j.1365-2710.2002.00430.x. [DOI] [PubMed] [Google Scholar]
- 16.Grimmett GR, Stirzaker DR. Probability and Random Processes. 2. New York: Oxford Science Publications; 1992. [Google Scholar]
- 17.Details of the regression analyses are described in Appendix 2, online as in Note 10.
- 18.See Appendix 3 online; ibid.
- 19.See Appendices 4 and 5 online; ibid.
- 20.Yin et al., “The Effect of the Medicare Part D”; and Lichtenberg and Sun, “The Impact of Medicare Part D.”
- 21.Pauly MV. Medicare Drug Coverage and Moral Hazard. Health Affairs. 2004;23(1):113–122. doi: 10.1377/hlthaff.23.1.113. [DOI] [PubMed] [Google Scholar]; Lichtenberg, Sun The Impact of Medicare Part D. doi: 10.1377/hlthaff.26.6.1735. [DOI] [PubMed] [Google Scholar]
- 22.Schneeweiss S, et al. Adherence to Statin Therapy under Drug Cost Sharing in Patients With and Without Acute Myocardial Infarction: A Population-Based Natural Experiment. Circulation. 2007;115(16):2128–2135. doi: 10.1161/CIRCULATIONAHA.106.665992. [DOI] [PubMed] [Google Scholar]; Shrank WH, et al. The Implications of Choice: Prescribing Generic or Preferred Pharmaceuticals Improves Medication Adherence for Chronic Conditions. Archives of Internal Medicine. 2006;166(3):332–337. doi: 10.1001/archinte.166.3.332. [DOI] [PubMed] [Google Scholar]
- 23.Soumerai SB, et al. Payment Restrictions for Prescription Drugs under Medicaid: Effects on Therapy, Cost, and Equity. New England Journal of Medicine. 1987;317(9):550–556. doi: 10.1056/NEJM198708273170906. [DOI] [PubMed] [Google Scholar]; Hsu J, et al. Unintended Consequences of Caps on Medicare Drug Benefits. New England Journal of Medicine. 2006;354(22):2349–2359. doi: 10.1056/NEJMsa054436. [DOI] [PubMed] [Google Scholar]
- 24.O’Grady KF, et al. The Impact of Cost Sharing on Emergency Department Use. New England Journal of Medicine. 1985;313(8):484–490. doi: 10.1056/NEJM198508223130806. [DOI] [PubMed] [Google Scholar]
- 25.McClellan MB. [(accessed 9 April 2007)];Hearing on Generic Drug Utilization in the Medicare Prescription Drug Benefit. testimony before the Senate Special Committee on Aging, 21 September 2006, http://aging.senate.gov/minority/_files/hr166mm.pdf.
- 26.Soumerai SB, et al. A Critical Analysis of Studies of State Drug Reimbursement Policies: Research in Need of Discipline. Milbank Quarterly. 1993;71(2):217–252. [PubMed] [Google Scholar]; Schneeweiss S, et al. Clinical and Economic Consequences of a Reimbursement Restriction of Nebulised Respiratory Therapy in Adults: Direct Comparison of Randomised and Observational Evaluations. BMJ. 2004;328(7439):560. doi: 10.1136/bmj.38020.698194.F6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soumerai, et al. A Critical Analysis [Google Scholar]
- 28.Schneeweiss S, et al. Quasi-Experimental Longitudinal Designs to Evaluate Drug Benefit Policy Changes with Low Policy Compliance. Journal of Clinical Epidemiology. 2002;55(8):833–841. doi: 10.1016/s0895-4356(02)00437-7. [DOI] [PubMed] [Google Scholar]
- 29.Safran, et al. Prescription Drug Coverage [Google Scholar]
- 30.Shrank WH, et al. The Effect of Transitioning to Medicare Part D Drug Coverage on Cardiac Medication Use in Seniors Dually Eligible for Medicare and Medicaid. Journal of the American Geriatrics Society. 2008;56(12):2304–2310. doi: 10.1111/j.1532-5415.2008.02025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schneeweiss, et al. Quasi-Experimental Longitudinal Designs [Google Scholar]
- 32.Hoadley, et al. An In-Depth Examination [Google Scholar]
- 33.OIG, Medicaid Pharmacy.