Abstract
We explored how stimulation of GABAA receptors at different times during conditioned taste aversion (CTA) acquisition or extinction influenced extinction. In Experiment 1, rats acquired a CTA to 0.3% saccharin-flavored water (SAC) when it followed an injection of lithium chloride (LiCl; 81.0 mg/kg, i.p.). Following conditioning, rats received extinction training in which the GABAA agonist muscimol (1.0 mg/kg, i.p.), or control (saline) injections, were administered either before or after each extinction trial. Muscimol hindered extinction when administered after extinction trials. Muscimol’s inhibitory effects may have impeded extinction learning by disrupting synaptic mechanisms required to consolidate information experienced during extinction training. In Experiment 2, we studied the effects of muscimol on CTA acquisition and subsequent extinction. Rats received muscimol (1.0 mg/kg, i.p.) either before or after CTA conditioning trials. Following CTA acquisition, all rats were given CTA extinction training without muscimol administration. All groups developed CTA, but the group that received muscimol before CTA conditioning trials extinguished rapidly in comparison to other treatment groups. Differences between muscimol’s effects on CTA conditioning and CTA extinction indicate that fear conditioning and extinction involve, to some degree, different neuronal mechanisms.
Keywords: gamma-aminobutyric acid, GABAA receptors, extinction, conditioned taste aversion, muscimol, learning
Introduction
Conditioned taste aversion (CTA) is the result of classical conditioning in which a novel taste (conditioned stimulus; CS) becomes associated with malaise or illness (unconditioned stimulus; US), resulting in subsequent avoidance of the taste (conditioned response; CR) (Garcia et al., 1955; Pavlov, 1927). Once a CTA is learned, repeated CS exposure without subsequent malaise reduces the occurrence of the CR; this process is known as extinction, and results in reacceptance of the once-aversive taste. CTA has provided a model for researchers to study both learning and extinction. Extinction research may be of particular practical benefit as investigating its neurological underpinnings holds promise for the development of new treatments for phobias, anxiety disorders, and post-traumatic stress disorder (PTSD) (Barad, 2005).
Several behavioral phenomena, including renewal (Bouton et al., 2006), reinstatement (Rescorla & Heth, 1975), and spontaneous recovery (Rosas & Bouton, 1996) give strong support to the theory that extinction is a form of new learning that interferes with, or overrides, the original CS+US association. Such learning generates an inhibitory response that is present simultaneously with the original CR (Rescorla, 1969). Biological evidence also supports the theory of extinction as new learning. Both CTA acquisition and extinction are blocked when anisomycin, a protein inhibitor, is injected into the insular cortex after conditioning or extinction trials, respectively (Bermúdez-Rattoni, 2004). Furthermore, Mickley et al. (2004) reported that c-Fos expression in the gustatory neocortex, a brain area that has been implicated in taste memory consolidation, does not return to its original state following CTA extinction, but to a new state that reflects the newly acquired information about the CS. Although extinction appears to be a form of new learning, the neural underpinnings of extinction and learning (i.e., original acquisition) may differ (for example, see Bahar et al., 2003; Lee et al., 2008).
A growing literature suggests that the brain neurotransmitter gamma-aminobutyric acid (GABA) and its receptors play a role in learning and extinction of conditioned fears (Davis & Myers, 2002). Muscimol, an analog of GABA, is a GABAA receptor agonist found in the caps of the psychoactive mushroom Amanita muscaria (Michelot & Melendez-Howell, 2003). In experimental contexts, systemic muscimol administration has been used to produce changes in learning retention. For example, Castellano and McGaugh (1990) showed that a single systemic post-training injection of muscimol disrupted retention of an inhibitory avoidance task. They also reported that the timing of the injection was critical, as injection prior to learning had no effect on consolidation.
The purpose of the current study was to extend this work to CTA by: (1) determining the effects of muscimol on both acquisition and extinction, and (2) further exploring how timing of the drug administration (i.e., before/after presentation of a CS/US) affects extinction. We employed a robust form of conditioning (CTA) that is normally resistant to extinction. In Experiment 1, CTA-conditioned animals received injections of muscimol either before or after extinction trials. In Experiment 2, animals received muscimol injections either before or after CTA conditioning trials and subsequent extinction behavior was observed. Our data suggest that the timing of the administration of the GABAA receptor agonist played a significant role in predicting the dynamics of CTA extinction.
Method
Experiment 1
Subjects
A total of 51 naive male Sprague-Dawley rats (mean weight ± SEM = 274.90 ± 14.58 g), supplied by Zivic Laboratories (Zelienople, PA) were used in this experiment. Animals were housed in individual plastic cages (44.45 cm long × 21.59 cm wide × 20.32 cm deep) with corncob bedding (Bed o’cobbs, The Andersons Industrial Products, Maumee, OH). A 12-hr light-dark cycle (lights on at 0600 hr) was maintained, and temperature was kept within 23–26°C. Rats also had free access to Purina Rat Chow (No. 5001, PMI Nutrition International, Brentwood, MO) for the duration of the study.
Procedures were approved by the Baldwin-Wallace College Institutional Animal Care and Use Committee. Animals were procured and cared for according to the recommendations in the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996) and in compliance with the Animal Welfare Act.
Group Nomenclature
The names of the experimental groups used in this study are based on a coding system. All groups were exposed to a CTA (conditioning) phase followed by an EXT (extinction) phase from which the name CTA+EXT is formed. The placement of Mus either before/after the CTA and/or EXT abbreviation denotes that a muscimol injection was given either before or after SAC exposures during the given experimental phase. For example, the name (Mus)CTA+EXT denotes the injection of muscimol before SAC exposure during the CTA phase of the experiment.
There were three additional control groups used in the first experiment that are denoted by the following names. The first group controlled for animals receiving explicitly unpaired presentations of the CS and US in lieu of the standard CTA training procedure and is named NoCTA. The second group controlled for any possible US effects of muscimol and is named MusCtrl. The third group, designated (Mus)SAC, was used to determine the extent of hypodipsia when muscimol was administered 30 min before SAC presentation (see Table 1 for a summary of group nomenclature).
Table 1.
Experiment 1: Summary of group names, numbers, and treatments.
| Group Designation | Number of subjects | Conditioning | Extinction Phase | |||||
|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | |||
| CTA+EXT | 12 | SAC1+LiCl2 | Water | SAC +LiCl | Water | SAC +LiCl | Water | SAC |
| CTA+EXT(Mus3)4 | 9 | SAC +LiCl | Water | SAC +LiCl | Water | SAC +LiCl | Water | SAC(Mus)7 |
| CTA+(Mus)EXT5 | 7 | SAC +LiCl | Water | SAC +LiCl | Water | SAC +LiCl | Water | (Mus)SAC8 |
| MusCtrl | 8 | SAC +Mus | Water | SAC +Mus | Water | SAC +Mus | Water | N/A9 |
| NoCTA6 | 10 | SAC | Water +LiCl | SAC | Water +LiCl | SAC | Water +LiCl | N/A |
| (Mus)SAC | 5 | (Mus) SAC | Water | (Mus) SAC | Water | (Mus) SAC | Water | (Mus)SAC |
SAC = 0.3% saccharin salt solution
LiCl = lithium chloride solution (81mg/ml) prepared in physiological saline; administered at a dose of 81mg/kg, i.p.
Mus = muscimol injection (1mg/ml) prepared in physiological saline; administered at a dose of 1mg/kg, i.p.
EXT(Mus) = muscimol was administered 45 min after SAC exposure throughout extinction
(Mus)EXT = muscimol was administered 30 min before SAC exposure throughout extinction
NoCTA = refers to explicitly unpaired (EU) treatment group
SAC(Mus) = muscimol was administered 45 min after SAC exposure
(Mus)SAC = muscimol was administered 30 min before SAC exposure
N/A = the extinction phase was not necessary since rats never acquired a CTA
Materials
All drugs and chemicals were purchased from the Sigma-Aldrich Chemical Company (St. Louis, MO). Muscimol powder was dissolved in a physiological saline vehicle to a final concentration of 1mg/ml and was administered at a dose of 1mg/kg (i.p.). This dose of muscimol was selected based on the previous literature which suggests that, in rodent models, 1 mg/kg, i.p. can alter retention of an inhibitory avoidance task without producing state-dependant effects (Castellano & McGaugh, 1990) or significant motor effects (e.g., catatonia; Mehta & Ticku, 1987). Lithium chloride (LiCl) was dissolved in physiological saline to produce a final concentration of 81mg/ml and was administered at a dose of 81mg/kg (i.p.). Saccharin salt was dissolved in deionized water to create a final 0.3% solution (SAC). SAC and LiCl concentrations/doses were selected based on previous experience with this regimen indicating that these parameters create a strong CTA that is resistant to rapid extinction (Mickley et al., 2007).
Conditioning Procedure
Animals were habituated to a 23 hr water deprivation schedule beginning two days prior to the first conditioning trial. Fluid consumption was recorded daily to the nearest tenth of a gram. On the first conditioning day, the water-deprived rats were given 30 min access to SAC. Following SAC exposure, the drinking bottle was removed, and animals assigned to the CTA groups received an injection of LiCl within 15 minutes of the bottle removal. Fifteen minutes after SAC bottles were removed, animals were given 30 min access to tap water to prevent dehydration. CTA animals received the CS-US pairings on 3 conditioning days (experimental days 1, 3, and 5). Interim days 2, 4, and 6 served as rest days on which the CTA animals received two 30 min presentations of water separated by a 15 min interval (replacing the LiCl injection period experienced on days 1, 3, and 5). Malaise due to LiCl injection may attenuate water consumption during the 30 min period following injection. Meachum and Bernstein (1990) reported that LiCl administration induced specific behavioral changes associated with malaise in rats within five to ten minutes following an i.p. injection, but that these changes in behavior only lasted for about one hour. However, rest days allowed for rats to rehydrate and negate any dehydration caused by this attenuation. To account for possible unconditioned stimulus properties of muscimol, rats in the MusCtrl group were given the same treatment as CTA animals, but a muscimol injection (1mg/kg, i.p.) was administered in lieu of the LiCl injection.
The NoCTA group received only SAC on days 1, 3, and 5 of conditioning and were injected with LiCl on the alternate days 2, 4, and 6. This explicitly unpaired procedure allowed the NoCTA animals to receive both the CS and US throughout conditioning without forming an association or subsequent aversion to SAC (Mickley et al., 2004; Mickley et al., 2007); this group was designed to allow us to estimate the residual effects of the SAC or LiCl exposures alone.
An additional group was used to determine the effects of muscimol exposure on SAC consumption over an extended time course (22 muscimol and SAC exposures over the course of 25 days). Following two days of 23 hr water deprivation, this group, designated as (Mus)SAC, received muscimol injections (1mg/kg; i.p.) 30 min prior to SAC exposure on days 1, 3, and 5. They received two 30 min presentations of water on days 2, 4, and 6 of the study, preceded by no injection. This group did not receive LiCl injections at any point in the study.
Extinction Procedure
After day 6 of the conditioning phase, the extinction phase of the study began. Animals received 30-min daily exposure to SAC. Fifteen minutes after SAC exposure, the animals received 30 min access to water to prevent dehydration.
During the extinction phase, rats were injected with muscimol (1mg/kg; i.p.). The time of administration was determined by each group assignment. CTA+(Mus)EXT animals received muscimol (1mg/kg; i.p.) 30 min prior to the presentation of SAC; CTA+EXT(Mus) animals received muscimol 45 min after SAC presentation had ended. CTA+EXT animals, which were controls and received physiological saline in lieu of muscimol, were randomly divided into two subgroups, one that received saline injections 30 min before and the other 45 min after SAC was removed from cages, i.e., in a manner that directly paralleled the timing of the muscimol injections.
For CTA+(Mus)EXT animals, a muscimol administration time-point of 30 min before SAC exposure was chosen to take into account the relatively short half-life of muscimol (Michelot & Melendez-Howell, 2003) and ensure that muscimol was still in the system at the time of SAC exposure but that any lethargy induced by the drug would not take effect during the bottle testing. When injected intravenously, muscimol exerts maximal effects on the brain between 30 and 60 minutes (Baraldi et al., 1979). Hypodipsia can occur within the first 30 min after systemic muscimol administration (1mg/kg) (Houston et al., 2002), which is another reason administration occurred 30 min before SAC was made available. An administration time of 45 min after SAC presentation was chosen for CTA+EXT(Mus) animals to parallel the injection period experienced by the CTA+(Mus)EXT group, while taking into account that the daily bottle testing procedure did not end until 45 min after SAC exposure. This procedure prevented disruption of the 30 min allotted for hydration.
After Day 19 of extinction, muscimol injections were terminated to determine the effects of drug cessation. The time of drug cessation was chosen based on our previous data indicating that non-drug-treated rats extinguished CTAs in 17.11 ± 3.01 (mean ± SEM) days (Mickley et al., 2004).
The (Mus)SAC animals received muscimol injections (1mg/kg; i.p.) every day 30 min prior to SAC exposure. Since they had acquired no CTA, they were drinking asymptotic amounts of SAC throughout the extinction phase. However, to parallel the number of exposures in the CTA+EXT(Mus) group, the data collection from these animals was also terminated after 19 consecutive muscimol and CS exposures.
Statistical Analysis
Extinction of CTA was defined as SAC consumption greater than or equal to 90% of the baseline (Mickley et al., 2004). Since pre-exposure to SAC would impede future CTA training by inducing latent inhibition, we could not record baseline SAC consumption in the actual experimental animals. Therefore, baseline SAC consumption was determined by averaging the amount of SAC consumption on the first day of asymptotic consumption (which was the third day of SAC exposure overall) from a separate group (N = 10) of similarly-sized rats not used in the current study (mean ± SEM = 17.57 ± 1.29 ml).
To analyze extinction data, we separated the time course of extinction into three phases, as originally established by Nolan et al. (1991): static, dynamic, and asymptotic (extinction). We compared SAC consumption between groups at each of these phases. The three phases correspond to different ranges of SAC consumption relative to baseline. SAC consumption less than 10% of baseline corresponds to the static phase; 10%–80% of baseline consumption corresponds to the dynamic phase; 80%–100% baseline consumption corresponds to the asymptotic phase (see Mickley et al. (2004) for an example of a CTA extinction curve). In the current experiment, extinction training ended when animals reached 90% of baseline SAC consumption, which operationally defined the specific asymptotic extinction criterion.
SPSS software (Chicago, IL) was used for all analyses. A repeated measures one-way analysis of variance (ANOVA) and subsequent Tukey Honestly Significant Difference (HSD) post-hoc tests were performed to analyze SAC consumption within and between groups during CTA conditioning (Kirk, 1982). A one-way ANOVA and Tukey HSD post-hoc tests were also used to analyze between-group differences in the total days to asymptotic extinction as well as the duration of the static and dynamic phases. Statistical significance was evaluated using an α = 0.05. Similar analyses were used for Experiment 2.
Results
As shown in Figure 1, SAC drinking rates of the CTA groups rapidly decreased over the three conditioning days and first day of extinction; this was in stark contrast to the increased drinking observed in theNoCTA animals. Also shown in the figure is a fairly steady daily consumption level (5 – 10 ml) in the MusCtrl group. A repeated-measures ANOVA revealed a significant drug treatment × treatment day interaction (drug treatment [CTA+(Mus)EXT, CTA+EXT(Mus), CTA+EXT, NoCTA, MusCtrl] × treatment day [Conditioning Day 1, 3, 5, or Extinction Day 1]) (F [12, 93] = 29.422, p < 0.001). Tukey HSD post-hoc comparisons were performed and significant differences were revealed in SAC consumption on the final CTA conditioning trial between the three groups designated as CTA, the MusCtrl, and the NOCTA animals (p < 0.05).
Figure 1.
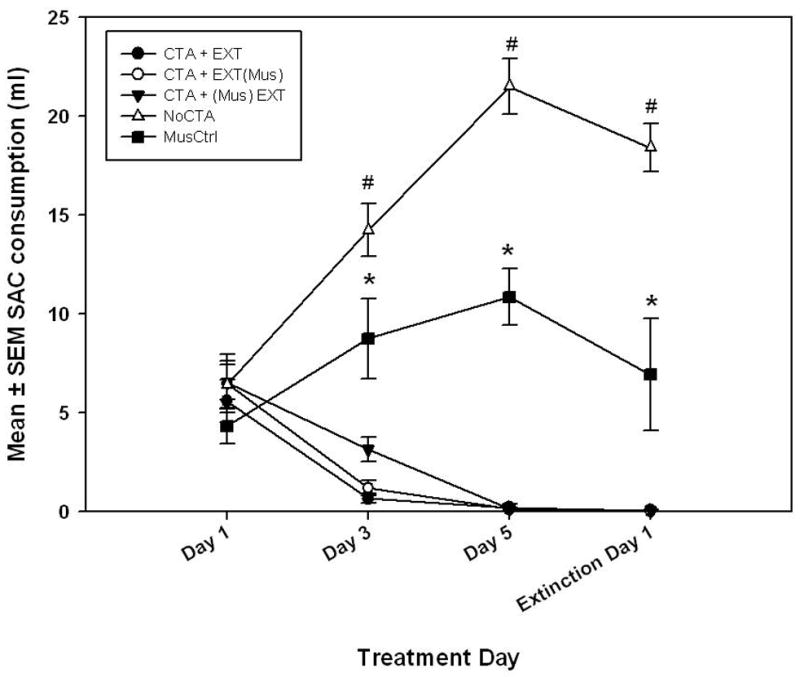
Experiment 1 SAC consumption data illustrating (depending on the treatment group) the development of a CTA, acceptance of SAC, or neither a significant rise nor decline in consumption over the three CS exposure days of conditioning and the first day of extinction, See Table 1 for group nomenclature. * = Significantly less than the NoCTA group and significantly greater than the CTA+EXT, CTA+EXT(Mus), and CTA+(Mus)EXT groups. # = Significantly greater than MusCtrl, CTA+EXT, CTA+(Mus)EXT, and CTA+EXT(Mus). α = 0.05. Variance indicators are SEM.
SAC consumption of CTA groups versus the NoCTA and MusCtrl groups continued to diverge throughout conditioning. Analysis of SAC consumption on the second day of conditioning showed significantly higher drinking levels in MusCtrl animals compared to the three CTA groups as well as significantly higher drinking levels in the NoCTA group compared to MusCtrl and the three CTA groups (F [4, 41]=32.587, p < 0.001; Tukey p < 0.01). Furthermore, a Tukey’s post hoc evaluation failed to reveal a significant difference among the three CTA groups’ SAC consumption on the second or third conditioning trials (Day 3 and Day 5). SAC consumption of MusCtrl animals on the third conditioning trial was significantly lower than the NoCTA group (p < 0.001), but significantly higher than the three CTA groups (F [4, 41] = 47.628, p < 0.001, all Tukey post hoc tests p < 0.001).
Similarly, comparisons revealed significantly higher drinking levels on Day 1 of extinction (the first CS-only exposure) in NoCTA rats as compared to MusCtrl and the three CTA groups. Likewise, there were significantly higher SAC drinking levels in the MusCtrl rats as compared to the three CTA groups (F [4, 41]=49.566, p < 0.001, all Tukey post hoc tests p < 0.001). Muscimol, therefore, did not create an aversion to the SAC as an unconditioned stimulus, nor did it allow neophobia to subside as in the NoCTA animals (see Discussion for an alternative explanation to this finding). SAC consumption of the (Mus)SAC control group resembled the NoCTA group, ruling out that muscimol has a hypodipsic effect at the 30 min interval used in the current study.
We used paired-samples t-tests to compare the MusCtrl group’s water consumption between the 30 minute hydration period following muscimol injection and the water consumed during 30 minute on the following rest day. The MusCtrl group consumed significantly less water on conditioning trial days 1 and 3 [t(7) = 6.45, p < .001, and t(7) = 4.70, p = .002, respectively]. Muscimol treatment produced significant hypodipsia in MusCtrl rats during their hydration period.
All rats that acquired a CTA continued to exhibit the aversion entering the extinction phase and then slowly began to reaccept SAC. However, CTA+EXT(Mus) animals, which received muscimol after the extinction trials, took significantly longer to reach asymptotic extinction compared to the CTA+EXT and CTA+(Mus)EXT animals (F [3,32]=17.651, p < 0.001, all Tukey post hoc tests p < 0.001). Giving muscimol before SAC presentation made no significant difference in total days to asymptotic extinction when compared to the control CTA+EXT animals.
The number of days to reach static, dynamic, and asymptotic phases of extinction was analyzed (see Figure 2). We previously reported that the duration of the static phase (but not the dynamic or asymptotic phases) varies significantly between treatment groups and accounts for the majority of the total days to asymptotic extinction. This experiment was no exception to that finding (refer to Mickley et al., 2007). The CTA+EXT(Mus) group spent significantly more time in the static phase of extinction compared to both the CTA+EXT group and the CTA+(Mus)EXT group (F [2, 25] = 7.928, p < 0.001, all Tukey post hoc tests p < 0.002). No group differences were revealed in the duration of the dynamic phase. Also, to see if the rate of extinction changed after cessation of muscimol on extinction day 19, we compared duration of the dynamic phase of extinction between animals that reached asymptotic extinction prior to day 20 with animals that extinguished their CTA after day 20. Comparisons between the CTA+EXT and CTA+EXT(Mus) groups (2-tailed t-test, a priori) revealed no significant differences between these groups.
Figure 2.
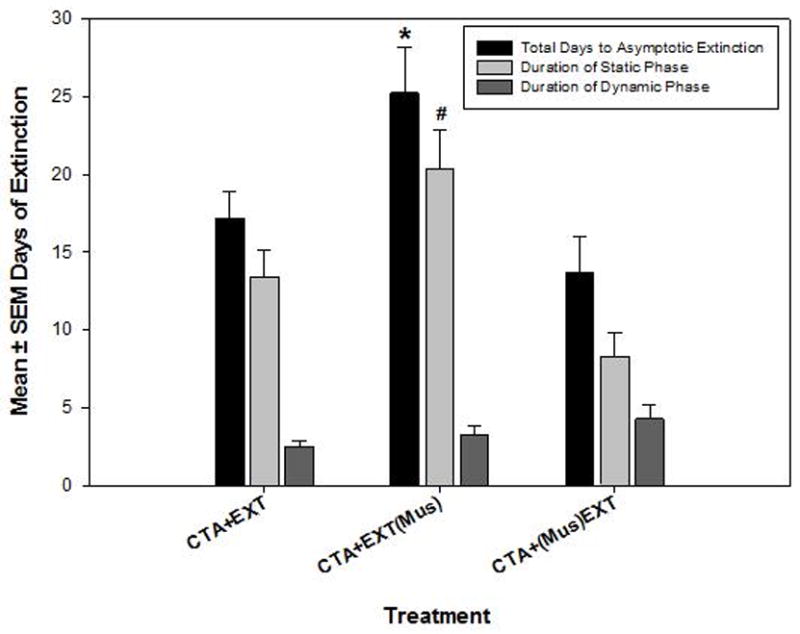
Experiment 1 extinction data showing the mean days of extinction training required to reach criterion for each phase of extinction. See Table 1 for group nomenclature * = Significantly more daily SAC exposures required to reach criterion for each phase of extinction compared to the CTA+(Mus)EXT group but significantly less time to reach each phase of extinction compared to the CTA+EXT(Mus) group. α = 0.05. Variance indicators are SEM.
The NoCTA and the MusCtrl groups displayed in Figure 1 were omitted from extinction analyses because SAC consumption never fell to the level of the control CTA+EXT group; these two groups never developed a CTA to be extinguished.
In summary, animals in Experiment 1 that received CTA conditioning demonstrated an aversion to SAC by the end of conditioning. Subsequently, only post-extinction trial injections of muscimol hindered extinction of a CTA. This effect was not due to inhibition of behavioral expression or muscimol acting as a US, but may instead be attributed to a disruption of extinction learning. Additionally, muscimol administered within 15 minutes following SAC exposure did not cause MusCtrl animals to develop CTA or reduce neophobia. Of equal importance, the (Mus)SAC group did not exhibit decreased SAC consumption when injected with muscimol 30 minutes before SAC presentation, ruling out hypodipsia as a possible explanation of our results.
Method
Experiment 2
The findings from Experiment 1 indicated that muscimol did indeed affect extinction learning depending on when (before or after CS presentation) the drug was administered. However, it remained unclear if retrograde amnesia or state-dependency could explain the observed patterns in SAC consumption and reacceptance we observed in the first study (Overton, 1984). We expected that if retrograde amnesia was an underlying explanation for our Experiment 1 observations, then animals given muscimol after CS exposure during CTA training would not acquire the CTA. Likewise, if state-dependency was an underlying explanation, then animals receiving muscimol injections during both the CTA and extinction training phases would exhibit a significant retardation in CTA extinction - provided they learned the original CTA.
Both acquisition and extinction of a CTA involve new learning (Bahar et al., 2003). Therefore, we wished to determine the generalizability of the effects of muscimol on CTA acquisition in addition to those demonstrated on CTA extinction in Experiment 1. In Experiment 2, we administered muscimol (1mg/kg, i.p.) either before or after CTA conditioning trials. Following conditioning, rats were allowed to extinguish their aversion to SAC. No muscimol was administered during extinction training in Experiment 2, with the exception of one group, which received muscimol before conditioning and extinction trials to test for state dependency.
Subjects
A total of 19 naive male Sprague-Dawley rats (mean weight ± SEM = 309.52 ± 3.32 g) supplied by Zivic Laboratories (Zelienople, PA) were used in this experiment. See Experiment 1 for animal housing and maintenance information.
Procedures were approved by the Baldwin-Wallace College Institutional Animal Care and Use Committee. Animals were procured and cared for according to the recommendations in the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996) and in compliance with the Animal Welfare Act.
Nomenclature
The group nomenclature system is similar to that used for Experiment 1. The placement of (Mus) within the base name CTA+EXT denotes the time point of muscimol administration throughout the experiment (before or after CS; during CTA and/or EXT training). To reiterate an example, (Mus)CTA+EXT signifies that animals received a muscimol injection prior to SAC exposures during CTA training only (see Table 2 for a summary of the group nomenclature and treatments).
Table 2.
Experiment 2: Summary of group names, numbers, and treatments.
| Group Designation | Number of subjects | Conditioning | Extinction Phase | |||||
|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | |||
| CTA+EXT | 4 | SAC1+LiCl2 | Water | SAC +LiCl | Water | SAC +LiCl | Water | SAC |
| (Mus)CTA4+EXT | 4 | (Mus3)SAC +LiCl | Water | (Mus)SAC +LiCl | Water | (Mus)SAC +LiCl | Water | SAC |
| CTA(Mus)5+EXT | 5 | SAC(Mus)+LiCl | Water | SAC(Mus)+LiCl | Water | SAC(Mus)+LiCl | Water | SAC |
| (Mus)CTA+ (Mus)EXT6 | 6 | (Mus)SAC +LiCl | Water | (Mus)SAC +LiCl | Water | (Mus)SAC+ LiCl | Water | (Mus)SAC |
SAC = 0.3% saccharin salt solution
LiCl = lithium chloride solution (81mg/ml) prepared in physiological saline; administered at a dose of 81mg/kg, i.p.
Mus = muscimol injection (1mg/ml) prepared in physiological saline; administered a dose of 1mg/kg, i.p.
(Mus)CTA = muscimol was administered 30 min before SAC exposure throughout conditioning
CTA(Mus) = muscimol was administered 45 min after SAC exposure throughout conditioning
(Mus)CTA+(Mus)EXT = muscimol was administered before SAC exposure throughout conditioning and extinction
Materials
Refer to Experiment 1 for chemical/drug supplier and preparation information for muscimol, LiCl, and SAC solutions.
Conditioning Procedure
Animals were habituated to a 23 hr water deprivation schedule and CTA training was administered as described in Experiment 1. However, various groups received muscimol injections either before or after CS exposure on CTA training days 1, 3, and 5. Rats in the (Mus)CTA+EXT group received a muscimol injection (1mg/kg; 1mg/ml; i.p.) 30 min before SAC exposure during the conditioning phase of the study. The (Mus)CTA+(Mus)EXT group received muscimol 30 min prior to SAC exposure during both the conditioning and extinction phases. Rats in the CTA(Mus)+EXT group received a muscimol injection 45 min following SAC exposure during the conditioning phase only. To control for handling and injection procedures, rats in the CTA+EXT group were randomly divided into two subgroups that received a saline injection either 30 min prior to SAC exposure or 45 min after SAC exposure.
Extinction Procedure
Animals received daily 30 min exposure to SAC until the extinction criterion was met (i.e., SAC reacceptance to 90% of baseline), followed by a 15 min interval (during which no liquid was available) and then 30 min access to water. The (Mus)CTA+(Mus)EXT groups also received muscimol injections 30 min prior to SAC presentation every day throughout extinction to control for any chance of muscimol state-dependency in which the drug-induced state would act as a context cue (Nakagawa et al., 1995). Animals that had previously received saline injections before CS exposure during conditioning were further divided at this point of the experiment and half were randomly selected to receive saline injections prior to each extinction trial as a control for the state-dependent group that received daily muscimol injections. The (Mus)CTA+EXT, CTA(Mus)+EXT, and CTA+EXT groups received no muscimol injections during the extinction phase of the study.
Results
In Experiment 2, all animals acquired a CTA as characterized by the rapid and successive decline in SAC consumption over the three conditioning trials and first extinction trial (see Figure 3). A repeated measures ANOVA (drug treatment [(Mus)CTA+EXT, CTA(Mus)+EXT, CTA+EXT, (Mus)CTA+(Mus)EXT] × treatment day [Conditioning Day 1, 2, 3, or Extinction Day 1]) showed a significant drug treatment × treatment day interaction across the three days of conditioning and first day of extinction (F [9, 45] = 5.986, p < 0.007). No significant differences in SAC consumption was observed among any groups on the second conditioning day (Day 3), final conditioning day (Day 5), and the first day of extinction. Furthermore, after three conditioning trials, SAC consumption for all groups was reduced to zero, indicating that all groups acquired a CTA.
Figure 3.
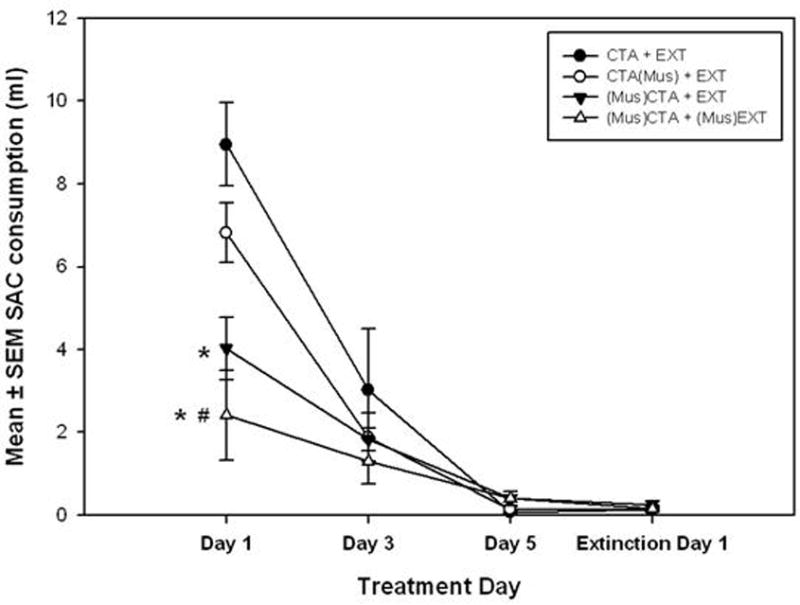
Experiment 2 SAC consumption data illustrating the development of a CTA over the three CS exposure days of conditioning and the first day of extinction. All treatment groups acquired a CTA. See Table 2 for group nomenclature. * = (Mus)CTA+EXT and (Mus)CTA+(Mus)EXT groups are significantly less than the CTA+EXT. # = (Mus)CTA+(Mus)EXT group is significantly less than the CTA(Mus)+EXT group. The CTA+EXT and CTA(Mus)+EXT groups appear to be significantly different on the first conditioning day due to the lack of overlapping error bars, but the difference is not statistically significant (F[3, 15] = 9.336, p=0.446). α = 0.05. Variance indicators are SEM.
A one-way ANOVA revealed significant differences in SAC consumption on the first day of conditioning between groups receiving muscimol prior to CS exposure and groups receiving either no muscimol or muscimol after CS exposure (F[3,15] = 9.366, p < 0.001). The (Mus)CTA+EXT and (Mus)CTA+(Mus)EXT groups both drank significantly less SAC on the first day of conditioning compared to the CTA+EXT control group, but only the (Mus)CTA+(Mus)EXT group drank significantly less SAC than the CTA(Mus)+EXT group according to Tukey post hoc tests (all Tukey post hoc tests p < 0.001) (see Figure 3).
Variability in the duration of extinction and phases of extinction, shown in Figure 4, were similar to those seen in Experiment 1 (compare to Figure 2). Analysis of the treatment-induced changes in duration of extinction and its phases revealed a significant difference in the duration of the static phase between treatment groups (F [3, 15] = 9.054, p < 0.001). Both the (Mus)CTA+EXT and (Mus)CTA+(Mus)EXT groups spent significantly less time in the static phase compared to the CTA+EXT group (Tukey p < 0.01 and p < 0.04, respectively) and the CTA(Mus)+EXT group (Tukey p < 0.003 and p < 0.013, respectively). Subsequently, a significant decrease was found via a one-way ANOVA and subsequent Tukey post hoc tests in the number of days required to reach asymptotic extinction in both groups that received muscimol before SAC presentation on conditioning days [(Mus)CTA+EXT and (Mus)CTA+(Mus)EXT] compared to the CTA+EXT group and compared to the CTA(Mus)+EXT (F [3, 15] = 9.514, p < 0.001, all Tukey post hoc tests p < 0.001).
Figure 4.
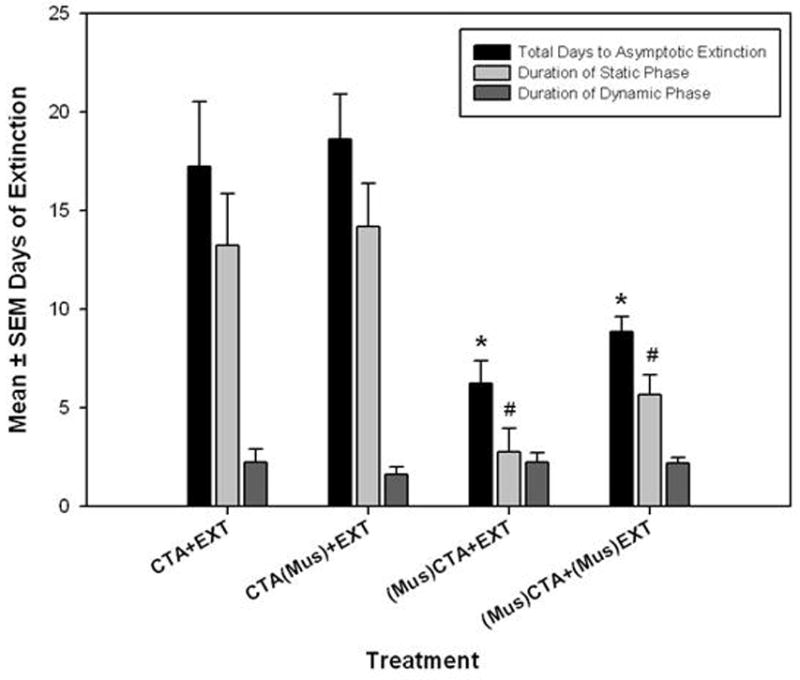
Extinction data showing the mean days of extinction training required to reach criterion for each phase of extinction. * = S ignificantly more SAC exposures required by both the CTA+EXT and CTA(Mus)+EXT groups to reach the end of the each phase of extinction compared to both the (Mus)CTA+EXT and (Mus)CTA+(Mus)EXT. The differences between (Mus)CTA+EXT and (Mus)CTA+(Mus)EXT were not significantly different. α = 0.05. Variance indicators are SEM.
There was no significant difference in the number of days to asymptotic extinction between animals not receiving muscimol and those receiving muscimol after SAC presentation on conditioning days. Furthermore, comparisons between the (Mus)CTA+EXT and the state dependent extinction (Mus)CTA+(Mus)EXT group revealed no significant difference in total days to reach asymptotic extinction, reducing the possibility that muscimol was exerting state dependant effects.
Discussion
The data from Experiment 1 revealed that a series of systemic muscimol injections following CTA extinction trials disrupt extinction learning. Rats that received post-extinction trial muscimol injections exhibited impeded extinction, specifically during the static phase. Once animals began to sample the SAC again (i.e., enter the dynamic phase), they extinguished their CTA at a rate similar to the other treatment groups. Moreover, cessation of post-extinction trial muscimol injections did not correspond with a rapid reacceptance of the once-aversive taste. Such findings suggest that muscimol was not inhibiting expression of extinction behavior, but impeding extinction learning itself.
These data are consistent with previous research reporting the ability of a single post-trial muscimol injection to block memory retention, while a single muscimol pre-trial injection had no effect on retention (Castellano & McGaugh, 1990). The current study provides additional data regarding chronic (i.e., successive) muscimol treatments on CTA extinction. Muscimol may only block extinction learning initially, as animals that received post-trial muscimol in the current study eventually extinguished, albeit slower than controls.
The slowed extinction observed in Experiment 1 may have been due to repeated retrograde amnesic effects (Rossato et al., 2004). Salinas and McGaugh (1995) produced such retrograde amnesic effects with muscimol in a reward shift paradigm, although the effect was seen for only one day (their methodology only used a single injection of muscimol during the experiment as opposed to repeated, daily administrations). Because of repeated retrograde amnesia, each extinction trial in the current study may have been perceived as a novel experience with unknown consequences, which prevented consolidation of the (SAC + no illness) association that normally develops during extinction.
Chronic muscimol treatment may have produced tolerance (leading to the abatement of its effects on consolidation), considering GABAergic drugs decrease post-synaptic sensitivity to GABA and its analogs (Biggio et al., 2003). However, the present study did not focus on tolerance factors (receptor regulation, binding changes, etc.) and correlating such changes to the interaction between muscimol administration and CTA extinction is worthy of future investigation. At this time, there is a dearth of literature on the effects of chronic muscimol treatment on animal models.
The impeded extinction observed in Experiment 1 was not due to malaise caused by muscimol, which would have essentially reconditioned rats to develop a CTA towards SAC. MusCtrl rats did not develop a CTA, providing further evidence that the effects of muscimol observed are acting upon extinction processes. These data are also consistent with Houston et al. (2002), who reported that systemic muscimol administration does not produce CTA. Apart from not developing a CTA from muscimol, the MusCtrl rats in Experiment 1 also did not show a reduction of neophobia over the course of the conditioning phase. Neophobia is the hesitation to consume novel substances, a behavior commonly observed in rats (Domjan & Gillan, 1977). Our data suggest that post-SAC consumption administration of muscimol also disrupts the consolidation of taste memory that is required for neophobia reduction (i.e., the development of a “safe taste” memory). In a manner consistent with our interpretation of the Experiment 1 extinction data, persistent neophobia may have occurred because every subsequent SAC exposure was perceived as a novel experience with unknown consequences.
An alternative explanation for the MusCtrl findings is that muscimol exhibited weak US properties, thereby causing consumption rates to neither decrease or increase over the course of this stage of the experiment. However, if a food is indeed toxic and causes CTA to develop, it would be evolutionarily unsound for an organism to continue ingesting the same amount of the particular food. One would predict that consumption rates would decrease. Such behavior is seen in the CTA literature and is consistent across different types of USs, such as LiCl (Mickley et al., 2004), radiation (Garcia et al., 1955), and hypertonic saline (Agüero et al., 1997).
MusCtrl rats exhibited hypodipsia during the conditioning stage of the experiment. They received water access within 15 minutes of muscimol injection, and subsequently, their consumption rates were significantly lower than when they received water but no muscimol. One may argue that muscimol-induced hypodipsia might influence drinking behavior on subsequent days because rats would be extra dehydrated. However, our procedure interleaved rest days, in which only water was given, with conditioning days to prevent dehydration from becoming a confounding variable for both MusCtrl rats and LiCl-treated rats. Importantly, this control group experienced hypodipsia due to the short latency between muscimol administration and water presentation; water presentation commenced within 15 minutes after muscimol injection. No hypodipsic effects were observed when the muscimol was administered 30 minutes before fluid presentation (based on the (Mus)SAC group’s drinking behavior).
Muscimol did not have the same effect on CTA acquisition as it did on CTA extinction. Rats that received muscimol after conditioning trials still developed a CTA, which required the same amount of extinction training as the CTA+EXT group for them to reach asymptotic extinction. On the contrary, muscimol treatment before CTA conditioning trials did reduce the number of SAC exposures required to reach asymptotic extinction, causing animals to extinguish rapidly in comparison to the other treatment groups. Receiving muscimol prior to both CTA conditioning trials and extinction trials, rats in the (Mus)CTA+(Mus)EXT treatment group extinguished at the same rate as rats in the (Mus)CTA+EXT group, suggesting that the effect was not due to state dependency. Additionally, our conditioning data suggest that muscimol does not cause the loss of taste sensation because (Mus)CTA+EXT and (Mus)CTA+(Mus)EXT rats perceived SAC on conditioning Days 3 and 5 and avoided the aversive taste. Both of these treatment groups also exhibited similar avoidance on the first day of extinction training, ruling out that pre-trial muscimol injection blocked expression of regular drinking behavior (i.e., data are indicative of CTA learning).
It may be likely that post-conditioning muscimol treatment does block CTA consolidation, but the timing used in the current experiment missed the critical period of time when consolidation occurred. This would suggest that CTA acquisition is consolidated rapidly, and the critical period that is vulnerable to muscimol’s effects subsides sometime within the first forty-five minutes after the CS+US pairing. The effects seen in the (Mus)CTA+EXT and (Mus)CTA+(Mus)EXT groups may actually be due to muscimol still exerting effects from the pre-trial injection after US presentation. The effects would be weaker compared to post-trial injections due to the drug’s metabolism (see Baraldi et al., 1979) over one hour (30 minutes between muscimol injection and CS presentation + 30 minutes between CS onset and US onset). Myers et al. (2006) have shown that commencing extinction within ten minutes of fear-conditioning causes erasure of the fear memory (although it could also be interpreted as a prevention of consolidation), while a twenty-four hour latency between acquisition and extinction leads to behavior indicative of new learning overriding the original association. Consolidation windows may vary between forms of learning, but all forms, including extinction, may have an initial window in which consolidation is vulnerable.
Extinction almost certainly represents new learning and involves protein synthesis (Bahar et al., 2003). However, the differences between muscimol’s effects on CTA conditioning and CTA extinction reported here indicate that fear conditioning and extinction involve, to some degree, different neuronal mechanisms as well. Similarly, recent data indicate that protein degradation is involved in the reorganization of retrieved memory and updated memory is reconsolidated by protein synthesis (Lee et al., 2008). Memory retrieval during the extinction process likely evokes a dynamic process that serves to incorporate new information into preexisting memories.
CTA acquisition may consolidate quickly due to the survival functions such learning provides. Organisms, including rats used in the current study, are predisposed to develop CTA, readily forming associations between taste and illness after one CS+US pairing (Gemberling & Domjan, 1982). Moreover, CTA acquisition can occur even with relatively long intervals between the CS and the US (Smith & Roll, 1967). CTA extinction trial outcomes may consolidate more slowly because it is evolutionarily conservative in terms of survival. An animal that extinguishes a CTA hastily may do so inaccurately with detrimental consequences. For example, an animal that has a CTA towards a particular poisonous food source may test the food again, in essence attempting to extinguish the CTA. If CTA extinction were to occur and consolidate rapidly, the animal may then consume large quantities of the food. If the food’s ill-effects (the US) were slow-acting, then the animal would die of poisoning due to the inaccurate extinction. Therefore, it may be the case that extinction trials consolidate more slowly to account for less contiguous USs as an evolved safety mechanism. This would also allow for a longer period of time in which extinction can be disrupted by muscimol. The current study only employed two time points (30 minutes before and 45 minutes after trials); future investigation is needed to further map muscimol’s time-dependent effects.
Muscimol before CS+US exposure did not block CTA formation. Behaviorally, these rats consumed very low SAC amounts similar to other CTA conditioned animals, but the intensity of CTA at a neural level is unknown. A floor effect may mask the detection of differing CTA intensities among groups by the end of conditioning. Moreover, it is hard to assess CTA strength by observing the rate of extinction alone because the extinction rate may also be a factor of extinction learning itself (Reilly & Bornovalova, 2005).
Rats in the (Mus)CTA+EXT and (Mus)CTA+(Mus)EXT treatment groups consumed less SAC than the other treatment groups on the first conditioning trial. Did this contribute to weaker conditioning? Previous research has shown that CTA conditioning strength is more dependent on the duration of CS exposure, not the quantity consumed (Barnfield & Clifton, 1989). Additionally, muscimol at the dose used in the current study did not cause hypodipsia when injected thirty minutes before fluid presentation. The (Mus)SAC control group in Experiment 1, which received muscimol thirty minutes before SAC exposure, consumed similar amounts of SAC compared the NoCTA group, which did not receive muscimol injections at all. In other labs, this hypodipsia was seen only during the first thirty minutes after a subcutaneous injection of muscimol (Houston et al., 2002). Subcutaneous injections yield slower drug effects than intraperitoneal injections used in the current study (Wellman, 1994), further supporting that thirty minutes was an ample period of time to allow any possible hypodipsia to subside. Lastly, CTA+(Mus)EXT rats in Experiment 1 did not exhibit hypodipsia under the influence of muscimol once they extinguished their CTA. Ultimately, the amount of SAC consumed on the first day of conditioning was not a potent predictor of the level of SAC consumed at the end of the conditioning procedure days later since all rats in the study were consuming near-zero amounts.
Some caution may be warranted in the interpretation of Experiment 2 where two of our groups contained relatively low Ns. In particular, groups CTA+EXT and (Mus)CTA+EXT each employed N=4. However, it should also be noted that the effects of primary interest in this study were detectable despite the low Ns in these groups. Specifically, as Figure 4 indicates, the “days of extinction” of the experimental groups that contained the fewest number of rats (CTA+EXT and (Mus)CTA+EXT were significantly different from one another. When non-significant effects were found (e.g., comparing CTA+EXT vs. CTA(Mus)+EXT or (Mus)CTA+EXT vs. (Mus)CTA+(Mus)EXT) the group “days of extinction” means were so similar that running of additional animals would not likely have produced significant differences and would have been a waste of animal resources.
The current study further implicates GABAergic mechanisms in the extinction of CTA. The data provided in the current study as well, as other research, suggest that GABA is involved in extinction, perhaps as a modulator of other neural mechanisms (e.g., Marsicano et al., 2002). Systemic muscimol administration decreases norepinephrine (NE) release in the basolateral amygdala (BLA), and this decrease is associated with reduced memory retention (for example, see Miranda & McGaugh, 2004). The BLA has been implicated in CTA extinction (Bahar et al., 2004), in particular, during the static phase of extinction in which the BLA exhibits increased activity (Mickley et al., 2004). In the current study, it is during the static phase that we observed muscimol’s effect on extinction. This further suggests that BLA has a critical role during the early stages of CTA extinction. Direct infusion of muscimol along with NE into the BLA does not produce the same consolidation decrement when muscimol is infused alone (Berlau & McGaugh, 2005), suggesting that noradrenergic influence supersedes GABAergic influence in the amygdala.
Uncovering GABA’s exact role in extinction could lead to the development of new pharmaceutical treatments for treating phobias, PTSD, and anxiety disorders; disorders in which pervasive anxiety/fear responses are resistant to extinction (Barad, 2005). Our data are confined to CTA – a particular defensive reaction to a learned fear. Further research will be needed to fully elucidate the extent of GABAergic influence on CTA, the acquisition and extinction of other fears, and more generally, learning.
Acknowledgments
The authors would like to acknowledge the following students and technicians for their contributions to this work: Haley Bartholomew, Orion Biesan, Sarah Clark, Jennifer Dunger, Sarah Frischmann, Sara Gombash, Nick Grisak, Jennifer Hardwick, Jennifer Huffman, Ivan Islamaj, Natalie Hogan, Nita Hoxha, Lorena Kanto, Kyle Ketchesin, Ye-Hyun Kim, Bruce Kinley, Clifford Raymond, Dave Revta, Nicole Schneider, & Beth Zanick.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agüero A, Gallo M, Arnedo M, Molina F, Puerto F. The functional relevance of medial parabrachial nucleus in intragastric sodium chloride-induced short-term (concurrent) aversive learning. Neurobiol Lear Mem. 1997;67:161–166. doi: 10.1006/nlme.1996.3749. [DOI] [PubMed] [Google Scholar]
- Bahar A, Dorfman N, Dudai Y. Short Communication: Amygdalar circuits required for either consolidation or extinction of taste aversion memory are not required for reconsolidation. Eur J Neurosci. 2004;19:1115–8. doi: 10.1111/j.0953-816x.2004.03215.x. [DOI] [PubMed] [Google Scholar]
- Bahar A, Samuel A, Hazvi S, Dudai Y. The amygdalar circuit that acquires taste aversion memory differs from the circuit that extinguishes it. Eur J Neurosci. 2003;17:1527–30. doi: 10.1046/j.1460-9568.2003.02551.x. [DOI] [PubMed] [Google Scholar]
- Barad M. Fear extinction in rodents: basic insight to clinical promise. Current Opinions in Neurobiol. 2005;15:710–15. doi: 10.1016/j.conb.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Baraldi M, Grandison L, Guidotti A. Distribution and metabolism of muscimol in the brain and other tissues of the rat. Neuropharmacol. 1979;18:57–62. doi: 10.1016/0028-3908(79)90009-1. [DOI] [PubMed] [Google Scholar]
- Barnfield AM, Clifton PG. Flavour aversions conditioned by dl-fenfluramine: A volume independent mechanism. Psychopharmacol. 1989;98:108–12. doi: 10.1007/BF00442015. [DOI] [PubMed] [Google Scholar]
- Berlau DJ, McGaugh JL. Enhancement of extinction memory consolidation: The role of the noradrenergic and GABAergic systems within the basolateral amygdala. Neurobiol of Learn & Mem. 2005;86:123–32. doi: 10.1016/j.nlm.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Bermúdez-Rattoni F. Molecular mechanisms of taste-recognition memory. Nature Reviews Neurosci. 2004;5:209–17. doi: 10.1038/nrn1344. [DOI] [PubMed] [Google Scholar]
- Biggio G, Dazzi L, Biggio F, Mancuso L, Talani G, Busonero F, Mostallino MC, Sanna E, Follesa P. Molecular mechanisms of tolerance to and withdrawal of GABAA receptor modulators. Eur Neuropsychopharmacol. 2003;13:411–23. doi: 10.1016/j.euroneuro.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Westbrook RF, Corcoran KA, Maren S. Contextual and temporal modulation of extinction: Behavioral and biological mechanisms. Biol Psych. 2006;60:352–60. doi: 10.1016/j.biopsych.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Castellano C, McGaugh JL. Effects of post-training bicuculline and muscimol on retention: Lack of state dependency. Behav & Neural Biol. 1990;54:156–64. doi: 10.1016/0163-1047(90)91352-c. [DOI] [PubMed] [Google Scholar]
- Davis M, Myers KM. The role of glutamate and gamma-aminobutyric acid in fear extinction: Clinical implications for exposure therapy. Biol Psychol. 2002;52:998–1007. doi: 10.1016/s0006-3223(02)01507-x. [DOI] [PubMed] [Google Scholar]
- Domjan M, Gillan DJ. Taste-aversion conditioning with expected versus unexpected drug treatment. J Exp Psychol: Animal Behav Processes. 1977;3:297–309. doi: 10.1037//0097-7403.3.4.297. [DOI] [PubMed] [Google Scholar]
- Garcia J, Kimeldorf DJ, Koelling RA. Conditioned aversion to saccharin resulting from exposure to gamma radiation. Science. 1955;122:157–58. [PubMed] [Google Scholar]
- Gemberling GA, Domjan M. Selective associations in one-day-old rats: Taste-toxicosis and texture-shock aversion learning. J Comp Physiol Psychol. 1982;96:105–13. doi: 10.1037/h0077855. [DOI] [PubMed] [Google Scholar]
- Houston AJ, Wong JCL, Ebenezer IS. Effects of subcutaneous administration of the γ-aminobutyric acidA receptor agonist muscimol on water intake in water-deprived rats. Physiol & Behav. 2002;77:445–50. doi: 10.1016/s0031-9384(02)00876-4. [DOI] [PubMed] [Google Scholar]
- Kirk RE. Experimental Design: Procedures for the Behavioral Sciences. Monterey, CA: Brooks/Cole; 1982. [Google Scholar]
- Lee SH, Choi JH, Lee N, Lee HR, Kim JI, Yu NK, Choi SL, Lee SH, Kim H, Kaang BK. Synaptic protein degradation underlies destabilization of retrieved fear memory. Science. 2008;319:1253–56. doi: 10.1126/science.1150541. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgänsberger W, Di Marzo V, Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–4. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- Meachum CL, Bernstein IL. Conditioned Responses to a taste conditioned stimulus paired with lithium chloride administration. BehavNeurosci. 1990;104:711–715. doi: 10.1037//0735-7044.104.5.711. [DOI] [PubMed] [Google Scholar]
- Metha AK, Ticu MK. Baclofen induces catatonia in rats. Neuropharmacology. 1987;25:1419–1423. doi: 10.1016/0028-3908(87)90108-0. [DOI] [PubMed] [Google Scholar]
- Michelot D, Melendez-Howell LM. Amantia muscaria: Chemistry, biology, toxicology, and ethnomycology. Mycol Res. 2003;107:131–46. doi: 10.1017/s0953756203007305. [DOI] [PubMed] [Google Scholar]
- Mickley GA, Kenmuir CL, McMullen CA, Yocom AM, Valentine EL, Dengler-Crish CM, Weber B, Wellman JA, Remmers-Roeber DR. Dynamic processing of taste aversion extinction in the brain. Brain Res. 2004;1061:79–89. doi: 10.1016/j.brainres.2004.04.071. [DOI] [PubMed] [Google Scholar]
- Mickley GA, Hoxha Z, Bacik S, Kenmuir CL, Wellman JA, Biada JM, DiSorbo A. Spontaneous recovery of a conditioned taste aversion differentially alters extinction-induced changes in c-Fos protein expression in rat amygdala and neocortex. Brain Res. 2007;1152:139–57. doi: 10.1016/j.brainres.2007.03.050. [DOI] [PubMed] [Google Scholar]
- Miranda MI, McGaugh JL. Enhancement of inhibitory avoidance and conditioned taste aversion memory with insular cortex infusions of 8-Br-cAMP: Involvement of the basolateral amygdala. Learn Mem. 2004;11:312–17. doi: 10.1101/lm.72804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, Ressler KJ, Davis M. Different mechanisms of fear extinction dependent on the length of time since fear acquisition. Learn Mem. 2006;13:216–23. doi: 10.1101/lm.119806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa Y, Ishibashi Y, Yoshii T, Tagashira E. Muscimol induces state-dependent learning in Morris water maze task in rats. Brain Res. 1995;681:126–30. doi: 10.1016/0006-8993(95)00303-8. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the care and use of laboratory animals. Washington (DC): National Academy Press; 1996. [Google Scholar]
- Nolan LJ, McCaughey SA, Giza BK, Rhinehart-Doty JA, Smith JC, Scott TR. Extinction of a conditioned taste aversion in rats: I. Behavioral effects. Physiol and Behav. 1997;61:319–23. doi: 10.1016/s0031-9384(96)00411-8. [DOI] [PubMed] [Google Scholar]
- Overton DA. State dependent learning and drug discriminations. In: Iversen LL, Iversen SD, Snyder SH, editors. Handbook of psychopharmacology. Vol. 18. New York: Plenum; 1984. [Google Scholar]
- Pavlov IP. Conditioned Reflexes. London: Oxford University Press; 1927. [Google Scholar]
- Reilly S, Bornovalova MA. Conditioned taste aversion and amygdala lesions in the rat: A critical review. Neurosci Biobehav Reviews. 2005;29:1067–88. doi: 10.1016/j.neubiorev.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Pavlovian conditioned inhibition. Psychol Bulletin. 1969;72:77–94. [Google Scholar]
- Rescorla RA, Heth CD. Reinstatement of fear to an extinguished conditioned stimulus. J Exp Psychol: Animal Behav Processes. 1975;104:88–96. [PubMed] [Google Scholar]
- Rosas JM, Bouton ME. Spontaneous recovery after extinction of a conditioned taste aversion. Animal Learn & Behav. 1996;24:341–8. [Google Scholar]
- Rossato JI, Bonini JS, Coitinho AS, Vianna MRM, Medina JH, Cammarota M, Izquierdo I. Retrograde amnesia induced by drugs acting on different molecular systems. Behav Neurosci. 2004;118(3):563–8. doi: 10.1037/0735-7044.118.3.563. [DOI] [PubMed] [Google Scholar]
- Salinas JA, Mcgaugh JL. Muscimol induces retrograde amnesia for changes in reward magnitude. Neurobiol of Learn Mem. 1995;63:277–85. doi: 10.1006/nlme.1995.1032. [DOI] [PubMed] [Google Scholar]
- Smith JC, Roll DL. Trace conditioning with x-rays as an aversive stimulus. Psychonomic Science. 1967;9:11–2. [Google Scholar]
- Wellman PJ. Laboratory Exercises in Physiological Psychology. 4. Needham Heights, MA: Allyn and Bacon; 1994. [Google Scholar]


