Abstract
A new, convenient pathway is developed to synthesize highly hydrolytically labile poly(ortho ester amide) (POEA) copolymers that overcomes some of the major weaknesses of the traditional methods of synthesizing poly(ortho esters) and their derivatives. A diamine monomer containing a built-in, stabilized ortho ester group was synthesized and was used for polycondensation with diacid esters, giving rise to a series of POEA copolymers with unique stimuli-responsive properties. The POEA undergoes temperature-responsive, reversible sol-gel phase transition in water. Phase diagrams of the POEA/H2O mixture reveal the concentration-dependent existence of different phases, including hydrogel and opaque or clear solution. Such behavior may be attributed to the temperature-dependent hydrogen-bonding involving the amide groups in the POEA backbone and hydrophobic interactions between POEA chains, and it is tunable by selecting diacid monomers with different chemical structures. The kinetics of POEA mass loss in physiological aqueous buffers and release of a model macromolecular drug, fluorescently labeled dextran, are nearly zero-order, suggesting predominantly surface-restricted polymer erosion. The rates of polymer erosion and drug release are much faster at pH 5.0 than pH 7.4. No cytotoxicity was found for the polymer extracts and the polymer degradation products at concentrations as high as 1 mg/ml. The normal morphology of fibroblasts cultured directly in contact with POEA films was not altered. These novel acid-labile temperature-responsive POEA copolymers may be potentially useful for a wide range of biomedical applications such as minimal invasive delivery of controlled-release drug formulations that respond to biological temperature and acidic-pH environments in cells and tissues.
Introduction
Over the past decades, a large variety of biodegradable polymers, such as polyesters,1,2 polyanhydrides,3,4 poly(ortho esters),5,6 polyacetals,7,8 polyphosphazene,9 and polyphosphoesters,10 have been synthesized and investigated for use as surgical tools, tissue prostheses, scaffolds for tissue engineering, and drug delivery systems.11 Despite much progress in the field, to meet the many challenges in modern medicine, there is an urgent need for developing novel polymeric materials with more sophisticated properties and degradation behavior that can be tailored to respond to multiple external stimuli such as temperature and pH.12–16
Biodegradable polymers that undergo surface-erosion are particularly attractive biomedical materials, due to the ability of achieving precise control over the rate of erosion and other time-dependent changes of material properties.17 The design principle of surface-eroding polymers involves building highly (hydrolytically) labile polymer backbone with tunable chain hydrophobicity, as exemplified in the development of polyanhydrides18 and poly(ortho esters).19 The synthesis of such polymers is challenging, because one has to create highly labile linkages from unstable monomers, while at the same time, suppress premature polymer degradation to allow for processing, sterilization, and long-term storage.5,6 To overcome these problems, we set out to demonstrate a new, efficient strategy of synthesis of highly hydrolytically labile polymers, using poly(ortho esters) (POE) as a proof-of-principle. We further show that this new strategy enables the generation of polymeric materials with a combination of unique responsive and erosion properties potentially useful for biomedical applications, i.e., hydrogels capable of temperature-dependent, reversible sol-gel phase transition and accelerated surface-erosion triggered by mildly acidic pH.
Four generations of POE have been reported by Heller and colleagues since the 1960s and have been the subject of numerous preclinical and clinical evaluations for use in orthopedic surgery, implantable medical devices, and controlled drug delivery.5,6 The chemical synthesis of POE is either through transesterification between an ortho ester and a diol, or through addition polymerization between a diol and a diketene acetal.19 Both methods have serious weaknesses. Transesterification requires high vacuum and high temperature, long reaction time, and the molecular weight of POE is often low and irreproducible. The addition polymerization method is more successful, however, a spiro diketene acetal, 3,9-diethylidene-2,4,8,10-tetraoxaspiro[5,5]undecane (DETOSU), is the only known monomer and is highly unstable – hydrolyzing rapidly with trace amount of water or isomerizing spontaneously during storage into an inactive form.19
Here we report a synthetic pathway to a new family of ortho-ester-containing copolymers, poly(ortho ester amides, POEA) (Scheme 1), to overcome the drawbacks of the traditional methods of POE synthesis. The AA-BB-type copolymers were synthesized by facile solution polycondensation between an acid-labile diamine with a built-in ortho ester bond and fatty diacid esters with different chain-length. These copolymers showed tunable temperature-responsive sol-gel transition behavior in water. Erosion of the POEA hydrogels and release of a model macromolecular drug were monitored in aqueous media at pH 7.4 and 5. Much faster polymer erosion and drug release were observed at the mildly acidic pH. The nearly zero-order kinetic of polymer erosion matched that of the drug release, suggesting surface-erosion of the POEA. Furthermore, cytotoxicity experiments indicated that the POEA polymers are compatible with cultured fibroblasts.
Scheme 1.
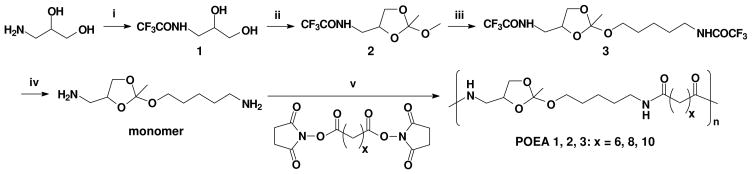
Synthesis of poly(ortho ester amides) (POEA). Reaction conditions: (i) ethyl trifluoroacetate, acetonitrile; ii) trimethyl orthoacetate, p-toluene sulfonic acid (p-TSA), acetonitrile; (iii) 5-trifluoroacetylamino-1-pentanol, pyridinium p-TSA; (iv) NaOH/H2O/THF; (v) triethylamine, DMF.
Experimental Section
Reagents and general methods
THF was distilled over sodium and benzophenone. Acetonitrile was dried by distillation over CaH2. Disuccinimidyl suberate, disuccinimidyl sebacate, disuccinimidyl dodecanoate were purchased from Molecular Biosciences, Inc. (Boulder, CO). All other reagents were purchased commercially and used without further purification unless otherwise specified. Elemental analysis was conducted on a Carlo Erba 1108 automatic analyzer. High-resolution mass spectra in electrospray (ESI) experiments were obtained on a Bruker MicrOTOF-Q mass spectrometer. The 1H and 13C- NMR spectra were recorded on a Varian Unity spectrometer (300 MHz), and chemical shifts were recorded in ppm. The molecular weight and polydispersity of polymers were determined by gel permeation chromatography (GPC) relative to poly(ethylene glycol) calibration using a Waters 1525 binary HPLC pump, a Waters 2414 differential refractometer, and a Waters YMC HPLC column. Methanol with 1% triethylamine (TEA) was the eluent with a flow rate of 1.0 mL/min at 35°C. All sample solutions were filtered through a 0.45-μm filter before injecting into the GPC system. Thermogravimetric analysis (TGA) was conducted on a Perkin Elmer TGA7 Thermogravimetric Analyzer with a heating rate of 20 °C/min under a nitrogen atmosphere.
2,2,2-Trifluoro-N-(2,3-dihydroxypropyl) acetamide (1)
To a stirred mixture of 3-amino-1,2-propanediol (18.80 g, 0.21 mol) in acetonitrile (120 mL) under argon was added dropwise ethyl trifluoroacetate (34.30 g, 0.24 mol). After 4 h, the solvent was evaporated, and the residue was dissolved in ethyl acetate (200 ml), washed with aqueous KHSO4 and brine, then dried over MgSO4, and concentrated to yield 34.90 g (91%) of 2,2,2-trifluoro-N-(2,3-dihydroxypropyl)-acetamide as colorless oil. 1H NMR (300MHz, CD3OD): δ (ppm) 3.27–3.29 (m, 2H, NH-CH2), 3.47–3.49 (m, 2H, CH2-OH), 3.70–3.78 (m, 1H, CH-OH), 7.60 (b, 1H, NH).
2,2,2-Trifluoro-N-(2-methoxy-2-methyl-[1,3]-dioxolan-4-ylmethyl) acetamide (2)
To a stirred mixture of 1 (10.16 g, 54.30 mmol), trimethyl orthoacetate (30.00 g, 0.25 mol), and acetonitrile (80 mL) was added p-toluene sulfonic acid (p-TSA; a trace amount). The mixture was reacted for 6 h at room temperature, followed by evaporation of most of volatile components. The residue was dissolved in ethyl acetate (250 ml), washed successively with saturated aqueous sodium hydrogen carbonate and brine, dried over MgSO4, and concentrated to yield 11.32 g (86%) of 2,2,2-trifluoro-N-(2-methoxy-2-methyl-[1,3] dioxolan-4-ylmethyl) acetamide as oil. 1H NMR (300MHz, CDCl3): δ (ppm) 1.67–1.69 (d, 3H, CH3), 3.27–3.30 (d, 3H, O-CH3), 3.37–3.72 (m, 2H, NH-CH2), 4.14–4.21 (m, 2H, O-CH2), 4.43–4.51 (m, 1H, O-CH), 6.86–7.89 (b, 1H, NH).
5-Trifluoroacetylaminopentanol
To a stirred mixture of 5-amino-1-pentanol (10.00 g, 96.94 mmol) in THF (100 mL) under argon was added dropwise ethyl trifluoroacetate (15.98 g, 112.45 mmol). After 4 h, the solvent was evaporated, and the residue was dissolved in ethyl acetate (200 ml), washed with aqueous KHSO4 and brine, then dried over MgSO4, and concentrated to yield 17.33 g (90%) of 5-trifluoroacetylaminopentanol as colorless oil. 1H NMR (300MHz, CDCl3): δ (ppm) 1.41–1.49 (m, 2H, CH2), 1.56–1.68 (m, 4H, CH2), 2.19 (b, 1H, O-H), 3.34–3.41 (q, 2H, CH-NH), 3.64–3.68 (q, 2H, CH-OH), 6.90 (b, 1H, NH).
2,2,2-Trifluoro-N-(2-(5′-trifluoroacetylaminopentyloxy)-2-methyl-[1,3]-dioxolan-4-ylmethyl) acetamide (3)
A mixture of 2 (4.18 g, 17.19 mmol), 5-trifluoroacetylaminopentanol (3.42 g, 17.19 mmol), and pyridinium p-toluene sulfonate (45.00 mg, 0.17 mmol) was heated at 130°C until no volatile component was distilled. After cooling to room temperature, the residue was purified with column chromatography (silica gel, ethyl acetate/hexane (1/1) as eluent) to yield 5.99 g (85%) of 2,2,2-trifluoro-N-(2-(5′-trifluoroacetylaminopentyloxy)-2-methyl-[1,3]dioxolan-4-ylmethyl) acetamide as oil. 1H NMR (300MHz, CDCl3): δ (ppm) 1.41–1.44 (t, 2H, CH2), 1.55–1.63 (m, 4H, CH2), 2.08–2.09 (d, 3H, CH3), 3.34–3.36 (m, 2H, NH-CH2), 3.61–3.65 (m, 2H, NH-CH2), 4.02–4.16 (m, 2H, O-CH2), 4.30–4.36 (m, 1H, O-CH), 6.75–6.91 (b, 1H, NH), 7.24–7.48 (b, 1H, NH).
4-Aminomethyl-2-aminopentyloxy-2-methyl-[1,3]-dioxolan (Monomer)
The trifluoroacetamide 3 (5.98 g, 14.57 mmol) was dissolved in THF (40 ml), and sodium hydroxide (1.6 M, 40 ml) was added. The mixture was vigorously stirred overnight, extracted with diethyl ether, dried over MgSO4, and evaporated. The residue was purified with column chromatography (silica gel, methanol/dichloromethane (1/10) as eluent) to yield 1.91 g (60%) of 4-aminomethyl-2-aminopentyloxy- 2-methyl-[1,3]-dioxolan as oil. 1H NMR (300MHz, CDCl3): δ (ppm) 1.45–1.71 (m, 9H, CH2, CH3), 2.67–2.90 (m, 4H, CH2-NH2), 3.48–3.50 (m, 1H, O-CH2), 3.58–3.62 (t, 2H, O-CH2), 3.71–3.72 (m, 1H, O-CH2), 4.06–4.16 (m, 1H, O-CH). 13C NMR (CDCl3, δ ppm): 22.02, 22.32, 23.57, 23.60, 29.63, 32.58, 33.32, 33.60, 44.72, 44.77, 62.21, 62.60, 67.19, 67.27, 78.44 (CH), 121.68, 121.90. Calcd: (C10H22N2O3) 218.3, found m/z 219.2 (M + H+), 241.2 (M + Na+). Anal. Calcd for (C10H22N2O3): C, 55.02; H, 10.16; N, 12.83. Found: C, 54.87; H, 10.06; N, 12.73.
Polymerization
The three copolymers (POEA1, 2 and 3) were synthesized using the same procedure of polycondensation, and the synthesis of POEA 1 is described here as an example: Disuccinimidyl suberate (0.34 g, 0.92 mmol) was added to a stirred mixture of monomer (0.20 g, 0.92 mmol), TEA (0.40 ml, 2.87 mmol), and dimethylformamide (DMF) (2.5 mL) under argon, reacted overnight at room temperature, and added dropwise to stirred diethyl ether. The precipitated polymer was collected, washed with diethyl ether repeatedly, and dried under high vacuum to yield 271 mg (83%) of POEA 1 as white powder. 1H NMR (300MHz, deuterated dimethyl sulfoxide (d-DMSO): δ (ppm) 1.15–1.41 (m, 20H, CH2), 1.92–1.97 (m, 3H, CH3), 2.43–2.44 (m, 4H, OC-CH2), 2.92 (m, 4H, NH-CH2), 3.28 (m, 4H, O-CH2), 4.29 (b, 1H, O-CH). 7.65 (b, 1H, NH). Anal. Calcd for (C18H32N2O5)n: C, 60.65; H, 9.05; N, 7.86. Found: C, 57.18; H, 8.75; N, 7.51. To demonstrate degradability of POEA, 30 mg of POEA 2 was stirred in 10 mL of 1 M hydrochloric acid for 1 hour at room temperature, and then adjusted to pH 7.4 with 1 M NaOH. The mixture was centrifuged at 4000 rpm for 30 minutes, and the obtained precipitate was dried overnight under vacuum. The molecular weight and polydispersity of the degradated product was determined by GPC.
Temperature-responsive Sol-gel Phase Transition
POEA was added to phosphate-buffered saline (PBS, pH 7.4) at 10 wt% and dissolved completely by heating to at least 80 °C. 50 μL of the solution was placed in a glass cuvette and sealed to prevent loss due to evaporation. The polymer solution was heated to >90°C, and was cooled at a constant rate of 5°C per minute, and the optical transmittance of the sample at 500 nm was recorded every 12 seconds using a DU 640B spectrophotometer (Beckman Coulter). Repeated cooling and heating using a thermostat-controlled water-bath was conducted to assess the reversibility of the sol-gel phase transition behavior.
Phase Diagram of POEA/H2O Mixtures
A series of POEA solutions in PBS (pH 7.4) with concentrations ranging from 5 to 35% was prepared by heating to complete dissolution. 0.4 mL of polymer solution was transferred to a microtube of 2 mL, placed in a water-bath, and cooled to room temperature. The sample was then heated stepwise every 1°C, held at each temperature point for 5 minutes to reach equilibrium, and the physical state of the sample was determined by inverting the tubes. The sol to gel transition manifested as the change from a fluidic solution to a non-fluidic gel. The procedure was repeated for all three POEA polymers.
pH-Dependent Erosion of POEA Hydrogels and Release Kinetics of FITC-dextran
POEA 2 (30 mg) and FITC-labeled dextran (FITC-dex, 70 kDa, Pierce, 1 mg) were dissolved by heating in 0.1 ml of phosphate buffer (20 mM, pH 7.4) in an Eppendorf tube, cooled to room temperature to form hydrogel, and vacuum-dried for 1 to 2 hours until constant weight. The FITC-dex-containing hydrogel was placed in 2 ml of neutral and acidic buffers − 20 mM phosphate buffer (pH 7.4) and 20 mM phosphate-citrate buffer (pH 5), respectively, and incubated at 37°C under mild agitation (60 rpm) using a platform shaker. The release medium was completely removed at predetermined time points, and 0.2 ml of the recovered buffer was used for quantifying the amount of released FITC-dex by fluorometry. The samples were excited at 490 nm, and the emission fluorescence intensity at 520 nm was determined using a Synergy HT microplate reader equipped with a 530 ± 15 nm band-pass filter (Bio-TEK). The equipment was calibrated using serially diluted standard solutions of FITC-dex. Cumulative release of FITC-dex at any given time point was calculated as the percentile of the total FITC-dex loaded into the hydrogels. After removal of the release medium, the remaining hydrogel was vacuum-dried and the dry weight measured. Polymer erosion was quantified by the time dependence of hydrogel dry weight normalized to the dry weight at time zero. Triplicate hydrogel samples were tested at each time point until day 14.
Determination of Cytotoxicity of Polymer Extract
The procedure is adapted from a method described in ISO 10993-5. POEA films were prepared by dissolving the polymers in ethanol at a concentration of 10 mg/mL and allowing the ethanol to evaporate overnight from the wells of a 96-well plate. Each well contained 50 μL of solution equivalent to 500 μg polymer. Cell culture media (200 μL, Dulbecco’s Modified Eagle Medium with 10% fetal bovine serum, 100 units/mL of penicillin/streptomycin, 2.5% glucose, pH 7.4) was then added to each well to extract the polymer films overnight at 37 °C with mild agitation. The polymer extract was transferred to a separate 96-well plate containing fibroblasts (NIH3T3) seeded at 5000 cells/well and was incubated at 37 °C in 5% CO2 for 24 hours before performing the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay to determine cell viability. To perform the assay, 20 μL of MTT stock solution (5 mg/mL in PBS) was added to the cells in each well and incubated for 4 hours. Cell culture media was then removed and replaced with 100 μL DMSO, and absorbance was read at 570 nm using a Synergy HT microplate reader (Bio-TEK). After subtracting background signal, cell viability (%) was calculated as the ratio between the absorbance of cells incubated with polymer extract and cells treated with culture media only.
Determination of Cytotoxicity of Polymer Degradation Products
POEA (6 mg) was added to 100 μL hydrochloric acid (1 N) to complete dissolution and incubated at 37°C overnight. Cell culture media (300 μL) was added and the pH was adjusted to 7 with NaOH (1 N). More cell culture media was added to a total volume of 600 μL with an equivalent polymer concentration of 10 mg/mL. This solution of polymer degradation products was then added to NIH 3T3 fibroblasts plated at 5000 cells/well in a 96-well plate in a series of dilutions. Cells were incubated for 24 hours and the MTT assay was performed to determine cell viability.
Cell Morphology by Direct Contact
The procedure is adapted from a method described in ISO 10993-5. POEA polymer films were prepared by dissolving polymers in ethanol at a concentration of 10 mg/mL, adding to a 6-well plate, and allowing the ethanol to evaporate overnight. NIH3T3 fibroblasts were seeded over the polymer film at 150,000 cells per well in 2 mL cell culture media and incubated for 24 hours at 37°C in 5% CO2. Following staining with a nucleus dye, Hoescht 333422 (Invitrogen, 5 μg/mL), for 30 minutes at 37 °C, cell morphology was examined using an Olympus BX60 upright microscope under polarized light. Cells cultured on tissue-culture-treated plates without exposure to polymer were used for comparison.
Results and Discussion
Synthesis and Characterization
The key acid-labile ortho ester diamine monomer was synthesized in four steps (Scheme 1): 2,2,2-trifluoro-N-(2,3-dihydroxypropyl) acetamide (1) was obtained by using 3-amino-1,2-propanediol as the starting material, and then reacted with trimethyl orthoacetate in the presence of catalytic p-toluenesulfonic acid to give the ortho ester 2. The compound 3 was easily obtained by transesterification between compound 2 and trifluoroacetylaminopentanol. The TFA-protecting groups of compound 3 were removed by aqueous sodium hydroxide in THF, and the acid-labile bifunctional monomer was easily obtained with the total yield of approximately 40%. The 1H and 13C NMR spectra confirmed that the monomer was pure and structurally correct (see the Supporting Information, Figure S1). This monomer is unique in the following aspects. First, the highly hydrolytically labile ortho ester is built into the monomer and is stabilized by the neighboring primary amines, allowing for easy handling and long-term storage. In fact, our NMR study confirmed that the monomer remained intact for at least one year at room temperature without desiccation (data not shown). Second, the diamine monomer can be copolymerized readily with any multifunctional amine-reactive monomers to produce a range of structurally and functionally diverse, biodegradable copolymers, for examples, ortho-ester-containing polyamides, polyurea, polyurethane, etc. Therefore, our new pathway of synthesis not only overcomes the drawbacks of the traditional method of synthesizing POE, but also opens up an array of possibilities that may lead to new biodegradable polymer structures and materials.
To demonstrate the feasibility of this strategy, we synthesized poly(ortho ester amides) (POEA) via polycondensation of the ortho ester diamine monomer with active esters of different aliphatic diacid (Scheme 1). Three polymers (POEA 1~3) were synthesized with high yield (Table 1) and their chemical structures were confirmed by 1H-NMR (Figure S2). The average molecular weight and distribution of the polymers was similar, ranging between 15,000 and 20,000 with polydispersity index of 1.8~2.0, as determined by GPC (Figure S3, Table 1). We found that the polymers could be hydrolyzed completely. For example, after treatment in concentrated acid (1 M HCl, 1 hour, room temperature), POEA 2 degraded to fragments with molecular weight of a few hundreds (Figure S3). TGA revealed that the polymers had relatively high thermal stability with initial degradation temperature in the range of 200~260°C (Figure S4, Table 1). With further optimization of the polymerization conditions, we expect that POEA with even higher molecular weight could be obtained.
Table 1.
Results of polymerization and characterization of polymers by GPC and TGA
| Polymer | Yield (%) | Mn (×10−4)a | Mw (×10−4)a | PDI | TGA (°C)b |
|---|---|---|---|---|---|
| POEA 1 | 82 | 1.50 | 2.70 | 1.80 | 202 |
| POEA 2 | 85 | 1.61 | 3.22 | 2.00 | 213 |
| POEA 3 | 86 | 2.00 | 3.82 | 1.91 | 257 |
Determined by GPC in methanol based on poly(ethylene glycol) standards.
Temperature at initial weight loss under nitrogen.
Temperature-responsive Sol-Gel Transition Behavior
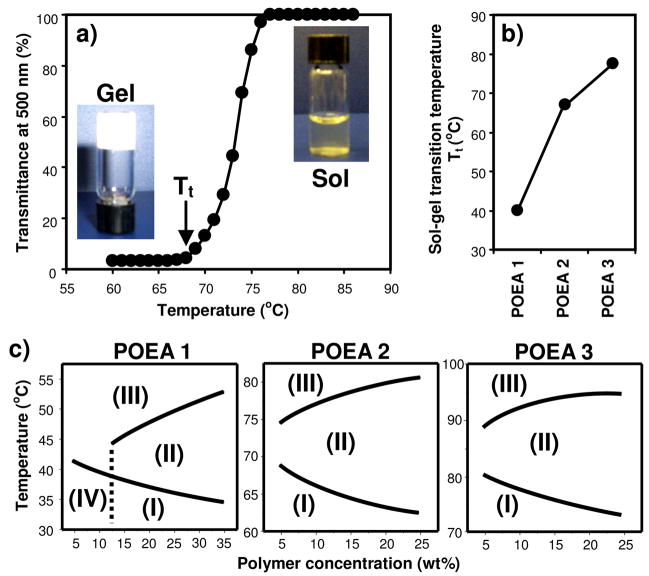
Surprisingly, we found that the POEA copolymers underwent temperature-responsive, reversible sol-gel phase transition in polar solvents such as water. As an example, the optical transparency of 10 wt% POEA 2 in PBS (pH 7.4) was recorded at 500 nm at different temperature (Figure 1a). The polymer dissolved completely as a slightly yellowish but clear solution at above 80°C. As the temperature was lowered, the polymer solution turned opaque, eventually solidifying to form an opaque gel at below 70°C. The sol-gel phase transition was fully reversible with little hysteresis. Apparently, both the amide groups with hydrogen-bonding potential and the hydrophobic segments within the POEA copolymers play important roles in facilitating hydrogel formation and temperature-responsive sol-gel transition.
Figure 1.
POEA copolymers undergo temperature-responsive, reversible sol-gel transition in aqueous medium. a) Temperature dependence of optical transmittance at 500 nm for 10 wt% POEA 2 aqueous mixture at pH 7.4. Insets are images of the hydrogel and solution phases. b) Dependence of sol-gel transition temperature (Tt) on polymer structure. c) Phase diagrams of POEA in PBS (pH 7.4). Phases: (I) hydrogel; (II) opaque solution; (III) clear solution; (IV) precipitate.
The temperature-responsive behavior of the POEA copolymers can be tuned by adjusting the number of methylene carbons in the diacid repeating unit, which affects the hydrophobicity of the polymer chain (Scheme 1). As shown in Figure 1b, the sol-gel transition temperature, Tt, increased as the number of methylene carbons in the polymers increased in the order of POEA 1 to 3, given that the polymers had similar average molecular weight and distribution. Phase diagrams of the polymers were constructed by equilibrating microtubes containing POEA solutions (5 to 35% in PBS, pH 7.4) at different temperature and observing the sol/gel state of the samples by inverting the tubes. All three polymers can exist as either hydrogel, opaque solution, or clear solution, in addition to POEA 1 precipitating at concentrations below 15% and at temperatures below 40 °C (Figure 1c). While the concentration dependence of phase transition is similar among POEA 1~3, the range of transition temperatures varies considerably, which appears to correlate with the difference in polymer hydrophobicity. Therefore, it is possible to tune the sol-gel phase transition of POEA over a wide range of temperatures, including the physiological temperature (~37 °C), through adjusting the chemical composition of the diacid monomers (Figure 1c).
A large number of noncovalently self-assembled polymeric hydrogels has been reported over the years, many of which are known to undergo temperature-responsive sol-gel transition in water and have been used as injectable systems for minimal invasive delivery of drugs and cells to humans.20,21 Some of these polymers, such as poly(N-isopropylacrylamide)22 and the Pluronic® block copolymers,23 are not biodegradable. Others, such as the ABA-block copolymers of polyethylene glycol (PEG) and polyesters,24,25 are degradable but not responsive to physiological pH. Recently there is much interest in designing materials that respond to mildly acidic pH environments such as those found in the endosome of cells, in solid tumors and other tissues inflicted with inflammatory diseases.26–31 As a result, several biodegradable polymer hydrogels combining temperature- and pH-sensitivities have been reported.32–35 These materials, however, respond to pH fluctuations through reversible protonation-deprotonation of chemical groups, leading to changes in solubility, rather than degradability, of the polymer chains. The POEA copolymers reported here are not only capable of temperature-responsive sol-gel transition, but also completely degradable in response to mildly acidic pH via the acid-labile ortho ester groups in the polymer backbone, which may be an advantage over non-degradable systems.
pH-dependent Erosion of POEA Hydrogels and Release Kinetics of FITC-dextran
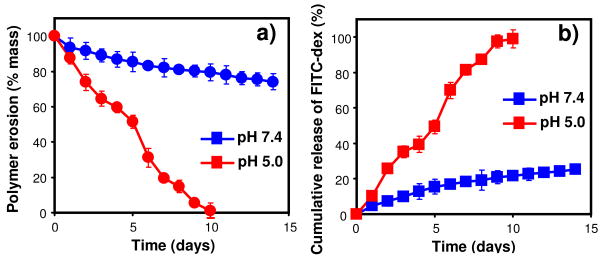
To demonstrate pH-responsive erosion of the acid-labile POEA copolymers and their utility in pH-triggered molecular delivery, we prepared a hydrogel of 30% POEA 2 loaded with 1% of a model drug, fluorescein isothiocyanate (FITC)-labeled dextran (FITC-dex, 70 kDa). The hydrogel was incubated at 37°C in aqueous buffers of pH 7.4 and 5.0. At different time points, the mass loss of hydrogel and the amount of FITC-dex released into the buffers were determined. The hydrogel lost approximately 20% of its mass after 14 days at physiological pH 7.4 but eroded completely by day-10 at mildly acidic pH 5.0 (Figure 2a). Accordingly, only 20% of the FITC-dex was released after 14 days at pH 7.4, whereas complete release was achieved by day-10 at pH 5 (Figure 2b). The process of hydrogel mass loss is nearly zero-order and matches the profile of release kinetics very well at both pHs, indicating that the release of FITC-dex is predominantly driven by surface-restricted polymer erosion.5,6,19 It was reported that PEG-POE graft copolymers could form temperature-responsive hydrogels with modest but poorly controlled pH-sensitivity.36 Synthesis of these graft copolymers required multiple steps from a highly unstable monomer (DETOSU) and the purification process was complicated.36 In contrast, the POEA copolymers described here can be synthesized from stable monomers in a single step, and the POEA hydrogels displayed tunable temperature-sensitivity and highly pH-sensitive surface-erosion, enabling precise control over drug release.
Figure 2.
pH-dependence of kinetics of a) polymer erosion/mass loss and b) release of FITC-dex from POEA 2 hydrogel (30%).
Cytotoxicity and Cell Morphology by Direct Contact
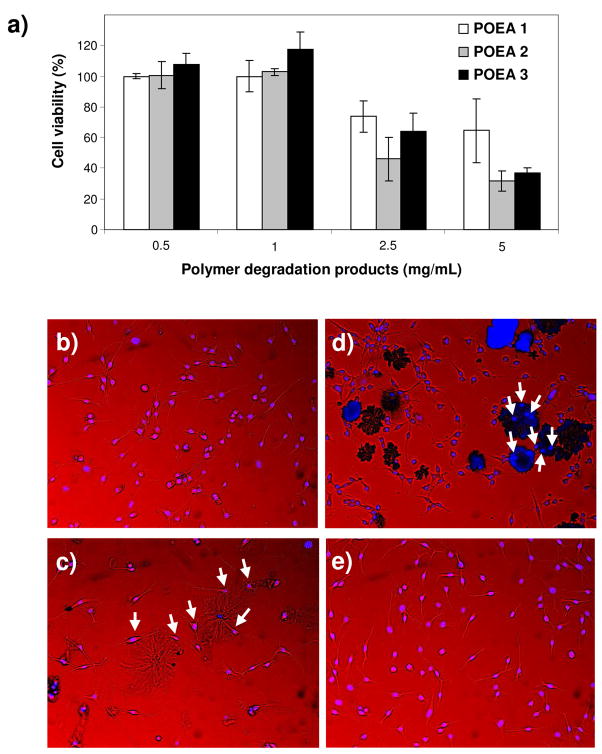
We established that the POEA copolymers are nontoxic and compatible with cells using several in vitro assays following the international regulatory guidelines for implantable biomaterials (ISO 10993-5). First, polymers were extracted with cell culture media for 24 hours. Fibroblasts (NIH3T3) were exposed to the polymer extract and found to be completely viable compared with cells treated with media only, based on a MTT cytotoxicity assay, suggesting that there was no cytotoxic leachable species in the polymers (Figure S5). To evaluate potential toxicity of POEA degradation products, the polymers were completely hydrolyzed in 1 N HCl, adjusted to pH 7, and incubated with fibroblasts for 24 hours. No toxicity was found for polymer concentration as high as 1 mg/mL. Only at even higher concentrations (e.g. 5 mg/mL) did the cell viability drop to below 50% (Figure 3a). These results are comparable or better than that of the poly(lactic-co-glycolic acids),27 a well-known biocompatible polymer widely used in biomedicine. Finally, morphology of fibroblasts cultured in direct contact with the POEA copolymers was examined. POEA films were prepared by dissolving polymers in ethanol, followed by casting into a cell culture plate and drying overnight. Fibroblasts were then cultured with the POEA films for 24 hours and observed with optical microscopy. The erosion of POEA was apparently much more rapid in the presence of cells than in buffer, perhaps due to a combination of cellular activity and mechanical disruption. Cells cultured with POEA films appeared normal and fully-spread (Figure 3b-d) and were morphologically indistinguishable to cells cultured on bioadhesive tissue culture plates (Figure 3e). In the cases of POEA 2 and 3, cells were also seen adhering to residual polymers (Figure 3c,d).
Figure 3.
Cell compatibility evaluation of POEA polymers. a) Viability of NIH3T3 fibroblasts exposed to degradation products of POEA for 24 hours determined by the MTT assay. b–d) Cell morphology after direct contact with POEA film for 24 hours. e) Cells cultured on tissue culture plate without exposure to polymer. Shown are merged white light and fluorescence images. Nuclei of cells were stained with Hoescht 333422 (in blue). Arrows point to cells adhering apparently to substantially degraded polymer residues. b) POEA 1, c) POEA 2, d) POEA 3. Magnification: 10x objective.
Conclusions
We report the synthesis of ortho-ester-containing copolymers as responsive, biocompatible, and biodegradable materials. This method is based on a unique ortho ester diamine monomer that overcomes the major weaknesses of the traditional synthesis of POE, and could be used to generate a large variety of novel polymer structures with unique properties via facile copolymerization with amine-reactive monomers. One type of such structure, POEA copolymers, forms hydrogels that undergo temperature-responsive reversible sol-gel transition in aqueous medium. Furthermore, the POEA hydrogels are highly acid-labile, capable of releasing a model drug through surface-erosion in response to mildly acidic pH. We envision that, with further optimization, POEA-based materials could be potentially useful for a wide range of biomedical applications as injectable controlled drug release devices, cell scaffolds, coatings of medical implants, and “smart” drug carriers in the forms of micro- and nano-particulates, capable of responding to biological temperature and acidic pH in subcellular compartments and inflammatory tissues.
Supplementary Material
Acknowledgments
This work is partially funded by the US National Institutes of Health (Grants R21CA121832, R01 CA129189), the Wallace H. Coulter Foundation (Early Career Translational Research Award), and the US Department of Defense. We thank Dr. Robert Tranquillo for use of his fluorescence microplate-reader.
Footnotes
Supporting Information Available. 1H and 13C-NMR spectra of the ortho ester diamine monomer, a representative 1H-NMR spectrum of POEA, GPC chromatograms of POEA, TGA thermograms of POEA, viability of NIH3T3 fibroblasts in the presence of POEA polymer extract. This material is available free of charge via the Internet at http://pubs.acs.org.
References and Notes
- 1.Oada M. Prog Polym Sci. 2002;27:87–133. [Google Scholar]
- 2.Zinn M, Withol B, Egli T. Adv Drug Deliv Rev. 2001;53:5–21. doi: 10.1016/s0169-409x(01)00218-6. [DOI] [PubMed] [Google Scholar]
- 3.Jain JP, Modi S, Domb AJ, Kumar N. J Controlled Release. 2005;103:541–563. doi: 10.1016/j.jconrel.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 4.Anastasiou TJ, Uhrich KE. Macromolecules. 2000;33:6217–6221. [Google Scholar]
- 5.Heller J, Barr J, Ng SY, Abdellauoi KS, Gurny R. Adv Drug Deliv Rev. 2002;54:1015–1039. doi: 10.1016/s0169-409x(02)00055-8. [DOI] [PubMed] [Google Scholar]
- 6.Heller J, Barr J. Biomacromolecules. 2004;5:1625–1632. doi: 10.1021/bm040049n. [DOI] [PubMed] [Google Scholar]
- 7.Tomlinson R, Heller J, Brocchini S, Duncan R. Bioconjugate Chem. 2003;14:1096–1106. doi: 10.1021/bc030028a. [DOI] [PubMed] [Google Scholar]
- 8.Paramonov SE, Bachelder EM, Beaudette TT, Standley SM, Lee CC, Dashe J, Fréchet JMJ. Bioconjugate Chem. 2008;19:911–919. doi: 10.1021/bc7004472. [DOI] [PubMed] [Google Scholar]
- 9.Cohen S, Bano MC, Visscher KB, Chow M, Allcock HR, Langer R. J Am Chem Soc. 1990;112:7832–7833. [Google Scholar]
- 10.Mao HQ, Leong KW. Adv Genet. 2005;53:275–306. [PubMed] [Google Scholar]
- 11.Nair LS, Laurencin CT. Prog Polym Sci. 2007;32:762–798. [Google Scholar]
- 12.Langer R, Tirrell DA. Nature. 2004;428:487–492. doi: 10.1038/nature02388. [DOI] [PubMed] [Google Scholar]
- 13.Lendlein A, Langer R. Science. 2002;296:1673–1676. doi: 10.1126/science.1066102. [DOI] [PubMed] [Google Scholar]
- 14.Yin X, Hoffman AS, Stayton PS. Biomacromolecules. 2006;7:1381–1385. doi: 10.1021/bm0507812. [DOI] [PubMed] [Google Scholar]
- 15.Dimitrov I, Trzebicka B, Muller AHE, Dworak A, Tsvetanov CB. Prog Polym Sci. 2007;32:1275–1343. [Google Scholar]
- 16.Bachelder EM, Beaudette TT, Broaders KE, Dashe J, Fréchet JM. J Am Chem Soc. 2008;130:10494–10495. doi: 10.1021/ja803947s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mano JF. Adv Eng Mater. 2008;10:515–527. [Google Scholar]
- 18.Göpferich A, Tessmar J. Adv Drug Deliv Rev. 2002;54:911–931. doi: 10.1016/s0169-409x(02)00051-0. [DOI] [PubMed] [Google Scholar]
- 19.Heller J. Adv Polym Sci. 1993;107:41–92. [Google Scholar]
- 20.Ruel-Gariépy E, Leroux J. Eur J Pharm Biopharm. 2004;58:409–426. doi: 10.1016/j.ejpb.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 21.Chitkara D, Shikanov A, Kumar N, Domb AJ. Macromol Biosci. 2006;6:977–990. doi: 10.1002/mabi.200600129. [DOI] [PubMed] [Google Scholar]
- 22.Hacker MC, Klouda L, Ma BB, Kretlow JD, Mikos AG. Biomacromolecules. 2008;9:1558–1570. doi: 10.1021/bm8000414. [DOI] [PubMed] [Google Scholar]
- 23.Packhaeuser CB, Schnieders J, Oster CG, Kissel T. Eur J Pharm Biopharm. 2004;58:445–455. doi: 10.1016/j.ejpb.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 24.Jeong B, Bae YH, Lee DS, Kim SW. Nature. 1997;388:860–862. doi: 10.1038/42218. [DOI] [PubMed] [Google Scholar]
- 25.Yu L, Ding J. Chem Soc Rev. 2008;37:1473–1481. doi: 10.1039/b713009k. [DOI] [PubMed] [Google Scholar]
- 26.Lee ES, Kim D, Youn YS, Oh KT, Bae YH. Angew Chem Int Ed. 2008;47:2418–2421. doi: 10.1002/anie.200704121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang C, Ge Q, Ting D, Nguyen D, Shen HR, Chen JZ, Eisen HN, Heller J, Langer R, Putnam D. Nature Mater. 2004;3:190–196. doi: 10.1038/nmat1075. [DOI] [PubMed] [Google Scholar]
- 28.Bae Y, Fukushima S, Harada A, Kataoka K. Angew Chem Int Ed. 2003;42:4640–4643. doi: 10.1002/anie.200250653. [DOI] [PubMed] [Google Scholar]
- 29.Lynn DM, Amiji MM, Langer R. Angew Chem Int Ed. 2001;40:1707–1710. [PubMed] [Google Scholar]
- 30.Lin S, Du F, Wang Y, Ji S, Liang D, Yu L, Li Z. Biomacromolecules. 2008;9:109–115. doi: 10.1021/bm7008747. [DOI] [PubMed] [Google Scholar]
- 31.Yang SC, Bhide M, Crispe IN, Pierce RH, Murthy N. Bioconjugate Chem. 2008;19:1164–1169. doi: 10.1021/bc700442g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shim WS, Yoo JS, Bae YH, Lee DS. Biomacromolecules. 2005;6:2930–2934. doi: 10.1021/bm050521k. [DOI] [PubMed] [Google Scholar]
- 33.Shim WS, Kim SW, Lee DS. Biomacromolecules. 2006;7:1935–1941. doi: 10.1021/bm0600567. [DOI] [PubMed] [Google Scholar]
- 34.He C, Kim SW, Lee DS. J Controlled Release. 2008;127:189–207. doi: 10.1016/j.jconrel.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 35.Determan MD, Guo L, Thiyagarajan P, Mallapragada SK. Langmuir. 2006;22:1469–1473. doi: 10.1021/la0527691. [DOI] [PubMed] [Google Scholar]
- 36.Schacht E, Toncheva V, Vandertaelen K, Heller J. J Controlled Release. 2006;116:219–225. doi: 10.1016/j.jconrel.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 37.Ignatius AA, Claes LE. Biomaterials. 1996;17:831–839. doi: 10.1016/0142-9612(96)81421-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.