Abstract
In a variety of vertebrates, highly aggressive individuals tend to have high social status and low serotonergic function. In the sex changing fish Lythrypnus dalli, serotonin (5-HT) may be involved as a mediator between the social environment and the reproductive system because social status is a critical cue in regulating sex change. Subordination inhibits sex change in L. dalli, and it is associated with higher serotonergic activity in other species. We tested the hypothesis that high serotonergic activity has an inhibitory effect on sex change. In a social situation permissive to sex change, we administered to the dominant female implants containing the serotonin precursor 5-hydroxytryptophan (5-HTP). In a social situation not conducive to sex change, we administered either the serotonin synthesis inhibitor p-chlorophenylalanine (PCPA) or the 5-HT1A receptor antagonist p-MPPI. After three weeks we used HPLC to measure brain levels of 5-HT and its metabolite 5-hydroxyindoleacetic acid (5-HIAA). We also performed PCPA, p-MPPI and fluoxetine injections in size-matched pairs of females to assess its effect on dominance status. Males and newly sex changed fish showed a trend for higher levels of 5-HIAA and 5-HT/5-HIAA ratio than females. The different implants treatments did not affect the probability of sex change. Interestingly, this species does not seem to fit the pattern seen in other vertebrates where dominant individuals have lower serotonergic activity than subordinates.
Keywords: protogyny, aggression, dominance, PCPA, p-MPPI, fluoxetine, 5-HTP
INTRODUCTION
Serotonin (5-hydroxytryptamine, 5-HT) is a neurotransmitter that mediates a wide variety of behavioral functions through its action in both the central and peripheral nervous systems. Aggressive behavior has been associated with serotonergic function [1–3] in a variety of taxa ranging from lobsters [4] to macaques [5]. In vertebrates, high serotonergic function is often associated with low levels of aggression and/or subordinate status. High serotonergic function has been induced in various studies by using 5-HT receptor agonists or selective serotonin reuptake inhibitors (SSRIs), which retains 5-HT for a longer period in the synapse. For example, in the lizard Anolis carolinensis, chronic SSRI administration to dominant males reversed their social status in a pair, and decreased their production of aggressive behavior [6,3]. In male song sparrows, administering the SSRI fluoxetine or a 5-HT1A receptor agonist decreased aggressive behavior [7]. Bluehead wrasses Thalassoma bifasciatum treated with fluoxetine, either chronically for 14 days or with one single injection, decreased their aggression towards an intruder [8]. Higher turnover of serotonin is commonly quantified by measuring the accumulation of its metabolite 5-hydroxyindoleacetic acid (5-HIAA). In fishes, brain levels of 5-HIAA were higher in subordinate than dominant individuals in both rainbow trout Oncorhyncus mykiss [9], and juvenile arctic charr Salvelinus alpinus [10]. This was shown to be the product of social interactions rather than intrinsic individual differences [11].
Similarly, there is evidence suggesting that low serotonergic function is associated with heightened aggression and/or social dominance. Lines of mice selected for high aggressiveness had lower serotonin levels in the prefrontal cortex than lines selected for low aggressiveness [12]. In stickleback Gasterosteus aculeatus, there was a negative correlation between 5-HT and levels of aggression against an intruder [13]. A single injection of the serotonin synthesis inhibitor, p-clorophenilalanine (PCPA), increased aggression towards a mirror in the firemouth cichlid Cichlasoma meeki [1].
The relationship between 5-HT and aggression is, however, quite complex and it is not clear whether they are causally related. For instance, 5-HT activity increases in both dominant and subordinate animals during a fight so its inhibitory action on aggression might be tightly linked to specific brain regions or might depend on the stage of aggressive contests [14,15]. Also, studies on mice have shown that the anti-aggressive effect of 5-HT1A and 5-HT1B agonists are actually due to their action on the pre-synaptic autoreceptors and therefore associated with a reduction rather than increase of 5-HT neurotransmission [16].
In addition to the relationship between the serotonergic system and aggressive behavior, there also is clear evidence for a link with reproductive function [17,18]. In rodents, 5-HT has an inhibitory effect on reproductive behavior [19,20]; and seems to be exerting this effect through the 5-HT1A receptors [21,22]. In fish, 5-HT can have both inhibitory and stimulatory actions on the reproductive system. For example, the yellow snapper Lutjanus argentiventris exhibits changes in telencephalic 5-HT and 5-HIAA levels over the course of the reproductive cycle with lowest levels during the spawning season [23]. In female rainbow trout, brain 5-HIAA levels were lowest during vitellogenesis and peaked during the periovulatory period [24]. Serotonin can facilitate the release of gonadotropins from the pituitary in the atlantic croaker Micropogonias undulates [25], sailfin molly Poecilia latipinna [26] and goldfish Carassius auratus [27,28]. Also, in tilapia Oreochromis mossambicus, treatment with estrogens or PCPA during development decreases 5-HT brain levels and produces a female biased sex ratio [29]. This suggests that the feminizing effect of estrogen during sex differentiation might be mediated through 5-HT action.
Many species of fish are capable of sex change and, in some, the social environment serves as the cue that controls the switch between sexes [30–35]. In many protogynous species, removing the male induces sex change in the dominant female. Because the serotonergic system is involved in the modulation of both aggressive and reproductive behavior, changes in serotonergic function could be responsible for translating a change in social context (e.g., dominance status and/or aggression) into physiological and neurobiological modifications that both initiate and maintain sex reversal. Some experimental evidence for a role of serotonin in the inhibition of sex change comes from the protogynous saddleback wrasse Thalassoma duperrey [36,37]. The SSRI sertraline inhibited gonadal sex change in a female in the presence of a smaller female, a situation usually conducive to sex change in this species [37].
To test the role of serotonin in the regulation of sex change, we used the bluebanded goby Lythrypnus dalli. At the functional level, this species is a sequential hermaphrodite because individuals exhibit only one behavioral sex at a time [38,39] but they are capable of bidirectional sex change and social status appears to play a critical role in regulating sexual transformations [35]. Because elevated serotonergic activity typically is associated with subordinate status in vertebrates, and because 5-HT can affect reproductive function, we hypothesized that female L. dalli, being the subordinate animals, would have higher serotonergic activity than males, and that this higher serotonergic activity inhibits them from changing sex. To test this hypothesis, we used both pharmacological and social manipulations of the sex-changing individual (see Table 1 for summary of treatments). We induced high 5-HT levels in a dominant female and predicted that this would inhibit sex change in a social environment permissive to sex change. We also induced low serotonergic function and predicted that this would induce sex change in a non-permissive environment. As a result of these experiments, we established that the general rule for serotonin and status in vertebrates with dominants having lower serotonergic activity than subordinates does not seem to apply to the bluebanded goby. Therefore, we also tested fish in pairs to determine whether acute 5-HT manipulations can affect social status or aggression levels in L. dalli, and whether differences in brain serotonergic activity exist between dominant and subordinate individuals. Although the focus of the work was on 5-HT and 5-HIAA, we also measured brain levels of norepinephrine (NE), dopamine (DA) and its metabolite because of their possible effect on social status [40,41] and sex change [37].
Table 1.
Summary of treatments for the implant experiment and predictions based on the hypothesis that high serotonergic function inhibits sex change.
| Treatment | Social environment | Serotonergic function | Expected sex change? |
|---|---|---|---|
| pMPPI (5-HT1A antagonist) | Non-permissive | Decrease | yes |
| PCPA (5-HT synthesis inhibitor) | Non-permissive | Decrease | yes |
| 5-HTP (5-HT precursor) | Permissive | Increase | no |
| Sham (wax only) | Permissive | No effect | yes |
METHODS
Study Organism
The bluebanded goby is a small benthic fish (20–45 mm adult standard length) that inhabits rocky reefs along Southern California and Baja California, Mexico [42]. Lythrypnus dalli establishes social hierarchies with a dominant male that defends a nest, and spawns with multiple females within a given season [43]. Fish used in our study were captured off the coast of Santa Catalina Island, CA (California Department of Fish & Game permit # 803036-03 to VL) during summer 2006 and 2007. The fish were held in a large 197 l (60 × 94 × 35 cm) holding tank until further processing; the holding tank was supplied continuously with seawater. Fish were housed either in indoor seawater tables at the Wrigley Institute of Environmental Studies on Catalina Island (implant experiment and fluoxetine/p-MPPI injection experiment) exposed to the natural summer light cycle through large windows or in aquaria at Georgia State University (PCPA injection experiment) on a 12:12 light/dark photoperiod. They were held at a temperature of 18–20°C.
The fish were fed brine shrimp twice daily. When setting up experimental groups in tanks, the sex of the fish was assessed by examining the genital papilla shape because males typically have thin and pointy papillae, while females have round, short and wide papillae [42,44]. At the start and end of each experiment, we took genital papilla pictures to measure the papilla length-to-width ratio. This ratio is a good indicator of papilla shape and therefore of sex, with female ratios being close to 1 and male ratios ≥ 1.4 [45]. At the end of the experiment the gonads were removed and photographed to confirm the final sex of the animal. Male L. dalli always possess an accessory gonadal structure (AGS), filled with mucous and/or sperm [46]. The presence of the AGS is a good indicator of functional sex in this species because individuals with rounded, female-like papilla might have some testicular tissue but lack AGS and they do not spawn as a male. The research conducted herein was approved by the Georgia State University IACUC protocol No. A06004 (0708) and University of Southern California IACUC protocol No. 10262.
Implant experiment
Social groups of one large male and three females were established in individual tanks. Males were at least 3 mm larger than the largest female in the tank to ensure their dominance. In each tank, the focal female was at least 3 mm larger than the other two females in her tank, to ensure that she would become the dominant female. Standard length ranges and averages (mean ± standard error) for the fish composing the social groups were: male 32.4–43.1 mm (36.8±0.6); largest female 29.2–33.1 mm (30.8±0.2); medium size female 25.5–28.9 mm (26.8±0.2); smallest female 23.2–26.1 mm (24.9±0.1). This type of hierarchy has been very successful at predicting sex change in the dominant female after male removal [47,35]. Five days after social group establishment, the dominant females were given one of the following treatments (all drugs were purchased from Sigma-Aldrich Co.): the serotonin precursor 5-hydroxytryptophan (5-HTP), the serotonin synthesis inhibitor para-chlorophenylalanine (PCPA), or the 5-HT1A receptor antagonist 4-Iodo-N-[2-[-4-(methoxyphenyl)-1-piperazinyl] ethyl]-N-2-pyridinylbenzamide hydrochloride (p-MPPI). The implants were made by melting beeswax and mixing it with the drug in a ratio of 1 mg of drug to 5 mg of wax [37], aspirating it into a thin silastic tubing (0.64 mm ID × 1.19 mm OD), peeling the tubing off, and cutting it into single 3 mm long implants. Control groups received implants made of beeswax only without any drug. The implants were inserted in the body cavity of the dominant female by anesthetizing with tricaine methanesulfonate (MS222) at a dosage of 150 mg/l, making a small incision, and closing the wound with veterinary glue (Nexaband® S/C). When the dominant female received either the 5-HTP implant or the control wax implant, the male was removed from the tank three days after the surgery to create a social situation conducive to sex change. When the dominant female received either the PCPA implant or the p-MPPI implant, we left the male in the tank to produce an inhibitory social environment; on the third day after the surgery, we put the hand net in these tanks to simulate the same disturbance to the fish as in the male removal treatments.
The fish were observed every day for 10 minutes and scored for aggressive acts performed and received. The aggressive behaviors collected were: approaches defined as movement towards another fish within 2 body lengths of distance; displacements defined as approaches that end with the subordinate fish moving away; attacks defined as very quick and aggressive displacements; threat displays defined as aggressive display consisting of raising the dorsal fin and opening the opercula when very close to another fish. We also recorded jerk swims: a typical courtship behavior that males or sex changing fish perform towards the females or around the nest [43,39]. Three weeks after male removal, animals were quickly moved with a hand-net from the tank into a beaker and killed via exposure to excess MS222 (1 g/l), the fish were decapitated, and their brains were rapidly removed, weighed fresh (± 0.1 mg), fast frozen on dry ice, and stored at − 80 °C until processing. Animals that no longer carried an implant in their body cavity were excluded from the analysis because they most likely lost it immediately or shortly after the surgery. The final sample size for each treatment is provided in Table 2. We also collected the brains of 6 of the males immediately after removing them from the experimental tanks and 10 untreated non-focal females at the end of the experiment.
Table 2.
Monoamine levels in the different treatments. Values are mean±standard error in pg/mg of fresh brain tissue. The number in parenthesis represents sample size.
| Male | Female | |||||
|---|---|---|---|---|---|---|
| Male | Wax | 5-HTP | Female | PCPA | p-MPPI | |
| 5-HT | 2104.52±181.23 (6) | 1777.59±167.79 (7) | 2071.38±221.96 (4) | 1860.56±140.38 (10) | 1488.42±198.53 (5) | 1823.96±198.53 (5) |
| 5-HIAA | 601.99±63.68 (6) | 574.14±58.96 (7) | 597.01±77.99 (4) | 414.49±49.33 (10) | 255.88±69.76 (5) | 383.39±69.76 (5) |
| 5-HIAA/5-HT | 0.294±0.024 (6) | 0.322±0.022 (7) | 0.276±0.029 (4) | 0.222±0.019 (10) | 0.169±0.026 (5) | 0.210±0.026 (5) |
| DA | 219.27±25.51 (6) | 207.90±23.61 (7) | 217.68±31.23 (4) | 266.58±19.76 (10) | 245.11±27.94 (5) | 205.87±27.94 (5) |
| DOPAC | 59.88±20.78 (5) | 44.31±12.16 (5) | 49.60±14.70 (3) | 65.84±17.89 (9) | 73.64±18.53 (5) | 71.68±13.62 (4) |
| DA/DOPAC | 0.252±0.078 (5) | 0.226±0.078 (5) | 0.270±0.101 (3) | 0.263±0.058 (9) | 0.288±0.078 (5) | 0.368±0.088 (4) |
| NE | 2592.41±245.86 (6) | 2141.85±229.69 (7) | 2150.60±361.14 (4) | 2310.13±184.41 (10) | 2343.86±129.84 (5) | 2305.21±129.18 (5) |
Injection experiments
We established pairs of size-matched females (standard length differences were ≤ 0.1 mm). After measurement, they were held singly in an isolation tank for 2 days to reduce the effect of previous social experience. On the third day, both fish were anaesthetized with MS222 and received an i.p. injection of a serotonergic compound. Within each pair, one fish received the drug in 0.9% saline solution (vehicle), and the other one received the same volume of vehicle. Ten pairs received PCPA (Sigma-Aldrich Co.) at a dose of 0.2 mg/g body mass [48], ten received fluoxetine (Xeredien, Valeas S.p.A.) at a dose of 10 μg/g body mass [8] and ten received p-MPPI (Sigma-Aldrich Co.) at a dose of 5 μg/g body mass. As there were no studies available on fish for p-MPPI, this dose was based on the range of concentrations that has a behavioral effect in rodents [49,50]. One fish in the p-MPPI treatment died so the final sample size in this treatment was 9 pairs. The fish were paired in an unfamiliar tank immediately after the PCPA injection or one hour after the fluoxetine or p-MPPI injection. Ten minute behavioral observations were performed immediately after pairing the fish, 3 hours later, and then once a day for the following five days. On the fifth day after the injection, the fish were sacrificed and brains were harvested as described in the implant experiment. In chicks [51], brain levels of 5-HT and 5-HIAA were still lower 5 days after a single i.p. injection of PCPA. We took pictures of the genital papillae and the gonads to determine final sexual phenotype. Brain monoamine levels were quantified only for the fish that received the PCPA treatment and their saline injected opponent (see below). Two samples from the HPLC analysis were unusable so we have brain measurements for N=18 animals (both the missing ones were saline injected and achieved dominant status).
Analysis of monoamines
Serotonin (5-HT), its metabolite 5-hydroxyindoleacetic acid (5-HIAA), norepinephrine (NE), dopamine (DA) and its metabolite 3,4-dihydroxyphenylacetic acid (DOPAC) were measured using high performance liquid chromatography (HPLC) with electrochemical detection [52–54]. The ratio of metabolite to transmitter 5-HIAA/5-HT is used as a measure of serotonergic activity. The brain samples were sonicated in 100 μl of sodium acetate buffer (pH 5.0) containing 5 × 10-8 M DHBA (9.4 pg/μl dihydroxybenzylamine; internal standard). Each sample was frozen, then thawed and centrifuged at 15000×g for 2 min. The supernatant was removed. We added 5 μl of ascorbic acid oxidase to each sample, and injected 45 μl of sample into a chromatographic system (Waters Associates, Milford, MA) and analyzed electrochemically with an LC-4B potentiostat (Bioanalytical Systems, West Lafayette, IN). The electrode potential was set at + 0.6 V with respect to an Ag/AgCl reference electrode. The mobile phase (pH=2.75, flow rate 1.8 ml min-1) consisted of 900 ml of deionized water, 250 mg NaOH, 18.63 mg Na2EDTA, 21 g citric acid, 0.85 ml TEA, 105 mg sodium octane sulfonic acid (SOS) and 25 ml acetonitrile.
Statistical analysis
Analysis of variance (ANOVA) was used in the implant experiment, to compare levels of neurotransmitters between fish that received different drugs when normality (Shapiro Wilk test) and homogeneity of variance were achieved (Levene’s test). A Wilcoxon test and the Kruskal Wallis test were used when these criteria were not met. Variance in levels of 5-HIAA in the implant experiment was not distributed homogenously so Kruskal Wallis was used to compare treatments. Wilcoxon was used in injection study to compare DA and NE levels. Tukey Kramer HSD was used as post hoc test following significant ANOVA results while Dunn’s multiple comparison test was used following Kruskal Wallis. A t-test was used to compare levels of neurotransmitters between PCPA and saline in the injection experiment. Paired t-test was used to compare values of papilla ratio for each animal at the start versus the end of the experiment. The monoamines and their metabolites were measured from the same brains for each animal so significant p-values were corrected with the sequential Dunn-Sidak adjustment to account for compounding of Type I error. Transformations of data are noted in results section if conducted.
RESULTS
Implant experiment
Social conditions inhibitory to sex change
Dominant females implanted with PCPA or p-MPPI did not change sex; they had an average final papilla ratio not significantly different from initial values (paired t test for PCPA: t4=0.99, p=0.38; initial=1.00±0.05; final=1.13±0.13; for p-MPPI: t4=−0.38, p=0.72; initial=1.00±0.06; final=0.99±0.02). These females had distinct ovaries and did not develop an AGS. As expected, all control subordinate females whose brain monoamine levels were quantified had an average final papilla length/width ratio not significantly different from initial values (paired t test: t9=0.80, p=0.45; initial=0.95±0.03; final=1.00±0.03). These females also had distinct ovaries and did not develop an AGS.
Social conditions permissive for sex change
Females that received sham and 5-HTP implants changed sex into males by the end of the experiment and their average final papilla ratio was significantly different from initial values (paired t test for sham: t6=10.21, p<0.0001; initial=1.01±0.03; final=2.74±0.16; for 5-HTP: t3=8.18, p=0.0038; initial=1.10±0.05; final=2.47±0.13). These females developed both testis and AGS. As expected, control males had testis and AGS and retained male typical papillae; there was no significant difference between initial and final papilla ratios for control males (paired t test: t5=2.35, p=0.065; initial=2.81±0.09; final=3.22±0.17).
Monoamine analysis
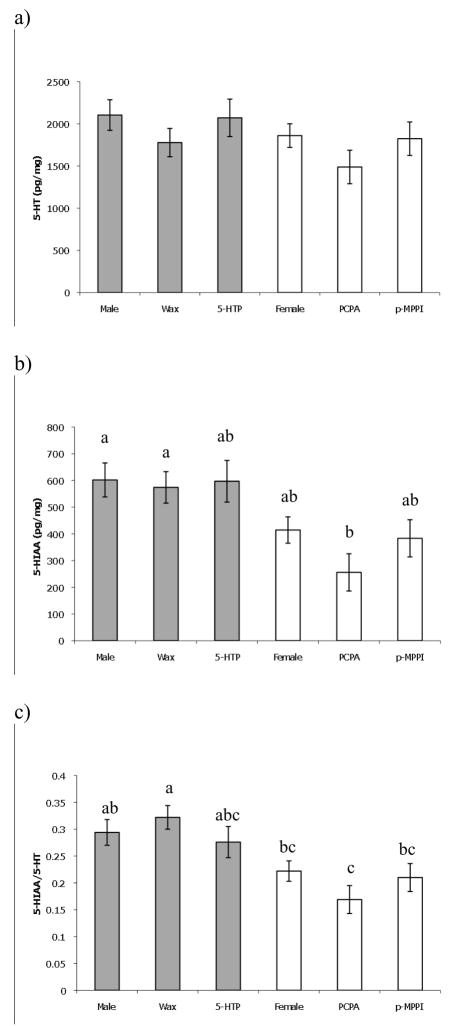
There was no significant difference (Table 2; Fig. 1a) in whole brain 5-HT levels between treatments (ANOVA: F5,31=1.30, p=0.29). There was a significant difference (Table 2; Fig. 1b) in 5-HIAA levels between treatments (Kruskal Wallis: χ52 =16.56, p=0.0054; sequential Dunn-Sidak αadj =0.0084). Animals treated with PCPA had 5-HIAA levels significantly lower than sham (Dunn’s multiple comparison: Q=3.119, Qcritical=2.936, p<0.05) and males (Q=4.438, Qcritical=2.936, p<0.05). There was a significant difference (Table 2; Fig. 1c) in 5-HIAA/5-HT ratio between treatments (ANOVA: F5,31=5.81, p=0.0007; sequential Dunn-Sidak αadj =0.0073). The 5-HIAA/5-HT ratio was higher in sham controls than in control females, p-MPPI, and PCPA treated animals (Tukey Kramer HSD p<0.05); 5-HIAA/5-HT ratio in males was higher than in PCPA treated animals (Tukey Kramer HSD p<0.05). There was no significant difference in DA (ANOVA: p=0.30, F5,31=1.26), DOPAC (ANOVA: p=0.65, F5,25=0.67), DOPAC/DA (ANOVA: p=0.66, F5,25=0.66), or NE (ANOVA: p=0.76, F5,31=0.52) across treatments (Table 2). DA, DOPAC and DOPAC/DA were natural log transformed to achieve normality.
Fig. 1.
Mean brain levels of 5-HT (a), 5-HIAA (b), 5-HIAA/5-HT (c) in animals that received implants and non-manipulated males and females. Bars represent standard errors. Gray bars are associated with treatments where the animals are males at the end of the experiment, and white bars with animals that are females. Different letters indicate significant difference (p<0.05).
There was no significant correlation between whole brain monoamine levels and rates of threat display, total aggression, displacements (natural log transformed) or courtship. For the correlation between monoamine levels and courtship rates we included only those animals that displayed courtship (control and 5-HTP groups). For rates of displacement received, we included only animals that remained female and were displaced (PCPA and p-MPPI). Rate of displacement received was calculated as numbers of displacements received per minute of observation and averaged over the whole experimental period. There was a significant negative correlation between rate of displacement received (Fig. 3) and 5-HT levels (p=0.040, r=−0.65, n=10) and a trend for a negative correlation between rate of displacement received and 5-HIAA levels (p=0.057, r=−0.62, n=10). There was no significant correlation between any of the behaviors and whole brain monoamine levels of control females and males.
Fig. 3.
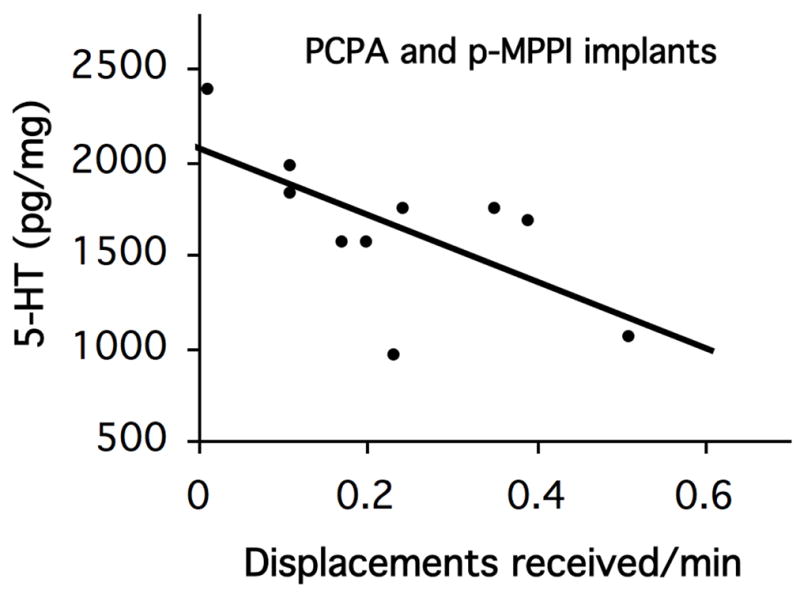
Correlation between 5-HT brain levels (pg/mg of fresh tissue) for fish that received PCPA and pMPPI implants and rate of displacements received/minute of behavioral observation (R2=0.428; p=0.0403; F=5.98, N=10).
Injection experiments
PCPA injection
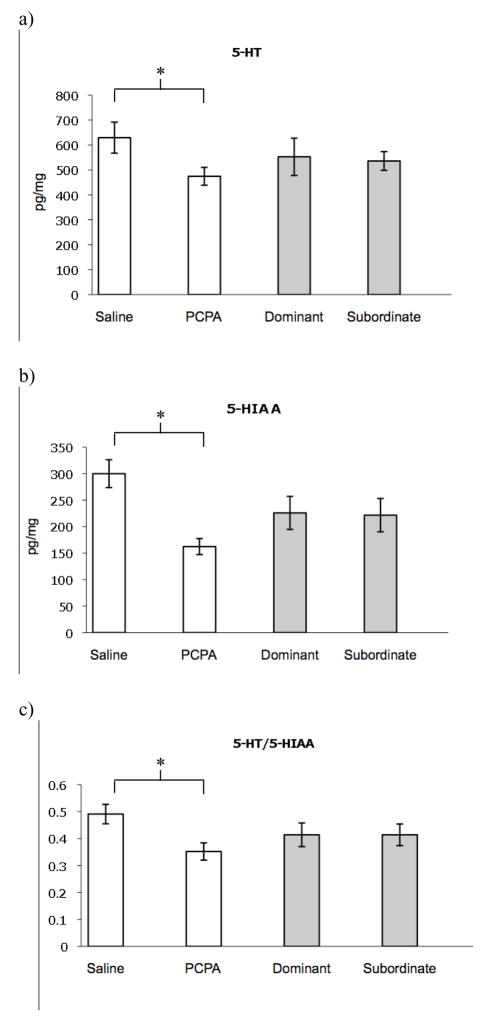
Of the ten animals that became dominant, seven received saline injections and three received PCPA injections. Papilla ratio did not change significantly for dominant (paired t test: t8=1.88, p=0.097; initial, 1.07 ±0.04; final, 1.23±0.06) or subordinate animals (paired t test: t9=−0.04, p=0.972; initial, 1.08 ±0.03; final, 1.08±0.05). Animals injected with PCPA had lower 5-HIAA (t=4.76, df=16, p=0.0002), 5-HT (t=2.26, df=16, p=0.038), and 5-HIAA/5-HT (t=2.85, df=16, p=0.012) than animals injected with saline vehicle (Table 3 and Fig. 2). When comparing animals that became subordinate versus dominant (Table 3 and Fig. 2), there were no significant differences in 5-HT (t=0.21, df=16, p=0.833), 5-HIAA (t=0.09, df=16, p=0.927), or 5-HT/5-HIAA (t=0.003, df=16, p=0.998). There were no significant differences in DA and NE levels between dominant and subordinate fish (Wilcoxon for NE: Z=0.400, p= 0.689; for DA: Z=0.934, p=0.350; Table 3) or between saline and PCPA treated fish (Wilcoxon for NE: Z=−0.133, p= 0.894; for DA: Z=−0.845, p=0.398).
Table 3.
Values are mean±standard error in pg/mg of fresh brain tissue.
| Saline injection (N=8) | PCPA injection (N=10) | Dominant (N=8) | Subordinate (N=10) | |
|---|---|---|---|---|
| 5-HT | 629.50±62.35 | 474.50±35.94 | 552.73±74.81 | 535.91±37.44 |
| 5-HIAA | 300.00±26.30 | 162.38±15.13 | 225.88±31.17 | 221.68±31.45 |
| 5-HIAA/5-HT | 0.49±0.04 | 0.35±0.03 | 0.41±0.04 | 0.41±0.04 |
| DA | 142.88±15.52 | 146.70±8.99 | 151.13±14.41 | 140.10±9.81 |
| NE | 3539.38±159.87 | 3808.70±287.79 | 3735.88±269.41 | 3651.50±238.31 |
Fig. 2.
Comparison of mean brain levels of 5-HT (a), 5-HIAA (b), and 5-HIAA/5-HT (c) in size-matched pairs of animals that received either PCPA or saline injections. White columns represent the comparison based on injection received, while gray columns represent the comparison based on social status. Values are measured in pg/mg of fresh tissue. Bars represent standard errors. The asterisk indicates significant difference (p<0.05).
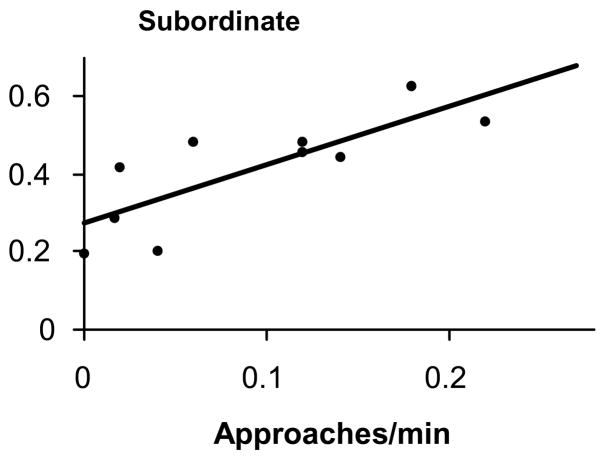
Whether we considered all the animals together, or dominant animals alone, there were no correlations between any of the behaviors and whole brain monoamine levels. For subordinate fish, regardless of injection type, there was a significant positive correlation between rate of approaches to the dominant and 5-HIAA levels (r=0.688, p=0.028, n=10) and between rate of approaches (Fig. 4) and 5-HIAA/5-HT (r=0.801, p=0.0054, n=10). The rate of approaches per minute was calculated for each daily observation and then averaged over the five days.
Fig. 4.
Correlation between brain serotonergic activity and rate of approaches/minute for fish that became subordinate after injection of either PCPA or saline (R2=0.641; p=0.0054; F=14.28).
Fluoxetine injection
Of the ten fish that became dominant, seven received fluoxetine injections and three received saline injections. The papilla ratio of dominant fish was significantly more male-like at the end of the experiment relative to the beginning (paired t test: t9=2.38, p=0.041; initial, 0.97 ±0.03; final, 1.13±0.08). The papilla of the subordinate fish did not change shape over the course of the experiment (paired t test: t8=0.38, p=0.713; initial, 0.96 ±0.04; final, 0.97±0.03). There was no significant difference between fluoxetine and saline treated animals in the mean rate of any of the behaviors.
p-MPPI injection
Of the nine fish that became dominant, four were injected with saline and five with the 5-HT1A receptor antagonist p-MPPI. The subordinate females did not show any significant change in papilla shape (paired t test: t8=−1.176, p=0.273; initial, 1.02 ±0.02; final, 0.99±0.02) while the papilla of dominant fish became more male-like at the end of the experiment (paired t test: t8=5.293, p=0.0007; initial, 0.95 ±0.03; final, 1.32±0.07). There was no significant difference between saline and p-MPPI treated animals in the mean rate of any of the behaviors.
DISCUSSION
Based on the existing literature in fishes, we predicted that male L. dalli, being the dominant individuals, would have lower serotonergic activity than females. The results from the implant experiment did not support this prediction, but rather showed a trend opposite to what we expected: although the difference was not significant, females showed a trend for lower levels of 5-HT, 5-HIAA and 5-HIAA/5-HT than males.
The experiment on size-matched pairs showed that serotonergic activity does not affect social status or aggressive behavior in L. dalli. In fact, none of the injections manipulating 5-HT levels affected female aggression levels or the outcome of the fight. This contrasts with the studies on firemouth cichlid (Cichlasoma meeki) where a single i.p. injection of PCPA increased aggression towards a mirror [1], and on bluehead wrasse where a single i.p. injection of fluoxetine decreased aggression towards an intruder presented in a glass container [8]. An important difference is that in those studies the fish could not physically interact. The lack of social resolution has been shown to have an impact in fish at least in terms of hormonal response. In male cichlids, aggressive behavior against their mirror image was not sufficient to increase androgen levels, which typically occurs following actual fights [55]. We know that in L. dalli physical interactions between individuals are important because the mere presence of a male behind a divider is not sufficient to inhibit sex change in the dominant female [47]. Low serotonergic function may predispose aggression before the fight [14,15] but its effect in determining the outcome of the fight can be overcome by matching the fish with an opponent that is superior in term of size or other characteristics. Therefore pharmacological manipulations of the serotonergic system might show their effect more readily if the animals cannot physically interact, or if they are size-matched. In the study on wrasse cited above [8], the fish do interact in the chronic treatment with fluoxetine and there is decreased aggression in the treated fish. However, in that study they used the intruder-resident paradigm, while we matched pairs of fish in a novel tank. Holding a territory is known to affect the outcome of fights and subsequent social status in a wide variety of organisms [56], suggesting that the use of different social contexts could explain some of the variation in the relationship between serotonergic function, aggression, and status.
On the other hand, there are other studies that, like ours, did not find an effect of serotonergic manipulations on aggression. For example, i.p. injection of a 5-HT1A antagonist into resident mice increased some threat behavior towards the intruder but did not affect the number of escalated aggressive acts such as chases and bite attacks [57]. In Siamese Fighting fish, Betta splendens, a single injection of a 5-HT1A antagonist, 3 daily injections of PCPA, or chronic fluoxetine for 14 days had no significant effects on aggression against a mirror [58]. In the same study though, a single injection of 5-HT and of a 5-HT1A agonist caused a reduction in aggression.
We found no differences in monoamine levels between dominant and subordinate animals in the size-matched pairs of fish. This contrasts results found in rainbow trout [9] and juvenile arctic charr [10]. There is the possibility that the difference in monoamine levels between dominant and subordinate animals is specific to certain regions of the brain [14] and that we missed it because we analyzed whole brains. A study on the bicolor damselfish P. partitus [59] provides some evidence against this argument. They let pairs interact during a daily observation session for 5 days, and found no difference between dominant and subordinate individuals in 5-HT, 5-HIAA or 5-HIAA/5HT in any brain region (brainstem, hypothalamus, telencephalon). Also, similar to our results, Winberg and colleagues [59] found that behavioral correlations were different depending on the social status. In subordinate P. partitus there was a significant positive correlation between 5-HIAA/5-HT ratio in the telencephalon and aggressive acts received. While in dominant animals, the positive correlation was between 5-HIAA/5-HT ratio in the hypothalamus and aggressive acts performed [59]. In our PCPA injection experiment, we found that subordinate animals, but not dominant ones, have brain 5-HIAA/5-HT that correlate positively with rate of approaches given. The behavior of a subordinate approaching the dominant could be interpreted as a form of inspection or challenge. Therefore, higher serotonergic activity in the subordinate may be associated with less stability in social rank. In the implant experiment, females treated with 5-HT synthesis inhibitor PCPA and 5-HT1A receptor antagonist p-MPPI in an inhibitory social environment, showed a significant negative correlation between rate of displacement received and 5-HT levels, and a trend for the negative correlation with 5-HIAA levels. Thus, the more the fish were displaced, the lower their 5-HT levels. This contrasts the work on other fishes included salmonids [10] but fits with the fact that male L. dalli show a trend for higher serotonergic activity than females because dominant males are never displaced.
L. dalli is a very social species that lives at high densities [43], and once a stable social hierarchy is established, it shows more ritualized behavior and less overt aggression. It is possible that, in a stable social environment, differences in monoamine levels between dominant and subordinate animals might not be as evident relative to situations in which the social hierarchy is being established, and more overt aggression is performed. The serotonergic system is closely associated with activation of the stress axis (Hypothalamus-Pituitary-Interrenal axis in fish) [15,60,13] so the effect of a fight on the serotonergic system of the subordinate might be more pronounced in other species of fish because being confined to a tank with a dominant aggressive animal could be more stressful than for L. dalli. For example, in pairs of artic charr differences between subordinate and dominant animals were detected even after 21 days of interactions [48]. In that study, 5-HIAA and 5-HIAA/5-HT ratio in subordinates increased after 1 day of social interaction and remained significantly higher than dominant fish until day 21 in telencephalon and brain stem. In that experiment, the subordinate animals seemed to be stressed because after hierarchy establishment on day 1, they took a position close to the surface or in a corner and lost weight. In our laboratory tanks L. dalli establishes a stable social hierarchy similar to what it would experience in the wild and shows normal social and feeding behaviors so being confined to the tank does not appear to be stressful to subordinate fish. Perhaps this could explain the lack of differences in serotonergic function between dominant and subordinate individuals in L. dalli. These ideas are consistent with a meta-analysis on primates showing that subordinates have higher cortisol levels only in species where their rank was associated with high physical or psychological stress [61].
None of our pharmacological manipulations affected sex change, and this contrasts with the study on T. duperrey, where an implant containing the SSRI sertraline inhibited sex change in a large female in the presence of a smaller female [36]. Larson and colleagues [36] did not quantify behavior so it is unclear whether the treatment caused subordination and therefore inhibited sex change or whether it had only a peripheral effect in the gonad. They administered a different SSRI than we used in the present study, and there also was a difference in interaction time. They allowed fish to interact for 8 weeks after sertraline treatment, while our fish interacted only for 5 days after fluoxetine injection. We chose not to use an SSRI to increase serotonergic function in the 3 week implant experiment because SSRIs have been shown to have other effects such as increasing levels of neurosteroids in the brain and plasma [62] opening up the possibility for non-specific or second order effects of the manipulations. In our implant experiment, we analyzed the brains 3 weeks after male removal so we cannot exclude the possibility that some monoaminergic changes might take place at the very initial steps of sex change. Females of saddleback wrasse have been analyzed at different times during sex change in pairs [36]: they found that 5-HIAA/5-HT in the POA, tuberal nucleus and dorsomedial zone were lowest in the first 3 days of interaction, and then increased and stayed elevated; while 5-HIAA/5-HT in the raphe nucleus was high at the beginning and then decreased. We are aware that some of the changes in brain serotonergic activity produced by our implants were modest, but the small size of the fish, limited implant size and thus final dosage. Therefore, there is still the possibility that higher doses could have had an impact on sex change, but they may not be physiologically relevant.
We predicted that the serotonergic system would inhibit sex change, so we increased serotonergic activity in females in situations permissive to sex change. Unexpectedly, we found that female L. dalli show a trend for lower serotonergic function than males, so it makes sense that the manipulations did not have an effect on sex change because higher serotonergic function seems to be associated with male status. To exclude the possibility that in this species 5-HT might facilitate rather than inhibit sex change, we gave a PCPA implant to 4 dominant females in social groups permissive to sex change: after 3 weeks from male removal, the focal female had low brain 5-HIAA levels but nevertheless changed sex into male (Lorenzi unpublished data). Since these treatments did not have any effect we did not further pursue the hypothesis that serotonin might facilitate sex change.
Regarding sex differences, our result that female have lower levels contrasts with a study in rats that showed females having higher whole brain 5-HT and 5-HIAA levels than males [63]. To our knowledge, there are not many studies looking at sex differences in serotonergic activity in fish, but in the bicolor damselfish (Pomacentrus partitus) there was no effect of sex on serotonergic activity when comparing dominant and subordinate animals [59]. It is interesting to note that in tilapia, O. mossambicus, decreasing 5-HT brain levels produces a female biased sex ratio [29]. Even if this applies only during sexual differentiation, the female condition in this case seems to be associated with low 5-HT levels, and this is consistent with our findings.
From the present study, it is clear that our serotonin manipulations were not sufficient to control sex change but we cannot exclude that the possibility that our doses were not high enough or that serotonin acts synergistically with other neurotransmitters or peptides to regulate sex change during adulthood. We did not see any significant difference in DA, DOPAC or NE among individuals so it is unlikely that any of these neurotransmitters alone, including serotonin, plays a major role. Also, further studies will need to test whether changes at the levels of the receptors rather than absolute levels of the transmitter are important to regulate sex change. There is evidence that receptors might play a role in modulating aggression levels. In rodents, aggressive strains have higher 5-HT1A autoreceptor sensitivity than docile strains [12], and non-aggressive individuals show a greater level of expression of 5-HT1A receptors in the midbrain [64].
In summary, direct comparisons across studies is difficult because of substantial between study variation in type of pharmacological manipulation, season and reproductive stage of the animals, experimental protocol, and social context. However, the results from ours and other studies suggest that the role of the serotonergic system in the modulation of aggressive behavior can vary widely in species with different social systems or in different social settings within a species (pairs vs. larger social groups). Thus, analyses that systematically alter these parameters may be needed to evaluate the general applicability of the association among low serotonergic function, aggression, social dominance and sex change.
Acknowledgments
This work was supported by the Center for Behavioral Neuroscience, an STC Program of the NSF under Agreement No. IBN-9876754 (to MSG and VL), an NSF grant IBO-0548567 (to MSG), a GSU Brains & Behavior seed grant (to MSG), a Rose Hill foundation internship (to VL), a NIH Grant P20 RR15567 (to CHS), and a South Dakota Board of Regents Fellowship (to REC). We would like to thank Bridget Wynn, Scott Wilson, Jeff Glenn and Yong Ah for helping with behavioral observations, the staff at USC Wrigley Institute for Environmental Studies for logistical assistance, and anonymous reviewers for valuable comments on the manuscript. Special thanks go to Charles Derby’s lab at GSU and in particular Cynthia Kicklighter for helping with HPLC analysis when validating the implants.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adams CF, Liley NR, Gorzalka BB. PCPA increases aggression in male firemouth cichlids. Pharmacol. 1996;53:328–30. doi: 10.1159/000139446. [DOI] [PubMed] [Google Scholar]
- 2.Edwards DH, Kravitz EA. Serotonin, social status and aggression. Curr Opin Neurobiol. 1997;7:812–819. doi: 10.1016/s0959-4388(97)80140-7. [DOI] [PubMed] [Google Scholar]
- 3.Larson ET, Summers CH. Serotonin reverses dominant social status. Behav Brain Res. 2001;121:95–102. doi: 10.1016/s0166-4328(00)00393-4. [DOI] [PubMed] [Google Scholar]
- 4.Kravitz EA. Serotonin and aggression: insights gained from a lobster model system and speculations on the role of amine neurons in a complex behavior. J Comp Physiol. 2000;186:221–38. doi: 10.1007/s003590050423. [DOI] [PubMed] [Google Scholar]
- 5.Howell S, Westergaard G, Hoos B, Chavanne TJ, Shoaf SE, Cleveland A, Snoy PJ, Suomi SJ, Higley JD. Serotonergic influences on life-history outcomes in free-ranging male rhesus macaques. Am J Primatol. 2007;69:851–865. doi: 10.1002/ajp.20369. [DOI] [PubMed] [Google Scholar]
- 6.Deckel AW. Behavioral changes in Anolis carolinensis following injection with fluoxetine. Behav Brain Res. 1996;78:175–82. doi: 10.1016/0166-4328(95)00246-4. [DOI] [PubMed] [Google Scholar]
- 7.Sperry TS, Thompson CK, Wingfield JC. Effects of acute treatment with 8-OH-DPAT and fluoxetine on aggressive behaviour in male song sparrows (Melospiza melodia morphna) J Neuroendocrinol. 2003;15:150–60. doi: 10.1046/j.1365-2826.2003.00968.x. [DOI] [PubMed] [Google Scholar]
- 8.Perreault HAN, Semsar K, Godwin J. Fluoxetine treatment decreases territorial aggression in a coral reef fish. Physiol Behav. 2003;79:719–724. doi: 10.1016/s0031-9384(03)00211-7. [DOI] [PubMed] [Google Scholar]
- 9.Winberg S, Lepage O. Elevation of brain 5-HT activity, POMC expression, and plasma cortisol in socially subordinate rainbow trout. Am J Physiol. 1998;274:R645–54. doi: 10.1152/ajpregu.1998.274.3.R645. [DOI] [PubMed] [Google Scholar]
- 10.Winberg S, Nilsson GE, Olsen KH. Social rank and brain levels of monoamines and monoamine metabolites in Artic charr, Salvelinus alpinus (L.) J Comp Physiol A. 1991;168:241–246. [Google Scholar]
- 11.Winberg S, Nilsson GE, Olsen KH. Changes in brain serotonergic activity during hierarchic behavior in Arctic charr (Salvelinus alpinus L. ) are socially induced. J Comp Physiol A. 1992;170:93–99. doi: 10.1007/BF00190404. [DOI] [PubMed] [Google Scholar]
- 12.Caramaschi D, de Boer SF, Koolhaas JM. Differential role of the 5-HT1A receptor in aggressive and non-aggressive mice: an across-strain comparison. Physiol Behav. 2007;90:590–601. doi: 10.1016/j.physbeh.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 13.Bell AM, Backstrom T, Huntingford FA, Pottinger TG, Winberg S. Variable neuroendocrine responses to ecologically-relevant challenges in sticklebacks. Physiol Behav. 2007;91:15–25. doi: 10.1016/j.physbeh.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 14.Summers CH, Korzan WJ, Lukkes JL, Watt MJ, Forster GL, Øverli Ø, Höglund E, Larson ET, Ronan PJ, Matter JM, Summers TR, Renner KJ, Greenberg N. Does serotonin influence aggression? Comparing regional activity before and during social interaction. Physiol Biochem Zool. 2005;78:679–94. doi: 10.1086/432139. [DOI] [PubMed] [Google Scholar]
- 15.Summers CH, Winberg S. Interactions between the neural regulation of stress and aggression. J Experim Biol. 2006;209:4581–4589. doi: 10.1242/jeb.02565. [DOI] [PubMed] [Google Scholar]
- 16.de Boer SF, Koolhaas JM. 5-HT1A and 5-HT1B receptor agonists and aggression: a pharmacological challenge of the serotonin deficiency hypothesis. Eur J Pharmacol. 2005;526:125–39. doi: 10.1016/j.ejphar.2005.09.065. [DOI] [PubMed] [Google Scholar]
- 17.Hull EM, Muschamp JW, Sato S. Dopamine and serotonin: influences on male sexual behavior. Physiol Behav. 2004;83:291–307. doi: 10.1016/j.physbeh.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 18.Fabre-Nys C. Steroid control of monoamines in relation to sexual behaviour. Rev Reprod. 1998;3:31–41. doi: 10.1530/ror.0.0030031. [DOI] [PubMed] [Google Scholar]
- 19.Malmnas CO, Meyerson BJ. p-chlorophenylalanine and copulatory behaviour in the male rat. Nature. 1971;232:398–400. doi: 10.1038/232398a0. [DOI] [PubMed] [Google Scholar]
- 20.Verma S, China GS, Mohan Kumar V, Singh B. Inhibition of male sexual behavior by serotonin application in the medial preoptic area. Physiol Behav. 1989;46:327–330. doi: 10.1016/0031-9384(89)90275-8. [DOI] [PubMed] [Google Scholar]
- 21.Kishitake M, Yamanouchi K. Effects of highly or relatively selective 5-HT 1A receptor agonists on lordosis in female rats. Zool Sci. 2003;20:1133–1138. doi: 10.2108/zsj.20.1133. [DOI] [PubMed] [Google Scholar]
- 22.Kishitake M, Yamanouchi K. Facilitatory effects of WAY-100635, a 5-HT1A receptor antagonist, on lordosis in female rats. Neurosci Letters. 2004;371:147–151. doi: 10.1016/j.neulet.2004.08.058. [DOI] [PubMed] [Google Scholar]
- 23.Hernandez-Rauda R, Aldegunde M. Changes in dopamine, norepinephrine and serotonin levels in the pituitary, telencephalon and hypothalamus during gonadal development of male Lutjanus argentiventris (Teleostei) Marine Biol. 2002;141:209–216. [Google Scholar]
- 24.Saligaut C, Salbert G, Bailhache T, Bennani S, Jego P. Serotonin and dopamine turnover in the female rainbow trout (Oncorhyncus mykiss) brain and pituitary: changes during the annual reproductive cycle. Gen Comp Endocrinol. 1992;85:261–268. doi: 10.1016/0016-6480(92)90010-h. [DOI] [PubMed] [Google Scholar]
- 25.Khan IA, Thomas P. Stimulatory effects of serotonin on maturational gonadotropin release in the atlantic croaker, Micropogonias undulatus. Gen Comp Endocrinol. 1992;88:388–396. doi: 10.1016/0016-6480(92)90233-a. [DOI] [PubMed] [Google Scholar]
- 26.Groves DJ, Batten TFC. Direct control of the gonadotroph in a teleost Poecilia latipinna. Gen Comp Endocrinol. 1986;62:315–326. doi: 10.1016/0016-6480(86)90122-x. [DOI] [PubMed] [Google Scholar]
- 27.Somoza GM, Yu KL, Peter RE. Serotonin stimulates gonadotropin release in female and male goldfish, Carassius auratus L. Gen Comp Endocrinol. 1988;72:374–382. doi: 10.1016/0016-6480(88)90159-1. [DOI] [PubMed] [Google Scholar]
- 28.Somoza GM, Peter RE. Effects of serotonin on gonadotropin and growth hormone release from in vitro perifused goldfish pituitary fragments. Gen Comp Endocrinol. 1991;82:103–110. doi: 10.1016/0016-6480(91)90301-l. [DOI] [PubMed] [Google Scholar]
- 29.Tsai CL, Wang LH, Chang CF, Kao CC. Effects of gonadal steroids on brain serotonergic and aromatase activity during the critical period of sexual differentiation in tilapia, Oreochromis mossambicus. J Neuroendocrinol. 2000;12:894–898. doi: 10.1046/j.1365-2826.2000.00536.x. [DOI] [PubMed] [Google Scholar]
- 30.Fishelson L. Protogynous sex reversal in the fish Anthias squamipinnis (Teleostei, Anthiidae) regulated by the presence or absence of a male fish. Nature. 1970;227:90–91. doi: 10.1038/227090b0. [DOI] [PubMed] [Google Scholar]
- 31.Robertson DR. Social control of sex reversal in a coral-reef fish. Science. 1972;177:1007–1009. doi: 10.1126/science.177.4053.1007. [DOI] [PubMed] [Google Scholar]
- 32.Shapiro DY. Social behavior, group structure and the control of sex reversal in hermaphroditic fish. Advan Study Behav. 1979;10:43–101. [Google Scholar]
- 33.Warner RR, Swearer SE. Social control of sex change in the bluehead wrasse Thalassoma bifasciatum (Pisces: Labridae) Biol Bull. 1991;181:199–204. doi: 10.2307/1542090. [DOI] [PubMed] [Google Scholar]
- 34.Godwin J, Luckenbach JA, Borski RJ. Ecology meets endocrinology: environmental sex determination in fishes. Evol Dev. 2003;5:40–9. doi: 10.1046/j.1525-142x.2003.03007.x. [DOI] [PubMed] [Google Scholar]
- 35.Rodgers EW, Earley RL, Grober MS. Social status determines sexual phenotype in the bidirectional sex changing bluebanded goby Lythrypnus dalli. J Fish Biol. 2007;70:1660–1668. [Google Scholar]
- 36.Larson ET, Summers CH. Monoaminergic changes associated with socially induced sex reversal in the saddleback wrasse. Neuroscience. 2003;119:251–263. doi: 10.1016/s0306-4522(03)00119-2. [DOI] [PubMed] [Google Scholar]
- 37.Larson ET, Norris DO, Grau EG, Summers CH. Monoamines stimulate sex reversal in the saddleback wrasse. Gen Comp Endocrinol. 2003;130:289–298. doi: 10.1016/s0016-6480(02)00622-6. [DOI] [PubMed] [Google Scholar]
- 38.St Mary CM. Sex allocation in a simultaneous hermaphrodite the blue-banded goby (Lythrypnus dalli): the effects of body size and behavioral gender and the consequences for reproduction. Behav Ecol. 1994;5:304–313. [Google Scholar]
- 39.Reavis RH, Grober MS. An integrative approach to sex change: social, behavioural and neurochemical changes in Lythrypnus dalli (Pisces) Acta Ethol. 1999;2:51–60. [Google Scholar]
- 40.Winberg S, Nilsson GE. Induction of social dominance by L-dopa treatment in Arctic charr. Neuroreport. 1992;3:243–246. doi: 10.1097/00001756-199203000-00006. [DOI] [PubMed] [Google Scholar]
- 41.Korzan WJ, Summers CH. Behavioral diversity and neurochemical plasticity: selection of stress coping strategies that define social status. Brain Behav Evol. 2007;70:257–66. doi: 10.1159/000105489. [DOI] [PubMed] [Google Scholar]
- 42.Wiley JW. Life histories and systematics of the western North American Gobies Lythrypnus dalli (Gilbert) and Lythrypnus zebra (Gilbert) San Diego Trans Soc Nat Hist. 1976;18:169–184. [Google Scholar]
- 43.Behrents KC. The comparative ecology and interactions between two sympatric gobies (Lythrypnus dalli and Lythrypnus zebra) University of Southern California; 1983. PhD thesis. [Google Scholar]
- 44.St Mary CM. Novel sexual patterns in two simultaneously hermaphroditic gobies Lythrypnus dalli and Lythrypnus zebra. Copeia. 1993;4:1062–1072. [Google Scholar]
- 45.Carlisle SL, Marxer-Miller SK, Canario AVM, Oliveira RF, Carneiro L, Grober MS. Effects of 11-ketotestosterone on genital papilla morphology in the sex changing fish Lythrypnus dalli. J Fish Biol. 2000;57:445–456. [Google Scholar]
- 46.Drilling CC, Grober MS. An initial description of alternative male reproductive phenotypes in the bluebanded goby, Lythrypnus dalli (Teleostei, Gobiidae) Environ Biol Fish. 2005;72:361–372. [Google Scholar]
- 47.Lorenzi V, Earley RL, Grober MS. Preventing behavioural interactions with a male facilitates sex change in female bluebanded gobies, Lythrypnus dalli. Behav Ecol Sociobiol. 2006;59:715–722. [Google Scholar]
- 48.Winberg S, Nilsson GE. Time course of changes in brain serotonergic activity and brain tryptophan levels in dominant and subordinate juvenile artic charr. J Exp Biol. 1993;179:181–195. [Google Scholar]
- 49.Markou A, Harrison AA, Chevrette J, Hoyer D. Paroxetine combined with a 5-HT(1A) receptor antagonist reversed reward deficits observed during amphetamine withdrawal in rats. Psychopharmacol. 2005;178:133–42. doi: 10.1007/s00213-004-2008-2. [DOI] [PubMed] [Google Scholar]
- 50.Harrison AA, Liem YT, Markou A. Fluoxetine combined with a serotonin-1A receptor antagonist reversed reward deficits observed during nicotine and amphetamine withdrawal in rats. Neuropsychopharmacol. 2001;25:55–71. doi: 10.1016/S0893-133X(00)00237-2. [DOI] [PubMed] [Google Scholar]
- 51.Buchanan CP, Shrier EM, Hill WL. Time-dependent effects of PCPA on social aggression in chicks. Pharmacol Biochem Behav. 1994;49:483–8. doi: 10.1016/0091-3057(94)90059-0. [DOI] [PubMed] [Google Scholar]
- 52.Renner KJ, Luine VN. Determination of monoamines in brain nuclei by high performance liquid chromatography with electrochemical detection: young vs. middle aged rats. Life Sci. 1984;34:2193–2199. doi: 10.1016/0024-3205(84)90320-5. [DOI] [PubMed] [Google Scholar]
- 53.Renner KJ, Luine V. Analysis of temporal and dose-dependent effects of estrogen on monoamines in brain nuclei. Brain Res. 1986;366:64–71. doi: 10.1016/0006-8993(86)91281-3. [DOI] [PubMed] [Google Scholar]
- 54.Emerson AJ, Kappenman DP, Ronan PJ, Renner KJ, Summers CH. Stress induces rapid changes in serotonergic activity: restraint and exertion. Behav Brain Res. 2000;111:83–92. doi: 10.1016/s0166-4328(00)00143-1. [DOI] [PubMed] [Google Scholar]
- 55.Oliveira RF, Carneiro LA, Canário AV. Behavioural endocrinology: no hormonal response in tied fights. Nature. 2005;437:207–208. doi: 10.1038/437207a. [DOI] [PubMed] [Google Scholar]
- 56.Hsu YY, Earley RL, Wolf LL. Modulation of aggressive behavior by fighting experience: mechanisms and contest outcomes. Biol Rev. 2006;81:33–74. doi: 10.1017/S146479310500686X. [DOI] [PubMed] [Google Scholar]
- 57.Bell R, Mitchell PJ, Hobson H. Effects of the 5-HT1A antagonist (+)-way-100135 on murine social and agonistic behavior. Pharmacol Biochem Behav. 1996;54:159–167. doi: 10.1016/0091-3057(95)02168-x. [DOI] [PubMed] [Google Scholar]
- 58.Clotfelter ED, O’Hare EP, McNitt MM, Carpenter RE, Summers CH. Serotonin decreases aggression via 5-HT1A receptors in the fighting fish Betta splendens. Pharmacol Biochem Behav. 2007;87:222–231. doi: 10.1016/j.pbb.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 59.Winberg S, Myrberg AA, Nilsson GE. Agonistic interactions affect brain serotonergic activity in an acanthopterygiian fish: The bicolor damselfish (Pomacentrus partitus) Brain Behav Evol. 1996;48:213–220. doi: 10.1159/000113199. [DOI] [PubMed] [Google Scholar]
- 60.Carpenter RE, Watt MJ, Forster GL, Øverli Ø, Bockholt C, Renner KJ, Summers CH. Corticotropin releasing factor induces anxiogenic locomotion in trout and alters serotonergic and dopaminergic activity. Hormones and Behavior. 2007;52:600–611. doi: 10.1016/j.yhbeh.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abbott DH, Keverne EB, Bercovitch FB, Shively CA, Mendoza SP, Saltzman W, Snowdon CT, Ziegler TE, Banjevic M, Garland T, Jr, Sapolsky RM. Are subordinates always stressed? A comparative analysis of rank differences in cortisol levels among primates. Horm Behav. 2003;43:67–82. doi: 10.1016/s0018-506x(02)00037-5. [DOI] [PubMed] [Google Scholar]
- 62.van Broekhoven F, Verkes RJ. Neurosteroids in depression: a review. Psychopharmacol. 2003;165:97–110. doi: 10.1007/s00213-002-1257-1. [DOI] [PubMed] [Google Scholar]
- 63.Carlsson M, Svensson K, Eriksson E, Carlsson A. Rat brain serotonin: biochemical and functional evidence for a sex difference. J Neur Transm. 1985;63:297–313. doi: 10.1007/BF01252033. [DOI] [PubMed] [Google Scholar]
- 64.Popova NK, Naumenko VS, Plyusnina IZ. Involvement of brain serotonin 5-HT1A receptors in genetic predisposition to aggressive behavior. Neurosci Behav Physiol. 2007;37:631–5. doi: 10.1007/s11055-007-0062-z. [DOI] [PubMed] [Google Scholar]