Abstract
Atrial natriuretic peptide (ANP) and nitric oxide (NO) are key regulators of ion and water transport in the kidney. Here, we report that these cGMP-elevating hormones stimulate Ca2+ reabsorption via a novel mechanism specifically involving type II cGMP-dependent protein kinase (cGK II). ANP and the NO donor, sodium nitroprusside (SNP), markedly increased Ca2+ uptake in freshly immunodissected rabbit connecting tubules (CNT) and cortical collecting ducts (CCD). Although readily increasing cGMP, ANP and SNP did not affect Ca2+ and Na+ reabsorption in primary cultures of these segments. Immunoblot analysis demonstrated that cGK II, and not cGK I, was present in freshly isolated CNT and CCD but underwent a complete down-regulation during the primary cell culture. However, upon adenoviral reexpression of cGK II in primary cultures, ANP, SNP, and 8-Br-cGMP readily increased Ca2+ reabsorption. In contrast, no cGMP-dependent effect on electrogenic Na+ transport was observed. The membrane localization of cGK II proved to be crucial for its action, because a nonmyristoylated cGK II mutant that was shown to be localized in the cytosol failed to mediate ANP-stimulated Ca2+ transport. The Ca2+-regulatory function of cGK II appeared isotype-specific because no cGMP-mediated increase in Ca2+ transport was observed after expression of the cytosolic cGK Iβ or a membrane-bound cGK II/Iβ chimer. These results demonstrate that ANP- and NO-stimulated Ca2+ reabsorption requires membrane-targeted cGK II.
Keywords: cortical collecting duct, connecting tubule, adenovirus, sodium transport, ENaC
In mammalian kidney, active Ca2+ reabsorption takes place primarily in the distal part of the nephron and involves the coordinated processes of passive Ca2+ influx across the luminal membrane by a still unidentified transporter, followed by diffusion through the cytosol of Ca2+ bound to calcium-binding proteins, and active extrusion across the opposing basolateral membrane (1–3). It is well established that this transcellular Ca2+ transport is under control of hormones, including parathyroid hormone (PTH), calcitonin, and 1,25(OH)2D3, but the regulatory mechanisms involved have not been completely elucidated. One established pathway for stimulating Ca2+ transport is the activation of adenylyl cyclase, accumulation of cAMP, and subsequent activation of cAMP-dependent protein kinase (cAK) (4). However, recent studies suggest a prominent role of protein kinase C (PKC) (5, 6), possibly in combination with cAK (7), in hormone-stimulated Ca2+ transport. Because cGMP-elevating hormones have been suggested to control ion and water transport processes in the intestine and kidney (8, 9), we have now investigated the possibility that cGMP may also have a role in kidney Ca2+ transport regulation. Importantly, cortical collecting duct cells have receptors and are considered a target for the action of natriuretic peptides (10).
cGMP cascade activation by NO and natriuretic peptides such as atrial natriuretic peptide (ANP) has been shown to stimulate natriuresis and diuresis in the kidney by increasing glomerular filtration rate, inhibiting renin release in juxtaglomerulosa cells, and inhibiting Na+ and water reabsorption in the collecting duct (9, 11). Downstream effectors of cGMP include cGMP-gated channels, cGMP-regulated phosphodiesterases (PDE), and cGMP-dependent protein kinases (cGK) (12–14). One or more of these targets may mediate cGMP effects on a given function. For example, in the inner medullary collecting duct, cGMP directly and indirectly, via cGK I, inhibits cyclic nucleotide-gated nonselective cation channels (15), thus decreasing Na+ reabsorption (16). Renin release from juxtaglomerular cells not only is stimulated by a mechanism involving cGMP-inhibited PDE (17), but also is inhibited by another mechanism involving cGK II (8, 18, 19). Kidney contains both major types of cGK (8), which are products of two separate genes (20–22). cGK II is a membrane-bound enzyme, whereas the closely related cGK I (consisting of α and β splice variants) is localized predominantly in the cytosol (22, 23). Both cGK forms have been shown to regulate ion channels, for example, cGK II activates the intestinal CFTR Cl− channel (23), whereas cGK I inhibits a renal cGMP-dependent cation channel (16). In addition, a membrane-bound cGK has been implicated in the stimulation of Ca2+-activated K+ channels present in the cortical collecting duct (24), and cGK I has direct and indirect effects on Ca2+-activated maxi-K+ (BKCa) channels in several cell types (25).
In the present study we investigated the ability of cGMP-elevating agents, including ANP and the NO-donor sodium nitroprusside (SNP), to modulate Ca2+ uptake in freshly isolated rabbit connecting tubules (CNT) and cortical collecting ducts (CCD) and Ca2+ and Na+ reabsorption in primary cultures thereof. Because endogenous cGK II was present in freshly isolated cells but proved to be down-regulated in these primary cultures, we performed transport measurements after adenoviral vector-mediated reexpression of cGK II, cGK I, and mutants of these cGKs, which possess different membrane-binding properties (23). Our results demonstrate that ANP and NO stimulate Ca2+ reabsorption in CNT/CCD cells by elevating cytosolic cGMP levels and the subsequent activation of membrane-bound cGK II.
MATERIALS AND METHODS
Chemicals and Antibodies.
Collagenase A and hyaluronidase were obtained from Boehringer Mannheim. All other chemicals, including rat ANP, d-amino-cys, d-Arg-8-vasopressin, 8-bromoguanosine cGMP (8-Br-cGMP), 8-Br-cAMP, 3-isobutyl-1-methylxanthine, SNP, and FITC-conjugated goat anti-rabbit IgG, were purchased from Sigma. Polyclonal cGK I and cGK II antibodies, raised against recombinant cGK I or cGK II expressed in Escherichia coli, were produced as reported previously (26). Characterization of anti-rabbit cortical collecting system mAb (R2G9) has been described previously (1). All stock solutions were stored at −20°C. Final vehicle concentrations never exceeded 0.1% (vol/vol).
Construction of cGK Mutants and Adenoviral Vectors.
A nonmyristoylated mutant of cGK II (G2A), constructed by mutation of Gly-2 to Ala (27), and a cGK II-cGK Iβ (cGK II/Iβ) chimer, consisting of the first 29 N-terminal amino acids of cGK II fused to the N terminus of full-length cGK Iβ, were made as described previously (23). Recombinant replication-deficient (E1 deletion) adenoviridae type 5 containing the coding sequences of rat cGK II (20), human cGK Iβ (28), the cGK II-G2A mutant, or the cGK II/Iβ chimer were prepared as described (23, 29). The titer of the adenoviral preparations was approximately 1 plaque-forming unit (pfu) per 500 particles.
Culture and Infection of Rabbit Kidney CNT and CCD Cells.
Rabbit kidney CNT/CCD tubules were immunodissected from the kidney cortex of New Zealand White rabbits (≈0.5 kg) by using antibody R2G9 and then placed in primary culture on permeable filters (0.33 cm2; Costar) as described in detail previously (1). The culture medium was a 1:1 mixture of DMEM and Ham’s F-12 medium (DME/F12; GIBCO) supplemented with 5% (vol/vol) decomplemented FCS/50 μg/ml of gentamicin/10 μl/ml of nonessential amino acids (GIBCO)/5 μg/ml of insulin/5 μg/ml of transferrin/50 nM hydrocortisone/70 ng/ml of prostaglandin E1/50 nM Na2SeO3/5 pM triiodothyronine and equilibrated with 5% CO2 at 37°C. Confluent monolayers were infected at day 3 of culture by adding culture medium (100 μl to apical and 600 μl to basolateral compartment) containing adenoviral vectors (5 × 109 particles per ml) and subsequently were used for transport assays at 2 days postinfection. As observed by confocal laser-scanning immunocytochemistry, an infection efficiency of more than 70% was reached for all constructs. Transepithelial potential difference and resistance were checked routinely before and after every transport measurement to confirm cell confluency and integrity of the monolayer.
45Ca2+ Uptake in Freshly Immunodissected CNT and CCD.
Freshly isolated tubules were resuspended in 300 μl medium containing 140 mM NaCl/1 mM MgCl2/1 mM CaCl2/20 μCi/ml 45Ca2+/10 mM Hepes/Tris, pH 7.4. Hormones and cGMP-elevating agents were added, and 45Ca2+ uptake was determined for 10 min at 37°C. Tubules were washed two times by centrifuging (200 × g for 5 min) in ice-cold stop buffer containing 140 mM NaCl/1 mM MgCl2/1 mM CaCl2/1.5 mM LaCl3/10 mM Hepes/Tris, pH 7.4, solubilized with 10% (wt/vol) SDS, dissolved in scintillation fluid, and counted for radioactivity.
Measurement of Transcellular Ca2+ Transport.
At 2 days postinfection, confluent monolayers of rabbit CNT/CCD cells growing on permeable filters were washed twice and preincubated in physiological salt solution (PSS) containing 140 mM NaCl/2 mM KCl/1 mM K2HPO4/1 mM MgCl2/1 mM CaCl2/5 mM glucose/5 mM l-alanine/5 μM indomethacin/10 mM Hepes/Tris, pH 7.4, for 15 min at 37°C. Subsequently, the monolayers were incubated in PSS (100 μl to apical and 600 μl to basolateral compartment), with or without drugs and hormones added to the apical and/or basolateral compartment, as indicated in the text, and after another 90 min transepithelial Ca2+ transport was measured. Controls consisting of identical concentrations of solvents (ethanol or dimethyl sulfoxide) used to dissolve various agents were without effect. At the end of the incubation period, 25-μl samples were removed in triplicate from the apical compartment and assayed for their Ca2+ concentration by using a colorimetric assay kit (Boehringer Mannheim). Under these experimental conditions, net apical-to-basolateral Ca2+ flux is linear with time for at least 3 hr (1). Ca2+ reabsorption was expressed in nmol⋅h−1⋅cm−2.
Measurement of Transcellular Short-Circuit Current.
Confluent monolayers were mounted between two half-chambers (area of 0.33 cm2) and bathed at 37°C with PSS. The solutions bathing the monolayer were connected via agar bridges and Ag-AgCl electrodes to a voltage-clamp current amplifier (Physiological Instruments, San Diego), and the short-circuit current (ISC) was recorded. The benzamil-sensitive component of the ISC was determined by addition of 10 μM benzamil to the apical compartment, as an estimate of transcellular Na+ transport (30).
Measurement of Intracellular cGMP Levels.
To assess the effects of hormones and drugs on intracellular cGMP levels, confluent monolayers were preincubated for 15 min in PSS with 100 μM 3-isobutyl-1-methylxanthine. Hormones and drugs were added to the apical and/or basolateral compartment. At 15 min, basolateral medium was discarded and the filters were excised and rapidly transferred to 100 μl of 0.1 M HCl to terminate the generation of cellular cGMP. The cell lysate was centrifuged (10 min, 20,000 × g), and the supernatant was neutralized with 20 μl of 0.5 M Tris, acetylated, and used for determination of cGMP by radioimmunoassay (31).
Immunoblotting.
Freshly immunodissected CNT/CCD tubules were frozen immediately in liquid nitrogen, thawed, collected by centrifugation at 100,000 × g for 30 min at 4°C in an airfuge (Beckman), and heated for 10 min at 100°C in SDS/PAGE sample buffer. CNT/CCD cells cultured on permeable filters were washed two times with PBS, and, subsequently, SDS/PAGE sample buffer was added directly to the cells. All samples (20 μg of protein each) were heated for 10 min at 100°, separated on 7.5% (wt/vol) SDS/PAGE gels, and blotted to nitrocellulose. Blots were incubated with cGK I or cGK II antibody (1:3,000), and immunoreactive protein was detected by using the enhanced chemiluminescence method as described by the manufacturer (Amersham). Expression of cGK proteins was quantitated by comparison of samples to standard amounts of purified bovine lung cGK I, or recombinant rat intestine cGK II expressed in and purified from Sf9 cells (32), by using the Molecular Imaging System GS-363 (Bio-Rad).
Reverse Transcription–PCR (RT-PCR).
Total RNA was isolated from rabbit intestinal mucosa and reverse-transcribed by oligo(dT) priming. The rabbit intestine cGK II cDNA obtained was amplified by using the oligonucleotide primers P1 and P2, described previously, with 35 PCR cycles (94°C, 45 s; 50°C, 45 s; 72°C, 1 min), followed by final elongation for 10 min at 72°C (20). The PCR product gave a single band of ≈700 bp that was subcloned in a TA cloning vector (Invitrogen). Three identical clones were sequenced by using fluorescent-labeled vector primers, T7 DNA polymerase, and the ALF Sequencer. The partial sequence of rabbit intestine cGK II was found to be 87% identical (at the nucleotide level) to the corresponding sequence of rat intestinal cGK II (unpublished observations). From this partial rabbit intestine cGK II sequence, new, specific oligonucleotide primers were designed for amplifying rabbit kidney cGK II. These were 5′-CCAAGCCAGAGACGAGCAGTA-3′ (sense, corresponding to nucleotides 857–877 of the rat intestine cGK II sequence) and 5′-TTCATCATCACGGTTCAGGTT-3′ (antisense, corresponding to nucleotides 1266–1286 of the rat intestine cGK II sequence). Total RNA was extracted from freshly isolated CNT/CCD tubules and primary cultures thereof by the method described by Chomczynski and Sacchi (33). RNA (2 μg) was used as starting material for RT-PCRs, as described previously (8). Briefly, RT-PCR amplification of rabbit kidney (CNT/CCD) cGK II was performed by using 35 PCR cycles (94°C, 1 min; 50°C, 1 min; 72°C, 1 min), followed by elongation for 10 min at 72°C. The PCR product gave a single band of 430 bp in size. Amplification of glyceraldehyde-3-phosphate dehydrogenase as a control was performed under similar conditions as described above by using primers of 5′-GCTGAACGGGAAACTCACTG-3′ and 5′-TCCACCACCCTGTTGCTGTA-3′. As a negative control, PCR also was carried out in the absence of reverse transcriptase.
Immunofluorescence Confocal Microscopy.
Monolayers on permeable filter supports were fixed in 1% (wt/vol) paraformaldehyde for 1 hr (3). Subsequently, the monolayers were incubated for 10 min in Tris-buffered saline (TBS; pH 7.6), containing 0.2% (vol/vol) Triton X-100/0.9% (wt/vol) NaCl/2.5 mM Tris/0.1% (wt/vol) BSA/0.01% (wt/vol) NaN3, and washed three times for 5 min in TBS. After blocking for 30 min in 10% (vol/vol) goat serum in TBS and rinsing thoroughly, the monolayers were incubated overnight at 4°C with antiserum against either cGK I or cGK II [1:200 diluted in 10% (vol/vol) goat serum in TBS]. After this incubation, the filters were rinsed three times and incubated for 1 hr at room temperature with FITC-conjugated goat anti-rabbit IgG [1:50 diluted in 10% (vol/vol) goat serum in TBS]. The filters were washed three times, dehydrated in methanol, and subsequently mounted in Mowiol (Hoechst Pharmaceuticals) and visualized by confocal laser-scanning microscopy (MRC-1000; Bio-Rad) by using a Nikon Diaphot microscope.
Statistics.
In all experiments the data are expressed as the mean ± SEM. Overall statistical significance was determined by ANOVA. In the case of significance (P < 0.05), individual groups were compared by contrast analysis according to Scheffé (34).
RESULTS
45Ca2+ Uptake Is Stimulated by ANP in Freshly Isolated CNT and CCD.
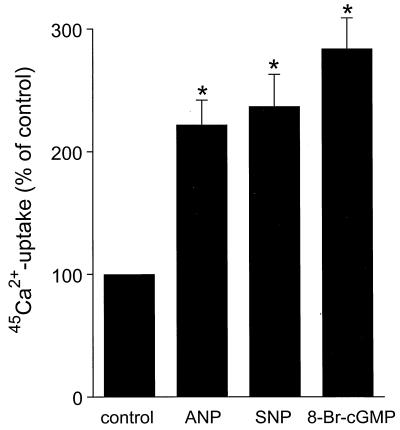
Fig. 1 shows that ANP (100 nM) significantly increased the uptake of 45Ca2+ in freshly isolated rabbit CNT and CCD tubules by more than a factor of 2 (P < 0.05). Because ANP generally acts through cGMP we subsequently tested the effects of 8-Br-cGMP (100 μM) and SNP (100 μM). Both cGMP-elevating agents increased the 45Ca2+ uptake to the same extent as ANP.
Figure 1.
Stimulation of 45Ca2+ uptake by ANP, SNP, and 8-Br-cGMP in freshly isolated CNT and CCD tubules. Freshly immunodissected CNT and CCD tubules were stimulated with ANP (100 nM), SNP (100 μM), and 8-Br-cGMP (100 μM) in the presence of tracer amount 45Ca2+ for 10 min at 37°C. Basal 45Ca2+ uptake is set at 100%, to which all values are related. Values are means ± SEM (n = 5). ∗, P < 0.05, significantly different from basal 45Ca2+ uptake.
ANP Increases Cytosolic cGMP Without Affecting Ca2+ Reabsorption in Confluent Monolayers of CNT and CCD.
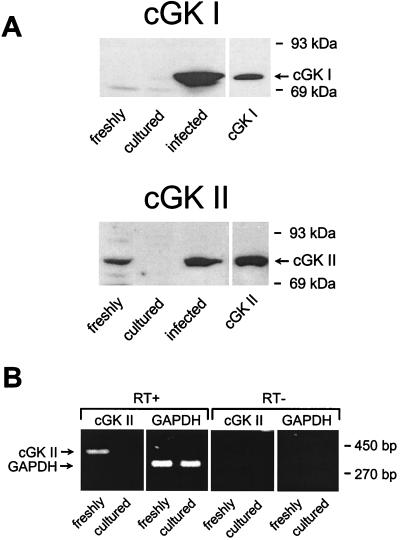
In primary cultures of immunodissected CNT and CCD, both ANP (100 nM, basolateral) and SNP (100 μM, both sides) significantly increased cytosolic cGMP levels 2.5-fold, from 23 ± 3 to 58 ± 7 and 58 ± 8 pmol cGMP⋅mg protein−1, respectively (n = 4, P < 0.05). However, despite their marked stimulation of cGMP, ANP, SNP, and 8-Br-cGMP (100 μM, both sides), each had no significant effect on Ca2+ reabsorption in these primary cultures (48 ± 3, 50 ± 3, and 47 ± 2 nmol⋅h−1⋅cm−2, respectively, versus a control value of 51 ± 2 nmol⋅h−1⋅cm−2; n = 4, P > 0.2). To investigate possible reasons for the lack of effect of cGMP on Ca2+ reabsorption, the expression of two important downstream mediators of cGMP action, i.e., cGK I and II, was measured by immunoblotting and RT-PCR both in freshly immunodissected CNT and CCD tubules and primary cultures thereof. As shown in Fig. 2, cGK I and II antibodies recognized purified cGK standards with apparent Mr of 76 and 86 kDa, respectively. Freshly immunodissected CNT and CCD tubules contained cGK II (30–50 ng⋅mg protein−1); however, this enzyme was no longer detectable after 5 days of culturing on permeable supports (Fig. 2A). In contrast, cGK I was not detectable in freshly isolated or cultured CNT and CCD cells (Fig. 2A). Consistent with the immunoblot data, cGK II mRNA was present in freshly immunodissected tubules and not detectable in cultured monolayers, demonstrating that the cGK II messenger was rapidly lost during culturing (Fig. 2B). Of note, equivalent amounts of glyceraldehyde-3-phosphate dehydrogenase mRNA were amplified in both RNA preparations, indicating that similar amounts of tissue were compared in the PCRs. The PCRs performed in the absence of reverse transcriptase were negative for cGK I and cGK II, demonstrating the absence of DNA contaminations.
Figure 2.
Analysis of endogenous and reexpressed cGK I and cGK II in CNT/CCD cells. (A) Immunoblots of samples (20 μg protein each) from homogenates of either freshly immunodissected CNT/CCD tubules, monolayers of cells cultured from these tubules, or monolayers infected with adenoviral-cGK constructs (described in Materials and Methods) were labeled with antibodies against cGK I or cGK II (A). Shown in the right lane of each blot are standards (2 ng) of either purified bovine lung cGK I or recombinant rat intestine cGK II. The 86-kDa protein endogenously present in freshly isolated cells was identified as cGK II, and this was confirmed with immunoblots by using an additional antibody independently raised against cGK II (data not shown). (B) cGK II mRNA was detected by RT-PCR in freshly isolated and cultured CNT/CCD cells. Samples of RT-PCR-derived cGK II (420 bp) and glyceraldehyde-3-phosphate dehydrogenase (309 bp) cDNA were analyzed on an ethidium bromide-stained 2% (wt/vol) agarose gel. PCR was carried out either with (RT+) or without (RT−) reverse transcriptase. RNA (2 μg) was used as starting material for RT-PCRs.
Reexpression of cGK II Enables ANP to Stimulate Ca2+ Reabsorption.
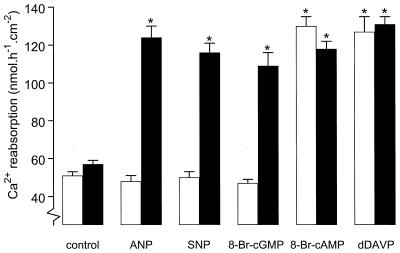
Recombinant adenoviral vectors, containing the coding sequence for rat cGK II, were used to reexpress cGK II in the primary CNT/CCD cultures, which had lost expression of the kinase. An expression level of 75–150 ng cGK II⋅mg protein−1, comparable to that of endogenous cGK II in freshly isolated cells, was observed (Fig. 2A). Reexpression of cGK II revealed the stimulatory potential of ANP on Ca2+ reabsorption (258 ± 13% above control values) (Fig. 3). Similar stimulation of Ca2+ reabsorption by other cGMP-elevating agents, such as 100 μM SNP (242 ± 10%) and 100 μM 8-Br-cGMP (227 ± 14%), also was observed. Of note, cGK II expression alone did not significantly affect basal Ca2+ transport.
Figure 3.
Transcellular Ca2+ transport in monolayers of CNT/CCD cells expressing cGK II. Cultured monolayers were either infected (solid bars) or not infected (open bars) with adenovirus for expressing cGK II. Two days after infection, transcellular Ca2+ transport was measured in the absence (control) and presence of either 100 nM ANP (basolateral), 100 μM SNP (both sides), 100 μM 8-Br-cGMP (both sides), 100 μM 8-Br-cAMP (both sides), or 10 nM d-Arg-8-vasopressin (basolateral). Values are means ± SEM (n = 6). ∗, P < 0.05, significantly different from the control values.
Previous studies have demonstrated that cAMP-elevating agents can also stimulate transcellular Ca2+ transport (2, 4, 6). However, reexpression of cGK II had no effect on the stimulation of Ca2+ reabsorption by either 8-Br-cAMP (100 μM, both sides) or the cAMP-elevating hormone d-Arg-8-vasopressin (10 nM, basolateral) (Fig. 3). To investigate the relationship between the classical cAMP-mediated and the novel cGMP-dependent pathway, the stimulatory effect of 8-Br-cAMP and 8-Br-cGMP, alone and in combination, on Ca2+ transport was studied. However, a combination of maximally effective doses of both stimulants did not further increase the rate of calcium transport (100 μM 8-Br-cAMP, 126 ± 6; 100 μM 8-Br-cGMP, 118 ± 5; combination of 8-Br-cAMP and 8-Br-cGMP, 123 ± 3 nmol⋅h−1⋅cm−2; n = 3, P > 0.2).
ANP Stimulation of Ca2+ Reabsorption Is Specifically Dependent on Membrane-Targeted cGK II.
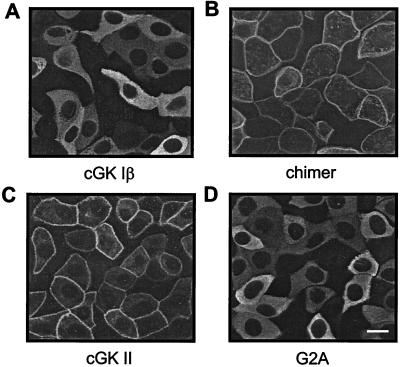
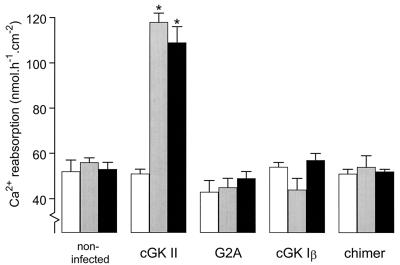
The specific properties of cGK II that might account for its stimulatory effect on Ca2+ reabsorption were investigated by using adenoviral-vector-expressed mutant kinases. cGK II, cGK I, and their modified constructs were compared with respect both to their subcellular localization (Fig. 4) and their ability to stimulate Ca2+ reabsorption in CNT/CCD cells (Fig. 5). Immunofluorescence confocal microscopy demonstrated that cGK II and a chimer (cGK II/Iβ), consisting of the first 29 N-terminal amino acids of cGK II linked to the N terminus of full-length cGK I, were positioned at the cell membrane (Fig. 4 C and B), whereas cGK Iβ and a myristoylation deficient (G2A) mutant of cGK II had a predominantly cytosolic localization (Fig. 4 A and D). The ability of the adenoviral-vector cGK constructs to mediate ANP-stimulated Ca2+ reabsorption was investigated by stimulating infected cell monolayers with 100 nM ANP (basolateral) and 100 μM 8-Br-cGMP (both sides). As demonstrated in Fig. 5, only cGK II-expressing cells displayed cGMP-dependent Ca2+ reabsorption. Prevention of myristoylation of cGK II (G2A mutant) resulted in the inability of this cGK to support Ca2+ reabsorption. cGK Iβ in its normal cytosolic localization did not activate Ca2+ reabsorption; however, unlike cGK II, membrane attachment in the form of the cGK II/Iβ chimer also did not result in such activity.
Figure 4.
Subcellular localization of cGK proteins expressed in CNT/CCD monolayers. Monolayers were infected with adenovirus for expressing either cGK Iβ (A), a chimer of the N terminus of cGK II linked to the N terminus of full-length cGK Iβ (B), cGK II (C), or a myristoylation-deficient (G2A) cGK II mutant (D). Two days after infection, immunolocalization of the cGK proteins was visualized by confocal laser-scanning microscopy. Membrane-associated (B and C) or cytosolic (A and D) localization was observed. Specificity of cGK immunostaining was confirmed by the absence of signal in noninfected monolayers (not shown). (Bar = 5 μm.)
Figure 5.
cGMP-stimulated Ca2+ reabsorption in CNT/CCD monolayers expressing cGK proteins of different membrane-association properties. Monolayers were noninfected or infected with adenovirus for expressing either cGK II, a myristoylation-deficient cGK II mutant (G2A), cGK Iβ, or a chimer of the N terminus of cGK II fused to the N terminus of full-length cGK Iβ. Two days after infection, monolayers either were not stimulated (open bars) or stimulated with 100 nM basolateral ANP (shaded bars) or 100 μM 8-Br-cGMP added to both sides (solid bars). Values are means ± SEM of three experiments. ∗, P < 0.05, significantly different from control values.
Na+ Reabsorption Is Not Affected by cGK.
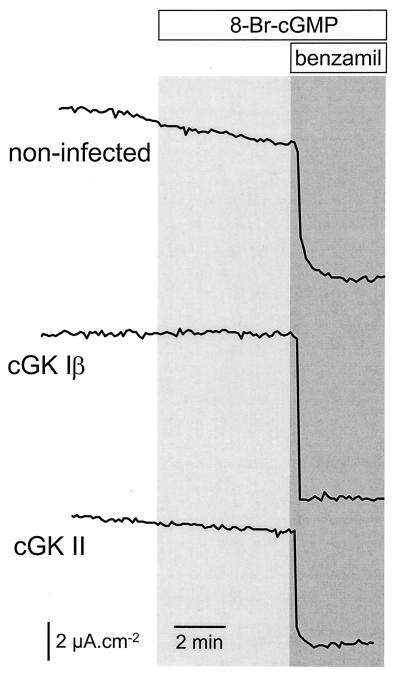
Transcellular Na+ transport across the CNT/CCD monolayers was measured as benzamil-sensitive ISC (Fig. 6). Whereas 10 μM benzamil (apical side) markedly reduced ISC, addition of 8-Br-cGMP (100 μM, both sides) had no significant effect on ISC either in uninfected or in cGK Iβ- or cGK II-infected monolayers. Na+ transport itself did not appear to be affected by the infection procedure as indicated by equivalent benzamil effects in all cases.
Figure 6.
Transcellular Na+ transport is not affected by cGK expression. Monolayers of CNT/CCD cells either were noninfected or infected with adenovirus for expressing cGK Iβ or cGK II. Two days after infection, the effect of 100 μM 8-Br-cGMP (both sides, for the period indicated in the figure) on the ISC was determined. Subsequently, transcellular Na+ transport was blocked by addition of 10 μM benzamil to the apical compartment. Representative traces of three experiments are shown.
DISCUSSION
The results of the present study demonstrate that ANP and NO stimulate Ca2+ reabsorption in rabbit CNT/CCD. This stimulatory effect is specifically mediated by membrane-targeted cGK II and is restricted to the process of Ca2+ reabsorption because electrogenic Na+ transport in these nephron segments was not regulated by a cGMP-dependent mechanism.
A detailed molecular characterization of cGMP-stimulated Ca2+ reabsorption was made possible by reintroducing different cGK gene products and mutants into primary cultures of CNT/CCD cells that had lost endogenous cGK. cGMP-stimulated Ca2+ transport was specifically mediated by cGK II but not cGK Iβ. The membrane localization of cGK II seemed to be a major determinant of its ability to activate Ca2+ reabsorption because the myristoylation-deficient cGK II mutant, which predominantly resided in the cytosol, was unable to mimic the effect of wild-type cGK II. The requirement of membrane targeting of cGK II for its ability to activate both the CFTR Cl− channel in intestinal cells (23) and, as we show here, Ca2+ reabsorption extends the emerging concept that compartmentalization of protein kinases and their substrates is a major determinant of their interaction under physiological conditions (35, 36). Expression of a membrane-bound cGK II/Iβ chimer did not result in restoration of cGMP-stimulatable Ca2+ transport, indicating that membrane anchoring alone is not sufficient to confer cGK Iβ activation of the Ca2+ transport process and underscoring further the specificity for cGK II in this process. In contrast, the membrane-bound cGK II/Iβ chimer partially phosphorylated and activated the CFTR Cl− channel, the only known physiological cGK II substrate characterized so far in intact cells, whereas cGK I did not (23, 37). Assuming that Ca2+ influx across the apical membrane is rate-limiting in transcellular Ca2+ transport, it is tempting to speculate that cGK II, by analogy with the CFTR Cl− channel, directly activates this transporter. This would concur with a distinct role for cGK II in regulating certain apical membrane processes, particularly electrolyte transport (22).
The second messengers, cGMP and cAMP, stimulated Ca2+ transport to the same maximal level, and their effects were not additive, suggesting that cGK II and cAK phosphorylate the same target in the process of Ca2+ transport. Indeed, both cGK II and cAK can phosphorylate similar consensus sequences consisting of a phosphorylatable serine or threonine (at P = 0) preceded at the P-2 and P-3 positions by basic amino acids (22). The observation that ANP and NO failed to stimulate Ca2+ transport in noninfected cells convincingly demonstrates that physiological activation of cGMP does not stimulate Ca2+ reabsorption by cross-activation of cAK.
In contrast to cGK II stimulation of Ca2+ reabsorption, cGK II did not affect Na+ transport in CNT/CCD cells. Thus, ANP inhibition of Na+ transport in general and, in particular, in the inner medullary collecting duct (IMCD) (38) does not seem to operate in CNT/CCD cells. ANP reduces amiloride-sensitive Na+ transport in IMCD by inhibiting Na+ channels via both a direct cGMP effect on the channel and an indirect effect mediated by cGK I (15, 16). In contrast, our results show that Na+ reabsorption in CNT/CCD cells, which occurs via a different type of Na+ channel, the so-called ENaC (epithelial sodium-selective channel), is not regulated by cGMP-elevating hormones. This is in agreement with another recent report (39) and consistent with the fact that ENaC contains no conserved cAK/cGK phosphorylation sites (38).
Our immunoblot results suggest that the cGK II concentration present in rabbit CNT/CCD cells is perhaps 10-fold lower than that previously measured in intestinal mucosa (29). Low levels of cGK II have also been observed in rat kidney juxtaglomerular cells, ascending thin limb, and proximal tubule brush borders, but not other nephron segments (8). It is not clear whether there are species differences between rat and rabbit expression of cGK II or whether immunodissection of CNT/CCD cells permitted optimization of cGK II detection in comparison with whole microdissected tubules. There is, however, some other independent, functional evidence for a membrane-bound cGK that can activate basolateral, Ca2+-dependent K+ channels in excised patches from rat CCD cells (24).
Comparison of CNT/CCD cells that had become deficient in cGK II with cells in which cGK II was reintroduced by using adenoviral vectors to rescue the in vitro knock-out system provided a unique opportunity to establish cGK II as a necessary mediator in the ANP/NO signal transduction pathway leading to cGMP-dependent Ca2+ reabsorption. Our analysis of cGK II expression revealed that the cGK II present in freshly isolated CNT/CCD cells was no longer detectable at the mRNA and protein level after 5 days of cell culturing. This demonstrates that cGK II gene expression is highly susceptible to being switched off during culturing of these cells, a characteristic of cGK I that has been observed at the protein level in endothelial (40) and smooth muscle cell cultures (41).
It has been suggested that the natriuretic effect of ANP is, in part, a result of an inhibition of the reabsorptive capacity of the proximal tubule, leading to increased urinary excretion of Na+, Ca2+, Mg2+, and PO43− (9). Thus, it is tempting to speculate that the Ca2+-sparing action of ANP in CNT/CCD may serve to reduce Ca2+ waste during ANP-induced natriuresis and, therefore, could be of considerable physiological importance. Because, in the present study, freshly isolated connecting tubules and cortical collecting ducts and primary cultures thereof were used, future investigations are needed to extrapolate our findings to the whole kidney level. cGK II and cGK I (as control) knock-out mice (42) may be useful for this purpose and for examining whether cGMP-dependent Ca2+ reabsorption requires exclusively cGK II.
Acknowledgments
We thank Anita Hartog for superb technical assistance with the immunocytochemistry. This work was supported in part by a grant from the Dutch Kidney Foundation (94.1348), the Deutsche Forschungsgemeinschaft (SFB 355), a fellowship to A.S. from the German Cardiology Society, and a fellowship to A.B.V. from the Netherlands Organization for Scientific Research.
ABBREVIATIONS
- cGK I and II
types I and II cGMP-dependent protein kinase, respectively
- cAK
of cAMP-dependent protein kinase
- ANP
atrial natriuretic peptide
- CNT
connecting tubules
- CCD
cortical collecting ducts
- SNP
sodium nitroprusside
- 8-Br-cGMP
8-bromoguanosine cGMP
- ISC
short-circuit current
- RT-PCR
reverse transcription–PCR
Note Added in Proof
While this report was being prepared, the epithelial calcium Ca2+ channel (ECaC) was cloned from rabbit CNT and CCD (43). ECaC is expressed exclusively in 1,25-dihydroxyvitamin D3-responsive epithelia. Sequence analysis revealed a protein of 730 aa with a classical ion channel structure based on six transmembrane spanning domains and a predicted hydrophobic stretch between transmembrane segment 5 and 6 indicated as the pore region. Within the C terminus of ECaC, two potential phosphorylation sites were found for cGK II. The serine at position 669 and the threonine at position 709 are part of a consensus phosphorylation sequence for cGK II and cAK. Future studies will reveal whether ECaC is indeed the final target for cGK II in the stimulatory effect of ANP on Ca2+ reabsorption in CNT and CCD.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Bindels R J M, Hartog A, Timmermans J A H, Van Os C H. Am J Physiol. 1991;261:F799–F807. doi: 10.1152/ajprenal.1991.261.5.F799. [DOI] [PubMed] [Google Scholar]
- 2.Friedman P A, Gesek F A. Physiol Rev. 1995;75:429–471. doi: 10.1152/physrev.1995.75.3.429. [DOI] [PubMed] [Google Scholar]
- 3.Van Baal J, Yu A, Hartog A, Willems P H G M, Lytton J, Bindels R J M. Am J Physiol. 1996;271:F985–F993. doi: 10.1152/ajprenal.1996.271.5.F985. [DOI] [PubMed] [Google Scholar]
- 4.Lau K, Bourdeau J E. J Biol Chem. 1989;264:4028–4032. [PubMed] [Google Scholar]
- 5.Hoenderop J G J, Hartog A, Willems P H G M, Bindels R J M. Am J Physiol. 1998;43:F736–F743. doi: 10.1152/ajprenal.1998.274.4.F736. [DOI] [PubMed] [Google Scholar]
- 6.Hoenderop J G J, De Pont J J H H M, Bindels R J M, Willems P H G M. Kidney Int. 1999;55:225–233. doi: 10.1046/j.1523-1755.1999.00228.x. [DOI] [PubMed] [Google Scholar]
- 7.Friedman P A, Coutermarsh B A, Kennedy S M, Gesek F A. Endocrinology. 1996;137:13–20. doi: 10.1210/endo.137.1.8536604. [DOI] [PubMed] [Google Scholar]
- 8.Gambaryan S, Häusler C, Markert T, Pöhler D, Jarchau T, Walter U, Haase W, Kurtz A, Lohmann S M. J Clin Invest. 1996;98:662–670. doi: 10.1172/JCI118837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gunning M E, Ingelfinger J R, King A J, Brenner B M. In: The Kidney. 5th Ed. Brenner B M, editor. Philadelphia: Saunders; 1996. pp. 626–712. [Google Scholar]
- 10.Millul V, Ardaillou N, Placier S, Baudouin B, Ronco P M. Kidney Int. 1997;51:281–287. doi: 10.1038/ki.1997.34. [DOI] [PubMed] [Google Scholar]
- 11.Ballermann B J, Zeidel M L. In: The Kidney: Physiology and Pathophysiology. 2nd Ed. Seldin D W, Giebisch G, editors. New York: Raven; 1992. pp. 1843–1884. [Google Scholar]
- 12.Schmidt H H H W, Lohmann S M, Walter U. Biochim Biophys Acta. 1993;1178:153–175. doi: 10.1016/0167-4889(93)90006-b. [DOI] [PubMed] [Google Scholar]
- 13.Lincoln T M, Cornwell T L. FASEB J. 1993;7:328–338. doi: 10.1096/fasebj.7.2.7680013. [DOI] [PubMed] [Google Scholar]
- 14.Francis S H, Corbin J D. Annu Rev Physiol. 1994;56:237–272. doi: 10.1146/annurev.ph.56.030194.001321. [DOI] [PubMed] [Google Scholar]
- 15.McCoy D E, Guggino S E, Stanton B A. Kidney Int. 1995;48:1125–1133. doi: 10.1038/ki.1995.396. [DOI] [PubMed] [Google Scholar]
- 16.Light D B, Corbin J D, Stanton B A. Nature (London) 1990;344:336–339. doi: 10.1038/344336a0. [DOI] [PubMed] [Google Scholar]
- 17.Kurtz A, Gotz K H, Hamann M, Wagner C. Proc Natl Acad Sci USA. 1998;95:4743–4747. doi: 10.1073/pnas.95.8.4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gambaryan S, Wagner C, Smolenski A, Walter U, Poller W, Haase W, Kurtz A, Lohmann S M. Proc Natl Acad Sci USA. 1998;95:9003–9008. doi: 10.1073/pnas.95.15.9003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wagner C, Pfeifer A, Ruth P, Hofmann F, Kurtz A. J Clin Invest. 1998;102:1576–1582. doi: 10.1172/JCI4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jarchau T, Häusler C, Markert T, Pöhler D, Vandekerckhove J, De Jonge H R, Lohmann S M, Walter U. Proc Natl Acad Sci USA. 1994;91:9426–9430. doi: 10.1073/pnas.91.20.9426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uhler M D. J Biol Chem. 1993;268:13586–13591. [PubMed] [Google Scholar]
- 22.Lohmann S M, Vaandrager A B, Smolenski A, Walter U, De Jonge H R. Trends Biochem Sci. 1997;22:307–312. doi: 10.1016/s0968-0004(97)01086-4. [DOI] [PubMed] [Google Scholar]
- 23.Vaandrager A B, Smolenski A, Tilly B C, Houtsmuller A B, Ehlert E M, Bot A G M, Edixhoven M, Boomaars W E M, Lohmann S M, De Jonge H R. Proc Natl Acad Sci USA. 1998;95:1466–1471. doi: 10.1073/pnas.95.4.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirsch J, Schlatter E. Pflügers Arch. 1995;429:338–344. doi: 10.1007/BF00374148. [DOI] [PubMed] [Google Scholar]
- 25.Alioua A, Tanaka Y, Wallner M, Hofmann F, Ruth P, Meera P, Toro L. J Biol Chem. 1998;273:32950–32955. doi: 10.1074/jbc.273.49.32950. [DOI] [PubMed] [Google Scholar]
- 26.Markert T, Vaandrager A B, Gambaryan S, Pöhler D, Häusler C, Walter U, De Jonge H R. J Clin Invest. 1995;96:822–830. doi: 10.1172/JCI118128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vaandrager A B, Ehlert E M, Jarchau T, Lohmann S M, De Jonge H R. J Biol Chem. 1996;271:7025–7029. doi: 10.1074/jbc.271.12.7025. [DOI] [PubMed] [Google Scholar]
- 28.Sandberg M, Natarajan V, Ronander I, Kalderon D, Walter U, Lohmann S M, Jahnsen T. FEBS Lett. 1989;255:321–329. doi: 10.1016/0014-5793(89)81114-7. [DOI] [PubMed] [Google Scholar]
- 29.Vaandrager A B, Tilly B C, Smolenski A, Schneider-Rasp S, Bot A G M, Edixhoven M, Scholte B J, Jarchau T, Walter U, Lohmann S M, Poller W C, De Jonge H R. J Biol Chem. 1997;272:4195–4200. doi: 10.1074/jbc.272.7.4195. [DOI] [PubMed] [Google Scholar]
- 30.Bindels R J M, Engbersen A M T, Hartog A, Blazer-Yost B L. Biochim Biophys Acta. 1996;1284:63–68. doi: 10.1016/0005-2736(96)00113-7. [DOI] [PubMed] [Google Scholar]
- 31.Vaandrager A B, van der Wiel E, Hom M L, Luthjens L H, De Jonge H R. J Biol Chem. 1994;269:16409–16415. [PubMed] [Google Scholar]
- 32.Pöhler D, Butt E, Meissner J, Müller S, Lohse M, Walter U, Lohmann S M, Jarchau T. FEBS Lett. 1995;374:419–425. doi: 10.1016/0014-5793(95)01168-e. [DOI] [PubMed] [Google Scholar]
- 33.Chomczynski P, Sacchi N. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 34.Snedecor G W, Cochran W G. Statistical Methods. Ames, IA: Iowa Univ. Press; 1974. [Google Scholar]
- 35.Monchly-Rosen D. Science. 1995;268:247–251. doi: 10.1126/science.7716516. [DOI] [PubMed] [Google Scholar]
- 36.Scott J D, McCarty S. Mol Endocrinol. 1994;8:5–11. doi: 10.1210/mend.8.1.8152430. [DOI] [PubMed] [Google Scholar]
- 37.French P J, Bijman J, Edixhoven M, Vaandrager A B, Scholte B J, Lohmann S M, Nairn A C, De Jonge H R. J Biol Chem. 1995;270:26626–26631. doi: 10.1074/jbc.270.44.26626. [DOI] [PubMed] [Google Scholar]
- 38.Garty H, Palmer L G. Physiol Rev. 1997;77:359–396. doi: 10.1152/physrev.1997.77.2.359. [DOI] [PubMed] [Google Scholar]
- 39.Schlatter E, Cermak R, Forssmann W G, Hirsch J R, Kleta R, Kuhn M, Sun D, Schafer J A. Am J Physiol. 1996;271:F1158–F1165. doi: 10.1152/ajprenal.1996.271.6.F1158. [DOI] [PubMed] [Google Scholar]
- 40.Draijer R, Vaandrager A B, Nolte C, De Jonge H R, Walter U, van Hinsbergh V W. Circ Res. 1995;77:897–905. doi: 10.1161/01.res.77.5.897. [DOI] [PubMed] [Google Scholar]
- 41.Cornwell T L, Lincoln T M. J Biol Chem. 1989;264:1146–1150. [PubMed] [Google Scholar]
- 42.Pfeifer A, Aszódi A, Seidler U, Ruth P, Hofmann F, Fässler R. Science. 1996;274:2082–2086. doi: 10.1126/science.274.5295.2082. [DOI] [PubMed] [Google Scholar]
- 43.Hoenderop J G J, van der Kemp A W C M, Hartog A, van de Graat S F J, van Os C H, Willems P H G M, Bindels R J M. J Biol Chem. 1999;274:8375–8378. doi: 10.1074/jbc.274.13.8375. [DOI] [PubMed] [Google Scholar]