Abstract
The sea lamprey recovers normal-appearing locomotion after spinal cord transection and its spinal axons regenerate selectively in their correct paths. However, among identified reticulospinal neurons some are consistently bad regenerators and only about 50% of severed reticulospinal axons regenerate through the site of injury. We previously suggested (Shifman and Selzer, 2000) that selective chemorepulsion might explain why some neurons are bad regenerators and others not. To explore the role of additional chemorepulsive axonal guidance molecules during regeneration, we examined the expression of the repulsive guidance molecule (RGM) and its receptor neogenin by in situ hybridization and quantitative PCR. RGM mRNA was expressed in the spinal cord, primarily in neurons of the lateral gray matter and in dorsal cells. Following spinal cord transection, RGM message was downregulated in neurons close (within 10 mm) to the transection at 2 and 4 weeks, although it was upregulated in reactive microglia at 2 weeks post-transection. Neogenin mRNA expression was unchanged in the brainstem after spinal cord transection, and among the identified reticulospinal neurons, was detected only in “bad regenerators, Neurons that are known to regenerate well never expressed neogenin. The downregulation of RGM expression in neurons near the transection may increase the probability that regenerating axons will regenerate through the site of injury and entered caudal spinal cord.
Keywords: axonal guidance, spinal cord regeneration, in situ hybridization, RGM, neogenin, lamprey, microglia
Introduction
Unlike mammals, lampreys show regeneration of axons and functional recovery after complete spinal cord transection (Rovainen, 1976, Selzer, 1978, Wood and Cohen, 1979). However, lamprey reticulospinal neurons differ in their regenerative abilities. While some are consistently “good regenerators”(regenerating 75% of the time or more), other neurons “bad regenerators” (regenerating less than 10–20% of the time)(Davis and McClellan, 1994, Jacobs, et al., 1997, Swain, 1989). In general, among identified reticulospinal neurons only about 50% regenerate through the site of injury.
The failure of axonal regeneration in the CNS of mammals has been ascribed primarily to the presence of inhibitory factors in the extracellular environment of the injured axon (Aguayo, et al., 1991, Caroni and Schwab, 1988, David and Aguayo, 1981) and glial scar prevention of axonal growth (Galtrey and Fawcett, 2007, Qiu, et al., 2000). Several inhibitory molecules are associated with myelin (Brittis and Flanagan, 2001, Huber and Schwab, 2000, McKerracher, et al., 1994) and in matrix molecules secreted by reactive astrocytes and oligodendrocytes (Fawcett and Asher, 1999, Fitch and Silver, 1997, Snow, et al., 1990). However, because the lamprey CNS lacks myelin (Bullock, et al., 1984), failure of many reticulospinal axons to regenerate cannot be attributed to myelin-derived growth inhibitors (e.g., MAG, MOG and Nogo). Moreover, lamprey spinal axons grow preferentially through a hemisection scar rather than around it (Lurie and Selzer, 1991), suggesting that reactive glial cells are supportive of axon regeneration. Therefore, other factors may be responsible for the heterogeneity in the regenerative abilities of lamprey reticulospinal neurons. Based on our previous data that showed upregulation of the netrin receptor UNC5 in “bad regenerating” reticulospinal neurons after spinal cord transection (Shifman and Selzer, 2000), we hypothesized that post-injury expression of some repulsive guidance molecules in the spinal cord and coordinated upregulation of their receptors in reticulospinal neurons could be responsible, at least in partial, for limiting the regenerative abilities of those neurons.
Research conducted during the last decade has identified several discrete classes of diffusible and transmembrane proteins that act as repellent cues in guiding axon growth during development and possibly during axon regeneration. Among them are the netrins, semaphorins, ephrins, slits and the newly described “repulsive guidance molecule” (RGM; reviewed in (Matsunaga and Chedotal, 2004, Moore and Kennedy, 2006)). RGM actually represents a new family of GPI membrane-bound proteins that play a critical role in axon guidance and other processes of neuronal development. RGMs contain a signal peptide, an RGD site, a von Willebrand factor (vWF) domain, a hydrophobic region, and a GPI (glycosylphosphatidylinositol) anchor (Monnier, 2002) and are divided into 3 classes – RGM A, RGM B and RGM C. Recent experimental data suggested that neogenin binds RGMs and act as their receptor, mediating intracellular signaling (Matsunaga and Chedotal, 2004, Wilson and Key, 2006, Yamashita, et al., 2007).
While involvement of semaphorins and netrins in restricting the ability of spinal cord axons to regenerate after injury (De Winter and Oudega M, 2002, Manitt, et al., 2006, Moreau-Fauvarque, et al., 2003, Pasterkamp and Verhaagen, 2001, Shifman and Selzer, 2000, Shifman and Selzer, 2007, Wehrle, et al., 2005) is well documented, the role of RGMs and their receptor neogenin in spinal cord regeneration has not received comparable attention. However, several recent articles described RGM expression after axonal injury (Doya, et al., 2006, Hata, et al., 2006, Schwab, et al., 2005, Schwab, et al., 2005).
To gain new insights into the function of RGM in axon regeneration, we used the highly accessible nervous system of larval sea lampreys (Petromyzon marinus), as a model system in which post-axotomy expression of RGM and its receptor neogenin could be studied in vivoat the single cell level. The lamprey brainstem contains several uniquely identifiable reticulospinal neurons, including the Mauthner neurons with crossed descending axons and several pairs of Müller cells whose axons descend ipsilaterally. Because their axons project almost the entire length of the spinal cord (Swain, et al., 1993), all these large reticulospinal neurons are axotomized by a high spinal cord transection. Moreover, because the regenerative abilities of these neurons have been quantified previously (Jacobs et al., 1997), post-axotomy changes in RGM and neogenin expression could be related to the known regenerative abilities of identified neurons.
MATERIALS AND METHODS
Animals
Wild-type larval lampreys (Petromyzon marinus), 12–14 cm in length (4–5 years old) and in a stable stage of neurological development, were obtained from streams feeding lake Michigan and maintained in fresh water tanks at 16°C until the day of surgery.
Spinal cord transection
Animals were anesthetized by immersion in 0.1% tricaine methanesulfonate, and the spinal cords exposed from the dorsal midline at the level of the fifth gill. Transection of the spinal cord was performed with Castroviejo scissors, after which the wound was allowed to air dry over ice for one hour. Each transected animal was examined 24 hours after surgery to confirm that there was no movement caudal to lesion site. A transection was tentatively considered complete if on stimulation of the head, an animal could move only its head and body rostral to the lesion. Animals were allowed to recover in fresh water tanks at room temperature. At the specified recovery times, animals were re-anesthetized, and the spinal cords and brains were removed for in situ hybridization or RNA extraction for real-time PCR analysis. Experiments were carried out on 100 lampreys that were either untransected (n = 20) or permitted to recover 2 weeks (n = 40) or 4 weeks (n = 40). Experiments were approved by the Institutional Animal Care and Use Committee at the University of Pennsylvania.
Riboprobe synthesis
Previously cloned cDNAs for lamprey neogenin and lamprey RGM were used as templates for the generation of digoxigenin-(DIG-) labeled sense and antisense riboprobes for in situ hybridization on spinal cord and brain wholemount preparations of control and spinal-transected animals. The neogenin (GenBank accession number AY744917) cRNA probe was transcribed from a 648-bp sequence spanning the intracellular domain (nucleotides 4052–4700) and the RGM probe was transcribed from a cloned sequence (nucleotides 1–581; GenBank accession number EU449948). Lamprey RGM and neogenin cDNAs cloned in pGEM-T Easy vector (Promega) were linearized with restriction enzymes and gel-purified. The DIG incorporation into probes was controlled by dot blots. The length and integrity of the probes was examined by gel electrophoresis. Sense RNA probes were used as controls.
Wholemount brain and spinal cord in situ hybridization
Wholemount preparations preserve three-dimensional information, which allows for the rapid and accurate identification of labeled cells. The lamprey spinal cord can be studied by whole-mount in situ hybridization, in part because of its flat shape and in part because it lacks myelin (Bullock, et al., 1984), making the entire CNS translucent. Hybridizations of DIG-labeled riboprobes to wholemounted lamprey brain and spinal cord, respectively, were performed using methods optimized for the lamprey as previously described (Shifman and Selzer, 2000). DIG-labeled sense RNA probes were used as internal controls and did not produce hybridization signals. Images were captured digitally using an Zeiss AxioCam CCD Video Camera attached to a Zeiss Axioskop microscope with AxioVision software, and scale bars were added. Images were imported into Adobe Photoshop CS2 (Adobe Systems, Inc., San Jose, CA). The images were cropped and adjusted for brightness and contrast, and labels were added.
Quantitative real-time RT-PCR assessment of RGM mRNA expression in the spinal cord after transection
Total RNA was extracted at 2 weeks or at 1 month after injury from rostral segments of spinal cord (including the injury epicenter), from segments 10 mm caudal to the transection, from the caudal remainder of the cord, and from sham-operated cords, using Trizol reagent (Invitrogen) according to the manufacturer’s instructions. RNA was suspended in diethylpyrocarbonate (DEPC)-treated water, followed by treatment with DNAse1 using the DNA-Free Kit (Ambion, TX, USA) to remove any traces of contaminating genomic DNA. The yield and purity of RNA were checked by spectrophotometric determination at 260 and 280 nm. Integrity of RNA was determined by the presence of 28S and 18S ribosomal RNA by electrophoresis of samples through 1.0% agarose gels. The first strand cDNA synthesis reaction from total RNA was catalyzed by Superscript II Reverse Transcriptase (Invitrogen) and random primers according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA).
Primer design
The DNA sequence of lamprey RGM (see above) and of lamprey Hypoxanthine-guanine phosphoribosyltransferase (HPRT 1; Genbank accession number FJ155927) were found using MegaBLAST programs to search a lamprey Trace Archive database that has been developed to store the raw genomic data underlying all of the sequences generated by genome projects. Cloning and sequencing of lamprey glyceraldehyde 3-phosphate dehydrogenase (GAPDH, Genbank accession number AAT70328) and 18S rRNA sequence (Genbank accession number M97575) was reported by others (Pancer, et al., 2004, Stock and Whitt, 1992). Sequences sharing homology with RGM, GAPDH, 18S rRNA and HPRT were amplified by RT-PCR. Only the open reading frame, or CDS coding for proteins, was chosen from these sequences. The primers were designed using Primer Express 3 software (Applied Biosystems CA, USA) according to the user’s manual. The specificity of primers was confirmed by homology search against the GenBank database. The sequences of primers and probes are shown in Table 1. All the primers were synthesized by IDT, Inc (Coralville, IA, USA). To test the specificity of primers for lamprey-specific genes, nonquantitative PCRs were performed using 2 μl of first-strand synthesis cDNA from lamprey spinal cord RNA. The 50-μl PCR reaction additionally contained the following components: 0.4 μM of each primer, 10 μl 5X Green GoTaq® Flexi Buffer (Promega, WI) and 1.25 U GoTaq® DNA polymerase (Promega, WI). The PCR reactions were run in a programmable themocycler GeneAmp® PCR System 9700 (Applied Biosystems) using an initial denature temperature of 95°C for 4 min, 35 cycles of 95°C for 45 s, 52°C for 45 s, 72°C for 1 min, and a final extension at 72°C for 10 min. A total of 20 μl of final PCR product was separated in a 1.5% agarose gel, stained with ethidium bromide, and photographed.
Table 1.
Sequence of primer pairs for the lamprey genes used for the real-time PCR
| Gene (GenBank Accession #) |
Sequence 5′–3′ | Fragment size(bp) |
|---|---|---|
| RGM (EU449948) | 96 | |
| Forward primer | CCTCGTCGACAACCCTTACC | |
| Reverse primer | AAGATGATGGTCAGCTTGTTGGT | |
| HPRT 1 (U03700) | 68 | |
| Forward primer | GCGCTCAACCGCAACAC | |
| Reverse primer | CAGTAGCTCTTGAGTCGGATGAAG | |
| 18S rRNA (M97575) | 75 | |
| Forward primer | GTAGTTGGTGGAGCGATTTGTCT | |
| Reverse primer | GGCCGCGTAGCTAGTTAGCA | |
| GAPDH(AAT70328) | 72 | |
| Forward primer | TGCAAAGCACGTCATCATCTC | |
| Reverse primer | TTCTCGTGGTTTACTCCCATCA |
For all gene expression studies using quantitative PCR it is necessary to compensate for differences between samples due to material losses, differences in RT yields and PCR inhibition. Normalization ideally should include an endogenous control gene that has constant expression in all the samples compared. There is no universal control gene, expressed at a constant level under all conditions and in all tissues. The best way to choose the proper reference gene is by running a panel of potential genes on a number of representative test samples. The gene(s) most appropriate for normalization are chosen in each case. We used the well-known housekeeping genes GAPDH, HPRT 1 and 18S rRNA in our preliminary experiments and found that GAPDH had the most constant expression in all the samples, and was therefore the best endogenous control gene.
Quantitative RT-PCR was performed at the Quantitative PCR Facility of Virus and Molecular Core Services of the University of Pennsylvania using an ABI PRISM 7500 Fast Real-Time PCR System (Applied Biosystems) under 9600 Emulation Mode using the following conditions: 50°C for 2 min, 95°C for 10 min, 40 cycles of 95°C for 15 s, 60°C for 1 min and 1 cycle 95°C for 15 s, 60°C for 1 min, 95°C for 15 s, 60°C for 15 s. The PCR reagents, except primers, were from the Power SYBR® Green PCR Master Mix (Applied Biosystems). The PCR for the target (RGM) and endogenous control (GAPDH) were performed in separate tubes in triplicate on cDNA samples in a MicroAmp Optical 96-well reaction plate (Applied Biosystems) according to manufacturer’s instructions. A Power SYBR® Green PCR Master Mix was mixed with 200 nM forward and reverse primer for RGM (300nM for GAPDH) and 10 ng cDNA. From this mixture, an aliquot of 20 μl was placed in each of three wells. Each plate contained no template control (NTC) in which DEPC water replaced the cDNA as well as 5 serial concentrations of cDNA standard to allow calculation of a standard curve.
Standard curve construction
Standard curves were prepared for both the target and the endogenous reference. Standard curves were based on RT-PCR of known quantities of cDNA synthesized from spinal cord RNA containing the sequences of interest. Each standard curve was generated based on five 10-fold serial dilutions of a cDNA (100, 10, 1, 0.1, 0.01ng), quantified by a spectrophotometer. The reaction kinetics was represented by an amplification curve, in which a region where the fluorescence increases exponentially was observed. The PCR cycle number at which the fluorescence crosses a threshold (CT value) can be related to the amount of starting templates. A standard curve plotting CT values against the logarithm of input DNA copy quantity (log attmoles) was constructed for each gene. The standard curves for all the genes analyzed were linear over a range of at least 0.00096–29.29.4 attmoles with a linear correlation coefficient > 0.987.
Data analysis
A relative standard curve method was used to quantify RGM mRNA at different times post-transection. NTC and DNA standards were run in the same plate with cDNA from injured spinal cord of each of the different sample regions (rostral, 10 mm caudal and caudal) at different times after injury. For each sample, the amount of target and endogenous reference was determined from the appropriate standard curve. The data were analyzed as described in “Relative Quantification Getting Started Guide” (Applied Biosystems). Results were analyzed by two-way ANOVA with Bonferroni post-hoc tests using Prism, version 5 (GraphPad, USA). Experiments were performed four times with each assay ran in triplicate per experiment and the results are shown as the fold-difference in the level of normalized RGM mRNA expression in control spinal cord and after transection.
Lectin histochemistry
To identify microglial cells, wholemounts were labeled with GSA isolectin I-B4 after RGM-labeled cells were revealed colorimetrically by in situ hybridization. The spinal cord wholemounts were washed in PBS and incubated with Fluorescein-labeled GSL I–isolectin B4 (5μg/ml Griffonia (Bandeiraea) simplicifolia lectin I GSL I-B4, Vector), which is a specific marker for microglia and labels D-galactose residues that are expressed by both resting and activated microglial cells (Boya, et al., 1991, Streit, 1990, Streit and Kreutzberg, 1987). Each step was followed by washing three times for 10 min with PBS. Wholemounts were coverslipped in VECTASHIELD® Mounting Medium (Vector, USA), and observed under a fluorescence microscope. Control for lectin staining consisted of (a) treating tissue prior to staining with α-galactosidase from coffee beans (Sigma) at 1 u/ml in phosphate-citrate buffer (pH 5.1) for 2 hours at 37°C; and (b) incubating with GSA I-B4 in the presence of 0.1 M melibiose (6-0-α-D-galactopyranosyl-D-glucose) to saturate lectin binding sites and prevent interaction with sugars of tissue components.
Cell countingand determination of microglia numbers
The numbers of GSA I-B4 and RGM-positive GSA I-B4-labeled cells were counted in the transected lamprey spinal cord and in unmanipulated control lamprey at different time points and locations. This was achieved by capturing five (approximately 210 μm × 180 μm) adjacent fields of view in the area of the highest microglia density at a magnification of × 400 beginning approximately 0.5 mm caudal (or rostral) to the center of the site of transection of the spinal cord, and continuing counting at 1.0 mm and 5 mm. Five fields of view were captured from each distance from the transection site (0.5; 1 and 5 mm) at 2 or 4 weeks post-transection and evaluated in the spinal cord of each animal. Our preliminary observation indicated that observed numbers of GSA I-B4-labeled microglial cells and RGM–labeled microglial cells were similar in caudal and rostral parts of transected spinal cord (up to 20 mm from the transection site). Therefore, we averaged the cell counts from rostral and caudal parts and presented them as one total cell count.
Statistics
The statistics were performed using Prism, version 5 (GraphPad, USA) applying a one way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test to analyze changes in the number of GSA I-B4-labeled cells after 2 weeks and 4 weeks post-lesion. A two way ANOVA followed by Bonferroni post-test was applied to analyze the number of RGM-positive GSA I-B4-labeled cells at 0.5 mm, 1mm and 5mm from the transection site after 2 and 4 weeks post-lesion and to compare numbers of RGM-positive GSA I-B4-labeled cells at each time point. Results are presented in bar graphs as means ± standard deviation.
RESULTS
RGM detection by PCR
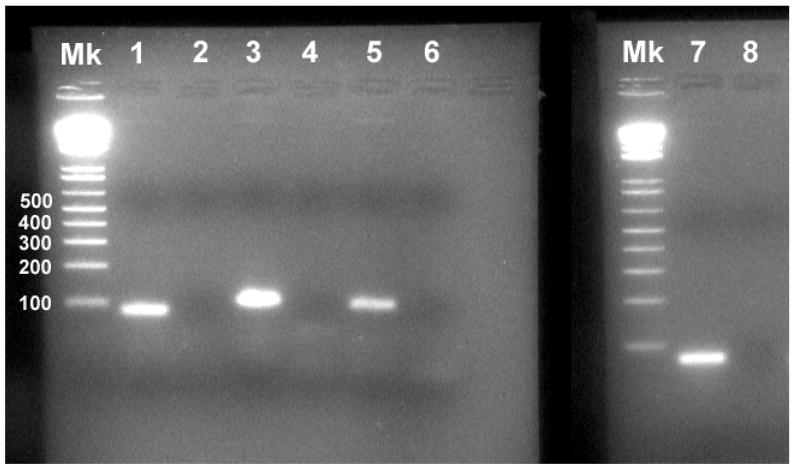
PCR using the primers for RGM and the lamprey versions of standard housekeeping genes yielded single bands of the expected sizes for all (Fig. 1). Of the housekeeping genes, GAPDH had the most constant expression in preliminary experiments (data not shown) and was selected as the control gene for quantitative RT-PCR of RGM.
Figure 1. PCR amplification of lamprey RGM cDNA.
Gel electrophoresis of PCR products from lamprey spinal cord cDNA yielded single bands at the expected sizes in base pairs (bp) for each target gene. No band was observed in the PCR products of the no template control (NTC). The RGM product was 96 bp, HPRT 1 was 68 bp, 18S rRNA was 75 bp and GAPDH was 72 bp. Molecular weight marker (Mk) was 1 Kb Plus (Invitrogen). Lanes are labeled as follows: #1 - 18S rRNA, #2 – NTC, #3 – RGM, #4 – NTC, #5 – GAPDH, #6 – NTC, #7 - HPRT 1, #8 – NTC.
Cellular localization of RGM mRNA expression in control spinal cord
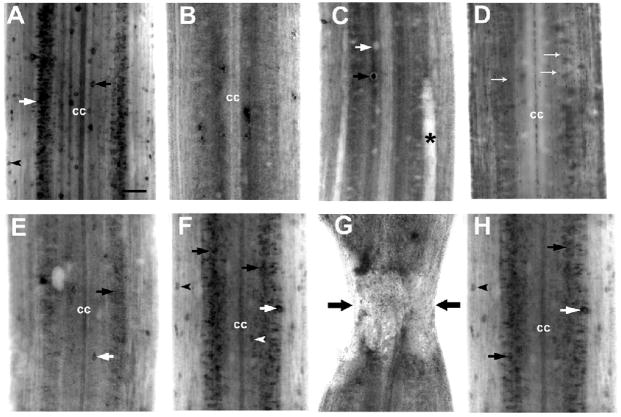
RGM mRNA was expressed throughout the spinal cord of control animals. Label was found in dorsal cells, edge cells and in medium sized neurons in the lateral grey matter (Fig. 2A).
Figure 2. Expression of RGM mRNA in lamprey spinal cord after spinal cord transection.
A, In situ hybridization in wholemount control spinal cord shows labeling for lamprey RGM in dorsal cells (black arrow), edge cells (black arrowhead) and in neurons of the spinal grey matter (white arrow). (cc) - central canal. B–F, RGM mRNA expression two weeks after spinal cord transection. Expression was strongly downregulated 500 μm rostral (B) and caudal (C) to the transection in neurons of the spinal gray matter. Only a few dorsal cells (black arrow) expressed RGM mRNA, while most dorsal cells stopped expressing it (the clear silhouette of a dorsal cell is indicated by the white arrow); asterisk – a swollen, degenerating Mauthner axon. D, Small cells (presumably microglia) throughout the spinal cord express RGM (white arrows). E, Expression increased with distance from the transection (10 mm caudal), but the intensity of RGM-specific in situ hybridization signal and the number of RGM-producing dorsal cells (white arrow) and neurons of the spinal grey matter (black arrow) were very small compared to control spinal cords. F, At distances more than 20 mm caudal to the lesion RGM mRNA expression patterns in neurons of the spinal grey matter (black arrows) resembled those in the uninjured control. Labeling was found primarily in dorsal cells (white arrowhead), edge cells (black arrowhead), lateral interneurons (white arrows) and neurons of the spinal gray matter (black arrows). G and H, RGM mRNA expression in spinal cord 1 month after transection. G, Absence of in situ hybridization signal for RGM in the transection site. H, Caudal to the transection (20 mm), RGM mRNA expression was present at reduced levels in medium sized neurons of the spinal grey matter (black arrows), in edge cells (black arrowhead) and lateral interneurons (white arrow). Scale bar, 100 μm (A).
RGM mRNA expression rostral and caudal to the transection
The spatiotemporal expression of RGM mRNA after complete spinal cord transection was analyzed by wholemount in situ hybridization at two and four weeks post-lesion. At 14 days, RGM mRNA expression was strongly downregulated in neurons of the spinal gray matter close to the transection site, both caudally and rostrally (Figs. 2B and C). Only a few dorsal cells continued to express RGM mRNA 0.5 mm caudal to the transection (Fig. 2C). Expression increased with distance from the transection, but the intensity of RGM-specific in situ hybridization signal and the number of RGM-expressing neurons were still noticeably reduced at 10 mm (Fig. 2E compared with Fig. 2A). The appearance of RGM expression patterns in the spinal cord region more than 20 mm from the lesion resembled those in uninjured controls (Fig. 2F). This labeling was found primarily in dorsal cells, edge cells and neurons of the spinal gray matter. In addition, many small cells (presumably microglia) throughout the spinal cord expressed RGM (Fig. 2D).
Four weeks after spinal cord injury, the transection site was easily identified in wholemount spinal cord preparations by the narrowing of the spinal cord and widening of the central canal (Fig. 2G). The transection zone was approximately 500 μm long, as described previously (Yin and Selzer, 1983). RGM mRNA expression was not detected at the lesion site (Fig. 2G) but at distances greater than 20 mm from transection, was present at reduced levels in medium sized neurons of the spinal gray matter, in dorsal cells and in lateral interneurons (Fig. 2H).
Identification of the non-neuronal RGM-expressing cell types in lamprey spinal cord after injury
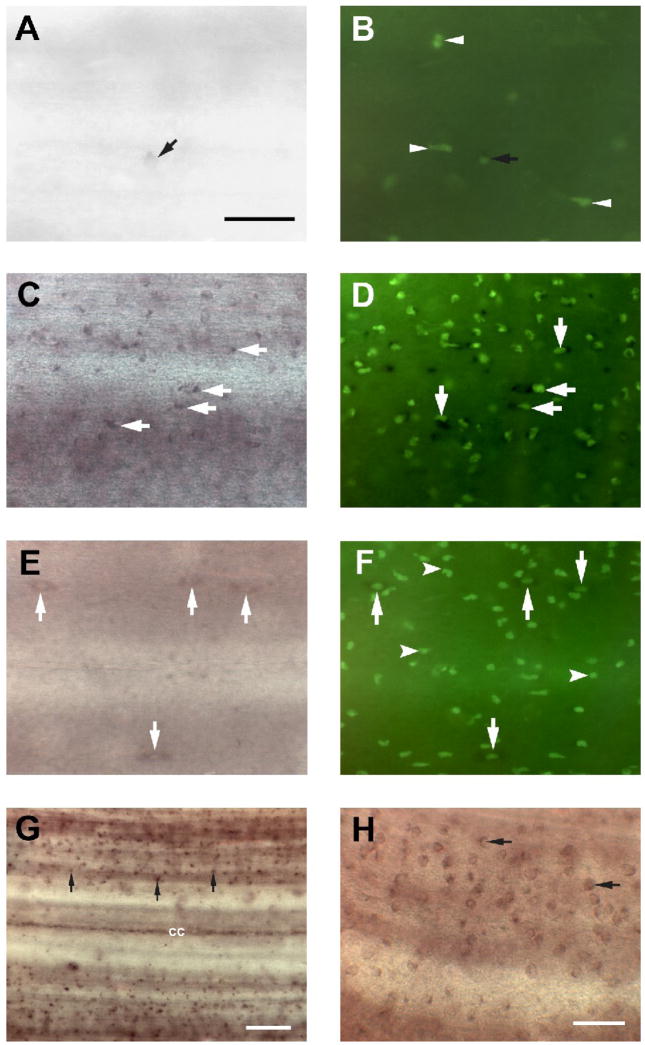
During our examination of RGM expression in the transected spinal cord, we detected populations of small, seemingly non-neuronal, cells that expressed RGM (Fig. 2D and Figs. 3C, G and H). By their sizes and shapes, they may be microglial cells (Boya, et al., 1991, Streit, 1990, Streit and Kreutzberg, 1987). They were located on the surface of the spinal cord (Fig. 3G), although some of them were located just below the cord surface.
Figure 3. Microglial activation after spinal cord transection includes RGM upregulation.
A, In situ hybridization in control spinal cord wholemount revealed the presence of small RGM-expressing cells (black arrow) on the surface of the spinal cord. Scale bar, 20 μm. B, Fluorescein-labeled GSL I–isolectin B4 histochemistry revealed several lectin-labeled cells in control spinal cord (white arrowheads) including RGM–expressing cells (black arrow). In controls, few isolectin B4-labeled profiles were RGM-positive. C, Two weeks after spinal cord transection, increased numbers of small, rounded cells (white arrows), reminiscent of activated microglia/macrophages, were labeled with the RGM probe, and a dense accumulation of reactive macrophages/microglial cells, as evidenced by increasing IB4 lectin reactivity, was seen (D). Most of the lectin-labeled cells co-expressed RGM mRNA (white arrows). E, Only a few RGM–expressing cells were detected in the spinal cord 1 month after injury. Some slender, elongated RGM-expressing cells (white arrows) resembled the macrophages/microglial cells seen in control spinal cord (Fig. 3A). F, At this time, numerous small, round macrophages/microglial cells (white arrowheads) could be detected by labeling with IB4 lectin in the spinal cord, but these were never labeled by the RGM probe. On the other hand, there were some larger, elongated RGM-expressing cells that also were labeled with IB4 lectin (white arrows). G and H, RGM-expressing cells exhibited very small rounded cell bodies (no larger than 5 μm) and possessed obvious non-neuronal morphology (black arrows). Scale bar, 20 μm(G, H).
Microglial cell activation after spinal cord transection
Fluorescein-labeled GSL I–isolectin B4, which is commonly used to identify macrophages/microglial cells in spinal cord and brain of mammals (Boya, et al., 1991, Streit, 1990, Streit and Kreutzberg, 1987), was used here to analyze the microglial response in the spinal cord 0.5–5 mm caudal and rostral to the lesion site at 14 days and 30 days after transection. To determine whether the RGM-labeled cells in the spinal cord are microglial cells, lectin histochemistry was performed after RGM in situ hybridization (Fig. 3A–H). In normal cord, few of the small, rounded RGM-expressing cells were detected on the surface of control spinal cord (Fig. 3A). These cells displayed morphological hallmarks of resting microglia (spindle shaped cell bodies and an elongated nucleus). Because these RGM-expressing cells were also labeled with IB4 lectin, (Fig. 3B), we identified these RGM-expressing cells as resting microglia. Moreover, several lectin-positive but RGM-negative cells were seen in control spinal cord, indicating that microglial cells might be present in control, untransected spinal cord (Fig. 3B). The specificity of the lectin histochemical staining was confirmed by the complete elimination of staining if the fluorescein-labeled GSL I–isolectin B4 was pre-incubated in the presence of 0.1 M melibiose, or if the tissue was treated with α-galactosidase from coffee beans (Sigma) prior to staining (data not shown).
Microglia expressing RGM mRNA increase after injury
Following injury, cellular elements of noticeably different shape were labeled in the spinal cord. A detailed analysis of changes in tissue adjacent to the injury is provided in Figure 3 and its legend. In the first two weeks post-transection, activated microglia were observed in the region rostral and caudal to the lesion center. These cells had very small rounded cell bodies and no cells larger than 5 μm or possessing obvious neuronal morphology were labeled with RGM (Fig. 3C, G and H). During this time, the number of RGM-expressing cells increased significantly 0.5–5 mm rostral and caudal to the injury site (Fig. 3C and Fig. 4B), accompanied by rapid accumulation of reactive microglial cells, as evidenced by increasing IB4 lectin reactivity (Fig. 3D and Fig. 4A). Most of the lectin-labeled cells also expressed RGM (Fig. 3D). Microglial RGM-expression was further examined 30 days after spinal cord transection. Although the localization, shapes and numbers of IB4 lectin-labeled microglial cells resembled those at 14 days (Figs. 3E, F and 4A), activated microglia that were also RGM-positive were largely absent from the spinal cord (Fig. 3E). RGM-expressing cells resembled the small spindle-shaped cells in control spinal cord (Fig. 3A and E) and because they were labeled by IB4 lectin they are likely to be resting microglial cells (Fig. 3F).
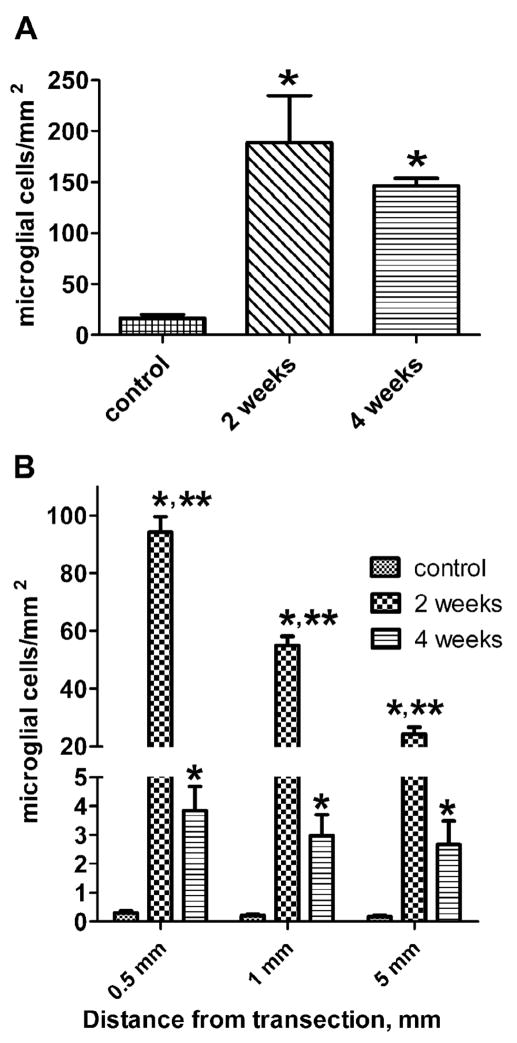
Figure 4. Microglial proliferation and RGM expression in response to spinal cord transection.
A, Increase in the number of fluorescein-labeled GSL I–isolectin B4 microglial cells in the spinal cord between 0.5 and 5 mm rostral and caudal to the lesion site at 14 and 30 days post-lesion. P-values are indicated as follows: * P < 0.05 compare to control. Differences between 14 days and 30 days numbers are not significant. Data are presented as mean with standard deviation and analyzed using a one way ANOVA followed by Tukey’s multiple comparison test. B, Temporal and spatial dependence of RGM expression in microglial cells post-transection. The number of RGM-expressing microglial cells at 2 and 4 weeks post-transection are compared between control and lesioned spinal cord 0.5, 1 and 5 mm from the transection. Control vs. 2 weeks: * P<0.001; control vs. 4 weeks: P>0.05, ns, not significant; 2 weeks vs. 4 weeks: ** P<0.001. Data are presented as means and standard deviations and analyzed using a two way ANOVA followed by a Bonferroni post-test.
Time course of increases in the numbers of RGM–expressing microglia/macrophages in the spinal cord post-transection
In lesioned spinal cords a significant accumulation of activated microglia was observed between 0.5 and 5 mm from the transection site at 14 days (p<0.05, mean=188.7/mm2, SEM= 46.3) and 30 days after injury (p<0.05, mean=146.5/mm2, SEM= 7.2) as compared to control tissue (mean=16.3/mm2, SEM=3.9; Fig. 4A). At 14 days post-transection, the proliferation of activated microglia contributed to an almost 12-fold increase (P<0.05) in total numbers of IB4 lectin-labeled cells, compared to control cords. At one month after injury, the numbers of IB4 lectin – stained microglia were still 9-fold greater than in controls cords.
In parallel with morphological signs of activation, reactive microglia appeared to upregulate RGM expression (Figs. 3C–F and 4B). A significant accumulation of RGM-expressing cells was seen two weeks after transection (Fig. 4B). This increase extended up to 10 mm caudal and rostral to the transection site but was not evident 20 mm from the transection. However, after 4 weeks of survival, the numbers of RGM-expressing cells decreased dramatically (Fig. 4B). The greatest numbers of these cells were detected close to transection site (0.5 mm), whereas at 1 mm and 5 mm from the transection cell counts decreased by almost 42% and 75% respectively (Fig. 4B). This uneven cell distribution was observed at 2 weeks but not at 4 weeks post-transection (Fig. 4B). However, the total numbers of activated microglia did not change significantly between 14 and 30 days after spinal cord transection (Fig. 4A).
RT-PCR determination of temporal and spatial changes in RGM mRNA expression after spinal cord transection
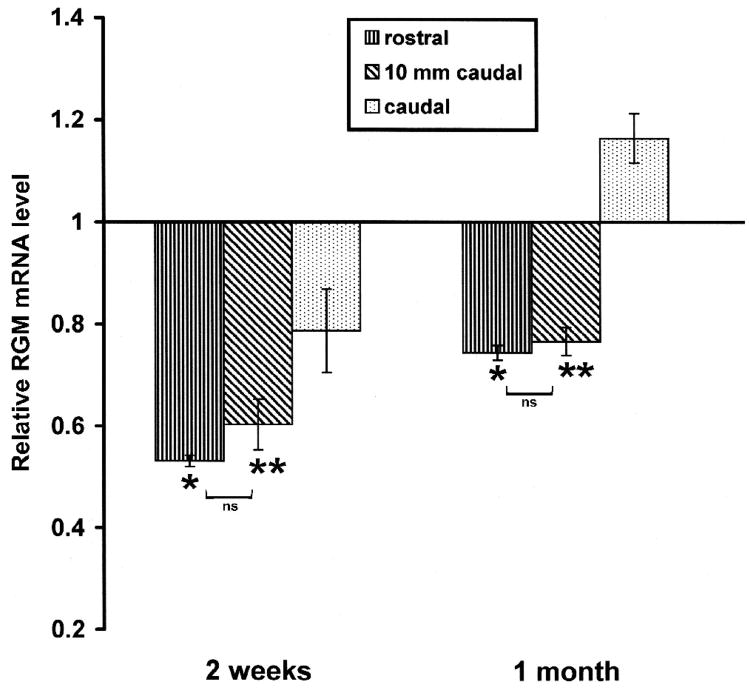
Our in situ expression data were confirmed by quantitative PCR, which showed downregulation of RGM expression 2 and 4 weeks after spinal transection. RGM mRNA was quantified at 2 weeks and 1 month after injury in segments of spinal cord representing the rostral part (including the injury epicenter), the first 10 mm caudal to the transection, and remainder of the cord caudal to it. Figure 5 shows the temporal changes in mRNA expression of RGM in these samples after transection. Two-way ANOVA indicated significant effects of both time and location after injury and of the interaction between these factors (location F=38.74; time F=225.68; interaction F=12.56. Significant effect with p < 0.001). In tissue rostral to the injury site, RGM mRNA was decreased by 47% at 2 weeks and 26% at 30 days. In tissue within 10 mm caudal to the transection, RGM mRNA decrease by 40% 2 weeks after injury and 24% at 30 days after injury. A different pattern was seen for the expression of RGM after transection in spinal cord more then 10 mm caudal to transection site. RGM expression was decreased by only 20% at 2 weeks post-transection and then was increased by 16% by 30 days.
Figure 5. Relative changes of mRNA expression of RGM post-transection in spinal cord rostral to the lesion (including the transection site), the first 10 mm caudal to the transection, and further caudal tissue.
The time course of relative changes of mRNA expression in spinal cord after injury was determined by quantitative real-time RT–PCR. Expression of RGM and GAPDH mRNA was measured in the presence of their respective primers and the PCR reaction was performed in triplicate. The level of RGM mRNA expression was calculated after normalizing against the GADPH mRNA level in each sample and is presented as the fold-difference in the level of RGM mRNA expression after transection compared with the untransected control sample, which was assigned a value of 1. Two-way ANOVA was used to compare experimental groups, with corrections for multiple post-hoc comparisons by the Bonferroni test. Non-significantly (ns) different: rostral vs 10 mm caudal - P >0.05; significantly different: * rostral vs caudal - P < 0.001; ** 10 mm caudal vs caudal - P < 0.01.
Changes in neogenin expression in control animals and after spinal cord transection
Neogenin is a member of the immunoglobulin superfamily of transmembrane receptors and a homologue of DCC, a netrin receptor that performs highly-conserved in vivo roles in cell migration and axon guidance. Like DCC, neogenin is a netrin receptor that is prominently expressed by differentiating neurons in the central nervous system. Recent experimental data suggested that neogenin bind RGM and act as it receptor mediating RGM signaling(Matsunaga and Chedotal, 2004, Yamashita, et al., 2007).
We used nonradioactive in situ hybridization to study neuronal expression of neogenin in lamprey brain neurons projecting to spinal cord. Neogenin mRNA was present most often in the cytoplasm of a several reticulospinal neurons, most prominently in the Mauthner neurons, B neurons of the bulbar region B1, B3 and B4 cells and in neurons of the isthmic region – I1 and I2 (Fig. 6A). Also two of mesencephalic neurons - M1 and M2 were labeled with neogenin probe (data not shown). Expression for any given neuron was variable from animal to animal and it was common to observe expression for neogenin in only one of a pair of neurons in an animal. There were virtually no other labeled cells. No labeling was observed when the sense RNA probe was used instead of the antisense probe.
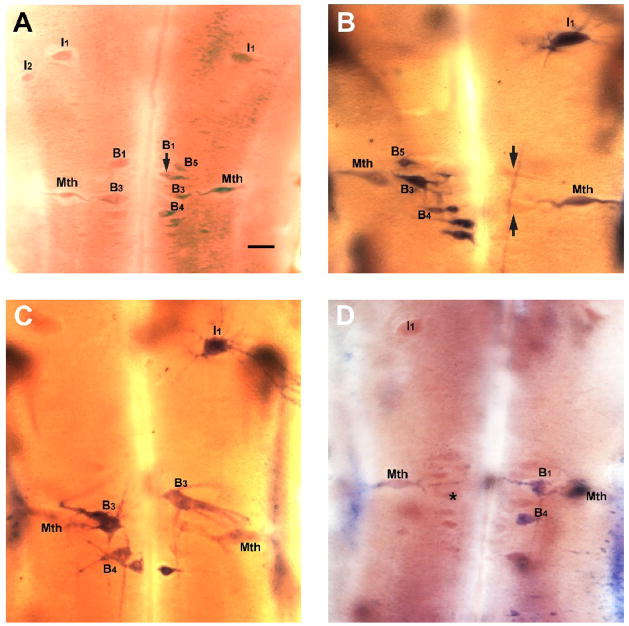
Figure 6. Expression of the RGM receptor neogenin in reticulospinal neurons after spinal cord transection.
A, In situ hybridization for neogenin in control animals. The rhombencephalon shows asymmetric neogenin expression in the Mauthner neurons (Mth), in the I1 and I2 neurons and in B neurons of the bulbar region (B1, B3, B4, B5). Scale bar – 100 μm. B, One week after transection, neogenin is expressed in the Mauthner neurons (Mth), I1 neuron and B neurons in the bulbar region. Note that neogenin expression was heterogeneous and was often stronger in one of a pair of cells or only one neuron was labeled. The silhouettes of several unlabeled B neurons are indicated by black arrows. C, At two weeks post-transection, neogenin is expressed in an I1 neuron and B neurons and very faintly in Mauthner neurons (Mth). D, At four weeks after transection, neogenin is expressed only in the B1 and B4 neurons. Note the shrinking Mauthner neurons leaving empty outlines around labeled neurons. The silhouette of an unlabeled B neuron is indicated by asterisk.
Changes in neogenin expression after spinal cord transection
In order to examine whether neogenin might be involved in the regenerative response of spinal projecting neurons, in situ hybridization for neogenin was performed at one, two and four weeks post-transection, consistent with the times when axons are dying back (first two weeks) and then regenerating through the proximal stump, respectively. The numbers of neogenin–expressing neurons did not change significantly at one, two and four weeks post-transection (Chi-square = 6.238, P = 0.1006, p> 0.05). As with expression in control animals, the level of receptor expression post-transection was variable, i.e. among cell types there was some degree of variability in expression levels at different times. However, beginning at four weeks post-transection, there was an apparent reduction in the intensity of staining of neogenin-expressing cells (Fig. 6D). Neurons were pale and appeared to be shrinking, which may indicate some degenerative processes or incipient neuronal death.
The expression of neogenin appeared to be limited to the Mauthner cell and a few other neurons that are known to regenerate poorly, specifically I1, B1 B3 and B4 (Jacobs, et al., 1997). On the other hand, some neurons that are known to regenerate well, such as the I3–I5, the auxiliary Mauthner, B2, B5 and B6 (Jacobs, et al., 1997) never showed neogenin message expression.
DISCUSSION
The present study demonstrates that the spinal cord of the large larval (4–5 years old) lamprey contains many cells that produce RGM, the expression of which is downregulated near the lesion after spinal cord transection. By contrast, RGM was up-regulated at the lesion site after spinal cord injury in rats (Hata, et al., 2006, Kyoto, et al., 2007, Schwab, et al., 2005). Since spinal-projecting axons regenerate in lampreys but not in mammals, RGM might play a role in inhibiting axonal regeneration after CNS injury, and thus posttraumatic downregulation of RGM expression in the lamprey spinal cord could be one reason why some of its axons regenerate. Interestingly, while there was little effect of transection on RGM expression remote (more than 20 mm) from the transection site, expression was decreased dramatically in neurons close to the lesion at 2 and 4 weeks post-transection, the period of most intense regeneration, when axons are growing back toward the transection site after initial retraction (Lurie and Selzer, 1991, Rovainen, 1976, Yin and Selzer, 1983).
Axotomy downregulates RGM expression in neurons
Some effects of spinal cord transection on expression of RGM could be explained as direct consequences of axotomy, e.g., the almost complete loss of RGM mRNA expression from the dorsal cells close to the transection site. These cells strongly expressed RGM in control animals. Dorsal cells are primary sensory neurons arrayed in two distinct rows on either side of the midline (Rovainen, 1967, Rovainen, 1979). Most project at least as far as the rostral spinal cord (Tang and Selzer, 1979) and are thus axotomized by the high-level spinal cord transection in the present study. Corresponding morphological and electrophysiological changes described previously in axotomized dorsal cells were maximal at 3 weeks and, like the loss of RGM expression, the intensities of those changes were also inversely related to the distance of the cell from the transection (Yin, et al., 1981).
The roles of RGM in embryonic development have been well described (Matsunaga and Chedotal, 2004) but their functions in the postnatal CNS are less clear. In mammals, the RGM receptor, neogenin displays sustained expression in adulthood (Keeling, et al., 1997, Meyerhardt, et al., 1997), when it might function in the maintenance of established connections by restricting spontaneous or aberrant axon sprouting. Several recent reports have described modulation of RGM expression by injury. Up-regulation of RGM at the lesion site after spinal cord injury was detected in rats neurons, neurites, blood-borne cells infiltrating the lesion, activated microglia/macrophages and some reactive astrocytes in areas of ongoing scar and oedema formation (Hata, et al., 2006, Kyoto, et al., 2007, Schwab, et al., 2005). Inhibition of RGM by a blocking antibody facilitated axon re-growth and improved locomotor behavior after spinal hemisection in adult rats (Hata, et al., 2006).
Reactive microglia upregulate RGM
The present findings suggest that in lamprey spinal cord, microglia are activated and increase in numbers for several weeks post-transection. In the normal adult CNS, microglia are distributed throughout the neural parenchyma and constitute a population of cells that proliferate and turn over slowly (Lawson, et al., 1992). Microglia are activated swiftly after spinal cord injury (Popovich, et al., 1997, Sroga, et al., 2003). Reactive microgliosis induced by acute neural injury includes massive, but usually transient expansion of the microglial cell population, induction of a wide range of myeloid markers, trophic factors, cytokines, free radicals and nitric oxide, and the acquisition of a phagocytic phenotype (Ladeby, et al., 2005, Streit, 1994, Streit, 2002). Macrophages/microglial cells may have important functions in axonal regeneration by secreting growth-inhibiting and/or -promoting molecules (Sandvig, et al., 2004, Streit, 1994, Streit, 1996, Streit, 2002, Streit, 2002).
In the present study, a marked increase in isolectin-B4 labeled cells was detected 14 day after a lesion and continued for at least another two weeks. Concurrently, expression of RGM mRNA in reactive microglial cells reached a maximum at 14 days post-injury and returned to pre-transection levels by 30 days. Our findings are in agreement with previous reports describing increased numbers of RGM-expressing macrophages/microglia after spinal cord injury in rodents and man (Hata, et al., 2006, Schwab, et al., 2005). However, in the present study, the reversal of RGM upregulation long before regeneration is complete, and the limitation of the reactive microgliosis to the surface of the cord, raise the question of how microglial expression of this GPI membrane-bound protein could be affecting regeneration axons located far from spinal cord surface. The lamprey glial scar differs from that in mammals. It consists of enlarged processes of glial cells whose cell bodies are located mainly in the proximal and distal stumps. There are no neuronal perikarya and relatively few glial cell bodies within the scar, except for the glial/ependymal cells reconstituting the enlarged central canal (Lurie and Selzer, 1991, Lurie and Selzer, 1991). Unlike the scar in lesioned mammalian spinal cord, which contains RGM-, Sema3- and/or netrin-expressing cells (Hata, et al., 2006, Pasterkamp, et al., 1999, Schwab, et al., 2005, Wehrle, et al., 2005), the transection scar in the lamprey spinal cord contained no cells that are positive for any of these chemorepulsive guidance molecules. This may partially explain previous observations that lamprey spinal axons grow preferentially through a hemisection scar rather than around it (Lurie and Selzer, 1991).
The in situ hybridization and quantitative real-time PCR experiments showed an overall reduction of RGM expression 2 and 4 weeks after injury in spinal cord areas close to the transection. Thus RGM expression in microglial cells was not enough to compensate for almost total disappearance of RGM–producing spinal cord neurons.
Possible role of RGM in regeneration
Axon regeneration in the lamprey spinal cord is incomplete (Lurie and Selzer, 1991, Selzer, 1978, Yin and Selzer, 1983). Fewer than 50% of severed axons grow into the distal stump within 10–12 weeks post-transection, and most of those grow less than 1 cm beyond the transection site (Lurie and Selzer, 1991, Lurie and Selzer, 1991, Rovainen, 1976, Yin and Selzer, 1983). The presence of chemorepulsive axonal guidance molecules RGM (this article) and Sema3 and Sema4 in lamprey spinal cord (Shifman and Selzer, 2006, Shifman and Selzer, 2007) and the down-regulation of netrin (Shifman and Selzer, 2007), which in some cases can act as a chemoattractant, could contribute to the regenerative failure of some lamprey axons after spinal cord transection however, further experiments will be needed to elucidate RGM role in spinal cord regeneration.
Acknowledgments
We thank Dr. Farida Shaheen, PhD for help with quantitative PCR. This work was supported by NIH grant R01 NS38537 to MES.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Aguayo AJ, Rasminsky M, Bray GM, Carbonetto S, McKerracher L, Villegas Pérez MP, Vidal Sanz M, Carter DA. Degenerative and regenerative responses of injured neurons in the central nervous system of adult mammals. Philos Trans R Soc Lond [Biol] 1991;331:337–343. doi: 10.1098/rstb.1991.0025. [DOI] [PubMed] [Google Scholar]
- 2.Boya J, Carbonell AL, Calvo JL, Borregon A. Microglial cells in the central nervous system of the rabbit and rat: cytochemical identification using two different lectins. Acta Anat (Basel) 1991;140:250–253. doi: 10.1159/000147064. [DOI] [PubMed] [Google Scholar]
- 3.Brittis PA, Flanagan JG. Nogo domains and a Nogo receptor: implications for axon regeneration. Neuron. 2001;30:11–14. doi: 10.1016/s0896-6273(01)00258-6. [DOI] [PubMed] [Google Scholar]
- 4.Bullock TH, Moore JK, Fields RD. Evolution of myelin sheaths: both lamprey and hagfish lack myelin. Neurosci Lett. 1984;48:145–148. doi: 10.1016/0304-3940(84)90010-7. [DOI] [PubMed] [Google Scholar]
- 5.Caroni P, Schwab ME. Two membrane protein fractions from rat central myelin with inhibitory properties for neurite growth and fibroblast spreading. J Cell Biol. 1988;106:1281–1288. doi: 10.1083/jcb.106.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.David S, Aguayo AJ. Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science. 1981;214:931–933. doi: 10.1126/science.6171034. [DOI] [PubMed] [Google Scholar]
- 7.Davis GR, McClellan AD. Extent and time course of restoration of descending brainstem projections in spinal cord-transected lamprey. J Comp Neurol. 1994;344:65–82. doi: 10.1002/cne.903440106. [DOI] [PubMed] [Google Scholar]
- 8.De Winter F, Oudega MLA, Hamers FP, Blits B, Ruitenberg MJ, Pasterkamp RJ, Gispen WH, Verhaagen J. Injury- induced class 3 semaphorin expression in the rat spinal cord. Exp Neurol. 2002;175:61–75. doi: 10.1006/exnr.2002.7884. [DOI] [PubMed] [Google Scholar]
- 9.Doya H, Ito T, Hata K, Fujitani M, Ohtori S, Saito-Watanabe T, Moriya H, Takahashi K, Kubo T, Yamashita T. Induction of repulsive guidance molecule in neurons following sciatic nerve injury. Journal of Chemical Neuroanatomy. 2006;32:74–77. doi: 10.1016/j.jchemneu.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Fawcett JW, Asher RA. The glial scar and central nervous system repair. Brain Res Bull. 1999;49:377–391. doi: 10.1016/s0361-9230(99)00072-6. [DOI] [PubMed] [Google Scholar]
- 11.Fitch MT, Silver J. Glial cell extracellular matrix: boundaries for axon growth in development and regeneration. Cell Tissue Res. 1997;290:379–384. doi: 10.1007/s004410050944. [DOI] [PubMed] [Google Scholar]
- 12.Galtrey CM, Fawcett JW. The role of chondroitin sulfate proteoglycans in regeneration and plasticity in the central nervous system. Brain Research Reviews. 2007;54:1–18. doi: 10.1016/j.brainresrev.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Hata K, Fujitani M, Yasuda Y, Doya H, Saito T, Yamagishi S, Mueller BK, Yamashita T. RGMa inhibition promotes axonal growth and recovery after spinal cord injury. J Cell Biol. 2006;173:47–58. doi: 10.1083/jcb.200508143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huber AB, Schwab ME. Nogo-A, a potent inhibitor of neurite outgrowth and regeneration. Biol Chem. 2000;381:407–419. doi: 10.1515/BC.2000.053. [DOI] [PubMed] [Google Scholar]
- 15.Jacobs AJ, Swain GP, Snedeker JA, Pijak DS, Gladstone LJ, Selzer ME. Recovery of neurofilament expression selectively in regenerating reticulospinal neurons. Journal of Neuroscience. 1997;17:5206–5220. doi: 10.1523/JNEUROSCI.17-13-05206.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keeling SL, Gad JM, Cooper HM. Mouse Neogenin, a DCC-like molecule, has four splice variants and is expressed widely in the adult mouse and during embryogenesis. Oncogene. 1997;15:691–700. doi: 10.1038/sj.onc.1201225. [DOI] [PubMed] [Google Scholar]
- 17.Kyoto A, Hata K, Yamashita T. Synapse formation of the cortico-spinal axons is enhanced by RGMa inhibition after spinal cord injury. Brain Research. 2007;1186:74–86. doi: 10.1016/j.brainres.2007.10.038. [DOI] [PubMed] [Google Scholar]
- 18.Ladeby R, Wirenfeldt M, Garcia-Ovejero D, Fenger C, Dissing-Olesen L, Dalmau I, Finsen B. Microglial cell population dynamics in the injured adult central nervous system. Brain Research Reviews. 2005;48:196–206. doi: 10.1016/j.brainresrev.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 19.Lawson LJ, Perry VH, Gordon S. Turnover of resident microglia in the normal adult mouse brain. Neuroscience. 1992;48:405–415. doi: 10.1016/0306-4522(92)90500-2. [DOI] [PubMed] [Google Scholar]
- 20.Lurie DI, Selzer ME. Axonal regeneration in the adult lamprey spinal cord. J Comp Neurol. 1991;306:409–416. doi: 10.1002/cne.903060305. [DOI] [PubMed] [Google Scholar]
- 21.Lurie DI, Selzer ME. The need for cellular elements during axonal regeneration in the sea lamprey spinal cord. Exp Neurol. 1991;112:64–71. doi: 10.1016/0014-4886(91)90114-r. [DOI] [PubMed] [Google Scholar]
- 22.Lurie DI, Selzer ME. Preferential regeneration of spinal axons through the scar in hemisected lamprey spinal cord. J Comp Neurol. 1991;313:669–679. doi: 10.1002/cne.903130410. [DOI] [PubMed] [Google Scholar]
- 23.Manitt C, Wang D, Kennedy TE, Howland DR. Positioned to inhibit: Netrin-1 and netrin receptor expression after spinal cord injury. Journal of Neuroscience Research. 2006;84:1808–1820. doi: 10.1002/jnr.21070. [DOI] [PubMed] [Google Scholar]
- 24.Matsunaga E, Chedotal A. Repulsive guidance molecule/neogenin: a novel ligand-receptor system playing multiple roles in neural development. Development, Growth and Differentiation. 2004;46:481–486. doi: 10.1111/j.1440-169x.2004.00768.x. [DOI] [PubMed] [Google Scholar]
- 25.McKerracher L, David S, Jackson DL, Kottis V, Dunn RJ, Braun PE. Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron. 1994;13:805–811. doi: 10.1016/0896-6273(94)90247-x. [DOI] [PubMed] [Google Scholar]
- 26.Meyerhardt JA, Look AT, Bigner SH, Fearon ER. Identification and characterization of neogenin, a DCC-related gene. Oncogene. 1997;14:1129–1136. doi: 10.1038/sj.onc.1200935. [DOI] [PubMed] [Google Scholar]
- 27.Monnier PP. RGM is a repulsive guidance molecule for retinal axons. Nature. 2002;419:392–395. doi: 10.1038/nature01041. [DOI] [PubMed] [Google Scholar]
- 28.Moore S, Kennedy T. Axon guidance during development and regeneration. In: Selzer MECS, Cohen LG, Duncan PW, Gage FH, editors. Textbook of Neural Repair and Rehabilitation. Cambridge University Press; Cambridge, UK: 2006. pp. 326–345. [Google Scholar]
- 29.Moreau-Fauvarque C, Kumanogoh A, Camand E, Jaillard C, Barbin G, Boquet I, Love C, Jones EY, Kikutani H, Lubetzki C, Dusart I, Chedotal A. The transmembrane semaphorin Sema4D/CD100, an inhibitor of axonal growth, is expressed on oligodendrocytes and upregulated after CNS lesion. J Neurosci. 2003;23:9229–9239. doi: 10.1523/JNEUROSCI.23-27-09229.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pancer Z, Amemiya CT, Ehrhardt GR, Ceitlin J, Gartland GL, Cooper MD. Somatic diversification of variable lymphocyte receptors in the agnathan sea lamprey. Nature. 2004;430:174–180. doi: 10.1038/nature02740. [DOI] [PubMed] [Google Scholar]
- 31.Pasterkamp RJ, Giger RJ, Ruitenberg MJ, Holtmaat AJ, De Wit J, De Winter F, Verhaagen J. Expression of the gene encoding the chemorepellent semaphorin III is induced in the fibroblast component of neural scar tissue formed following injuries of adult but not neonatal CNS. Mol Cell Neurosci. 1999;13:143–166. doi: 10.1006/mcne.1999.0738. [DOI] [PubMed] [Google Scholar]
- 32.Pasterkamp RJ, Verhaagen J. Emerging roles for semaphorins in neural regeneration. Brain Research Reviews. 2001;35:36–54. doi: 10.1016/s0165-0173(00)00050-3. [DOI] [PubMed] [Google Scholar]
- 33.Popovich PG, Wei P, Stokes BT. Cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats. Journal of Comparative Neurology. 1997;377:443–464. doi: 10.1002/(sici)1096-9861(19970120)377:3<443::aid-cne10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 34.Qiu J, Cai D, Filbin MT. Glial inhibition of nerve regeneration in the mature mammalian CNS. Glia. 2000;29:166–174. [PubMed] [Google Scholar]
- 35.Rovainen CM. Physiological and anatomical studies on large neurons of central nervous system of the sea lamprey (Petromyzon marinus). II. Dorsal cells and giant interneurons. J Neurophysiol. 1967;30:1024–1042. doi: 10.1152/jn.1967.30.5.1024. [DOI] [PubMed] [Google Scholar]
- 36.Rovainen CM. Regeneration of Müller and Mauthner axons after spinal transection in larval lampreys. Journal of Comparative Neurology. 1976;168:545–554. doi: 10.1002/cne.901680407. [DOI] [PubMed] [Google Scholar]
- 37.Rovainen CM. Neurobiology of lampreys. Physiol Rev. 1979;59:1007–1077. doi: 10.1152/physrev.1979.59.4.1007. [DOI] [PubMed] [Google Scholar]
- 38.Sandvig A, Berry M, Barrett LB, Butt A, Logan A. Myelin-, reactive glia-, and scar-derived CNS axon growth inhibitors: Expression, receptor signaling, and correlation with axon regeneration. Glia. 2004;46:225–251. doi: 10.1002/glia.10315. [DOI] [PubMed] [Google Scholar]
- 39.Schwab JM, Conrad S, Monnier PP, Julien S, Mueller BK, Schluesener HJ. Spinal cord injury-induced lesional expression of the repulsive guidance molecule (RGM) European Journal of Neuroscience. 2005;21:1569–1576. doi: 10.1111/j.1460-9568.2005.03962.x. [DOI] [PubMed] [Google Scholar]
- 40.Schwab JM, Monnier PP, Schluesener HJ, Conrad S, Beschorner R, Chen L, Meyermann R, Mueller BK. Central Nervous System Injury-Induced Repulsive Guidance Molecule Expression in the Adult Human Brain. Arch Neurol. 2005;62:1561–1568. doi: 10.1001/archneur.62.10.1561. [DOI] [PubMed] [Google Scholar]
- 41.Selzer ME. Mechanisms of functional recovery and regeneration after spinal cord transection in larval sea lamprey. J Physiol (Lond) 1978;277:395–408. doi: 10.1113/jphysiol.1978.sp012280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shifman MI, Selzer ME. Expression of Netrin Receptor UNC-5 in Lamprey Brain; Modulation by Spinal Cord Transection. Neurorehabilitation and Neural Repair. 2000;14:49–58. doi: 10.1177/154596830001400106. [DOI] [PubMed] [Google Scholar]
- 43.Shifman MI, Selzer ME. In situ hybridization in wholemounted lamprey spinal cord: localization of netrin mRNA expression. Journal of Neuroscience Methods. 2000;104:19–25. doi: 10.1016/s0165-0270(00)00316-2. [DOI] [PubMed] [Google Scholar]
- 44.Shifman MI, Selzer ME. Semaphorins and their Receptors in Lamprey CNS: cloning, phylogenetic analysis and developmental changes during metamorphosis. J Comp Neurol. 2006;497:115–132. doi: 10.1002/cne.20990. [DOI] [PubMed] [Google Scholar]
- 45.Shifman MI, Selzer ME. Differential expression of Class 3 and 4 semaphorins and netrin in the lamprey spinal cord during regeneration. The Journal of Comparative Neurology. 2007;501:631–646. doi: 10.1002/cne.21283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Snow DM, Lemmon V, Carrino DA, Caplan AI, Silver J. Sulfated proteoglycans in astroglial barriers inhibit neurite outgrowth in vitro. Exp Neurol. 1990;109:111–130. doi: 10.1016/s0014-4886(05)80013-5. [DOI] [PubMed] [Google Scholar]
- 47.Sroga JM, Jones TB, Kigerl KA, McGaughy VM, Popovich PG. Rats and mice exhibit distinct inflammatory reactions after spinal cord injury. J Comp Neurol. 2003;462:223–240. doi: 10.1002/cne.10736. [DOI] [PubMed] [Google Scholar]
- 48.Stock DW, Whitt GS. Evidence from 18S ribosomal RNA sequences that lampreys and hagfishes form a natural group. Science. 1992;257:787–789. doi: 10.1126/science.1496398. [DOI] [PubMed] [Google Scholar]
- 49.Streit W. An improved staining method for rat microglial cells using the lectin from Griffonia simplicifolia (GSA I-B4) J Histochem Cytochem. 1990;38:1683–1686. doi: 10.1177/38.11.2212623. [DOI] [PubMed] [Google Scholar]
- 50.Streit WJ. The role of microglia in regeneration. Eur Arch Otorhinolaryngol. 1994:S69–70. doi: 10.1007/978-3-642-85090-5_19. [DOI] [PubMed] [Google Scholar]
- 51.Streit WJ. The role of microglia in brain injury. Neurotoxicology. 1996;17:671–678. [PubMed] [Google Scholar]
- 52.Streit WJ. Microglia and the response to brain injury. Ernst Schering Res Found Workshop. 2002:11–24. doi: 10.1007/978-3-662-05073-6_2. [DOI] [PubMed] [Google Scholar]
- 53.Streit WJ. Microglia as neuroprotective, immunocompetent cells of the CNS. Glia. 2002;40:133–139. doi: 10.1002/glia.10154. [DOI] [PubMed] [Google Scholar]
- 54.Streit WJ, Kreutzberg GW. Lectin binding by resting and reactive microglia. J Neurocytol. 1987;16:249–260. doi: 10.1007/BF01795308. [DOI] [PubMed] [Google Scholar]
- 55.Swain GP. PhD Dissertation. Northeastern University; 1989. CNS Regeneration and Behavioral Recovery Following Spinal Cord Transection in the Sea Lamprey Petromyzon Marinus. [Google Scholar]
- 56.Swain GP, Snedeker JA, Ayers J, Selzer ME. The cytoarchitecture of spinal-projecting neurons in the brain of the larval sea lamprey. Journal of Comparative Neurology. 1993;336:194–210. doi: 10.1002/cne.903360204. [DOI] [PubMed] [Google Scholar]
- 57.Tang D, Selzer ME. Projections of lamprey spinal neurons determined by the retrograde axonal transport of horseradish peroxidase. J Comp Neurol. 1979;188:629–645. doi: 10.1002/cne.901880408. [DOI] [PubMed] [Google Scholar]
- 58.Wehrle R, Camand E, Chedotal A, Sotelo C, Dusart I. Expression of netrin-1, slit-1 and slit-3 but not of slit-2 after cerebellar and spinal cord lesions. European Journal of Neuroscience. 2005;22:2134–2144. doi: 10.1111/j.1460-9568.2005.04419.x. [DOI] [PubMed] [Google Scholar]
- 59.Wilson NH, Key B. Neogenin interacts with RGMa and Netrin-1 to guide axons within the embryonic vertebrate forebrain. Developmental Biology. 2006;296:485–498. doi: 10.1016/j.ydbio.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 60.Wood MR, Cohen MJ. Synaptic regeneration in identified neurons of the lamprey spinal cords. Science. 1979;206:344–347. doi: 10.1126/science.482943. [DOI] [PubMed] [Google Scholar]
- 61.Yamashita T, Mueller BK, Hata K. Neogenin and repulsive guidance molecule signaling in the central nervous system. Current Opinion in Neurobiology. 2007;17:29–34. doi: 10.1016/j.conb.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 62.Yin HS, Selzer ME. Axonal regeneration in lamprey spinal cord. J Neurosci. 1983;3:1135–1144. doi: 10.1523/JNEUROSCI.03-06-01135.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yin HS, Wellerstein KK, Selzer ME. Effects of axotomy on lamprey spinal neurons. Experimental Neurology. 1981;73:750–761. doi: 10.1016/0014-4886(81)90210-7. [DOI] [PubMed] [Google Scholar]