Abstract
Arsenic is an established lung carcinogen, however, the carcinogenic mechanisms are currently under investigation. Phosphorylation of the epidermal growth factor receptor (EGFR) has been reported with arsenic exposure in bladder cells. EGFR is a tyrosine kinase transmembrane receptor that regulates important processes in carcinogenesis, including cell survival, cell cycle progression, tumor invasion, and angiogenesis. We investigated the mechanisms of EGFR pathway activation by levels of arsenic relevant to human exposure scenarios both in vitro using cultured lung epithelial cells, and in lung tumors samples from New England Lung Cancer Study participants. Toenail arsenic levels were used as an internal biomarker of arsenic exposure. Our in vitro data suggest that arsenic increases levels of the EGFR ligand, heparin binding-EGF, and activate EGFR phosphorylation in the lung. Downstream of EGFR, arsenic exposure increased pERK and cyclin D1 levels. These effects were inhibited by treatment of cultured cells with the EGFR tyrosine kinase inhibitor, Tarceva (erlotinib). In a consecutive series of human lung tumor specimens, pEGFR protein levels were higher in subjects with elevated toenail arsenic levels compared to those with low exposure (odds ratio adjusted for other factors, OR 4.1 (95% confidence interval 1.1–15.6) (p = 0.04). These data suggest that arsenic exposure may stimulate EGFR pathway activation in the lung. Moreover, the tumors that arise in arsenic-exposed individuals also exhibit signs of EGFR pathway dysregulation. Further work is needed to assess the clinical utility of targeting the EGFR pathway in subgroups of lung cancer patients who have been exposed to elevated levels of arsenic.
Keywords: epidermal growth factor, lung cancer, arsenic, cyclin D1, human
Arsenic is an established lung, skin and bladder carcinogen (IARC, 2004), however the precise mechanisms by which arsenic acts in these tissues are currently under investigation (Abernathy et al., 1999). Arsenic has been shown to potentiate the genotoxicity of other organic mutagen-carcinogens, particularly polycyclic aromatic hydrocarbons (PAHs) including benzo(a)pyrene (BAP) (ATSDR, 1999; Rossman, 2003). Rats treated with arsenic and BAP sustained 84% of their DNA adduct burden after 27 days, compared with only a 13% burden sustained for rats treated with BAP alone (Tran et al., 2002). Additionally, there is compelling evidence that arsenic carcinogenesis is synergistic with tobacco (IARC, 2004). Chronic arsenic exposure from consumption of contaminated drinking water has been associated with lung cancer risk in epidemiologic studies conducted in Taiwan (20+ mg/l × years), Chile (200–400 μg/l, 2–5μM), and Bangladesh (> 599 μg/l, 8μM) (reviewed in Navarro Silvera and Rohan, 2007). In Chile, subjects born during the years when Antofagasta city water levels were approximately 875 μg/l, 12μM and exposed in early childhood had a lung cancer standardized mortality ratio of 7.0 (95% confidence interval [CI] 5.4–8.9) (Smith et al., 2006). Primarily due to geologic sources of contamination, drinking water arsenic levels are above the current recommended maximum contaminant level of 10 μg/l in several areas of the United States including the Northeast and Southwest (Karagas et al., 2002; NRC, 2001). In these U.S. populations chronic consumption of arsenic-contaminated drinking water over a period of years has been associated with elevated risk of skin cancer and of bladder cancer among smokers (Karagas et al., 2001; Steinmaus et al., 2003; Karagas et al., 2004). While high levels of arsenic consumption are clearly associated with lung cancer risk and interact with smoking, the risk for exposed U.S. populations and the involvement of smoking remains understudied (Celik et al., 2008).
Lung cancer is the most common type of cancer worldwide, accounting for 12.6% of all incident cancer cases. Lung cancer accounted for more deaths than any other type of cancer in the United States in 2007. Of all cancer deaths, 31% were due to lung cancer in men and 26% were due to lung cancer in women (ACS, 2007). The overall five year survival rate is only 15%, and varies dramatically by stage (49% for localized, 16% for regional and 2% for distant) (Jemal et al., 2004). Unfortunately, distant disease is the most common stage at presentation (ACS, 2007). Tobacco smoking is responsible for the majority of lung cancer cases (87%) (ACS, 2007). Passive exposure to tobacco smoke is associated with a reported 20–25% increased risk of lung cancer (Travis et al., 2004). Since 15–20% of lung cancers in women and 5–10% of lung cancers in men occur in never smokers, it is also important to consider other etiologic factors and carcinogenic mechanisms in nonsmokers (Zaridze et al., 1998).
The mechanisms of arsenic carcinogenesis are an active area of investigation. At a biochemical level, arsenic can disrupt zinc finger containing protein structures (Witkiewicz-Kucharczyk and Bal, 2006) and binds to sulfhydryl groups and cysteinyl residues (Suzuki et al., 2008). Arsenic induces chromosomal abberations, aneupolidy, and micronuclei formation, but does not directly interact with DNA and is not a strong mutagen (Rossman, 2003). Arsenic also induces formation of reactive oxygen and nitrogen species (Smith et al., 2001). The known biological effects of arsenic include endocrine disruption, altered DNA repair, altered cell signaling, altered cell cycle kinetics, and alterations in proliferative response (reviewed in Rossman, 2003).
The epidermal growth factor receptor (EGFR) is a tyrosine kinase transmembrane receptor in the ErB family of receptors expressed on the surface of epithelial cells (Kari et al. 2003). A previous study demonstrated EGFR activation in lung cells at very high levels of arsenite (500μM) (Wu et al. 1999). Further work has demonstrated that both sodium arsenite and monomethylarsonous acid (MMA(III)) also induced EGFR phosphorylation in bladder cell lines (Eblin et al., 2007; Simeonova et al., 2002). EGFR is overexpressed in lung tumors and precancerous lesions, and is known to induce tumor formation in animal studies. This overexpression occurs in many tumors without gene amplification, supporting the hypothesis that exposures can modify EGFR levels (Hilbe et al., 2003).
EGFR regulates important processes in carcinogenesis, including cell survival, cell cycle progression, tumor invasion, and angiogenesis. Ligands including EGF, transforming growth factor-α (TGF-α) and Amphiregulin (Areg) bind to EGFR activating signal transduction pathways that upregulate transcription factors leading to growth stimulation (Lin et al., 2001). The EGFR pathway mediates its effects by regulating the expression of a number of target genes (Kari et al., 2003). We hypothesize that some of the biologic effects of arsenic exposure may mechanistically involve modifying signaling through the EGFR pathway. Several lines of evidence indicate that cyclin D1 is a downstream regulator in the EGFR pathway (Reissmann et al., 1999; Lin et al., 2001; Moriuchi et al., 2001; Petty et al., 2004). EGFR tyrosine kinase inhibitors (EGFR-TKI) suppress cell growth, arrest cells in G1, and decrease cyclin D1 expression in cell cultures. Therapeutic concentrations of EGFR-TKI were associated with decreased cyclin D1 and tumor necrosis in EGFR-TKI responsive patients in a small lung clinical trial (Petty et al., 2004). Cell culture studies show that EGF stimulates Ras/MAPK activity and cyclin D1 expression (Moriuchi et al., 2001). EGFR contains a transactivation domain and can bind to AT-rich consensus sites, including those found in cyclin D1, and activate transcription (Lin et al., 2001). Chromatin immunoprecipitation assays demonstrated that nuclear EGFR is associated with the cyclin D1 promoter in vivo (Lin et al., 2001). Arsenic exposure is associated with elevated cyclin D1 levels in some, but not all cell culture models (Rossman et al., 2001; Souza et al., 2001; Vogt and Rossman, 2001; Wei et al., 2002).
Defects in cell cycle control may contribute to tumorigenesis and tumor progression by allowing cells to overcome cell cycle checkpoints. Cell cycle arrest normally occurs in response to a DNA lesion to allow repair of the damaged DNA and therefore, lack of arrest decreases repair efficiency and fidelity (Sancar et al., 2004). Overexpression of cyclin D1 results in more cyclin D1- cdk4 or 6 heterodimeric complexes that phosphorylate the growth suppressor pRb. Inactivation allows Rb to release its repression of the transcription factors E2F and DP allowing them to induce transcription of cyclin E and other genes that promote cell cycle progression (Fu et al., 2004).
Therapeutic efficacy with EGFR-TKIs that block EGFR activation is observed clinically and are a recent Food and Drug Administration (FDA)–approved treatment for non–small cell lung cancer (NSCLC) (reviewed in Herbst et al., 2004). Despite this, responsiveness to this class of drugs varies dramatically between patients, and reliable predictors of the most appropriate patient subgroups remain to be identified. Motivated by the availability of these EGFR pathway inhibitors, this study of lung epithelial cells investigates the mechanisms involved in EGFR pathway activation by arsenic exposure at contamination levels relevant to the U.S. population. We test the efficacy of EGFR inhibitors in blocking arsenic-induced pathway activation and then assess the relationship between EGFR alterations in human lung tumors and biomarker-based arsenic exposure status.
MATERIALS AND METHODS
Cell culture.
Human bronchial epithelial cells (Beas-2B, ATCC; Rockville, MD) were grown to postconfluence in 6- or 12-well plates (Corning Costar; Corning, NY) on VPM matrix, as previously described (Andrew and Barchowsky, 2000; Andrew et al., 2001a, b; Barchowsky et al., 2002). The cultures were maintained in LHC-9 medium (Biofluids Inc.; Rockville, MD) at 37°C under an atmosphere of 5% CO2/95% air. Cells were then switched to KBM medium without EGF (Clonetics; Cambrex Bio Science Walkersville, Inc., Walkersville, MD) 72 h before treatment. Cells were exposed to sodium arsenite (Sigma, St Louis, MO) (0.01–10μM, which is equivalent to 0.75–750 μg/l) for a period of 24 h before harvesting for RNA isolation or protein. Tarceva (erlotinib) was a kind gift from OSI pharmaceuticals (Farmingdale, NY). EGF (50 ng/ml) was used as a positive control (Sigma). Cycloheximide (CHX) (30 μg/ml) (Sigma) was used to inhibit protein synthesis.
Gene expression analysis.
RNA was harvested using Trizol reagent (Gibco/BRL, Life Technologies, Gaithersburg, MD) followed by DNase digestion using DNAfree (Ambion, Inc., Austin, TX) according to the manufacturer's instructions and quantitated by spectrophotometric absorbance at 260 nm. Real-time reverse transcription PCR (RT-PCR) was performed using gene specific primers and reagents (ABI, Foster City, CA) using the ABI PRISM sequence detection system and software. Briefly, total RNA (0.5 μg) was reverse transcribed using 100 U M-MLV reverse transcriptase in a mixture with oligo-dT and dNTPs according to the instructions provided with the Qiagen Omniscript kit (Qiagen; Valencia, CA). Samples were reverse transcribed in a MJ Research PTC-100 thermocycler (MJ Research, Inc., Watertown, MA) for 60 min at 44°C and the reaction terminated by heating to 95°C for 10 min. Expression of HBEGF (heparin-binding EGF-like growth factor; ABI, GenBank GeneID 1839), Cyclin D1 (GenBank GeneID 595), TGF-α (GenBank 7039), and amphiregulin (GenBank 374) were assessed by real-time PCR using 10 ng total RNA, 400nM primers, 200nM probe, and TaqMan Universal PCR Master Mix (ABI). The figures are representative of at least two experiments performed with n = 3 individual cultures. Relative quantitation was performed using a standard curve consisting of serial dilutions of pooled sample cDNA from the same source as the test RNA with each plate. Relative expression levels of each gene were normalized to 18s rRNA or GAPDH (ABI).
Protein levels.
The levels of EGFR, p-EGFR, and Cyclin D1 proteins were assessed by immunoblotting using sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) to resolve proteins from whole cell lysates. Each figure is representative of a minimum of two experiments performed with an n = 3 individual cultures. Cells were rinsed with ice cold stop buffer (10 mmol/l Tris-HCl, pH 7.4, 10 mmol/l ethylenediaminetetraacetic acid, 5 mmol/l EGTA, 100 mmol/l NaF, 200 mmol/l sucrose, 100 μmol/l Na-orthovanadate, 5 pyrophosphate, 4 μg/ml leupeptin, 4 μg/ml soybean trypsin inhibitor, 1 mmol/l benzamidine, 20 μmol/l calpain inhibitor 1, 100 mU/ml aprotinin, and 100 μmol/l phenylmethylsulphonylflouride). The stop buffer was then replaced with a minimal volume of 2× SDS-PAGE buffer (62.5mM Tris-HCl, pH 6.8, 10% glycerol, 2% SDS, 5% β-mercaptoethanol, 0.05% (wt/vol) bromophenol blue). The lysates were boiled for 5 min and clarified by centrifugation at 13,000 rpm for 10 min. Equal amounts of cell lysate were then resolved by electrophoresis on 8–12% SDS-polyacrylamide gels. Electrophoresis was performed at constant voltage (200 V), then the resolved proteins were transferred from the polyacrylamide gel to polyvinylidene difluoride membrane (PVDF, Immobilon-P, Millipore, Bedford, MA) by semi-dry transfer (Hoeffer Semiphor, San Fransisco, CA) for 1 h at constant current (32 mA/minigel) using transfer buffer (25mM Tris, 192mM glycine, 20% [vol/vol] methanol, 0.01% SDS). To eliminate nonspecific interactions of antibodies with the membrane, the PVDF membrane was blocked with TTBS (10mM Tris-HCl, pH 8.0, 150mM NaCl, 0.05% Tween-20) containing 5% milk (7.5 g/150 ml) for 1 h at room temperature or overnight at 4°C. Membranes were incubated with the primary EGFR, p-EGFR antibody specific to Tyr 1173, pSTAT1 (Tyr701) (Cell Signaling Technology, Danvers, MA) diluted 1:1000, or the primary Cyclin D1 antibody diluted 1:500, the pAKT (Ser473), or the pERK 1/2 (Thr202/Tyr204) antibodies diluted 1:1000 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) in TTBS overnight at 4°C. B-actin was used as a loading control diluted 1:100,000 in TTBS for 1 h (Sigma). The membranes were washed three times with TTBS. The EGFR and p-EGFR membranes were incubated with horseradish peroxidase (HRP)–linked goat anti-rabbit IgG (Santa Cruz Biotechnology, Inc.) 1:2000 in TTBS with 1% milk (0.3 g/30 ml) for 1 h at room temperature. The Cyclin D1 membrane was incubated with HRP-linked goat anti-mouse (Santa Cruz Biotechnology, Inc.) 1:3000 in TTBS with 1% milk (0.3 g/30 ml). The Secondary for B-actin was HRP-linked goat anti-mouse at 1:6000 (Bio-Rad Laboratories, Inc., Richmond, CA). After three washes with TTBS, protein bands were visualized by enhanced chemiluminescence using the Amersham ECL Plus Western Blotting Detection system (GE Healthcare, Piscataway, NJ) and film (Lumi-Film, Roche Molecular Biochemicals, Indianapolis, IN).
Lung tumors.
Lung cancer cases include individuals with histologically confirmed NSCLC, newly diagnosed between 1/1/05 and 12/1/06 who were 25–74 years of age and residents of the New England Lung Cancer Study area (ten contiguous counties of New Hampshire and Vermont) at the time of diagnosis. We obtained signed consent to access tumor specimens from these cases and received institutional human subjects approval. Immunohistochemistry was performed on a slide cut from a master lung cancer Tissue Microarray block containing tissue samples from 68 subjects using an antibody to p-EGFR or EGFR (Cell Signaling Technology, Danvers, MA). The intensity and percent of cells with membranous staining in each tumor was scored by the study pathologist, who was blinded to the arsenic exposure status of the subjects. For logistic regression analysis, positive p-EGFR staining was defined as more than 20% of cells staining at greater than 1+ intensity, which represented the top 10th percentile of staining. Toenail clipping samples collected at the time of interview were analyzed for arsenic (As, μg/g) by collision cell inductively coupled plasma mass spectrometry (ICP-MS) (7500c, Agilent, Santa Clara, CA). At the Dartmouth Trace Metals core facility. The detection limit for arsenic was approximately 0.003 μg/g.
Statistical analysis.
Statistical analysis for gene expression and immunoblotting was performed using the ANOVA procedure with Newman-Keuls post-test (Figs. 3–5) or unpaired, two-sided t-test (Fig. 6) in GraphPad PRISM (GraphPad Software, La Jolla, CA). We considered p values less than 0.05 to be statistically significant. We conducted logistic regression analyses for positive vs. negative p-EGFR staining lung tumors in relation to arsenic exposure. Analyses were adjusted for age, gender, tumor histology, and pack-years of smoking using SAS 9.1.3 (SAS Institute, Cary, NC).
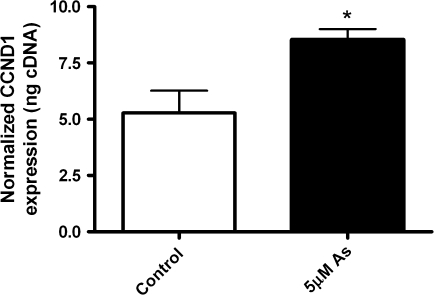
FIG. 3.
Cells were exposed to arsenic (5μM) for 3 h and Cyclin D1 (CCND1) gene expression was assessed using real-time PCR. Bars represent the mean and SD. *Statistically significant from control group, or from arsenic group (*p < 0.05).
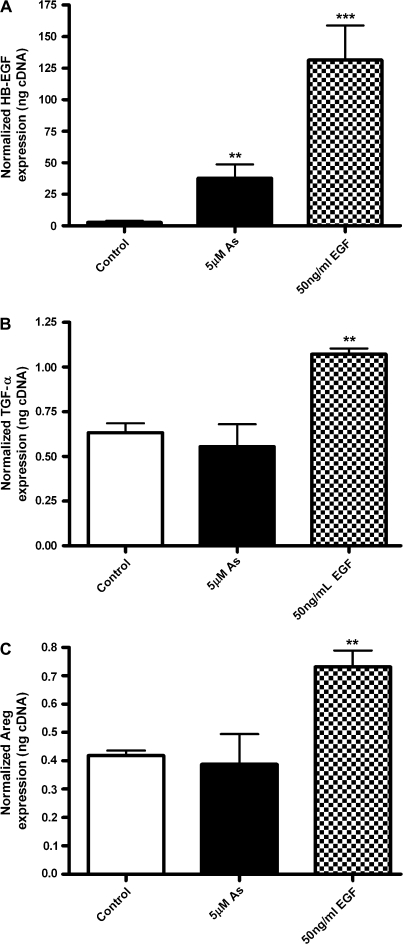
FIG. 4.
Arsenic induces HB-EGF, but not the other EGFR ligands TGF-α and Areg. Cells were exposed to arsenic (5μM) for 4 h. EGF (50 ng/ml) was used as a positive control. RNA was harvested and real-time PCR assays were performed for expression of (A) HB-EGF, (B) TGF-α, (C) amphiregulin (AREG). Bars represent the mean and SD. *Statistically significant from control group, or from arsenic group (*p < 0.05, **p < 0.01, ***p < 0.001).
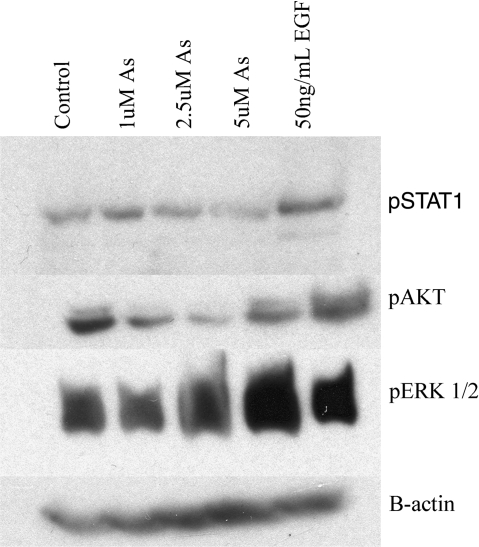
FIG. 5.
Arsenic induces phosphorylation of ERK and STAT, but not AKT. Cells were exposed to arsenic (1, 2.5, 5μM) for 5 h. The classical ligand EGF (50 ng/ml) was used as a positive control. Western blots were performed using antibodies to pSTAT1, p-ERK1/2, pAKT, or B-actin.
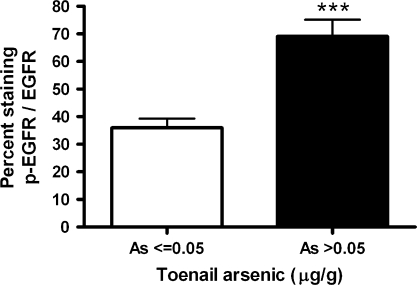
FIG. 6.
Toenail arsenic is associated with p-EGFR protein levels in human lung tumors. Immunohistochemical staining and scoring were performed on lung tumor tissue samples from 68 consenting cases using an antibody to p-EGFR or EGFR. The percent of cells staining with 1+ intensity was plotted by subject's toenail level of arsenic, as an internal biomarker of exposure (n = 68 subjects, upper 75th percentile As, ≤0.05 μg/g vs. > 0.05 μg/g). Bars represent the mean and standard error. Statistically significant difference between high and low arsenic exposure (*p = 0.04).
RESULTS
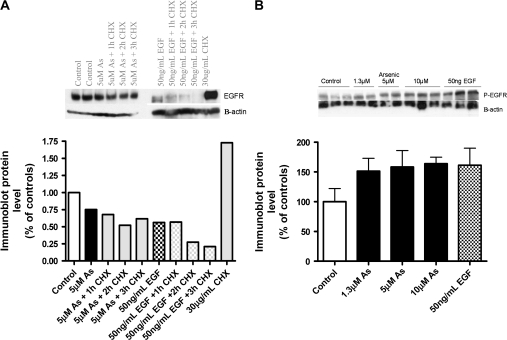
Our experiments consistently showed EGFR degradation following EGF, but not arsenic treatment (Figs. 1 and 2, row 1 EGFR). Treatment with the cycloheximide to inhibit protein synthesis further supported the observation that EGFR levels stay steady following arsenic exposure, while they degrade within the 3 h of EGF treatment. Protein synthesis inhibition alone (cycloheximide only) does not affect EGFR levels, suggesting that the receptor is not degraded unless activated by EGF (Fig. 1A). Figure 1B shows increased levels of the phosphorylated form of EGFR following treatment with arsenic (1.3, 5, and 10μM) or the positive control, EGF.
FIG. 1.
EGFR levels following arsenic or EGF exposure. (A) Cells were exposed to 5μM arsenic or EGF (50 ng/ml) 3 h, with or without the protein synthesis inhibitor CHX (30 μg/ml) for 1, 2, or 3 h. Immunoblots were performed using antibodies to EGFR and B-actin. Densitometry was performed to quantitate the protein levels. (B) Cells were exposed to 1, 5, 10 μM arsenic or EGF (50 ng/ml) for 3 h. Immunoblots were performed using antibodies to p-EGFR and B-actin. Densitometry was performed to quantitate the protein levels.
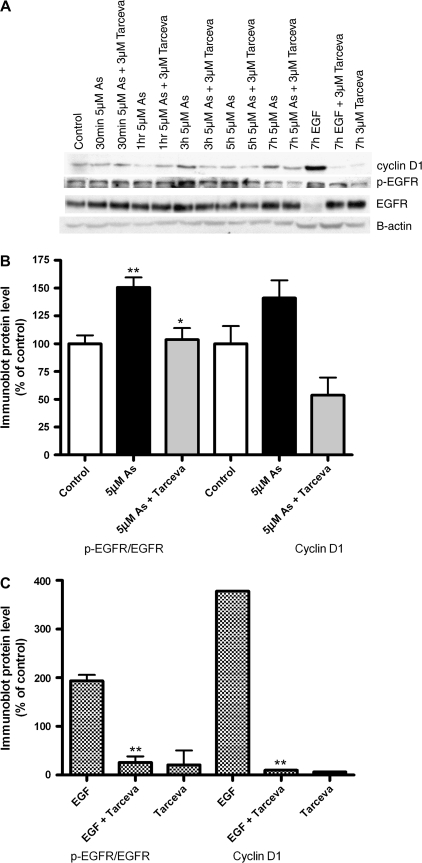
FIG. 2.
EGFR tyrosine kinase inhibition by Tarceva blocks arsenic induced p-EGFR and cyclin D1. (A) Cells were exposed to arsenic (5μM) for 30 min–5 h, with or without 3μM of the EGFR-TKI Tarceva (erlotinib). The classical ligand EGF (50 ng/ml) 7 h was used as a positive control. Immunoblots were performed using antibodies to EGFR, phospho-EGFR (p-EGFR), cyclin D1 (CCND1) and B-actin. (B) Densitometry was performed on this and other immunoblots (n = 3) to quantitate the protein levels at 3 h. Bars represent the mean and SD of the ratio of p-EGFR to EGFR, as well as Cyclin D1. (C) The ratio of p-EGFR to EGFR, as well as Cyclin D1 protein levels quantitated by densitometry for EGF treated samples. *Statistically significant from control group, or from arsenic group (*p < 0.05, **p < 0.01, ***p < 0.001).
Cells treated with 5μM sodium arsenite showed increased levels of EGFR phosphorylation (p-EGFR at Tyr 1173) at 3 and 5 h by immunoblot (Fig. 2, row 2, p-EGFR). The classical ligand, EGF induced EGFR phosphorylation and degradation of the receptor. We did not see changes in other phosphorylation sites in response to arsenic, including Tyr 992, 845, 1068 (data not shown). This effect was inhibited by pretreatment with the FDA-approved EGFR-TKI drug Tarceva at clinically achievable doses. The total level of EGFR protein in Beas-2B human bronchial epithelial cells was modestly increased following arsenic exposure (Fig. 2A). We observed a significant increase in EGFR mRNA levels with EGF, but not with arsenic exposure (data not shown).
We then assessed the effects of arsenic on the cell cycle regulator cyclin D1, since it can be regulated by the EGFR pathway (Lin et al., 2001). Levels of cyclin D1 protein were elevated following 3 h arsenic and EGF (Fig. 2A, row 1 CCND1). The positive control, EGF also induced a large increase in cyclin D1 levels. Pretreatment with the EGFR-TKI, Tarceva blocked the effects of arsenic (3 h) and EGF on cyclin D1. As reported previously, arsenic exposure increased cyclin D1 gene expression levels to those that were significantly higher than controls at 5μM for 3 h (Fig. 3).
We hypothesized that arsenic activates EGFR phosphorylation via one of its classical ligands. As shown in Figure 4A, arsenic induced the expression of the EGFR pro-ligand HB-EGF, as did EGF itself. Neither TGF-α (Fig. 4B), nor Areg (Fig. 4C) levels were modified by arsenic exposure at 4 h, yet they were induced by the positive control EGF.
We also investigated several possible signal transduction pathways immediately downstream from EGFR. We did not observe a dose-responsive increase in p-STAT1 with arsenic exposure. The positive control EGF did increase pSTAT1 levels (Fig. 5, row 1). Likewise, AKT was not significantly phosphorylated in response to either arsenic or EGF treatment, and actually decreased at the 1 and 2.5μM arsenic doses (Fig. 5, row 2). Arsenic exposure led to increased protein levels of p-ERK that were strongest at the 5μM dose (Fig. 5, row 3).
We then investigated the relation between arsenic exposure and EGFR pathway activation by immunohistochemistry in a consecutive series of human lung tumors. Figure 6 shows a significantly higher percent of cells staining for phospho-EGFR/EGFR in the human lung tumor specimens for subjects with elevated toenail arsenic concentration (as an internal biomarker of exposure) (n = 68, p = 0.04). Although EGFR levels were slightly elevated in the high arsenic group, the difference was not statistically significant. We also assessed the association between high levels of p-EGFR staining and arsenic exposure by logistic regression, with adjustment for tumor histology, AJCC stage classification, age, gender, and pack-years of smoking. Arsenic exposure (> 0.05 μg/g) was associated with higher p-EGFR staining OR 4.1 (95% CI 1.1–15.6) (based on upper 75th percentile, n = 22) in comparison to subjects with low exposure (As ≤ 0.05 μg/g, n = 46).
DISCUSSION
EGFR overexpression and pathway activation is observed in lung tumors and induces tumor formation in animal models. Some of these effects may be mediated through induction of the cell cycle promoter, cyclin D1 (Petty et al., 2003). Our data demonstrate for the first time that the EGFR pathway is activated in the human lung in response to the moderate levels of arsenic that are observed in contaminated U.S. drinking water. We used toenail arsenic levels as a long-term internal biomarker of exposure that is highly correlated with drinking water levels (Karagas et al., 2000). Our in vitro studies demonstrate that arsenic-induced phosphorylation of this receptor is prevented by pretreatment with the EGFR-TKI erlotinib/Tarceva, an FDA approved treatment for NSCLC.
Our data are consistent with the EGFR activation that was observed previously following very high level arsenic exposure (500μM) in a study of lung cells (Wu et al. 1999). Another study reported that arsenic (50μM) phosphorylated both EGFR and ERK in urothelial cells, however they did not see activation of the Tyr 1173 auto-phosphorylation site (Simeonova et al., 2002). These bladder cells also showed increased EGFR levels following long-term incubation with MMA(III) and EGFR phosphorylation within an hour of exposure (Eblin et al., 2007). Arsenic-exposed keratinocytes (200μM) showed AKT, but not EGFR activation (Souza et al., 2001), however, EGFR activation was observed in another keratinocyte study with either 100μM arsenite or 800μM arsenate (Tanaka-Kagawa et al. 2003). Arsenic trioxide (20μM) also increased p21 expression in A431 cells via activation of the EGFR/ERK pathway (Huang et al., 2006; Liu and Huang, 2006).
We observed that activation of EGFR signaling by the classical endogenous ligand, EGF, triggers rapid receptor degradation. In contrast, arsenic induced phosphorylation of the receptor at a more subtle level than EGF and the receptor was not degraded in response to activation by arsenic. Previous studies have documented that EGFRs are mono-ubiquinated soon after activation and transported to lysosomes for degradation (Lin et al., 2006; Shen et al., 2007). EGF-triggered receptor degradation is mediated by cbl, which recognizes EGFR phosphorylation and acts as the E3 ubiquitin ligase. The cdc42-associated tyrosine kinase ACK1, later colocalizes with EGFR following receptor phosphorylation on vesicles during endocytosis and acts as a ubiquitin-binding protein (Shen et al., 2007). Our phospho-specific EGFR antibody detected the phosporylated form of the receptor. Despite an increase in EGFR mRNA levels with EGF treatment, this rapid receptor protein degradation causes the total EGFR protein level in the cell to decrease. Consistent with our in vitro studies, the lungs of arsenic-exposed lung cancer patients showed slightly higher total levels of EGFR, while pEGFR levels were significantly higher than nonexposed patients.
Classical EGFR ligands include HB-EGF, TGF-α and Areg (Kari et al., 2003). Arsenic exposure increased HB-EGF levels, suggesting that this may be an upstream mediator of EGFR pathway activation. We did not observe any effect of arsenic on TGF-α or Areg. Identifying the specific downstream target genes that are regulated by the EGFR pathway is an area of active investigation. Consistent with previous studies in other cell types, both arsenic and EGF exposure increased p-ERK levels downstream of p-EGFR (Huang et al., 2006; Liu and Huang, 2006; Tanaka-Kagawa et al., 2003). These increases were inhibited by EGFR-TKI treatment. Several studies provide evidence that cyclin D1 is regulated by EGFR signaling (Lin et al., 2001; Moriuchi et al., 2001; Petty et al., 2004; Reissmann et al., 1999). Our data strongly support the hypothesis that arsenic increases cyclin D1 levels in part via EGFR pathway activation, and that this effect is inhibited by EGFR-TKI treatment. Previous studies have also suggested a role for Src in EGFR signaling to downstream target genes (Huang et al., 2006; Simeonova et al., 2002). Recent evidence also suggested that EGFR can regulate vascular endothelial growth factor (VEGF) expression via HIF-1α and Sp1. Although the hypothesized EGFR regulated genes, including VEGF, as well as p21 and HIF-1α were induced by arsenic, we did not observe EGFR-TKI inhibition of this effect (Huang et al., 2006; Liu and Huang, 2006; Pore et al., 2006).
Human plasma levels of the EGFR extracellular domain were significantly increased with arsenic exposure in a Bangladeshi population (Li et al., 2007). Likewise, we observed higher EGFR and p-EGFR protein levels in the lung tumor tissue of arsenic-exposed compared to nonexposed patients. Our in vitro work demonstrating a dose-responsive increase in total EGFR protein and mRNA levels further support this finding and suggest that arsenic-induced activation of EGFR pathway signaling in lung epithelial cells can be inhibited by treatment with EGFR-TKIs (Gschwind et al., 2004; Pao and Miller, 2005). The EGFR signaling in response to repeated drinking water arsenic exposure could lead to chronic EGFR pathway activation that could feed tumor development. This mechanism of carcinogenesis due to chronic pathway activation may be akin to that found in the lung tumors of individuals who have specific activating mutations in the tyrosine kinase domain of the EGFR (Paez et al., 2004; Pedersen et al., 2005). Additional studies are required to confirm this hypothesis.
Limitations of our lung cancer study include a small patient population and an observational study design. Nevertheless, EGFR tyrosine kinase inhibition has shown clinical utility in patient response and improved survival specifically among subsets of patients who have EGFR driven tumors (Clark et al., 2006). Data from the current study suggest that arsenic-associated lung tumors may also have EGFR pathway activation. Further work is needed to assess the clinical utility of targeting the EGFR pathway in subgroups of lung cancer patients who have been exposed to elevated levels of arsenic.
FUNDING
Grant numbers (CA099500, CA102327, P42 ES007373, P20 RR018787); the Institutional Development Award Program of the National Center for Research Resources, National Institutes of Health (NIH); the National Cancer Institute, NIH; and the National Institute of Environmental Health Sciences, NIH.
Acknowledgments
Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. We do not have any competing financial interests.
References
- Abernathy CO, Liu YP, Longfellow D, Aposhian HV, Beck B, Fowler B, Goyer R, Menzer R, Rossman T, Thompson C, et al. Arsenic: health effects, mechanisms of actions, and research issues. Environ. Health Perspect. 1999;107:593–597. doi: 10.1289/ehp.99107593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ACS. Lung Cancer (Non-Small Cell) 2007. Vol. 2007. American Cancer Society, Atlanta, GA. Available from: www.cancer.org. [Google Scholar]
- Andrew A, Barchowsky A. Nickel-induced plasminogen activator inhibitor-1 expression inhibits the fibrinolytic activity of human airway epithelial cells. Toxicol. Appl. Pharmacol. 2000;168:50–57. doi: 10.1006/taap.2000.9009. [DOI] [PubMed] [Google Scholar]
- Andrew AS, Klei LR, Barchowsky A. AP-1-dependent induction of plasminogen activator inhibitor-1 by nickel does not require reactive oxygen. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001a;281:L616–L623. doi: 10.1152/ajplung.2001.281.3.L616. [DOI] [PubMed] [Google Scholar]
- Andrew AS, Klei LR, Barchowsky A. Nickel requires hypoxia-inducible factor-1 alpha, not redox signaling, to induce plasminogen activator inhibitor-1. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001b;281:L607–L615. doi: 10.1152/ajplung.2001.281.3.L607. [DOI] [PubMed] [Google Scholar]
- ATSDR. Toxicological Profile for Arsenic. Atlanta, GA: Agency for Toxic Substances and Disease Registry (ATSDR); 1999. [PubMed] [Google Scholar]
- Barchowsky A, Soucy NV, O'Hara KA, Hwa J, Noreault TL, Andrew AS. Novel pathway for nickel-induced interleukin-8 expression. J Biol. Chem. 2002;277:24225–24231. doi: 10.1074/jbc.M202941200. [DOI] [PubMed] [Google Scholar]
- Celik I, Gallicchio L, Boyd K, Lam TK, Matanoski G, Tao X, Shiels M, Hammond E, Chen L, Robinson KA, et al. Arsenic in drinking water and lung cancer: A systematic review. Environ. Res. 2008;108:48–55. doi: 10.1016/j.envres.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Cheng T, Morris J, Koirtyohann S, Spate V, Baskett C. Study of the correlation of trace elements in carpenter's toenails. J. Radioanal. Cucl. Chem. 1995;195:31–42. [Google Scholar]
- Clark GM, Zborowski DM, Santabarbara P, Ding K, Whitehead M, Seymour L, Shepherd FA. Smoking history and epidermal growth factor receptor expression as predictors of survival benefit from erlotinib for patients with non-small-cell lung cancer in the National Cancer Institute of Canada Clinical Trials Group Study BR.21. Clin. Lung Cancer. 2006;7:389–394. doi: 10.3816/clc.2006.n.022. [DOI] [PubMed] [Google Scholar]
- Eblin KE, Bredfeldt TG, Buffington S, Gandolfi AJ. Mitogenic signal transduction caused by monomethylarsonous acid in human bladder cells: Role in arsenic-induced carcinogenesis. Toxicol. Sci. 2007;95:321–30. doi: 10.1093/toxsci/kfl160. [DOI] [PubMed] [Google Scholar]
- Fu M, Wang C, Li Z, Sakamaki T, Pestell RG. Minireview: Cyclin D1: Normal and abnormal functions. Endocrinology. 2004;145:5439–5447. doi: 10.1210/en.2004-0959. [DOI] [PubMed] [Google Scholar]
- Gschwind A, Fischer OM, Ullrich A. The discovery of receptor tyrosine kinases: targets for cancer therapy. Nat. Rev. Cancer. 2004;4:361–370. doi: 10.1038/nrc1360. [DOI] [PubMed] [Google Scholar]
- Herbst RS, Fukuoka M, Baselga J. Gefitinib—A novel targeted approach to treating cancer. Nat. Rev. Cancer. 2004;4:956–965. doi: 10.1038/nrc1506. [DOI] [PubMed] [Google Scholar]
- Hilbe W, Dirnhofer S, Oberwasserlechner F, Eisterer W, Ammann K, Schmid T, Hilbe G, Thaler J, Woll E. Immunohistochemical typing of non-small cell lung cancer on cryostat sections: Correlation with clinical parameters and prognosis. J. Clin. Pathol. 2003;56:736–41. doi: 10.1136/jcp.56.10.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HS, Liu ZM, Ding L, Chang WC, Hsu PY, Wang SH, Chi CC, Chuang CH. Opposite effect of ERK1/2 and JNK on p53-independent p21(WAF1/CIP1) activation involved in the arsenic trioxide-induced human epidermoid carcinoma A431 cellular cytotoxicity. J. Biomed. Sci. 2006;13:113–125. doi: 10.1007/s11373-005-9040-z. [DOI] [PubMed] [Google Scholar]
- IARC. Some Drinking-Water Disinfectants and Contaminants, Including Arsenic. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. World Health Organization, International Agency for Research on Cancer Lyon, France: 2004. pp. 39–267. [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, Feuer EJ, Thun MJ. Cancer statistics, 2004. CA Cancer J. Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- Karagas MR, Stukel TA, Morris JS, Tosteson TD, Weiss JE, Spencer SK, Greenberg ER. Skin cancer risk in relation to toenail arsenic concentrations in a US population-based case-control study. Am. J. Epidemiol. 2001;153:559–565. doi: 10.1093/aje/153.6.559. [DOI] [PubMed] [Google Scholar]
- Karagas MR, Stukel TA, Tosteson TD. Assessment of cancer risk and environmental levels of arsenic in New Hampshire. Int. J. Hyg. Environ. Health. 2002;205:85–94. doi: 10.1078/1438-4639-00133. [DOI] [PubMed] [Google Scholar]
- Karagas MR, Tosteson TD, Blum J, Klaue B, Weiss JE, Stannard V, Spate V, Morris JS. Measurement of low levels of arsenic exposure: a comparison of water and toenail concentrations. Am. J. Epidemiol. 2000;152:84–90. doi: 10.1093/aje/152.1.84. [DOI] [PubMed] [Google Scholar]
- Karagas MR, Tosteson TD, Morris JS, Demidenko E, Mott LA, Heaney J, Schned A. Incidence of Transitional Cell Carcinoma of the Bladder and Arsenic Exposure in New Hampshire. Cancer Causes Control. 2004;15:465–472. doi: 10.1023/B:CACO.0000036452.55199.a3. [DOI] [PubMed] [Google Scholar]
- Kari C, Chan TO, Rocha de Quadros M, Rodeck U. Targeting the epidermal growth factor receptor in cancer: apoptosis takes center stage. Cancer Res. 2003;63:1–5. [PubMed] [Google Scholar]
- Li Y, Chen Y, Slavkovic V, Ahsan H, Parvez F, Graziano JH, Brandt-Rauf PW. Serum levels of the extracellular domain of the epidermal growth factor receptor in individuals exposed to arsenic in drinking water in Bangladesh. Biomarkers. 2007;12:256–265. doi: 10.1080/13547500601133939. [DOI] [PubMed] [Google Scholar]
- Lin Q, Yang W, Cerione RA. Measurement of epidermal growth factor receptor turnover and effects of Cdc42. Methods Enzymol. 2006;406:614–625. doi: 10.1016/S0076-6879(06)06048-4. [DOI] [PubMed] [Google Scholar]
- Lin SY, Makino K, Xia W, Matin A, Wen Y, Kwong KY, Bourguignon L, Hung MC. Nuclear localization of EGF receptor and its potential new role as a transcription factor. Nat. Cell Biol. 2001;3:802–808. doi: 10.1038/ncb0901-802. [DOI] [PubMed] [Google Scholar]
- Liu ZM, Huang HS. As(2)O(3)-induced c-Src/EGFR/ERK signaling is via Sp1 binding sites to stimulate p21(WAF1/CIP1) expression in human epidermoid carcinoma A431 cells. Cell Signal. 2006;18:244–255. doi: 10.1016/j.cellsig.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Moriuchi A, Hirono S, Ido A, Ochiai T, Nakama T, Uto H, Hori T, Hayashi K, Tsubouchi H. Additive and inhibitory effects of simultaneous treatment with growth factors on DNA synthesis through MAPK pathway and G1 cyclins in rat hepatocytes. Biochem. Biophys. Res. Commun. 2001;280:368–373. doi: 10.1006/bbrc.2000.4063. [DOI] [PubMed] [Google Scholar]
- Navarro Silvera SA, Rohan TE. Trace elements and cancer risk: a review of the epidemiologic evidence. Cancer Causes Control. 2007;18:7–27. doi: 10.1007/s10552-006-0057-z. [DOI] [PubMed] [Google Scholar]
- NRC. Arsenic in Drinking Water. Washington, DC: National Academy Press; 2001. [Google Scholar]
- Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- Pao W, Miller VA. Epidermal growth factor receptor mutations, small-molecule kinase inhibitors, and non-small-cell lung cancer: Current knowledge and future directions. J. Clin. Oncol. 2005;23:2556–2568. doi: 10.1200/JCO.2005.07.799. [DOI] [PubMed] [Google Scholar]
- Pedersen MW, Pedersen N, Damstrup L, Villingshoj M, Sonder SU, Rieneck K, Bovin LF, Spang-Thomsen M, Poulsen HS. Analysis of the epidermal growth factor receptor specific transcriptome: Effect of receptor expression level and an activating mutation. J. Cell Biochem. 2005;96:412–427. doi: 10.1002/jcb.20554. [DOI] [PubMed] [Google Scholar]
- Petty WJ, Dragnev KH, Dmitrovsky E. Cyclin D1 as a target for chemoprevention. Lung Cancer. 2003;41(Suppl. 1):S155–S161. doi: 10.1016/s0169-5002(03)00159-4. [DOI] [PubMed] [Google Scholar]
- Petty WJ, Dragnev KH, Memoli VA, Ma Y, Desai NB, Biddle A, Davis TH, Nugent WC, Memoli N, Hamilton M, et al. Epidermal growth factor receptor tyrosine kinase inhibition represses cyclin D1 in aerodigestive tract cancers. Clin. Cancer Res. 2004;10:7547–7554. doi: 10.1158/1078-0432.CCR-04-1169. [DOI] [PubMed] [Google Scholar]
- Pore N, Jiang Z, Gupta A, Cerniglia G, Kao GD, Maity A. EGFR tyrosine kinase inhibitors decrease VEGF expression by both hypoxia-inducible factor (HIF)-1-independent and HIF-1-dependent mechanisms. Cancer Res. 2006;66:3197–3204. doi: 10.1158/0008-5472.CAN-05-3090. [DOI] [PubMed] [Google Scholar]
- Reissmann PT, Koga H, Figlin RA, Holmes EC, Slamon DJ. Amplification and overexpression of the cyclin D1 and epidermal growth factor receptor genes in non-small-cell lung cancer. Lung Cancer Study Group. J. Cancer Res. Clin. Oncol. 1999;125:61–70. doi: 10.1007/s004320050243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman TG. Mechanism of arsenic carcinogenesis: an integrated approach. Mutat. Res. 2003;533:37–65. doi: 10.1016/j.mrfmmm.2003.07.009. [DOI] [PubMed] [Google Scholar]
- Rossman TG, Uddin AN, Burns FJ, Bosland MC. Arsenite is a cocarcinogen with solar ultraviolet radiation for mouse skin: an animal model for arsenic carcinogenesis. Toxicol. Appl. Pharmacol. 2001;176:64–71. doi: 10.1006/taap.2001.9277. [DOI] [PubMed] [Google Scholar]
- Sancar A, Lindsey-Boltz LA, Unsal-Kaccmaz K, Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu. Rev. Biochem. 2004;73:39–85. doi: 10.1146/annurev.biochem.73.011303.073723. [DOI] [PubMed] [Google Scholar]
- Shen F, Lin Q, Gu Y, Childress C, Yang W. Activated Cdc42-associated kinase 1 is a component of EGF receptor signaling complex and regulates EGF receptor degradation. Mol. Biol. Cell. 2007;18:732–742. doi: 10.1091/mbc.E06-02-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeonova PP, Wang S, Hulderman T, Luster MI. c-Src-dependent activation of the epidermal growth factor receptor and mitogen-activated protein kinase pathway by arsenic. Role in carcinogenesis. J. Biol. Chem. 2002;277:2945–2950. doi: 10.1074/jbc.M109136200. [DOI] [PubMed] [Google Scholar]
- Smith AH, Marshall G, Yuan Y, Ferreccio C, Liaw J, von Ehrenstein O, Steinmaus C, Bates MN, Selvin S. Increased mortality from lung cancer and bronchiectasis in young adults after exposure to arsenic in utero and in early childhood. Environ. Health Perspect. 2006;114:1293–1296. doi: 10.1289/ehp.8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KR, Klei LR, Barchowsky A. Arsenite stimulates plasma membrane NADPH oxidase in vascular endothelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001;280:L442–L449. doi: 10.1152/ajplung.2001.280.3.L442. [DOI] [PubMed] [Google Scholar]
- Souza K, Maddock DA, Zhang Q, Chen J, Chiu C, Mehta S, Wan Y. Arsenite activation of P13K/AKT cell survival pathway is mediated by p38 in cultured human keratinocytes. Mol. Med. 2001;7:767–772. [PMC free article] [PubMed] [Google Scholar]
- Steinmaus C, Yuan Y, Bates MN, Smith AH. Case-control study of bladder cancer and drinking water arsenic in the western United States. Am. J. Epidemiol. 2003;158:1193–1201. doi: 10.1093/aje/kwg281. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Naranmandura H, Hirano S, Suzuki KT. Theoretical calculations and reaction analysis on the interaction of pentavalent thioarsenicals with biorelevant thiol compounds. Chem. Res. Toxicol. 2008;21:550–553. doi: 10.1021/tx700346z. [DOI] [PubMed] [Google Scholar]
- Tanaka-Kagawa T, Hanioka N, Yoshida H, Jinno H, Ando M. Arsenite and arsenate activate extracellular signal-regulated kinases 1/2 by an epidermal growth factor receptor-mediated pathway in normal human keratinocytes. Br. J. Dermatol. 2003;149:1116–1127. doi: 10.1111/j.1365-2133.2003.05704.x. [DOI] [PubMed] [Google Scholar]
- Tran HP, Prakash AS, Barnard R, Chiswell B, Ng JC. Arsenic inhibits the repair of DNA damage induced by benzo(a)pyrene. Toxicol. Lett. 2002;133:59–67. doi: 10.1016/s0378-4274(02)00088-7. [DOI] [PubMed] [Google Scholar]
- Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC, editors. Pathology and Genetics of Tumors of the Lung, Pleura, Thymus and Heart. Lyon, France: IARC Press; 2004. [Google Scholar]
- Vogt BL, Rossman TG. Effects of arsenite on p53, p21 and cyclin D expression in normal human fibroblasts—A possible mechanism for arsenite's comutagenicity. Mutat. Res. 2001;478:159–168. doi: 10.1016/s0027-5107(01)00137-3. [DOI] [PubMed] [Google Scholar]
- Wei M, Wanibuchi H, Morimura K, Iwai S, Yoshida K, Endo G, Nakae D, Fukushima S. Carcinogenicity of dimethylarsinic acid in male F344 rats and genetic alterations in induced urinary bladder tumors. Carcinogenesis. 2002;23:1387–1397. doi: 10.1093/carcin/23.8.1387. [DOI] [PubMed] [Google Scholar]
- Witkiewicz-Kucharczyk A, Bal W. Damage of zinc fingers in DNA repair proteins, a novel molecular mechanism in carcinogenesis. Toxicol. Lett. 2006;162:29–42. doi: 10.1016/j.toxlet.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Wu W, Graves LM, Jaspers I, Devlin RB, Reed W, Samet JM. Activation of the EGF receptor signaling pathway in human airway epithelial cells exposed to metals. Am. J. Physiol. 1999;277:L924–L931. doi: 10.1152/ajplung.1999.277.5.L924. [DOI] [PubMed] [Google Scholar]
- Zaridze D, Maximovitch D, Zemlyanaya G, Aitakov ZN, Boffetta P. Exposure to environmental tobacco smoke and risk of lung cancer in non-smoking women from Moscow, Russia. Int. J. Cancer. 1998;75:335–338. doi: 10.1002/(sici)1097-0215(19980130)75:3<335::aid-ijc1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]