Abstract
Previous studies have demonstrated that the stress response induced by some drugs and chemicals contributes in a predictable way to alteration of particular immunological parameters in mice. It has not been determined if mice can become tolerant or habituated with regard to the stress response and consequent immunological effects. Addressing this issue was the purpose of the present study. Mice were dosed daily for 28 days with atrazine, ethanol, propanil, or subjected to restraint, which are known to induce neuroendocrine stress responses and thereby to alter several immunological parameters. On day 29, a blood sample was taken and the spleen was removed for analysis of cellular phenotypes, differential cell counts (for blood), and natural killer (NK) cell activity. Corticosterone concentration at various times after dosing (or restraint) was also measured. Comparison of these results with results from previous studies with a single acute exposure revealed that the corticosterone response was almost completely absent in mice treated with ethanol, reduced in mice treated with restraint and propanil, and for atrazine the response was the same as noted for acute exposure. In most cases, the changes in immunological parameters were consistent with expectations based on these corticosterone responses. However, in a few cases (e.g., NK cell activity), it was clear that there were effects not mediated by stress. These results indicate that the nature of the stressor determines whether mice become tolerant with regard to the stress response and consequent immunological effects. This finding has practical implications for safety testing in mice.
Keywords: stress, corticosterone, immunotoxicology, chronic, acute
Previous studies have demonstrated that the stress response induced by drugs and chemicals can be used to quantitatively predict changes in immunological parameters (Pruett and Fan, 2001; Pruett et al., 1999, 2000a,b, 2003; Schwab et al., 2005; Spies et al., 2006). For ethanol, it has been confirmed using other methods that all the effects evaluated, except suppression of natural killer (NK) cell activity (Weiss et al., 1996; Wu and Pruett, 1996, 1997), are mediated by corticosterone, which is increased due to the stress response caused by ethanol (Lolait et al., 2007). Thus, corticosterone was also used as an indicator of the magnitude of the stress response in the present study. The purpose of previous studies was to understand stress-mediated immunosuppression both mechanistically and quantitatively. The International Committee on Harmonization S8 Guidance Document specifies that regulators will not accept assertions that immunological effects are mediated by nonspecific stress responses, unless there is credible evidence for such a response (Hastings, 2005). These quantitative assessments of stress-mediated changes in selected immunological parameters along with evaluation of corticosterone in the serum or urine can provide such evidence (Pruett et al., 2008).
Considering these findings, it became important to determine if mice adapt to chemical-induced stress responses such that stress-induced immunosuppression would not occur following a typical 28-day exposure regimen. The working hypothesis tested here is that habituation or tolerance will diminish the stress response during the 28-day exposure resulting in smaller effects on some (but not all) immunological parameters than noted previously for acute exposure.
MATERIALS AND METHODS
Mice.
C57Bl/6 x C3H F1 mice (B6C3F1) female mice were used in this study so results could be compared with results from previous acute exposure experiments in which these mice were used. Females were selected for the original studies because male mice housed three or more per cage often fight, which causes unpredictable stress responses that could affect the immunological parameters under investigations. The mice were 8–12 weeks old when used in experiments and were allowed to recover from shipping stress for at least two weeks before use. Mice were housed in groups of five in a temperature and humidity controlled environment, and they were given food (Purina Lab Chow, Purina Mills, Inc., Richmond, IN) and tap water ad libitum. Mice were obtained from Charles River Labs through the National Cancer Institute's Animal Program. All procedures involving mice were conducted in accord with the National Institutes of Health Guide and the regulations of the Institutional Animal Care and Use Committee of Louisiana State University Health Sciences Center in Shreveport.
Stressors and administration of stressors.
Restraint stress was accomplished by restraining mice in a 50 ml conical centrifuge with a lengthwise slit to allow ventilation and allow the tail to be used to guide the mouse into the tube. The cap was replaced and the tubes were placed in the home cage for the indicated periods of time. Atrazine was administered intraperitoneally in corn oil, as in previous studies (Pruett et al., 2003). Ethanol was prepared as a 32% (wt/vol) solution in sterile purified water and administered by gavage, as in previous studies (Pruett et al., 2003). Propanil was administered intraperitoneally in corn oil, as in our previous study (Schwab et al., 2005). The rationale for using corn oil and intraperitoneal administration is that this is a vehicle and route commonly used in studies sponsored by the National Toxicology Program (Anonymous, 2006). Because such studies are used in risk assessment and the results of this study were designed to be useful in the risk assessment process, it seemed reasonable to use this vehicle and route. Blood samples for corticosterone analysis were obtained by decapitation 1, 2, and 4 h after administration of stressors. Later time points depended on previous results from a single acute exposure and were as follows: restraint, 9 h; ethanol, 12 h; propanil, 12 h; and atrazine 6 h. For analysis of immunological end points, a blood sample and the spleen were removed from each mouse 24 h after the last exposure to a stressor. The dosages used in the current 28-day study were within the range used in previous acute studies.
Corticosterone assay.
Mice were bled by decapitation within 3 min of the time that the cage was removed from the animal room. Serum was obtained, and corticosterone was quantified using a radioimmunoassay kit (Diagnostic Products Corp., Los Angeles, CA).
Immunological assays.
Immunological assessments were done on day 29, 1 day (24 h) after the last treatment with stressor. Organ weights were measured to the nearest mg, and single cell suspensions were prepared by pressing the spleen or thymus between the frosted ends of sterile microscope slides. Blood samples were collected in heparin microtainer tubes by retroorbital bleeding under halothane anesthesia. Blood smears were prepared and stained with DiffQuick stain. Differential counts were done manually. A 150-μl portion of anti-coagulated blood sample was labeled with fluorescent antibodies to allow flow cytometric determination of leukocyte phenotypes. For blood samples, the following antibodies were used: anti-pan NK phycoerythrin, anti-CD4-fluorescein isothiocyanate, anti-CD8-Cychrome, anti-B220-phycoerythrin, and anti-major histocompatibility complex (MHC) class II-fluorescein isothiocyanate (BD Pharmingen, San Diego, CA). The same antibodies were used to label splenic or thymic lymphocytes, which were prepared as noted above. To determine the percentage of B lymphocytes with MHC II expression, two color analysis was used, and a gate was set using B220 positive cells and only these cells were analyzed for MHC II. Results are reported as MHC II expression per B cell (expressed as percentage of control) to account for loss of MHC II mediated solely by loss of B lymphocytes. Thus, the values reported reflect decreases in MHC II expression per B cell, not just changes in B cell percentages.
NK cell activity was measured using a 51Cr release assay with Yac-1 target cells exactly as described in our previous study (Wu et al., 1994).
Statistical analysis.
For most end points, results are expressed as percent of naive control values to facilitate comparison of 28-day exposures in this study with acute exposures in previous studies. The absolute values for all parameters were similar in this study to those noted in previous studies (data not shown), but for most end points, plotting data normalized to naive control values yielded a clearer comparison between acute and chronic dosing than the use of absolute values. Comparison of means for the control and all dosage groups were determined using ANOVA followed by the Newman-Keuls post hoc test as implemented by Prism 4.0 software (GraphPad, San Diego, CA). Regression lines described by the data were calculated, and the differences between the slope and elevation for the lines derived from acute and chronic treatment of mice were determined using Prism 4.0 software. In cases in which the slope was substantially different, differences in elevation could not be calculated by this method, and this is indicated in the figures by noting only the difference in slope.
RESULTS
Corticosterone Response
The treatments used in this study have been previously shown to induce a stress response sufficient to significantly affect several immunological parameters 12–24 h after a single exposure (Pruett and Fan, 2001; Pruett et al., 1999, 2000a,b, 2003, 2005). The corticosterone response and immunological parameters were consistent in all of these studies, and data were obtained over a period of several years at two different locations. One experiment was done three times at two locations, and the data were essentially superimposable. In fact, the later papers in this series report the use of data obtained earlier to predict the effects of chemicals on immunological parameters on the basis of the area of the corticosterone concentration versus time curve. These predications were remarkably accurate, indicating that the corticosterone response to stressors and the immunological parameters assessed in this study provide data that are sufficiently consistent to allow comparison of previous results (using acute stressors) with current results (28-day exposure to stressors). The chemicals used in this study serve as surrogates for drugs and chemicals such as those that are routinely tested for immunotoxicity as part of safety testing mandated by the U.S. Environmental Protection Agency (EPA) and Food and Drug Administration. The EPA Guidance Document specifies a high dose near the maximum tolerated dose, and this is also commonly done in safety testing of drugs (Anonymous, 1998). Many chemicals induce stress responses at such dosages (Pruett et al., 1999), so evaluating the involvement these responses in immunological changes is necessary for appropriate interpretation of these types of results.
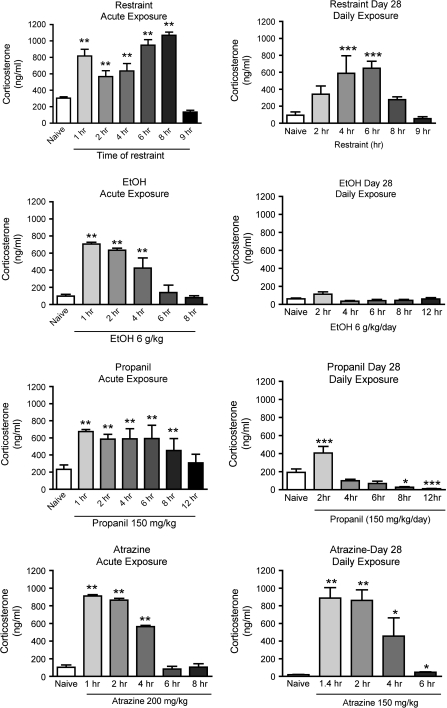
Results shown in Figure 1 indicate that 28-day atrazine exposure does not cause tolerance or habituation. The corticosterone response was very similar after the 28-day exposure as observed after a single exposure. In contrast, a 28-day exposure to ethanol completely abrogated the corticosterone response. Exposure to propanil for 28 days substantially decreased the corticosterone response, and exposure to restraint stress for 28 days only moderately decreased the corticosterone response. Thus, the corticosterone response after the 28-day exposure regimen was: atrazine >> restraint > propanil > ethanol.
FIG. 1.
Corticosterone concentrations in serum samples obtained following acute or 28-day exposure to stressors. Mice were bled at the indicated times after administration of restraint, ethanol (EtOH), propanil, or atrazine. Corticosterone was quantified by radioimmunoassay. In graphs labeled acute exposure, corticosterone was measured after the first administration of the stressor. Results for acute exposure were obtained in previous studies that have been published, and they are shown here to facilitate comparison with the effects of daily exposure. In the graphs labeled Day 28 Daily Exposure, samples were obtained from mice after the 28th daily administration of stressor. Values shown are means ± SEM (n = 5 mice/group). Values significantly different from naive (untreated) control mice are indicated by *p < 0.05, **p < 0.01, or ***p < 0.001.
White Blood Cell Count and Differential Blood Count
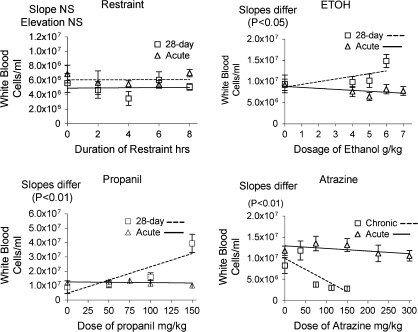
Restraint has little effect on white blood cell (WBC) counts following one exposure or after 28 daily exposures (Fig. 2). The effects of chronic and acute administration of propanil and ethanol suggest that the compounds per se cause an increase in WBC counts, which is not observed following acute exposure. These effects generally correspond to the effects of these agents on corticosterone responses, in that it would be expected that the higher concentrations of corticosterone following acute dosing would tend to counteract the tendency to increase WBC counts manifested following prolonged dosing. Atrazine suppresses WBC counts following both acute and 28-day exposures, which is consistent with its effects on corticosterone concentrations. However, the suppression is greater with 28-day exposure, possibly indicating cumulative effects.
FIG. 2.
WBC counts were counted using an electronic cell counter (Coulter Electronics, Hialeah, FL) 12 h after the last administration of stressor. Values shown were calculated in comparison to the mean of the control (naive) group (= 100%). Values for acute exposure were published previously (Schwab et al., 2005) and are shown here for comparison. A statistical routine implemented by Prism 4.0 software was used to determine if differences in either slope or intercept were significant. If the slope was significantly different for two lines, evaluation of elevation is excluded. Values shown are means ± SEM for groups of five mice each.
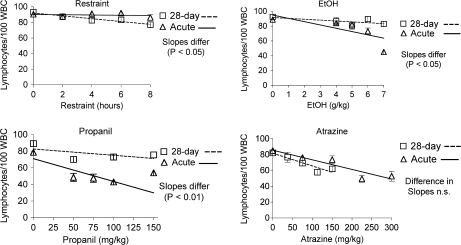
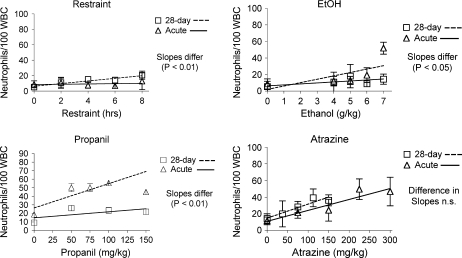
Differential blood leukocyte counts indicated that acute exposure to stressors tended to increase the percentage of neutrophils and decrease the percentage of lymphocytes in the blood (Figs. 3 and 4). The exception was restraint stress, for which acute exposure had no effect and chronic exposure tended to increase neutrophils and decrease lymphocytes. Even though mice became partially habituated to the corticosterone-inducing effects of restraint (Fig. 1), the effect of 28 days of daily restraint was slightly greater than the acute effect. However, both effects were considerably less than the effects of acute exposure to any of the other stressors. Interestingly, a similar pattern (28-day treatment caused greater effects than acute treatment) was associated with restraint with regard to some lymphocyte phenotypes (see the following section). The results for propanil and ethanol were consistent with the corticosterone response to these compounds (i.e., chronic treatment had less effect than acute). Thus, there was little effect following 28 days of exposure to propanil or ethanol, but atrazine caused essentially the same increase in neutrophils and decrease in lymphocytes after 28 doses as it did after 1 dose, which was consistent with its effects on corticosterone.
FIG. 3.
Differential blood lymphocyte counts. Differential counts were determined manually using stained blood smears and compared with previously published data for acute exposures (Schwab et al., 2005). Group size and statistical analysis were as noted in the legend for Figure 2.
FIG. 4.
Differential blood neutrophil counts. Differential counts were determined manually using stained blood smears and compared with previously published data for acute exposures (Schwab et al., 2005). Statistical methods and group sizes were as noted in the legend for Figure 2.
Lymphocyte Phenotypes in the Blood and Spleen
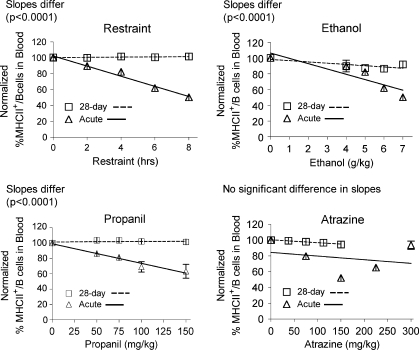
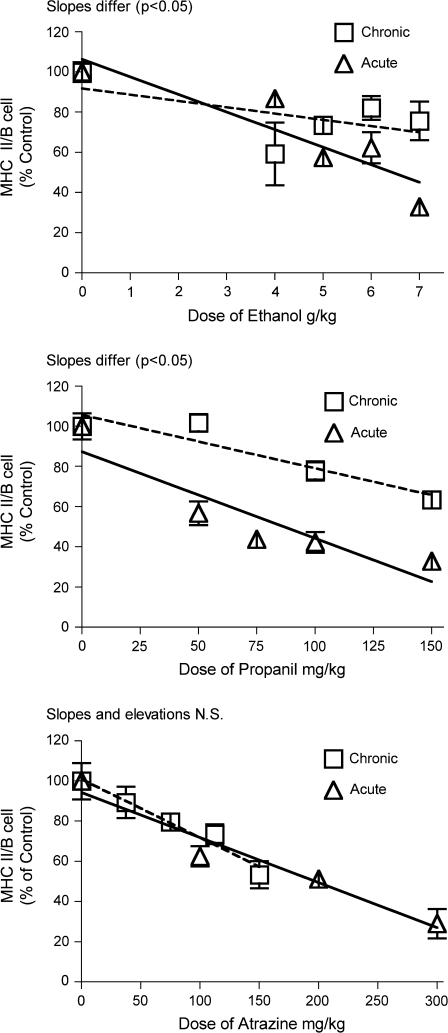
Flow cytometry was used to evaluate the percentage of lymphocytes with surface markers for particular cell types. The expression of MHC class II protein on B lymphocytes was evaluated for cells from the spleen and the blood. Previous studies indicated that the effect of acute stress on this parameter could be very accurately predicted based entirely on the overall corticosterone exposure (measured by the area under the concentration vs. time curve) for all stressors tested (Pruett et al., 2003; Schwab et al., 2005). Results from previous studies for acute exposures are shown here to facilitate comparison of acute and chronic effects. It should be emphasized that the parameters measured and the effects of stressors on them are remarkably robust (Pruett et al., 1999), suggesting that differences between results of acute and chronic exposure are unlikely to represent experimental or biological variability that is not related to the treatment regimen. The results for 28-day exposure were as expected based on corticosterone responses. Effects of 28-day treatments were less than the effects of a single treatment for restraint, ethanol, and propanil but about the same for atrazine. A similar pattern was observed for peripheral blood B cells and B cells in the spleen (Figs. 5 and 6). Results from a previous study demonstrate that the decreased percentage of MHC II high expressing B cells was not due to loss of B cells, but decreased MHC II expression on B cells (Pruett et al., 1999, 2003). It is also important to note that a previous study demonstrates that stress-induced inhibition of MHC II expression on B cells is mediated entirely by corticosterone (Weiss et al., 1996).
FIG. 5.
Percentage of MHC class II expression by B lymphocytes in the blood. Blood lymphocytes were identified by flow cytometry after staining with fluorescent-labeled antibodies specific for CD45RA (B220) and MHC class II. Values shown here are expressed as the percentage of MHC expression per B cell normalized so that the control group = 100%. Values for acute exposure were published previously and are shown here for comparison (Schwab et al., 2005). Group size and statistical analysis were as noted in the legend for Figure 2.
FIG. 6.
MHC class II expression on B lymphocytes in the spleen. Splenic lymphocytes were identified by flow cytometry after staining with fluorescent-labeled antibodies specific for CD45RA (B220) and MHC class II. Values shown here are expressed as the percentage of MHC expression per B cell normalized so that the control group = 100%. Values for acute exposure were published previously and are shown here for comparison (Pruett et al., 2003). Group size and statistical analysis were as noted in the legend for Figure 2.
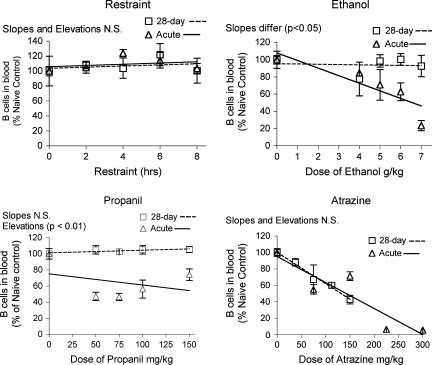
The percentage of B lymphocytes in the blood changed in a manner consistent with the observed changes in corticosterone for all three chemicals (Fig. 7). Exposure to ethanol and propanil for 28 days caused little or no decrease in the percentage of B cells in the blood, whereas acute exposure caused a substantial decrease. This was consistent with the near complete (ethanol) and partial (propanil) habituation caused by 28-day exposure, which was associated with decreased corticosterone responses. In contrast, atrazine caused virtually identical effects on B cell percentage in the blood following 28-day or acute treatment, as was the case for corticosterone responses as well. Restraint did not affect the percentage of B lymphocytes following acute or 28-day exposure. A similar pattern was observed in the spleen (Fig. 8).
FIG. 7.
Changes in the percentage of B lymphocytes in the blood. Blood lymphocytes were identified by flow cytometry after staining with fluorescent-labeled antibodies specific for CD45RA (B220). Values shown here are expressed as the percentage of B cells in blood normalized so that the control group = 100%. Values for acute exposure were published previously and are shown here for comparison (Schwab et al., 2005). Group size and statistical analysis are as described in the legend of Figure 2.
FIG. 8.
Changes in the percentage of B lymphocytes in the spleen. Splenic lymphocytes were identified by flow cytometry after staining with fluorescent-labeled antibodies specific for CD45RA (B220). Values shown here are expressed as the percentage of B cells in blood normalized so that the control group = 100%. Values for acute exposure were published previously and are shown here for comparison (Pruett et al., 2003). Group size and statistical analysis were done as noted in the legend for Figure 2.
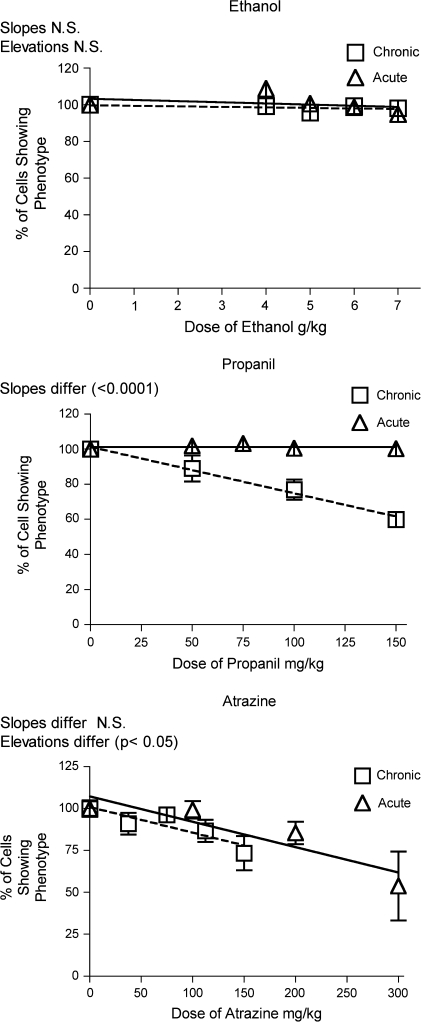
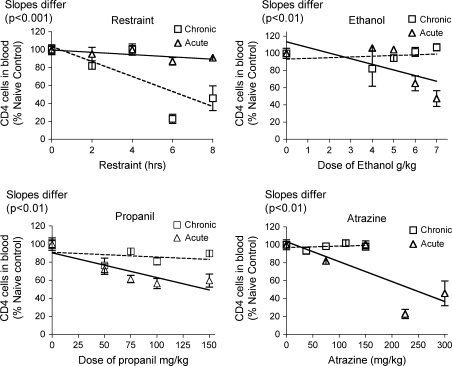
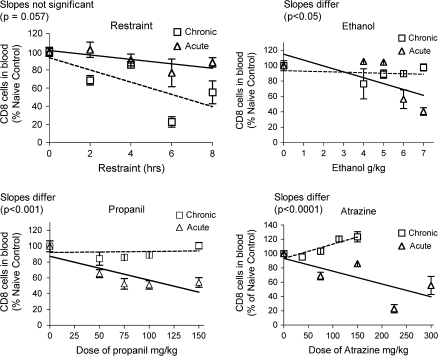
The results for T lymphocytes were not consistent with the chemical-induced effects on corticosterone production. For both CD4+ and CD8+ T cells, acute treatment with all three chemicals caused a dose dependent decrease in percentage of these cells in blood (Figs. 9 and 10). However, 28-day treatment had little effect or actually increased the percentage of T cells in the blood. The latter effect was caused by atrazine, even though atrazine had the same effect on corticosterone whether administered for 28 days or 1 day. Restraint for 28-days decreased the T cell percentage in the blood, whereas acute restraint caused a smaller decrease. This was opposite the effects of these treatments on the corticosterone response, which was smaller for the 28-day than for the acute treatment.
FIG. 9.
Changes in CD4+ T lymphocytes in the blood. Changes in the percentage of Blood Th lymphocytes were identified by flow cytometry after staining with fluorescent-labeled antibodies specific for CD4. Values shown here are expressed as the percentage of CD4+ T cells in blood normalized so that the control group = 100%. Values for acute exposure were published previously and are shown here for comparison (Schwab et al., 2005). Group size and statistical analysis were as described in the legend of Figure 2.
FIG. 10.
Changes in CD8+ lymphocytes in the blood. Changes in the percentage of CD8+ lymphocytes in the blood were identified by flow cytometry after staining with fluorescent-labeled antibodies specific for CD8. Values shown here are expressed as the percentage of CD8+ T cells in blood normalized so that the control group = 100%. Values for acute exposure were published previously and are shown here for comparison (Schwab et al., 2005). Group size and statistical analysis were as described in the legend of Figure 2.
NK Cell Activity
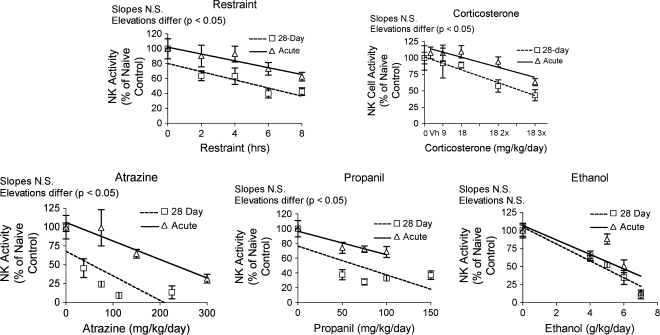
In general, changes in NK cell activity were not consistent with the observed changes in corticosterone response. All stressors decreased NK cell activity in a dose responsive manner, but chronic exposure caused greater decreases in the case of atrazine, propanil, and restraint (Fig. 11). This pattern is different from the pattern of corticosterone induction associated with prolonged exposure, which caused continuing increases of corticosterone in mice treated with atrazine but a significantly smaller increases than acute exposure in mice treated with propanil and restraint. This suggests mechanisms other than stress mediator production are responsible for the effects of some of these compounds on NK cells. Results for acute and chronic ethanol exposure were very similar, again suggesting a minimal role for corticosterone in the effects of acute or chronic exposure. This is consistent with previous studies, in which corticosterone was demonstrated to explain a relatively small portion of the suppression of NK cell activity by the chemical stressor ethanol (Wu and Pruett, 1997).
FIG. 11.
Changes in NK cell activity of splenic lymphocytes. Changes in the NK cell activity of splenocytes were assessed using a 51Cr-release assay using YAC-1 tumor target cells. Values shown here are expressed as lytic units normalized so that the mean value for the control group is 100%. Values for acute exposure were published previously and are shown here for comparison (Schwab et al., 2005). Group size and statistical analysis were as described in the legend of Figure 2.
DISCUSSION
Habituation or tolerance is particularly relevant in safety testing of drugs or chemicals when the ICH S8 Guidance Document for immunotoxicity testing is considered. That document indicates that changes in immunological parameters generally associated with stress (e.g., thymic atrophy, increased percentage of neutrophils in the blood, or decreased percentage of lymphocytes in the blood) will not be considered sufficient to indicate a nonspecific stress response as the cause of the immunological changes (Hastings, 2005). Additional evidence that the effects are mediated by stress will be required. Corticosterone and/or its metabolites in the urine can be used as a direct indicator of stress responses (Pruett et al., 2007, 2008). Those studies indicate that tolerance to the stress response to some chemicals may be dose dependent and can require more than 7 days of dosing to occur. However, high dosages of ethanol can induce tolerance with regard to the stress response within 10 days (Pruett et al., 1998). The mechanism(s) of tolerance or habituation could include adaptation to psychological stress associated with perceived aversive effects of the compound, induction of metabolizing enzymes leading to more rapid clearance of the compound (this has been reported for propanil) (Takeuchi et al., 2008), or adaptation of the targets of stress mediators making them less susceptible to effects of stressors. Any of these or a combination of them could occur and will be the subject of future investigations. The clearance rate for these compounds has obvious implications for these results. The clearance rate of ethanol has been reported in our previous study, and it corresponds quite well to the pattern of elevated corticosterone (clearance is complete by 8 h and corticosterone values return to normal at or shortly after this) (Carson and Pruett, 1996). Ethanol can induce metabolizing enzymes leading to more rapid clearance, and this may have occurred in this study. Kinetics of atrazine absorption and clearance in rats suggest a similar time frame as for ethanol (Timchalk et al., 1990). Clearance data for propanil are not available, but the pattern of corticosterone response suggests it is similar to that for ethanol or atrazine.
Changes in most of the immunological parameters after 28 days of exposure correspond quite well with the degree of habituation or tolerance with regard to the corticosterone response. It is particularly important that a parameter for which it was previously shown that stress-mediated effects are mediated entirely by corticosterone, MHC class II protein expression on B cells (Weiss et al., 1996), was affected just as expected on the basis of the observed corticosterone responses. This suggests that the different pattern observed for the effects of the stressors on other immunological parameters is based on either direct effects of the compounds on that parameter or on mediators other than corticosterone.
Ethanol, which caused nearly complete habituation with regard to the corticosterone response, did not affect most immunological parameters after 28 days of exposure. However, 28-day exposure to ethanol did inhibit NK cell activity. This is consistent with previous results indicating that only a small portion of inhibition of NK cell activity by ethanol is caused by stress mediators following acute ethanol exposure (Wu and Pruett, 1997). Similar results were noted for most stressors and most immunological parameters. However, in some cases (e.g., changes in subpopulations of lymphocytes in the blood), restraint yielded essentially opposite results compared with other stressors (28-day exposure caused greater change than acute exposure). This occurred even though mice partially adapted to daily restraint by producing less corticosterone at the end of the 28-day exposure. Collectively these results suggest that corticosterone is not the primary mediator of the stress-related effects of restraint. Even when comparing acute exposures only, restraint produced different effects on some parameters than exogenous corticosterone or chemical stressors (Pruett et al., 2003). This suggests that there are quantitative or qualitative differences in stress mediators (other than corticosterone) induced by restraint as compared with most chemical stressors.
The tendency toward increased neutrophils and decreased lymphocytes observed here is commonly reported in experimental animals and humans exposed to stressors (Bessey et al., 1984; Brenner et al., 1998; Harris et al., 1995; Hou et al., 1996). Again, the effects of restraint did not fit the pattern of effects induced by the chemical stressors, suggesting qualitative differences between the stress response to restraint and most chemical stressors. It is possible that restraint induces stress mediators in a manner that is quantitatively or qualitatively different from the stress response induced by chemicals. It is interesting that the pattern of the corticosterone response after 28-day exposure to restraint is different from that of all other stressors in that the peak corticosterone concentrations occur at 4–6 h (instead of 1–2 h). This pattern is different than for acute exposure to restraint, for which the peak is 8 h. This likely represents habituation to the neurogenic stress caused by restraint (Lu et al., 1998).
The present study was not designed to determine whether changes in specific immunological parameters were mediated by corticosterone, but to determine if the corticosterone response to chemicals diminishes in a 28-day exposure study. The role of corticosterone in alteration of some immunological parameters has been determined for ethanol and propanil (Cuff et al., 1996; Weiss et al., 1996), and changes in some immunological parameters were found to be mostly or completely mediated by corticosterone. More compounds will need to be evaluated to determine if the results here are representative, but if they are, this would suggest that tolerance or habituation is common in drugs or chemicals that are immunotoxic due to induction of a stress response. A new finding here is that two of three chemicals tested caused a much smaller corticosterone response after 28 days of treatment than after a single treatment. This is associated with smaller changes in most immunological parameters. Those for which this pattern was not observed presumably are affected by a mechanism other than the chemical-induced stress response (e.g., NK cell inhibition by ethanol).
These results have implications with regard to safety testing of drugs and chemicals. Evaluation of the concentration of corticosterone and metabolites in urine at the beginning and end of a 28-day exposure regimen would be necessary to determine if acute immunological effects were associated with increased corticosterone concentrations and could be stress mediated and whether immunological effects at the end of the exposure period were associated with increased corticosterone concentrations or not (Pruett et al., 2008). If corticosterone elevation was still observed after a 28-day exposure, it would be clear that at least some of the immunological effects mediated by the compound could be stress mediated. Clearly, more chemicals need to be tested to determine if the three tested here are representative. If they are, it would be expected that most chemicals and drugs would cause habituation or tolerance and that stress would be a less likely explanation for immunological changes after 28 days of treatment than after acute exposure. This would indicate that 28-day exposures are more appropriate for evaluation of immunotoxicity than acute studies, because the longer exposure is more likely to reveal immunotoxicity that does not result from a stress response. Such stress responses and consequent immunological effects could be misinterpreted as immunotoxicity, whereas humans would not typically be exposed to sufficient dosages of agents being evaluated to cause a nonspecific stress response.
FUNDING
National Institutes of Health (R01ES09158 and R01ES013708).
References
- Anonymous. Health Effects Test Guidelines OPPTS 870.7800 Immunotoxicity, pp. 1–11. 1998 U.S. Environmental Protection Agency, Research Triangle Park, NC. [Google Scholar]
- Anonymous. NTP toxicology and carcinogenesis studies of 2,3,4,7,8-pentachlorodibenzofuran (PeCDF) (CAS No. 57117-31-4) in female Harlan Sprague-Dawley rats (gavage studies) Natl. Toxicol. Program Tech. Rep. Ser. 2006;525:1–198. [PubMed] [Google Scholar]
- Bessey PQ, Watters JM, Aoki TT, Wilmore DW. Combined hormonal infusion simulates the metabolic response to injury. Ann. Surg. 1984;200:264–281. doi: 10.1097/00000658-198409000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner I, Shek PN, Zamecnik J, Shephard RJ. Stress hormones and the immunological responses to heat and exercise. Int. J. Sports Med. 1998;19:130–143. doi: 10.1055/s-2007-971895. [DOI] [PubMed] [Google Scholar]
- Carson EJ, Pruett SB. Development and characterization of a binge drinking model in mice for evaluation of the immunological effects of ethanol. Alcohol Clin. Exp. Res. 1996;20:132–138. doi: 10.1111/j.1530-0277.1996.tb01055.x. [DOI] [PubMed] [Google Scholar]
- Cuff CF, Zhao W, Nukui T, Schafer R, Barnett JB. 3,4-dichloropropionanilide-induced atrophy of the thymus: Mechanisms of toxicity and recovery. Fundam. Appl. Toxicol. 1996;33:83–90. doi: 10.1006/faat.1996.0145. [DOI] [PubMed] [Google Scholar]
- Harris JG, Flower RJ, Perretti M. Endogenous corticosteroids mediate the neutrophilia caused by platelet-activating factor in the mouse. Eur. J. Pharmacol. 1995;283:9–18. doi: 10.1016/0014-2999(95)00274-o. [DOI] [PubMed] [Google Scholar]
- Hastings KL. S8 Immunotoxicity Studies for Human Pharmaceuticals. 2005. http://www.fda.gov/OHRMS/DOCKETS/98fr/2005d-0022-gdl0001.doc. Accessed April 27, 2009. [Google Scholar]
- Hou FY, Coe CL, Erickson C. Psychological disturbance differentially alters CD4+ and CD8+ leukocytes in the blood and intrathecal compartments. J. Neuroimmunol. 1996;68:13–18. doi: 10.1016/0165-5728(96)00055-0. [DOI] [PubMed] [Google Scholar]
- Lolait SJ, Stewart LQ, Roper JA, Harrison G, Jessop DS, Young WS, III, O'Carroll AM. Attenuated stress response to acute lipopolysaccharide challenge and ethanol administration in vasopressin V1b receptor knockout mice. J. Neuroendocrinol. 2007;19:543–551. doi: 10.1111/j.1365-2826.2007.01560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu ZW, Song C, Ravindran AV, Merali Z, Anisman H. Influence of a psychogenic and a neurogenic stressor on several indices of immune functioning in different strains of mice. Brain Behav. Immun. 1998;12:7–22. doi: 10.1006/brbi.1997.0510. [DOI] [PubMed] [Google Scholar]
- Pruett SB, Collier SD, Wu W-J. Ethanol-induced activation of the hypothalamic-pituitary-adrenal axis in a mouse model for binge drinking: Role of Ro15-4513-sensitive gamma aminobutyric acid receptors, tolerance, and relevance to humans. Life Sci. 1998;63:1137–1146. doi: 10.1016/s0024-3205(98)00375-0. [DOI] [PubMed] [Google Scholar]
- Pruett SB, Collier S, Wu W-J, Fan R. Quantitative relationships between the suppression of selected immunological parameters and the area under the corticosterone concentration vs. time curve in B6C3F1 mice subjected to exogenous corticosterone or to restraint stress. Toxicol. Sci. 1999;49:272–280. doi: 10.1093/toxsci/49.2.272. [DOI] [PubMed] [Google Scholar]
- Pruett SB, Fan R. Quantitative modeling of suppression of IgG1, IgG2a, IL-2, and IL-4 responses to antigen in mice treated with exogenous corticosterone or restraint stress. J. Toxicol. Environ. Health. 2001;62:175–189. doi: 10.1080/009841001458299. [DOI] [PubMed] [Google Scholar]
- Pruett SB, Fan R, Myers LP, Wu W-J, Collier SD. Quantitative analysis of the neuroendocrine-immune axis: Linear modeling of the effects of exogenous corticosterone and restraint stress on lymphocyte subpopulations in the spleen and thymus in female B6C3F1 mice. Brain Behav. Immun. 2000a;14:270–287. doi: 10.1006/brbi.2000.0605. [DOI] [PubMed] [Google Scholar]
- Pruett SB, Fan R, Zheng Q, Myers LP, Hébert P. Modeling and predicting selected immunological effects of a chemical stressor (3,4-dichloropropionanilide) using the area under the corticosterone concentration versus time curve. Toxicol. Sci. 2000b;58:77–87. doi: 10.1093/toxsci/58.1.77. [DOI] [PubMed] [Google Scholar]
- Pruett SB, Fan R, Zheng Q, Myers LP, Hebert P. Modeling and predicting immunological effects of chemical stressors: Characterization of a quantitative biomarker for immunological changes caused by atrazine and ethanol. Toxicol. Sci. 2003;75:343–354. doi: 10.1093/toxsci/kfg200. [DOI] [PubMed] [Google Scholar]
- Pruett SB, Hébert P, Lapointe JM, Reagan W, Lawton M, Kawabata TT. Characterization of the action of drug-induced stress responses on the immune system: Evaluation of biomarkers for drug-induced stress in rats. J. Immunotoxicol. 2007;4:28–35. doi: 10.1080/15476910601115150. [DOI] [PubMed] [Google Scholar]
- Pruett SB, Lapointe J-M, Reagan W, Lawton M, Kawabata TT. Urinary corticosterone as an indicator of stress-mediated immunological changes in rats. J. Immunotoxicol. 2008;5:17–22. doi: 10.1080/15476910801897474. [DOI] [PubMed] [Google Scholar]
- Pruett SB, Zheng Q, Schwab C, Fan R. Sodium methyldithiocarbamate inhibits MAP kinase activation through toll-like receptor 4, alters cytokine production by mouse peritoneal macrophages, and suppresses innate immunity. Toxicol. Sci. 2005;87:75–85. doi: 10.1093/toxsci/kfi215. [DOI] [PubMed] [Google Scholar]
- Schwab CL, Fan R, Zheng Q, Myers LP, Hebert P, Pruett SB. Modeling and predicting stress-induced immunosuppression in mice using blood parameters. Toxicol. Sci. 2005;83:101–113. doi: 10.1093/toxsci/kfi014. [DOI] [PubMed] [Google Scholar]
- Spies C, Eggers V, Szabo G, Lau A, von Dossow V, Schoenfeld H, Althoff H, Hegenscheid K, Bohm B, Schroeder T, et al. Intervention at the level of the neuroendocrine-immune axis and postoperative pneumonia rate in long-term alcoholics. Am. J. Respir. Crit. Care Med. 2006;174:408–414. doi: 10.1164/rccm.200506-907OC. [DOI] [PubMed] [Google Scholar]
- Takeuchi S, Iida M, Yabushita H, Matsuda T, Kojima H. In vitro screening for aryl hydrocarbon receptor agonistic activity in 200 pesticides using a highly sensitive reporter cell line, DR-EcoScreen cells, and in vivo mouse liver cytochrome P450-1A induction by propanil, diuron and linuron. Chemosphere. 2008;74:155–165. doi: 10.1016/j.chemosphere.2008.08.015. [DOI] [PubMed] [Google Scholar]
- Timchalk C, Dryzga MD, Langvardt PW, Kastl PE, Osborne DW. Determination of the effect of tridiphane on the pharmacokinetics of [14C]-atrazine following oral administration to male Fischer 344 rats. Toxicology. 1990;61:27–40. doi: 10.1016/0300-483x(90)90004-z. [DOI] [PubMed] [Google Scholar]
- Weiss PA, Collier SD, Pruett SB. Role of glucocorticoids in ethanol-induced decreases in expression of MHC class II molecules on B cells and selective decreases in spleen cell number. Toxicol. Appl. Pharmacol. 1996;139:153–162. doi: 10.1006/taap.1996.0154. [DOI] [PubMed] [Google Scholar]
- Wu W-J, Pruett SB. Suppression of splenic natural killer cell activity in a mouse model for binge drinking. I. Direct effects of ethanol and its major metabolites are not primarily responsible for decreased natural killer cell activity. J. Pharmacol. Exp. Ther. 1996;278:1325–1330. [PubMed] [Google Scholar]
- Wu W-J, Pruett SB. Involvement of catecholamines and glucocorticoids in ethanol-induced suppression of splenic natural killer cell activity in a mouse model for binge drinking. Alcohol Clin. Exp. Res. 1997;21:1030–1036. [PubMed] [Google Scholar]
- Wu W-J, Wolcott RM, Pruett SB. Ethanol decreases the number and activity of splenic natural killer cells in a mouse model for binge drinking. J. Pharmacol. Exp. Ther. 1994;271:722–729. [PubMed] [Google Scholar]