Abstract
The second messenger, 3′,5′-cyclic adenosine monophosphate (cAMP), is known to be modulated in taste buds following exposure to gustatory and other stimuli. Which taste cell type(s) (Type I/glial-like cells, Type II/receptor cells, or Type III/presynaptic cells) undergo taste-evoked changes of cAMP and what the functional consequences of such changes are remain unknown. Using Fura-2 imaging of isolated mouse vallate taste cells, we explored how elevating cAMP alters Ca2+ levels in identified taste cells. Stimulating taste buds with forskolin (Fsk; 1 μm) + isobutylmethylxanthine (IBMX; 100 μm), which elevates cellular cAMP, triggered Ca2+ transients in 38% of presynaptic cells (n= 128). We used transgenic GAD-GFP mice to show that cAMP-triggered Ca2+ responses occur only in the subset of presynaptic cells that lack glutamic acid decarboxylase 67 (GAD). We never observed cAMP-stimulated responses in receptor cells, glial-like cells or GAD-expressing presynaptic cells. The response to cAMP was blocked by the protein kinase A inhibitor H89 and by removing extracellular Ca2+. Thus, the response to elevated cAMP is a PKA-dependent influx of Ca2+. This Ca2+ influx was blocked by nifedipine (an inhibitor of L-type voltage-gated Ca2+ channels) but was unperturbed by ω-agatoxin IVA and ω-conotoxin GVIA (P/Q-type and N-type channel inhibitors, respectively). Single-cell RT-PCR on functionally identified presynaptic cells from GAD-GFP mice confirmed the pharmacological analyses: Cav1.2 (an L-type subunit) is expressed in cells that display cAMP-triggered Ca2+ influx, while Cav2.1 (a P/Q subunit) is expressed in all presynaptic cells, and underlies depolarization-triggered Ca2+ influx. Collectively, these data demonstrate cross-talk between cAMP and Ca2+ signalling in a subclass of taste cells that form synapses with gustatory fibres and may integrate tastant-evoked signals.
Taste buds are tight clusters of neuroepithelial sensory cells embedded in the oral epithelium. Most cells in mammalian taste buds appear morphologically similar – fusiform and extending from the basement membrane to the mucosal surface – but they comprise functionally distinct classes. Some taste cells express taste G protein-coupled receptors (GPCRs) for sweet, bitter or umami tastants (Zhao et al. 2003). Such taste cells correspond to ultrastructurally defined ‘Type II’ cells (Yee et al. 2001) and the relationship was further elaborated by combining morphological markers with functional analyses (Ogura et al. 2002; Clapp et al. 2006; DeFazio et al. 2006; Tomchik et al. 2007). Because Type II cells display the primary taste GPCR-mediated Ca2+ responses to tastants, we have termed them ‘receptor cells’ (DeFazio et al. 2006). The Type III cells of taste buds possess specialized chemical synapses, synthetic enzymes for aminergic neurotransmitters and proteins involved in vesicular release (Yang et al. 2000; Yee et al. 2001; Yang et al. 2004; DeFazio et al. 2006; Dvoryanchikov et al. 2007). These cells, which we term ‘presynaptic cells’, may serve to integrate taste quality information within the taste bud (Tomchik et al. 2007). Taste stimulation evokes Ca2+ transients in receptor cells and this activates TrpM5 channels in receptor cells (Liu & Liman, 2003), eventually triggering the release of neurotransmitters. When taste buds are stimulated with tastants, receptor cells secrete ATP (Huang et al. 2007; Romanov et al. 2007) while presynaptic cells release serotonin and noradrenaline (Huang et al. 2005; Huang et al. 2007; Huang et al. 2008).
In addition to Ca2+ elevation evoked by taste stimulation, mammalian taste buds also display alterations in cAMP levels upon exposure to sweet (Striem et al. 1991; Trubey et al. 2006), bitter (Yan et al. 2001) and umami (Ninomiya et al. 2000; Abaffy et al. 2003) taste stimuli. It is not known whether these changes in cAMP are the direct consequence of taste receptor activation, or are generated indirectly, i.e. following sensory transduction or synaptic signalling within the taste bud (see Roper, 2007). Taste cells express several isoforms of adenylate cyclase (Abaffy et al. 2003; Trubey et al. 2006) and phosphodiesterase (Spickofsky et al. 1994; McLaughlin et al. 1994; Moriyama et al. 2002), enzymes that synthesize and degrade cAMP, respectively, and hence dynamically regulate cAMP concentrations. In summary, the intracellular machinery for cAMP signalling is present in taste buds and cAMP changes occur following gustatory stimulation.
Yet, we do not know the effector pathways that utilize cAMP in taste cells, nor whether such pathways interact or cross-talk with Ca2+ signals. Hence, we tested whether altering taste cell cAMP levels affects intracellular Ca2+ levels. By imaging Ca2+ changes in isolated cells and tastebuds, we found that certain taste cells respond to cAMP elevation with a Ca2+ transient. We identified these as a subset of presynaptic cells (Type III cells). We tested the pharmacology of the cAMP-triggered responses and also employed single-cell RT-PCR to identify the pore-forming subunits of voltage-gated Ca2+ channels expressed in these same cells. These independent methods confirm that while all Type III cells express P/Q-type Ca2+ channels (as typical for presynaptic sites), a defined subset also express L-type Ca2+ channels. The latter channels provide a mechanism for interaction between the cAMP and Ca2+ signalling pathways in these presynaptic cells. Specifically, elevated cAMP levels result in elevated cytoplasmic Ca2+, which may then regulate other cellular processes such as transmitter secretion.
Methods
Ethical approval
All animals were housed and handled following the National Institutes of Health's Guidelines for the Care and Use of Laboratory Animals and the American Veterinary Medical Association's AVMA Guidelines on Euthanasia. Mice were killed with CO2 inhalation and cervical dislocation before the tongue was excised to isolate taste buds. All procedures were approved by the University of Miami Animal Care and Use Committee.
Animals, tissues and physiological buffers
Adult C57/Bl6 mice and two transgenic mouse strains were used. Transgenic mice expressed green fluorescent protein (GFP) under control of either the GAD67 promoter (GAD-GFP; Chattopadhyaya et al. 2004), or the PLCβ2 promoter (PLCβ2-GFP; Kim et al. 2006), thereby labelling presynaptic cells or receptor cells, respectively.
To isolate taste buds and taste cells, a mix of 1 mg ml−1 collagenase A (Roche Corp., Indianapolis, IN, USA), 2.5 mg ml−1 dispase II (Roche), and 1 mg ml−1 trypsin inhibitor in Tyrode buffer was injected under the circumvallate and foliate taste papillae. Tyrode buffer consisted of (in mm): 139 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 Hepes, 10 glucose, 10 sodium pyruvate, and 5 NaHCO3; pH 7.2, 318–323 mosmol l−1. After 20–25 min at room temperature, the lingual epithelium, enriched in taste buds, was peeled away, incubated in EGTA-containing Tyrode buffer (in mm: 139 NaCl, 5 KCl, 2 EGTA, 10 Hepes, 10 glucose, 10 sodium pyruvate, and 5 NaHCO3; pH 7.2; 318–323 mosmol l−1) for 10–15 min and then returned to Tyrode buffer. Taste buds and cells were drawn into glass pipettes with gentle suction and transferred to a glass coverslip, pre-coated with Cell-Tak (BD Biosciences, San Jose, CA, USA) to hold cells firmly in place.
Taste cells were depolarized by stimulation with 50 mm KCl Tyrode buffer (50 mm KCl substituted for 50 mm NaCl to maintain osmolarity). To elevate cAMP in taste cells, we bath applied 1 μm forskolin (Fsk) or forskolin combined with 100 μm 3-isobutyl-1-methylxanthine (IBMX) (both from Sigma). The concentration of forskolin was chosen to be below values reported to inhibit potassium currents non-specifically in taste cells (>5 μm) (Herness et al. 1997). This is important because non-specifically inhibiting resting K+ channels might be expected to depolarize taste cells and produce secondary affects.
Ca2+ imaging
Isolated taste buds were loaded with 4 μm fura-2AM for 30 min at room temperature and washed in Tyrode buffer for 20–30 min. The recording chamber, containing taste buds and cells, was then mounted on an Olympus (Tokyo, Japan) IX70 inverted microscope. Cells were perfused with Tyrode buffer, nominally Ca2+-and Mg2+-free Tyrode buffer, or experimental solutions all at a rate of 1 ml min−1. Images were captured at a rate of 0.1 Hz using sequential excitation at 340 and 380 nm and a longpass (≥510 nm) emission filter. The ratio of emission intensities (F340/F380) was calculated using Imaging Workbench version 5.1 software (INDEC Biosystems, Mountain View, CA, USA). Ca2+ signals were plotted as the ratio of F340/F380 minus the mean ratio for 100 s before each stimulus (i.e. prestimulus baseline). Peak values above the prestimulus baseline (i.e. ΔF340/F380) were used to quantify statistical significance (2-tailed t-tests and/or ANOVA) using Prism v.5.0 (GraphPad Software, San Diego, CA, USA).
RNA preparation and RT-PCR
RT-PCR was carried out either on linear-amplified RNA (for pools of 10 cells) or directly on RNA from individual cells (for functionally characterized cells).
For the initial screen, we collected GFP(+) taste cells from circumvallate papillae transgenic mice and transferred each cell into lysis buffer (Absolutely RNA Nanoprep kit, Stratagene, La Jolla, CA, USA). We then grouped the cell lysates into pools, each of which contained 10 cells. Three of the pools were GFP(+) cells from GAD-GFP mice, three pools were GFP(+) cells from PLCβ2-GFP mice. Total RNA was isolated from each pool of 10 cells using the Nanoprep kit and was used as template for reverse transcription and T7 linear RNA amplification using the Message BOOSTER cDNA Synthesis kit for qPCR (Epicentre, Madison, WI, USA) as described previously (DeFazio et al. 2006). In brief, first strand cDNA synthesis was primed using a T7-Oligo(dt) anchor-primer, RNaseH digestion was followed by second strand cDNA synthesis. Amplified antisense RNA (aRNA) was then transcribed in vitro using T7 RNA polymerase, purified on a MinElute column (Qiagen, Valencia, CA, USA) and subjected to a second round of reverse transcription using random hexamers. Finally, 0.1% of the cDNA (representing aRNA from a pool of 10 cells) was used as template for PCR (40 cycles).
To analyse gene expression in functionally defined cells, we performed sequential Ca2+ imaging, cell collection, and RT-PCR. Individual imaged cells were collected using 15 μm diameter glass pipettes and were expelled into microfuge tubes containing 60 μl of lysis buffer (Nanoprep kit) containing 1 μg of poly-inosinic acid as a carrier. RNA isolation and cDNA synthesis with SuperScript III (Invitrogen Corp., Carlsbad, CA, USA) was as previously reported (DeFazio et al. 2006). RT-PCR (45 cycles) on cDNA representing a fraction of each single cell was performed in 20 μl in parallel with positive (taste buds) and negative (water in place of template) control reactions.
Two pairs of primers were tested for each of Cav2.1 and Cav1.2 (Table 1). Primer pairs designated ‘2’ were designed to span at least one intron to avoid amplifying genomic DNA. These primers were used on cDNA from individual cells. The primer pairs designated ‘1’ in Table 1 were designed for greater sensitivity, were located at the 3′ ends of the respective mRNAs and were used in RT-PCR from linear-amplified RNAs (where genomic DNA contamination is not a concern). Conditions for PCR were 95°C for 5 min, followed by cycles (30 s each at 94°C, annealing temperature, and 72°C) using QIAGEN Taq DNA Polymerase. The specificity of each PCR product was confirmed by DNA sequencing.
Table 1.
RT-PCR primers
| Protein | Gene | cDNA accession no. | Forward Primer (5′–3′) | Reverse Primer (5′–3′) | Product bp | Anneal temp. |
|---|---|---|---|---|---|---|
| β-Actin | Actb | NM_007393 | CACCCTGTGCTGCTCACC | GCACGATTTCCCTCTCAG | 328 | 58°C |
| PLCβ2 | Plcb2 | NM_177568 | GAGCAAATCGCCAAGATGAT | CCTTGTCTGTGGTGACCTTG | 163 | 60°C |
| SNAP25 | Snap25 | NM_011428 | GGCAATAATCAGGATGGAGTAG | AGATTTAACCACTTCCCAGCA | 310 | 58°C |
| Cav1.2 α1C L-type | Cacna1c | NM_009781 | (1) CAGGGATCTATGGCAGGAAT | (1) AGTCGCAACATGCTGACAAG | 253 | 58°C |
| (2) ACTGCTGAGGAGGAGTTGGA | (2) GTTGGCATTGTTGATGTTGG | 294 | 59°C | |||
| Cav2.1 α1A P/Q type | Cacna1a | NM_007578 | (1) CTCTGGGCCGATACACTGAT | (1) GGGATGATGATGATGGTGGT | 164 | 58°C |
| (2) CCAGCAGAGAACCAGAGGAG | (2) GCTCAGATCTGTCCCCAAAC | 258 | 62°C | |||
| Cav2.2 α1B N-type | Cacna1b | AF042317 | CGAATTGGCTCTGACCCTTA | CCAGTGCTGAGTCCCAAAGT | 212 | 58°C |
| Cav3.2 α1H T-type | Cacna1h | NM_021415 | CCTCGTGGTGATTCAGGTTT | GAAGGATGCCGAGTGATGAT | 292 | 58°C |
Two pairs of primers were used for each, Cav1.2 and Cav2.1. See Methods for details on their use.
Results
Presynaptic cells respond to cAMP elevation
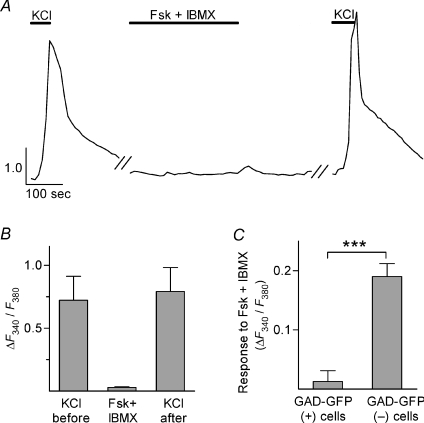
Isolated mouse vallate tastebuds were loaded with Fura-2AM and ΔF340/F380 (peak F340/F380 less prestimulus baseline) was measured as an indicator of change in intracellular Ca2+. We elevated cAMP in taste cells (Abaffy et al. 2003) by bath-applying a low concentration of forskolin (Fsk, 1 μm), an activator of adenylate cyclase, and 3-isobutyl-1-methylxanthine (IBMX, 100 μm) a non-selective phosphodiesterase inhibitor. We selected this low concentration of Fsk to avoid non-specific actions on taste cell potassium channels (Herness et al. 1997). A subset of taste cells responded to Fsk+IBMX with an increase in [Ca2+]i (Fig. 1A). These cells also responded to KCl depolarization with an increase in [Ca2+]i (Fig. 1A and B), thus identifying them as presynaptic cells (Type III cells) (Medler et al. 2003; DeFazio et al. 2006). In total, 38% of presynaptic cells (49 of 128) responded to Fsk+IBMX. Interestingly, applying KCl after Fsk+IBMX often produced a [Ca2+]i signal that was significantly larger than that evoked by the initial application of KCl (Fig. 1B). This suggested that Ca2+ channels in these cells may undergo a change that is slow to reverse. When applied together, Fsk and IBMX produced a significantly greater Ca2+ signal (n= 15 cells) than either Fsk (n= 10) or IBMX (n= 7) alone (Fig. 1C).
Figure 1. Elevating cAMP elicits Ca2+ transients in presynaptic cells (Type III cells).
A, Ca2+ imaging (with Fura-2) shows a presynaptic cell responding to bath applications of KCl (50 mm) and Fsk+IBMX (1 μm Fsk, 100 μm IBMX). Durations of applications are shown by horizontal bars above traces. Y-axis scale bar, F340/F380. In this and subsequent figures, each treatment began only after a complete recovery to baseline following the washout of the previous treatment. B, mean of peak ΔF340/F380 (i.e. F340/F380 minus prestimulus baseline, see Methods) from 19 presynaptic cells that responded to Fsk+IBMX (as in A). Note that responses to KCl were somewhat augmented following Fsk+IBMX application (P= 0.0004, paired t-test; n= 19 cells). C, Ca2+ responses to either Fsk or IBMX alone were of significantly lower magnitude than to Fsk+IBMX (ANOVA with Bonferroni's correction for multiple comparisons; P < 0.01; n= 10, 7, 15 cells respectively).
A subset of presynaptic cells (Type III cells) express GAD67, the 67 kDa isoform of glutamic acid decarboxylase (DeFazio et al. 2006). Using a GAD-GFP line of transgenic mice (Chattopadhyaya et al. 2004; Tomchik et al. 2007), we explored whether this subset of GAD-expressing presynaptic cells responds to Fsk+IBMX. As expected, KCl depolarization consistently stimulated Ca2+ transients in GFP-expressing taste cells from GAD-GFP mice. But, Fsk+IBMX stimulation did not evoke Ca2+ transients in these same GAD-GFP(+) cells (Fig. 2A and B). In marked contrast, GFP-lacking presynaptic cells in GAD-GFP mice responded to Fsk+IBMX (Fig. 2C). These cells also were identified as presynaptic cells by their responses to KCl. The findings indicate that elevating intracellular cAMP only evoked Ca2+ responses in GAD(−) presynaptic cells.
Figure 2. Only a subset of presynaptic cells (Type III cells), those lacking GAD expression, respond to cAMP elevation.
Transgenic mice expressing GFP from the GAD1 promoter were used to identify GAD-expressing, i.e. GAD-GFP(+), and GAD-lacking, i.e. GAD-GFP(–), presynaptic cells. A, Ca2+ responses from a GAD-GFP(+) presynaptic cell, stimulated with KCl (50 mM) and Fsk + IBMX, as in Fig 1. The absence of responses evoked by Fsk+IBMX was typical for GAD-GFP(+) presynaptic cells. Y-axis scale bar, F340/F380. B, summary of data for GAD-GFP(+) presynaptic cells (n= 12 cells). Peak responses here and in subsequent bar graphs were quantified as in Fig. 1B. C, summary of Fsk+IBMX-evoked responses in GAD-GFP(+) and GAD-GFP(–) presynaptic cells (t-test; P < 0.0001; n= 14 GFP(+) and 19 GFP(–) presynaptic cells).
We next tested whether Fsk+IBMX evoked Ca2+ transients in receptor cells (Type II cells). Receptor cells were identified by the expression of GFP in taste buds from PLCβ2-GFP transgenic mice (Kim et al. 2006). Applying Fsk+IBMX never elicited significant changes of in [Ca2+]i in receptor cells (n= 36 cells, data not shown). Thus, the ability of Fsk+IBMX to stimulate Ca2+ transients in taste bud cells appears to be limited to a subset of presynaptic cells (Type III cells). (We cannot rule out that higher concentrations of Fsk might evoke responses in receptor cells. But if they did, such responses could not readily be distinguished from non-selective actions of high Fsk on K+ channels; Herness et al. 1997.)
The response to cAMP is an influx of Ca2+ mediated by protein kinase A
Next, we investigated the source of Ca2+ underlying the response to cAMP elevation in presynaptic cells. Ca2+ responses evoked by Fsk+IBMX were significantly reduced by eliminating Ca2+ from the bathing solution (Fig. 3A and B), indicating that elevating cAMP in these cells causes an influx of Ca2+. This Ca2+ influx was most strikingly demonstrated by briefly removing extracellular Ca2+ during the course of Fsk+IBMX responses (Fig. 3C).
Figure 3. Fsk+IBMX-evoked responses are produced by Ca2+ influx.
A, sequential responses from a presynaptic cell to bath-applied Fsk+IBMX, recorded in the presence and absence of extracellular Ca2+. Y-axis scale bar, F340/F380. B, summary of the effect of removing extracellular Ca2+ on Fsk+IBMX-evoked responses (two-tailed t-test; P= 0.002; n= 5 cells). C, response of a presynaptic cell when perfusion was momentarily changed to buffer containing nominally 0 Ca2+ (grey bar) during elevation of cAMP. Y-axis scale bars in A and C are F340/F380.
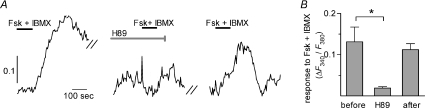
In many cells, cAMP activates protein kinase A (PKA) to phosphorylate intracellular targets, including Ca2+ channels. Thus, we tested whether Ca2+ responses evoked by Fsk+IBMX depend on PKA. Incubating taste cells with the PKA inhibitor H89 (10 μm), significantly reduced Ca2+ responses to Fsk+IBMX (P= 0.03; paired t-test; n= 5 cells; Fig. 4A and B). The effects of H89 were reversible (Fig. 4B). These data show that PKA activity is necessary for cAMP to generate a Ca2+ influx.
Figure 4. Fsk+IBMX-evoked Ca2+ influx in presynaptic cells requires PKA.
A, responses to Fsk+IBMX were reversibly inhibited by incubating cells with the PKA inhibitor H89 (10 μm, 10 min.). Bars above traces show application of Fsk+IBMX and/or of H89. Y-axis scale bar, F340/F380. B, summary of effects of H89 on Fsk+IBMX-evoked responses (paired t-test; P= 0.03; n= 5 cells).
Ca2+ influx occurs through voltage-gated Ca2+ channels
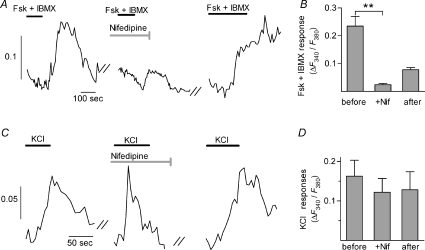
Presynaptic cells express several voltage-gated Ca2+ channels (Medler et al. 2003; DeFazio et al. 2006). Thus, we characterized which Ca2+ channels were involved in the responses to Fsk+IBMX. For these experiments, we used taste buds from GAD-GFP mice and examined KCl-responsive cells that were either GFP(+) or GFP(–). Nifedipine (10 μm), which is a selective blocker of L-type Ca2+ channels, strongly inhibited Fsk+IBMX responses (P= 0.001; paired t-test; n= 7 cells; Fig. 5A and B). The effects of nifidepine were incompletely reversed upon washout. Despite blocking responses to Fsk+IBMX, nifedipine did not affect Ca2+ responses to KCl (n= 6 cells; Fig. 5C and D). Similar results were obtained with verapamil (50 μm), a less specific L-type channel blocker (data not shown). In contrast, ω-agatoxin IVA (300 nm), a blocker of P/Q-type Ca2+ channels, diminished KCl-evoked responses in all presynaptic cells (GFP(+) and (−), alike) but did not affect Fsk+IBMX responses (Fig. 6). The effects of ω-agatoxin on KCl-evoked responses were reversible. Finally, ω-conotoxin GVIA (800 nm), an N-type Ca2+ channel blocker, did not affect Ca2+ responses evoked by either KCl or Fsk+IBMX (Fig. 6C).
Figure 5. L-type Ca2+ channels mediate responses to Fsk+IBMX in presynaptic cells.
A, representative trace from a presynaptic cell stimulated with Fsk+IBMX before, during, and after incubation in the L-type channel blocker nifedipine (10 μm, 10 min, grey bar). Black bars show application of Fsk+IBMX, as in Fig. 1. B, summary of effects of nifedipine on responses to Fsk+IBMX (P= 0.001; n= 7 cells) C, representative trace from another presynaptic cell showing lack of effects of nifedipine (10 μm, 10 min) on responses to KCl. Bars show application of KCl. Y-axis scale bars in A and C are F340/F380. D, summary of effects of nifedipine on KCl responses (n= 6 cells).
Figure 6. P/Q-type Ca2+ channels mediate KCl responses in presynaptic cells.
A, representative trace of a presynaptic cell stimulated sequentially with KCl and Fsk+IBMX before, during, and after incubation with the P/Q-type blocker ω-agatoxin IVA (300 nm). X-axis calibration bar in all panels represents 100 s; Y-axis scale bar is F340/F380. B, summary of effects of agatoxin. The response to KCl was reduced (paired t-test; P < 0.0001; n= 14 cells) while the response to Fsk+IBMX in the same cells was not significantly changed. C, incubating presynaptic cells for 30 min in 800 nmω-conotoxin GVIA, a blocker of N-type Ca2+ channels, had no effect on the response to either KCl or Fsk+IBMX (paired t-test; n= 9 cells).
In sum, these data suggest that P/Q-type Ca2+ channels in all presynaptic cells mediate Ca2+ influx in response to KCl depolarization, but L-type Ca2+ channels underlie the response to cAMP elevation in Fsk+IBMX-sensitive presynaptic cells. N-type Ca2+ channels do not appear to contribute to either of the observed Ca2+ responses.
Gene expression profiling confirms the identify of Ca2+ channels in presynaptic cells
To provide an independent characterization of the Ca2+ channels involved in taste cell responses, we carried out RT-PCR on identified taste cells. Ca2+ channels have been categorized pharmacologically and physiologically into the L, N, T, P/Q and R types. The pore-forming (α1) subunits for these channels comprise a large sequence family (Catterall et al. 2005). L-type channels are formed by one of several alternative subunits: Cav1.1, Cav1.2, Cav1.3 or Cav1.4. The P/Q-, N-and R-type channels derive respectively from Cav2.1, Cav2.2 and Cav2.3 subunits. Finally, the low-threshold activated T-type channels are formed from subunits Cav3.1, Cav3.2 or Cav3.3.
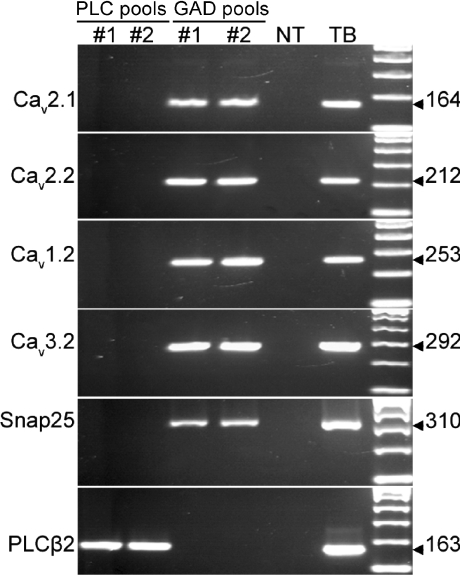
We isolated taste cells from two different strains of transgenic mice – those expressing GFP under promoters for either PLCβ2 or GAD67. GFP-labelled taste cells from PLCβ2 mice are receptor cells (DeFazio et al. 2006; Kim et al. 2006); those from GAD-GFP mice are presynaptic cells (Tomchik et al. 2007). We collected GFP-labelled cells into pools – three pools of receptor cells, each pool containing 10 PLCβ2-GFP positive cells, and three pools of presynaptic cells, each pool containing 10 GAD-GFP positive cells. RNA from each pool served as a template for linear RNA amplification followed by RT-PCR. We confirmed the lack of cross-contamination of cell types in our samples as follows. SNAP-25 was detected only in the pools of GAD-GFP cells; PLCβ2 was seen only in PLCβ2-GFP cells. We then tested for Ca2+ channel transcripts in each pool. PCR products of sizes expected for Cav1.2 (L-type), Cav2.1 (P/Q-type), Cav2.2 (N-type), and Cav3.2 (T-type) were obtained from each of the three GAD-GFP pools, but not from any of the PLCβ2-GFP pools (Fig. 7). These data verify that voltage-gated Ca2+ channels are expressed only in presynaptic cells, not in receptor cells, and specifically identify P/Q-, N-and L-type Ca2+ channels in GAD-expressing presynaptic cells.
Figure 7. Presynaptic cells (Type III cells) express pore-forming subunits Cav1.2 (L-type), Cav2.1 (P/Q type), Cav2.2 (N-type) and Cav3.2 (T-type) of voltage-gated Ca2+ channels.
Each column of lanes represents RT-PCR on equal aliquots of cDNA from a pool of 10 cells. Three pools of GFP(+) cells from PLCβ2-GFP mice (PLC pools) and from GAD-GFP mice (GAD pools) were tested. Two of the three are shown here. Taste buds (TB) and non-taste epithelium (NT) served as positive and negative controls respectively for RT-PCR. Each cDNA was analysed for each of the transcripts. Predicted lengths (in base pairs) of PCR products are shown to the right. As previously reported (DeFazio et al. 2006), the remaining Ca2+ channel α1 subunits are not prominently expressed in taste buds.
As reported above, however, presynaptic cells that lack GAD expression selectively responded to Fsk+IBMX. Thus, we combined functional imaging and single cell RT-PCR to refine our characterization of Ca2+ channels in presynaptic cells. First, we confirmed that cells identified following Ca2+ imaging would yield single cell cDNA of sufficient quality for the analysis. Hence, we dissociated taste cells from GAD-GFP mice (Fig. 8A), loaded them with Fura-2, and functionally imaged them. We collected individual cells that showed responses to KCl (thereby identifying them as presynaptic cells), extracted RNA, and analysed expression of the P/Q-type channel subunit, Cav2.1, by single cell RT-PCR. We analysed 19 KCl-responsive taste cells (i.e. Presynaptic cells). All 19 cells expressed the presynaptic cell marker, SNAP25, and most (14 of 19) also expressed Cav2.1 as previously reported (DeFazio et al. 2006). These findings are consistent with our observation that ω-agatoxin blocks the ubiquitous KCl responses in presynaptic cells (Fig. 6).
Figure 8. Presynaptic cells that lack GAD expression preferentially express L-type (α1C) channels.
A, taste buds from GAD-GFP mice were dissociated into individual cells and loaded with Fura-2. Cells were viewed under bright field and fluorescence illumination. The images are shown overlaid. Scale bar, 50 μm. B, responses of a GAD-GFP(+) and a GAD-GFP(−) taste cell (arrows in A) to bath-applied KCl. Ca2+ responses evoked by KCl serve as a functional marker for presynaptic cells (Type III cells). Responses from GAD-GFP(+) cells were typically larger than from GAD-GFP(–) cells, as in these examples. Y-axis scale bar, F340/F380. C, individual presynaptic cells, collected after imaging, were tested by RT-PCR. Of the 18 cells analysed, 5 representative cells are shown. D, aggregate data on RT-PCR based expression of voltage-gated Ca2+ channels. All 18 cells expressed SNAP25 (not shown), as expected for presynaptic cells. GAD-GFP(+) and (–) cells showed significantly different incidence of Cav1.2 Ca2+ channel expression (P= 0.015; Fisher's exact test).
As expected, depolarization-triggered responses were detected in both GFP(+) and GFP(−) taste cells (Fig. 8B). Thus, we isolated 18 additional presynaptic cells. Of these, 10 were GAD-GFP(+) and 8 were GAD-GFP(−). We divided each single-cell cDNA into aliquots to assay for expression of several genes: SNAP25 (as a positive control), Cav2.1 and Cav1.2, both of which were implicated in KCl or Fsk+IBMX responses by our pharmacological dissection (Figs 5 and 6). We did not assay for Cav2.2 (which produces N-type channels) as its mRNA appeared to be less abundant, and our pharmacological analysis did not suggest a substantial contribution of N-type channels to the Ca2+ transients we examined here. As expected for presynaptic cells, all 18 cells expressed SNAP25. Most cells also expressed Cav2.1 (Fig. 8C and D). The incidence of Cav2.1 expression was not significantly different across GAD-GFP(+) and GAD-GFP(−) presynaptic cells (P= 0.44; Fisher's exact test; Fig. 8D). In striking contrast, Cav1.2 was detected in nearly all (7 of 8) GAD-GFP(−) cells, but only in 20% (2 of 10) of the GAD-GFP(+) cells (Fig. 8D). This difference was significant (P= 0.015; Fisher's exact test). The molecular distribution of Ca2+ channel subunits is entirely consistent with our pharmacologically based interpretation (Fig. 5) that Fsk+IBMX responses are mediated via L-type (Cav1.2) channels and that such responses are primarily in GAD-GFP(−) cells (Fig. 2).
Discussion
The findings show that approximately one-third of presynaptic cells (Type III cells) respond to forskolin stimulation with an influx of Ca2+ through L-type voltage gated Ca2+ channels (Cav1.2). These cells are characterized by the absence of glutamic acid decarboxylase (GAD), the biosynthetic enzyme for the neurotransmitter GABA, and thus, GAD-GFP labelling cannot be used to identify forskolin-sensitive taste cells. The similar fractions of presynaptic cells that lack GAD (∼30%; Tomchik et al. 2007) and that show cAMP-induced Ca2+ elevation (38%, here) suggest that GAD-lacking presynaptic cells may represent a functionally distinct set of cells within the taste bud. Bernhardt et al. (1996) also reported Ca2+ influx into some rat taste bud cells following forskolin stimulation, but these cells were not defined with respect to either molecular markers or other functional properties. The implication of our findings is that in certain presynaptic cells, an increase in cAMP elevates intracellular Ca2+, thereby linking the two major second messenger systems – cAMP and Ca2+– in taste buds. Our results implicate L-type Ca2+ channels and PKA as mediators of this linkage. A distinctively different interplay between cAMP and Ca2+ occurs in the other major cell type in taste buds. In the receptor cells (Type II cells), cAMP-and PKA-mediated phosphorylation of Ca2+-release machinery, likely to be PLCβ2 and IP3R3, damps taste-evoked Ca2+ signals (Clapp et al. 2008).
Voltage-gated Ca2+ channels are composed of a principal pore-forming (α1) subunit and a number of accessory (α2δ, β and γ) subunits that regulate the functional properties of the channels (reviewed in Catterall, 2000; Ertel et al. 2000; Catterall et al. 2005). Of the high-threshold channels, N-and P/Q-type channels typically are located at presynaptic terminals where they mediate the influx of Ca2+ that triggers vesicular release of transmitters (Westenbroek et al. 1995). Indeed, the P/Q channel subunit, Cav2.1 (also called α1A) interacts directly with the synaptic vesicle machinery, including SNAP25 (Rettig et al. 1996). We note that both Cav2.1 and SNAP25 are expressed in most presynaptic cells (Fig. 8; and DeFazio et al. 2006). The Cav1.2 subunit (also called α1C) produces high-threshold L-type currents, and is central to excitation–contraction coupling in cardiac and smooth myocytes and excitation–secretion coupling in pancreatic β-cells. In neurons, Cav1.2 is principally located in the soma and proximal dendritic compartments (Hell et al. 1993), where it is thought to orchestrate aspects of synaptic integration as well as activity-dependent regulation of gene expression. The various α1 subunits are differentially effective in triggering transmitter vesicle fusion in neurons, with Cav2.1 (P/Q-type) and Cav2.2 (N-type) being efficient, Cav2.3 (R-type) less so, and Cav1.2 (L-type) being ineffective for transmitter release (Mochida et al. 2003). T-type channels require much smaller depolarizations for activation (i.e. are low threshold) compared to the L-, P/Q-or N-types and employ Cav3.n family subunits. These channels are found in many neurons and other cells and the rapidly inactivating, transient currents serve to regulate firing patterns and shape action potentials (Perez-Reyes, 2003). We previously reported (DeFazio et al. 2006) that of the seven high-threshold voltage-gated Ca2+ channels, only three are expressed in a taste bud-selective pattern – Cav1.2 (also called α1C, and producing L-type currents), Cav2.1 (α1A, P/Q-type currents) and Cav2.2 (α1B, N-type currents). Here, we have refined our previous analyses by showing the broad expression of Cav2.1 across presynaptic cells, and the restricted expression pattern of Cav1.2.
In a subset of presynaptic cells, we report that forskolin stimulation activates PKA to enhance Ca2+ influx through L-type voltage-gated Ca2+ channels. Regulation of L-type (especially Cav1.2), but not P/Q-type, channels by PKA phosphorylation is well documented in cardiac and skeletal muscle, neurons, and chromaffin cells (reviewed in McDonald et al. 1994). In patch-clamp recordings, some cells in frog and rat taste buds are strongly depolarized by cAMP elevation, an effect attributed to PKA-mediated block of a K+ current (Tonosaki & Funakoshi, 1988; Avenet et al. 1988; Cummings et al. 1993, 1996). In intact tongue preparations, membrane-permeant cAMP analogues produce bursts of action potentials in taste buds and/or afferent nerves (Cummings et al. 1993). Thus, we postulate that cAMP and PKA, acting on both K+ and Ca2+ channels accounts for the cAMP-triggered Ca2+-influx that we have recorded here. The block of K+ channels and ensuing action potentials would produce a strong depolarization; P/Q-type and PKA-phosphorylated L-type channels would together produce an influx of calcium. In contrast, the depolarization induced by 50 mm KCl in our experiments is more modest (−25 mV calculated) and slow in onset due to bath perfusion. This would fail to trigger action potentials (Gilbertson et al. 2001). The activation threshold for P/Q channels tends to be 10–30 mV more negative than for L channels, depending on which β and α2d subunits are co-expressed (e.g. Stea et al. 1994; Bourinet et al. 1994). Hence, KCl may preferentially activate the lower-threshold P/Q channels, resulting in Ca2+ influx that is mostly insensitive to nifedipine.
Our study does not address the source of cAMP elevation in these taste cells. The taste-selective Gα protein, α-gustducin, is believed to decrease cAMP when activated. This primarily derives from its sequence similarity to Gα-transducin (McLaughlin et al. 1992) and its ability to stimulate retinal phosphodiesterase in vitro (Ruiz-Avila et al. 2000). However, Gα-gustducin is expressed only in a subset of receptor cells (Type II cells) (Clapp et al. 2001; Perez et al. 2002). Hence, gustducin would not be expected to modulate cAMP in presynaptic cells. One possibility is that cAMP is synthesized when ligands, other than taste stimuli, act on taste cell GPCRs. For instance, catecholaminergic fibres penetrate into mouse taste buds and probably secrete noradrenaline (Dvoryanchikov et al. 2007). β-Adrenergic receptors, some of which couple via Gαs to cAMP elevation, might be a physiological trigger for cAMP-PKA-mediated Ca2+ influx in presynaptic cells (Herness et al. 2002). Taste cells also express other GPCRs known to modulate cAMP, including receptors for neuropeptide Y, glucagon-like peptide-1 and serotonin (Kaya et al. 2004; Zhao et al. 2005; Shin et al. 2008).
In chromaffin cells, elevating cAMP and activating PKA enhances catecholamine secretion (Carabelli et al. 2003; Polo-Parada et al. 2006). Indeed, membrane-permeant cAMP, applied globally to taste epithelium was shown to enhance some taste-evoked afferent nerve responses in the rat (Lyall et al. 2002). Our findings may thus suggest a role for cAMP signalling in modulating the secretion of amine neurotransmitters from presynaptic cells (Type III cells). Presynaptic cells in situ are broadly tuned and respond to stimulation with multiple taste qualities (Tomchik et al. 2007). These cells express synaptic proteins, form specialized chemical synapses with afferent nerve fibres and release serotonin and noradrenaline (Yang et al. 2000; Clapp et al. 2006; DeFazio et al. 2006; Huang et al. 2007; Huang et al. 2008). How these findings relate to a coherent picture of signal processing in taste buds, however, remains to be determined.
The question that remains is does taste signalling for sweet, bitter and umami in taste receptor cells (Type II cells) lead to cAMP changes in adjacent presynaptic cells (Type III cells), and if so, does this modulate transmitter release from presynaptic cells? Recent evidence demonstrates that sweet, bitter and umami tastes stimulate receptor cells to secrete ATP and that secreted ATP excites adjacent presynaptic cells (Huang et al. 2007). However, whether ATP stimulation of presynaptic cells leads to changes in cAMP, and whether cAMP elevation alters transmitter release remain unknown at the present time.
Acknowledgments
This work was supported by grants from the National Institutes of Health: F31DC007591 (C.D.R.), R01DC000374 (S.D.R.), and R01DC006021 and R01DC006308 (N.C).
Glossary
Abbreviations
- cAMP
3′,5′-cyclic adenosine monophosphate
- Fsk
forskolin
- GAD
glutamic acid decarboxylase 67
- GFP
green fluorescent protein
- GPCR
G protein-coupled receptor
- IBMX
3-isobutyl-1-methylxanthine
- PLCβ2
phospholipase Cβ2
Author Contributions
C.R. carried out functional imaging experiments, analysed data, and drafted portions of the manuscript; G.D. conducted the molecular biological experiments, analysed data and drafted portions of the manuscript; S.R. and N.C. analysed and interpreted data and critically revised the manuscript. All four authors participated in the conception and design of the study and approved the final version of the manuscript to be published. All experiments were carried out at the University of Miami School of Medicine in Miami, FL, USA.
References
- Abaffy T, Trubey KR, Chaudhari N. Adenylyl cyclase expression and modulation of cAMP in rat taste cells. Am J Physiol Cell Physiol. 2003;284:C1420–C1428. doi: 10.1152/ajpcell.00556.2002. [DOI] [PubMed] [Google Scholar]
- Avenet P, Hofmann F, Lindemann B. Signalling in taste receptor cells: cAMP-dependent protein kinase causes depolarization by closure of 44 pS K-channels. Comp Biochem Physiol A Comp Physiol. 1988;90:681–685. doi: 10.1016/0300-9629(88)90684-6. [DOI] [PubMed] [Google Scholar]
- Bernhardt SJ, Naim M, Zehavi U, Lindemann B. Changes in IP3 and cytosolic Ca2+ in response to sugars and non-sugar sweeteners in transduction of sweet taste in the rat. J Physiol. 1996;490:325–336. doi: 10.1113/jphysiol.1996.sp021147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourinet E, Charnet P, Tomlinson WJ, Stea A, Snutch TP, Nargeot J. Voltage-dependent facilitation of a neuronal α1C L-type calcium channel. EMBO J. 1994;13:5032–5039. doi: 10.1002/j.1460-2075.1994.tb06832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carabelli V, Giancippoli A, Baldelli P, Carbone E, Artalejo AR. Distinct potentiation of L-type currents and secretion by cAMP in rat chromaffin cells. Biophys J. 2003;85:1326–1337. doi: 10.1016/S0006-3495(03)74567-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol Rev. 2005;57:411–425. doi: 10.1124/pr.57.4.5. [DOI] [PubMed] [Google Scholar]
- Chattopadhyaya B, Di CG, Higashiyama H, Knott GW, Kuhlman SJ, Welker E, Huang ZJ. Experience and activity-dependent maturation of perisomatic GABAergic innervation in primary visual cortex during a postnatal critical period. J Neurosci. 2004;24:9598–9611. doi: 10.1523/JNEUROSCI.1851-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp TR, Medler KF, Damak S, Margolskee RF, Kinnamon SC. Mouse taste cells with G protein-coupled taste receptors lack voltage-gated calcium channels and SNAP-25. BMC Biol. 2006;4:7. doi: 10.1186/1741-7007-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp TR, Stone LM, Margolskee RF, Kinnamon SC. Immunocytochemical evidence for co-expression of Type III IP3 receptor with signaling components of bitter taste transduction. BMC Neurosci. 2001;2:6. doi: 10.1186/1471-2202-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp TR, Trubey KR, Vandenbeuch A, Stone LM, Margolskee RF, Chaudhari N, Kinnamon SC. Tonic activity of Gα-gustducin regulates taste cell responsivity. FEBS Lett. 2008;582:3783–3787. doi: 10.1016/j.febslet.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings TA, Daniels C, Kinnamon SC. Sweet taste transduction in hamster: sweeteners and cyclic nucleotides depolarize taste cells by reducing a K+ current. J Neurophysiol. 1996;75:1256–1263. doi: 10.1152/jn.1996.75.3.1256. [DOI] [PubMed] [Google Scholar]
- Cummings TA, Powell J, Kinnamon SC. Sweet taste transduction in hamster taste cells: evidence for the role of cyclic nucleotides. J Neurophysiol. 1993;70:2326–2336. doi: 10.1152/jn.1993.70.6.2326. [DOI] [PubMed] [Google Scholar]
- DeFazio RA, Dvoryanchikov G, Maruyama Y, Kim JW, Pereira E, Roper SD, Chaudhari N. Separate populations of receptor cells and presynaptic cells in mouse taste buds. J Neurosci. 2006;26:3971–3980. doi: 10.1523/JNEUROSCI.0515-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvoryanchikov G, Tomchik SM, Chaudhari N. Biogenic amine synthesis and uptake in rodent taste buds. J Comp Neurol. 2007;505:302–313. doi: 10.1002/cne.21494. [DOI] [PubMed] [Google Scholar]
- Ertel EA, Campbell KP, Harpold MM, Hofmann F, Mori Y, Perez-Reyes E, Schwartz A, Snutch TP, Tanabe T, Birnbaumer L, Tsien RW, Catterall WA. Nomenclature of voltage-gated calcium channels. Neuron. 2000;25:533–535. doi: 10.1016/s0896-6273(00)81057-0. [DOI] [PubMed] [Google Scholar]
- Gilbertson TA, Boughter JD, Jr, Zhang H, Smith DV. Distribution of gustatory sensitivities in rat taste cells: whole-cell responses to apical chemical stimulation. J Neurosci. 2001;21:4931–4941. doi: 10.1523/JNEUROSCI.21-13-04931.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hell JW, Westenbroek RE, Warner C, Ahlijanian MK, Prystay W, Gilbert MM, Snutch TP, Catterall WA. Identification and differential subcellular localization of the neuronal class C and class D L-type calcium channel alpha 1 subunits. J Cell Biol. 1993;123:949–962. doi: 10.1083/jcb.123.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herness MS, Sun XD, Chen YS. cAMP and forskolin inhibit potassium currents in rat taste receptor cells by different mechanisms. Am J Physiol Cell Physiol. 1997;272:C2005–C2018. doi: 10.1152/ajpcell.1997.272.6.C2005. [DOI] [PubMed] [Google Scholar]
- Herness S, Zhao FL, Kaya N, Lu SG, Shen T, Sun XD. Adrenergic signalling between rat taste receptor cells. J Physiol. 2002;543:601–614. doi: 10.1113/jphysiol.2002.020438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YA, Maruyama Y, Roper SD. Norepinephrine is coreleased with serotonin in mouse taste buds. J Neurosci. 2008;28:13088–13093. doi: 10.1523/JNEUROSCI.4187-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. The role of pannexin 1 hemichannels in ATP release and cell-cell communication in mouse taste buds. Proc Natl Acad Sci U S A. 2007;104:6436–6441. doi: 10.1073/pnas.0611280104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YJ, Maruyama Y, Lu KS, Pereira E, Plonsky I, Baur JE, Wu D, Roper SD. Mouse taste buds use serotonin as a neurotransmitter. J Neurosci. 2005;25:843–847. doi: 10.1523/JNEUROSCI.4446-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya N, Shen T, Lu SG, Zhao FL, Herness S. A paracrine signaling role for serotonin in rat taste buds: expression and localization of serotonin receptor subtypes. Am J Physiol Regul Integr Comp Physiol. 2004;286:R649–R658. doi: 10.1152/ajpregu.00572.2003. [DOI] [PubMed] [Google Scholar]
- Kim JW, Roberts C, Maruyama Y, Berg S, Roper S, Chaudhari N. Faithful expression of GFP from the PLCβ2 promoter in a functional class of taste receptor cells. Chem Senses. 2006;31:213–219. doi: 10.1093/chemse/bjj021. [DOI] [PubMed] [Google Scholar]
- Liu D, Liman ER. Intracellular Ca2+ and the phospholipid PIP2 regulate the taste transduction ion channel TRPM5. Proc Natl Acad Sci U S A. 2003;100:15160–15165. doi: 10.1073/pnas.2334159100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall V, Alam RI, Phan TH, Phan DQ, Heck GL, DeSimone JA. Excitation and adaptation in the detection of hydrogen ions by taste receptor cells: a role for cAMP and Ca2+ J Neurophysiol. 2002;87:399–408. doi: 10.1152/jn.00331.2001. [DOI] [PubMed] [Google Scholar]
- McDonald TF, Pelzer S, Trautwein W, Pelzer DJ. Regulation and modulation of calcium channels in cardiac, skeletal, and smooth muscle cells. Physiol Rev. 1994;74:365–507. doi: 10.1152/physrev.1994.74.2.365. [DOI] [PubMed] [Google Scholar]
- McLaughlin SK, McKinnon PJ, Margolskee RF. Gustducin is a taste-cell-specific G protein closely related to the transducins. Nature. 1992;357:563–569. doi: 10.1038/357563a0. [DOI] [PubMed] [Google Scholar]
- McLaughlin SK, McKinnon PJ, Spickofsky N, Danho W, Margolskee RF. Molecular cloning of G proteins and phosphodiesterases from rat taste cells. Physiol Behav. 1994;56:1157–1164. doi: 10.1016/0031-9384(94)90360-3. [DOI] [PubMed] [Google Scholar]
- Medler KF, Margolskee RF, Kinnamon SC. Electrophysiological characterization of voltage-gated currents in defined taste cell types of mice. J Neurosci. 2003;23:2608–2617. doi: 10.1523/JNEUROSCI.23-07-02608.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochida S, Westenbroek RE, Yokoyama CT, Itoh K, Catterall WA. Subtype-selective reconstitution of synaptic transmission in sympathetic ganglion neurons by expression of exogenous calcium channels. Proc Natl Acad Sci U S A. 2003;100:2813–2818. doi: 10.1073/pnas.262787299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama K, Bakre MM, Ahmed F, Spickofsky N, Max M, Margolskee RF. Assaying G protein-phosphodiesterase interactions in sensory systems. Methods Enzymol. 2002;345:37–48. doi: 10.1016/s0076-6879(02)45005-7. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Nakashima K, Fukuda A, Nishino H, Sugimura T, Hino A, Danilova V, Hellekant G. Responses to umami substances in taste bud cells innervated by the chorda tympani and glossopharyngeal nerves. J Nutr. 2000;130:950S–953S. doi: 10.1093/jn/130.4.950S. [DOI] [PubMed] [Google Scholar]
- Ogura T, Margolskee RF, Kinnamon SC. Taste receptor cell responses to the bitter stimulus denatonium involve Ca2+ influx via store-operated channels. J Neurophysiol. 2002;87:3152–3155. doi: 10.1152/jn.2002.87.6.3152. [DOI] [PubMed] [Google Scholar]
- Perez CA, Huang L, Rong M, Kozak JA, Preuss AK, Zhang H, Max M, Margolskee RF. A transient receptor potential channel expressed in taste receptor cells. Nat Neurosci. 2002;5:1169–1176. doi: 10.1038/nn952. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes E. Molecular physiology of low-voltage-activated t-type calcium channels. Physiol Rev. 2003;83:117–161. doi: 10.1152/physrev.00018.2002. [DOI] [PubMed] [Google Scholar]
- Polo-Parada L, Chan SA, Smith C. An activity-dependent increased role for L-type calcium channels in exocytosis is regulated by adrenergic signaling in chromaffin cells. Neurosci. 2006;143:445–459. doi: 10.1016/j.neuroscience.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Rettig J, Sheng ZH, Kim DK, Hodson CD, Snutch TP, Catterall WA. Isoform-specific interaction of the α1A subunits of brain Ca2+ channels with the presynaptic proteins syntaxin and SNAP-25. Proc Natl Acad Sci U S A. 1996;93:7363–7368. doi: 10.1073/pnas.93.14.7363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanov RA, Rogachevskaja OA, Bystrova MF, Jiang P, Margolskee RF, Kolesnikov SS. Afferent neurotransmission mediated by hemichannels in mammalian taste cells. EMBO J. 2007;26:657–667. doi: 10.1038/sj.emboj.7601526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper SD. Signal transduction and information processing in mammalian taste buds. Pflugers Arch. 2007;454:759–776. doi: 10.1007/s00424-007-0247-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Avila L, Ming D, Margolskee RF. An in vitro assay useful to determine the potency of several bitter compounds. Chem Senses. 2000;25:361–368. doi: 10.1093/chemse/25.4.361. [DOI] [PubMed] [Google Scholar]
- Shin YK, Martin B, Golden E, Dotson CD, Maudsley S, Kim W, Jang HJ, Mattson MP, Drucker DJ, Egan JM, Munger SD. Modulation of taste sensitivity by GLP-1 signaling. J Neurochem. 2008;106:455–463. doi: 10.1111/j.1471-4159.2008.05397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spickofsky N, Robichon A, Danho W, Fry D, Greeley D, Graves B, Madison V, Margolskee RF. Biochemical analysis of the transducin-phosphodiesterase interaction. Nat Struct Biol. 1994;1:771–781. doi: 10.1038/nsb1194-771. [DOI] [PubMed] [Google Scholar]
- Stea A, Tomlinson WJ, Soong TW, Bourinet E, Dubel SJ, Vincent SR, Snutch TP. Localization and functional properties of a rat brain α1A calcium channel reflect similarities to neuronal Q-xsand P-type channels. Proc Natl Acad Sci U S A. 1994;91:10576–10580. doi: 10.1073/pnas.91.22.10576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striem BJ, Naim M, Lindemann B. Generation of cyclic AMP in taste buds of the rat circumvallate papilla in response to sucrose. Cell Physiol Biochem. 1991;1:46–54. [Google Scholar]
- Tomchik SM, Berg S, Kim JW, Chaudhari N, Roper SD. Breadth of tuning and taste coding in mammalian taste buds. J Neurosci. 2007;27:10840–10848. doi: 10.1523/JNEUROSCI.1863-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonosaki K, Funakoshi M. Cyclic nucleotides may mediate taste transduction. Nature. 1988;331:354–356. doi: 10.1038/331354a0. [DOI] [PubMed] [Google Scholar]
- Trubey KR, Culpepper S, Maruyama Y, Kinnamon SC, Chaudhari N. Tastants evoke cAMP signal in taste buds that is independent of calcium signaling. Am J Physiol Cell Physiol. 2006;291:C237–C244. doi: 10.1152/ajpcell.00303.2005. [DOI] [PubMed] [Google Scholar]
- Westenbroek RE, Sakurai T, Elliott EM, Hell JW, Starr TVB, Snutch TP, Catterall WA. Immunochemical identification and subcellular distribution of the α1A subunits of brain calcium channels. J Neurosci. 1995;15:6403–6418. doi: 10.1523/JNEUROSCI.15-10-06403.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W, Sunavala G, Rosenzweig S, Dasso M, Brand JG, Spielman AI. Bitter taste transduced by PLC-β2-dependent rise in IP3 and α-gustducin-dependent fall in cyclic nucleotides. Am J Physiol Cell Physiol. 2001;280:C742–C751. doi: 10.1152/ajpcell.2001.280.4.C742. [DOI] [PubMed] [Google Scholar]
- Yang R, Crowley HH, Rock ME, Kinnamon JC. Taste cells with synapses in rat circumvallate papillae display SNAP-25-like immunoreactivity. J Comp Neurol. 2000;424:205–215. doi: 10.1002/1096-9861(20000821)424:2<205::aid-cne2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Yang R, Stoick CL, Kinnamon JC. Synaptobrevin-2-like immunoreactivity is associated with vesicles at synapses in rat circumvallate taste buds. J Comp Neurol. 2004;471:59–71. doi: 10.1002/cne.20021. [DOI] [PubMed] [Google Scholar]
- Yee CL, Yang R, Bottger B, Finger TE, Kinnamon JC. “Type III” cells of rat taste buds: immunohistochemical and ultrastructural studies of neuron-specific enolase, protein gene product 9.5, and serotonin. J Comp Neurol. 2001;440:97–108. doi: 10.1002/cne.1372. [DOI] [PubMed] [Google Scholar]
- Zhao FL, Shen T, Kaya N, Lu SG, Cao Y, Herness S. Expression, physiological action, and coexpression patterns of neuropeptide Y in rat taste-bud cells. Proc Natl Acad Sci U S A. 2005;102:11100–11105. doi: 10.1073/pnas.0501988102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJ, Zuker CS. The receptors for mammalian sweet and umami taste. Cell. 2003;115:255–266. doi: 10.1016/s0092-8674(03)00844-4. [DOI] [PubMed] [Google Scholar]