Abstract
The mechanisms by which the nucleus accumbens integrates afferent input from limbic and cortical structures have been influential in the development of models of psychiatric disorders such as schizophrenia. Previous studies of the response of nucleus accumbens (Nacb) cells to the stimulation of afferent inputs from hippocampus (HC) and prefrontal cortex (PFC) have demonstrated that PFC throughput can be modulated by preceding HC input. Examination of the post-synaptic potential size has suggested, however, that summation of these inputs is sublinear. All studies to date examining Nacb integration of inputs via stimulation of afferents have been performed in the anaesthetized rat. The present experiments compare the response of Nacb cells to different combinations of PFC and HC stimulation in awake and isoflurane-anaesthetized rats that were chronically implanted with both stimulating and recording electrodes. The results of these experiments suggest that summation of afferent input in the Nacb of the awake rat is predominantly sublinear, with only a minority of neurons demonstrating modulation of PFC inputs by the HC in the awake or the anaesthetized animal. The response profile of many cells changed during anaesthesia when compared to the awake condition, and on average showed suppression to PFC input 50 and 150 ms following HC stimulation while under deep isoflurane anaesthesia. These results suggest that sublinear integration of afferent input from the PFC and HC is the dominant mode of integration of Nacb cells in the awake animal, which has implications for corticostriatal models of psychiatric dysfunction.
The nucleus accumbens (Nacb) is part of the ventral striatum and receives input from most of the limbic system and related structures including the prefrontal cortex (PFC), the hippocampus (HC), the amygdala and the thalamus (Pennartz et al. 2004). The predominant cells in the Nacb are the medium spiny neurons (MSP), which integrate inputs from a subset or all of the afferent structures depending on their location in the Nacb (Finch, 1996; Groenewegen et al. 1999). The Nacb also receives a large dopaminergic projection from the ventral tegmental area (Swanson, 1982), and strong experimental evidence supports the notion that dopamine (DA) modulation in the Nacb is involved in the processing of reward-related information, salience, novelty, as well as motivational state (Salamone & Correa, 2002; Kelley, 2004; Kalivas & Volkow, 2005).
Previous studies examining the integration of afferent input in the ventral striatum have focused on the integration of PFC and HC inputs and have utilized intracellular recordings in the anaesthetized animal (O’Donnell & Grace, 1995; Goto & O’Donnell, 2002). MSP cells under anaesthetized conditions or in slices in vitro (Vergara et al. 2003), have a bimodal membrane potential predominantly determined by their afferent inputs, a hyperpolarized or ‘down’ state and a depolarized or ‘up’ state (Wilson & Kawaguchi, 1996). These states are similar to the slow oscillations first described in cerebral cortex and thalamus which characterize slow wave sleep (Steriade et al. 1993) but which are not present in the awake state (Steriade, 2001). These membrane states in the Nacb MSP neurons have been suggested to function as a binary gate that allows the flow of information only while in the depolarized state (O’Donnell & Grace, 1995; Grace, 2000). One of the most important functional consequences of the gating models is that alterations in gating have been proposed as part of the pathophysiology of cognitive disorders such as schizophrenia (Grace, 2000).
The HC has been proposed as the mediator of the state transitions by promoting the transition to the ‘up’ state, which would then allow information from other areas, particularly the PFC, to flow through the Nacb (O’Donnell & Grace, 1995; Grace, 2000). Stimulation of the fimbria-fornix (FF) was demonstrated to shift MSP cells to the depolarized state, at which point the PFC stimulation could then induce spike output. However, more recent studies of excitatory post-synaptic potentials (EPSP) have demonstrated sublinear summation in the MSP neurons, suggesting that spike output may also sum sublinearly (Goto & O’Donnell, 2002). There have also been recent reports that stimulation of the PFC alone can induce spike responses in the Nacb under anaesthesia, suggesting that the response to PFC inputs is not exclusively HC dependent (Gruber et al. 2009).
In order to examine the rules of input integration in Nacb MSP cells in the awake rat, we implanted recording electrodes into the rat Nacb, and stimulating electrodes in the PFC and FF (or ventral HC). We then stimulated these afferents at different amplitudes and in different orders while recording extracellularly in the Nacb. These experiments were then repeated under deep isoflurane anaesthesia in order to test whether the response profiles of the Nacb cells are affected by anaesthesia.
Rather than a robust gating phenomenon, we found that most Nacb cells in the awake animal integrate inputs sublinearly, with a small subset displaying supralinear responses. The results were similar under isoflurane anaesthesia, but with a drastically reduced response level to most stimulations. These results are in agreement with previous sublinear summation reported earlier for EPSPs. However, the results suggest that in the awake animal spike output in response to input from the HC and the PFC summates sublinearly, which may require a revision of hypotheses based upon supralinear summation in the Nacb.
Methods
Surgery
Surgery was performed under University of Pennsylvania institutional animal care and use committee (IACUC) approved protocols. Rats were given ketaprofen (s.c.) prior to surgery, bupivicaine (s.c.) locally at the incision site, and were anaesthetized during surgery with isoflurane inhalation anaesthesia. Post-operative care included ketaprophen (s.c.) for analgesia.
Chronic recordings
Tetrodes were constructed from tungsten wire (12.5 μm diameter) coated with Form-Var insulation (California Fine Wire, Grover Beach, CA, USA) and plated with gold to an impedance of 300–500 kΩ. These were loaded into a drive (Neuralynx, AZ, USA) capable of holding 12 individually moveable tetrodes (Jog et al. 2002) and connected to a custom-designed electrode interface board (EIB-57, Neuralynx) that allowed for stimulation as well as recording in the same animal. The drive was implanted into male Sprague–Dawley rats (n= 6) under isoflurane anaesthesia such that the centre of the cannula (1 mm diameter) through which the tetrodes are driven was located at 1.4 mm antero-posterior (AP) from bregma and 0.9 mm medio-lateral (ML). This location ensured that advancement of tetrodes by 6.4–7.4 mm from the cortical surface positioned all tetrodes in the Nacb, which was later confirmed by passing current through each tetrode and verifying the location with histology. Descent records of the electrode travel were kept as well. Tetrodes were adjusted by at least 300 μm each day in order to avoid recording from the same cell on consecutive sessions. A separate lower impedance (50 kΩ) reference wire was advanced into the white matter and was used as the reference for all tetrode channels.
The drive was connected to a unity-gain headstage (ERP-27, Neuralynx) and then an 80-channel commutator (Neuralynx) that allowed for free rotation of the cables when torque was applied by the rat. The channels from the commutator are connected to the Cheetah Data Acquisition system on which spike channels were acquired at 30 kHz, while local field potentials were acquired at 3 kHz.
Stimulation
Electrical stimulation was delivered in a bipolar configuration through pairs of tungsten electrodes (FHC, ME, ∼50 kΩ) affixed together with dental acrylic and having tip separations of 500 μm in both the dorso–ventral (DV) axis and ML axis for the PFC and the ventral HC electrodes, and 500 μm offset only in the ML axis for the electrode placed in the fimbria-fornix (FF). Two bipolar stimulating electrodes were implanted in each rat. In five rats the PFC electrodes were at the border of the infralimbic and prelimbic cortex (3.2 AP, 0.6 ML, −4.2 DV) (Paxinos & Watson, 1998) and in the FF (−1.4 AP, 1.6 ML −4.0 DV). In two additional rats the FF stimulating electrode was substituted for an electrode located in the ventral CA1 (−6.0 AP, 5.3 ML, −6.8 DV) (Fig. 1). To assure positioning of the FF electrode during surgery, we stimulated through it while recording the well-known LFP response it evokes in HC (Boeijinga et al. 1993). All stimulating electrode locations were verified histologically by the electrode tracks and by small lesions induced by current (50 μA for 5 s) 3–4 days prior to killing of the animal.
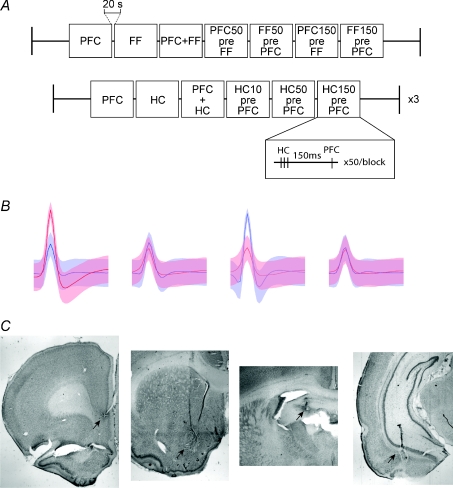
Figure 1. Stimulation protocols, example units, and electrode locations.
A, afferent areas of the nucleus accumbens were stimulated in blocks. The first protocol was tested under both awake and isoflurane conditions, while the second was repeated at three different stimulation amplitudes in the awake condition only. In the first protocol, either the fimbria-fornix (FF), the prefrontal cortex (PFC), both, or one followed by the other 50 or 150 ms apart, were stimulated. These were done in blocks of 50, with 5 s between stimuli and 20 s between blocks. In the second protocol, hippocampal CA1 (HC) or PFC were stimulated separately or simultaneously, or the HC was stimulated 10, 50 and 150 ms prior to the PFC. An example of one of the stimulations is inset, demonstrating the three HC stimulations (100 Hz) prior to the PFC stimulation with a 150 ms separation. B, an example of two cells recorded from the same tetrode, with each of four wires represented. The standard deviation of the waveform average is signified by the shaded areas. Each waveform is 1 ms in length. C, the locations of lesions from implanted stimulation and recording electrodes (arrows). From left to right: bipolar stimulating electrode at the border of the pre-and infra-limbic cortex, tetrode locations in the Nacb, bipolar stimulating electrode in the fimbria-fornix, and the ventral hippocampus (see Methods).
Rats were kept awake and free to move in a small enclosure (∼30 cm by 30 cm) during stimulation and recording. Electrical stimulation was applied as blocks of 50 stimuli (Fig. 1). Stimulation blocks were applied in the following order: PFC alone, FF alone, PFC and FF simultaneously, PFC 50 ms prior to FF (PFC50preFF), FF 50 ms prior to PFC (FF50prePFC), PFC 150 ms prior to FF (PFC150preFF) and FF 150 ms prior to PFC (FF50prePFC). For the blocks with paired stimulation, the first area was stimulated with short trains of three stimuli at 100 Hz at half the intensity of single stimuli (in order to avoid inducing epileptiform activity). The stimulation protocol for animals implanted in the ventral CA1 was similar, but was modified as follows: the PFC stimulation prior to the HC was removed, and an additional condition of HC stimulation 10 ms prior to the PFC was added (awake condition only). An interstimulus interval of 5 s was utilized to avoid plasticity (verified by stable evoked LFP response over time – data not shown), and 20 s elapsed between each block of stimulations. Stimulation amplitude was set at 1.0 mA for both electrodes in order to elicit large responses in the FF/PFC stimulations across all animals, and ranged from 0.25 mA to 1.0 mA in the CA1/PFC stimulation experiments. All stimulations were 200 μs in duration. Stimulation protocols were written in MATLAB (Natick, MA, USA), and combined with a LabView interface to a NIDAQ A/D board (National Instruments, Austin, TX, USA) and applied through a stimulus isolation unit (Model PS106, Grass Instruments, Warwick, RI, USA). Stimulation current was passed through the commutator directly to the stimulating electrode via a custom headstage interface board (EIB-57, Neuralynx).
Data analysis
Waveforms crossing set thresholds (55 μV) were captured via the A/D card and analysed off-line. Potential cells were first identified using automated clustering software utilizing peak and trough feature sets (KlustaKwik). These clusters were then examined manually for waveform shape in order to discard non-cell clusters, and combine clusters that captured waveforms from the same cell. These clusters were further refined by hand upon examination of the interspike intervals (Supplementary Fig. S2) (SpikeSort3D, Neuralynx). An example of spike waveforms on the four wires of a single tetrode is demonstrated in Fig. 1B. Most tetrodes had one to three separable cells. Clusters that were not easily separable were discarded to avoid contamination of one cell with another. To quantify the spike responses to the various stimulation protocols we constructed peri-stimulus time histograms (PSTHs) using MATLAB routines. Responses only within 30 ms of the stimulation were binned. Statistical analysis was performed using Friedman's non-parametric test followed by Dunn's post test.
Results
Our goal was to quantify the interaction of afferent inputs to the nucleus accumbens (Nacb) originating in prefrontal cortex (PFC) and hippocampus (HC). More specifically, we wanted to test the hypothesis that hippocampal inputs give rise to a short time window during which prefrontal cortex inputs to the Nacb become suprathreshold for spike generation. According to this hypothesis, the HC acts as a gate for PFC throughput. We stimulated electrically both afferent structures at different time intervals while recording single neurons in the Nacb using tetrodes. Brief (200 μs) current pulses were delivered to the medial portion of the PFC and the fimbria-fornix (FF) or ventral CA1 (HC) of chronically implanted rats (n= 4, PFC/FF; n= 3, PFC/HC). Stimuli were delivered to each structure in isolation or to both structures at interstimulus intervals of 0, 10, 50 and 150 ms (Fig. 1 and Supplementary Fig. S1). To agree with published experimental protocols (O'Donnell & Grace, 1995) and to maximize the possibility of observing gating, we used short trains of three stimuli at 100 Hz instead of single shocks for the first stimulated structure in all paired protocols. In addition, because previous studies of gating in Nacb have been conducted under anaesthesia, all our stimuli were applied during the waking state and then repeated under isoflurane anaesthesia in each animal in order to compare the two conditions. Cells in the Nacb that had a response ratio of greater than 10% to either FF or PFC stimulation in the awake condition were included in the analysis (n= 49, PFC/FF; n= 67, PFC/HC). The mean frequency during the period prior to stimulation was 2.50 ± 0.62 Hz.
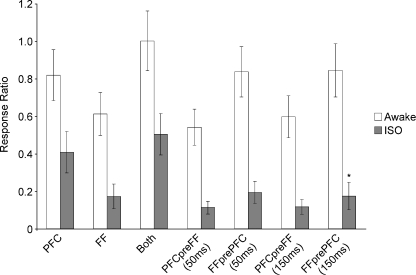
For the population of Nacb cells responding to PFC and FF studied in the awake condition (n= 49; Fig. 2), the average response magnitude to PFC (0.82 ± 0.13 spikes stimulus−1) and FF (0.62 ± 0.11 spikes stimulus−1) alone were not different from each other (P > 0.05). The mean population response to simultaneous stimulation of FF and PFC (1.00 ± 0.16 spikes stimulus−1) demonstrated sublinear summation (Fig. 2). Furthermore, the response magnitude to simultaneous activation was not significantly larger than PFC alone (P > 0.05), while it was larger than the response to the FF alone (P < 0.001). In the awake animal, none of the paired stimulation conditions at 50 or 150 ms intervals caused significant changes in the responses to the PFC or FF alone (Fig. 2).
Figure 2. Mean number of responses (±s.e.m.) per stimulation for each stimulation condition (46 cells).
Cells responded to both PFC and FF stimulation, but then when stimulated together the addition of the responses was sublinear, and not significantly different from PFC alone. Responses to stimulation of FF prior to PFC, or PFC prior to FF at 50 or 150 ms did not alter the mean response to the later stimulation when compared to the response to this stimulation alone. Isoflurane anaesthesia (ISO) significantly reduced responses to all stimulation protocols (P < 0.001). Under isoflurane anaesthesia, stimulation of FF prior to PFC significantly reduced the response to the PFC at 150 ms compared to PFC alone (*P < 0.05).
Under isoflurane anaesthesia, the mean frequency of spontaneous firing prior to the initiation of the stimulation protocols was drastically reduced to 0.16 Hz. In addition, the average population responses to single PFC stimuli (0.40 ± 0.11 spikes stimulus−1) or FF (0.17 ± 0.11 spikes stimulus−1) were significantly reduced (P < 0.001), as compared to the awake animal (n= 49 cells). The response to the simultaneous stimulation of both afferents was not significantly different from that to the PFC alone (P > 0.05), and therefore was highly sublinear. As in the awake condition, the response to the simultaneous stimulation of both structures was significantly higher than that to the FF alone (P < 0.001). Prior stimulation of the PFC at both intervals did not significantly change the response to FF stimulation. Surprisingly, and in contrast to previous reports, the response to the PFC was not facilitated but was instead significantly suppressed (P < 0.05, Fig. 2) by the prior stimulation of FF at a 150 ms interval.
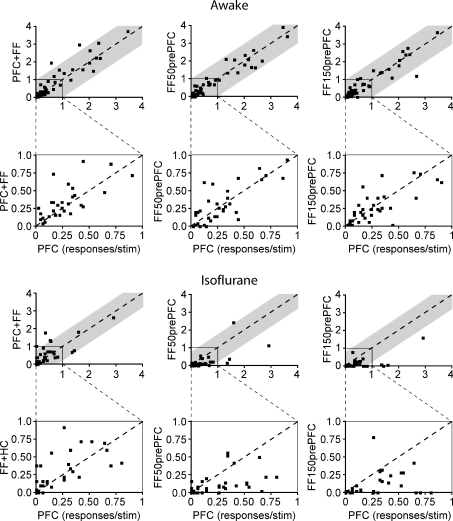
The population effects described above may hide the existence of subpopulations of Nacb cells that are effectively gated by the preceding HC/FF or PFC input but do not appear in the total population average. In order to examine individual cell responses included in the population measures, and to assess changes between the awake and anaesthetized condition, individual cell response magnitudes for different stimulation protocols were plotted against the response to the PFC alone (Fig. 3). Responses above the diagonal line indicate an increase in response magnitude, while responses below the main diagonal indicate a decrease. The grey shaded areas represent one standard deviation of the PFC responses (s.d.= 0.95 awake, 0.77 isoflurane). In such plots, gating of PFC responses would be observed as a disproportionate increase in response magnitude for the combined stimulus compared to PFC alone, particularly for very small or zero values of the PFC response. Instead, for all three types of combined stimuli in the awake state (Fig. 3) the responses to combined stimuli were clustered along the main diagonal indicating little effect of the preceding FF stimulation. More importantly, for the small responses to PFC stimulation, where gating is more likely, the combined response was never above 1 s.d. from response alone. Thus, our results demonstrate that, as with the average population plots, there were no cases of clear gating by the HC over PFC responses. Instead, we observed a ceiling effect for response magnitude in which the response to both structures was always very similar to PFC alone regardless of interval. In contrast to the awake condition, under isoflurane anaesthesia the PFC responses were largely suppressed by the preceding FF stimulation at both preceding intervals as shown by the majority of the points lying below the main diagonal (n= 3, FF50prePFC; n= 5, FF150prePFC).
Figure 3. Individual cell responses to PFC stimulation plotted against different stimulation conditions under awake and isoflurane states.
Cells with identical responses to the PFC condition lie along the line, while cells above the line responded more to the compared condition than the PFC alone and cells below the line were suppressed compared to the PFC response. The grey shading represents one standard deviation of the PFC response in each condition. Below each condition, responses have been plotted again up to a maximum of one in order to better show the distribution of the responses in the lower range. Responses to PFC stimulation alone are plotted against those preceded by FF stimulation at the two intervals (50 and 150 ms). The majority of cells are clustered around the main diagonal, indicating little effect of the preceding FF stimulation. Results were similar under anaesthesia, but distribution of cell responses below the diagonal demonstrates the suppression of the PFC response by prior FF stimulation under anaesthesia.
In order to test whether the sublinear responses were due to a ceiling effect, and also to compare the direct stimulation of ventral hippocampal CA1 (HC) to the FF, animals (n= 3) with stimulating electrodes in the HC were stimulated in the awake condition. In this protocol, stimulation currents were set at 25%, 50% or 100% of a maximal current (1.0 mA). Exploring increasing intensities was necessary since the HC represents a spatially spread input source compared to the tight axonal bundle represented by the FF, which can be stimulated very efficiently even at low intensities. The mean frequency of firing of the cells during the period prior to the stimulation protocol was 3.15 ± 0.48 Hz (not significantly different from the previous results). Latencies of the responses to stimulation were not significantly different between the stimulation intensities, and were therefore combined together for reporting. The latencies of the PFC and HC stimulation were comparable to previous reports, and the combination of PFC and HC stimulation did not significantly change the latency of the responses compared to PFC alone (PFC = 11.4 ± 3.3 ms (mean ±s.d.), HC = 14.8 ± 2.76 ms, both = 12.68 ± 2.78 ms, HC10prePFC =10.22 ± 3.28 ms, HC50prePFC = 11.41 ± 3.15 ms, HC150prePFC = 11.56 ± 3.34 ms; O’Donnell & Grace, 1995).
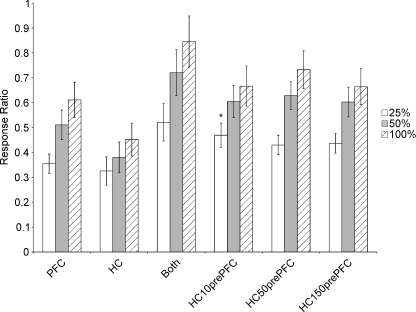
The responses to the stimulation protocols that included the HC (n= 67 cells) were similar to those with FF stimulation (Fig. 4). Summation of the response to the simultaneous stimulation of HC and PFC was highly sublinear. When the HC stimulation was presented at the lowest intensity 10 ms prior to the PFC, there was a significant increase in response magnitude compared to the PFC stimulation alone (P < 0.001). However, this difference was no longer significant when the intensity of HC stimulation was increased, or when the delay by which HC preceded PFC was increased to 50 or 150 ms.
Figure 4. Mean responses per stimulations (±s.e.m.) of cells (n= 67) to three different amplitudes of stimulation and multiple stimulation conditions of the PFC and the ventral hippocampus (HC) in the awake animal.
As stimulation amplitude is increased, there is an increase in responses under most conditions, suggesting there was not a ceiling effect in the previous PFC/FF experiments. Within stimulation amplitudes, stimulating the HC 50 or 150 ms prior to the PFC did not significantly increase the number of responses. When stimulated 10 ms prior to the PFC, the HC did not significantly affect the PFC responses except at 25% of maximal stimulation (*P < 0.001).
Discussion
The Nacb integrates information from a large number of limbic areas, and is considered one of the primary areas where motivation and reward information are incorporated into behavioural responses (Pennartz et al. 1994; Kelley, 2004). Due to the prominence of the PFC in working memory tasks, and of the HC in spatial location and memory formation, the integration of the input from these areas and their contributions to the output of the medium spiny projection (MSP) cells of the Nacb has been a major focus of prior research. Previous studies examining this integration have used electrical stimulation of the afferents to the Nacb in the in vivo anaesthetized preparation (O’Donnell & Grace, 1995; Goto & O’Donnell, 2002; Goto & Grace, 2005). In order to examine the summation of inputs from those afferent structures in the cells of the Nacb in the awake rat, we implanted recording electrodes in the Nacb and stimulating electrodes in the PFC and FF or ventral hippocampus. Our results indicate that the predominant mode of integration of inputs to the Nacb from these afferent areas is sublinear, and that there is not a dominant gating phenomenon between these areas in the awake rat. To the best of our knowledge, this is the first time that stimulation of multiple afferent structures has been utilized in the awake animal to determine the integration properties of the MSP neurons.
One of the hallmarks of MSP spontaneous behaviour under anaesthesia is the bimodal distribution of the membrane potential (Wilson & Groves, 1981; O’Donnell & Grace, 1995). This bimodality refers to the tendency of the membrane to be in one of two states: a hyperpolarized (‘down’) state dominated by an inwardly rectifying potassium current, and a more depolarized (‘up’) state dominated by synaptic and A-type potassium currents during which action potentials occur (Wilson & Kawaguchi, 1996; Stern et al. 1997). Abnormal gating between afferent structures modulated by these state transitions has been proposed to mediate the pathophysiology of schizophrenia (O’Donnell & Grace, 1995; Grace, 2000). It remains an open question whether these state transitions are meaningful in the awake animal. One study of the dorsal striatum in awake monkeys used timing of responses and computational modelling to suggest that up and down states exist in the awake animal (Kitano et al. 2002). Other studies have suggested that desynchronization of the cortical inputs to mimic the awake state may leave the cells in a permanent depolarized state in the awake animal (Kasanetz et al. 2002). A recent set of experiments describing intracellular recordings in awake rats in a head-fixed preparation demonstrated that the transition from waking to slow-wave sleep leads to a transition from a unimodal membrane potential in the awake animal, to a bimodal membrane potential reminiscent of the bimodality visualized under anaesthesia (Mahon et al. 2006). These results are in agreement with previously published intracellular recordings from cortical and thalamic cells in non-anaesthetised animals demonstrating the absence of slow oscillations (up and down states) in the awake animal, and their presence in the onset stages and throughout slow wave sleep (Steriade, 2001). However, rapid cortical activation states have recently been demonstrated in the awake mouse during whisking while recording intracellularly from two cells in cortex simultaneously (Poulet & Petersen 2008), suggesting that these transitions may play a role in sensory processing.
The first examination of the interaction of the PFC and HC inputs in the Nacb demonstrated that the loss of the FF input via transection removed the bimodal nature of the membrane potential in the subset of cells that exhibited this behaviour (O’Donnell & Grace, 1995). The PFC did not fire the Nacb cells unless the cell was already in the depolarized state, or if a number of stimuli delivered to the FF induced a depolarized state. This behaviour was noted in a subset of the cells examined. Since transection of the FF removed the transitions between states it was presumed that HC input is critical for state transitions, and for bringing the cells to the depolarized state. However, we have demonstrated that in the awake and the isoflurane-anaesthetized animal, stimulation of the PFC is capable of inducing firing in the majority of cells examined in the Nacb, suggesting that there is no need for a different afferent area to induce a state transition prior to afferent input from the PFC. Isoflurane anaesthesia significantly reduced responses, but the PFC and FF stimulation were still capable of eliciting responses regularly in these cells. Other more recent studies have also suggested that the PFC alone is capable of firing Nacb neurons when stimulated (Gruber et al. 2009).
Previous examinations of the subthreshold EPSPs generated by the PFC and HC and their interactions have also demonstrated sublinear responses, suggesting that summation of spike output may also be sublinear (Goto & O’Donnell, 2002). Another finding from this same study examined the interaction of the limbic inputs with a 100 ms offset between those inputs and the PFC. In each case, when the PFC was stimulated prior to the HC or BLA inputs, there was a suppression of the EPSP response to the other inputs. However, when the order of stimulation was reversed, and the limbic inputs were stimulated first, there was no change in the PFC-elicited EPSP. This indicates that the interactions of these areas may be more complex than a simple gating phenomenon based on state transitions.
Our results are a measure of spike output, and are therefore difficult to compare directly to these findings. However, the main previous finding that the summation of the EPSPs is sublinear was consistent with what we observed in the spike output. Our demonstration of suppression of spike output by the HC stimulation prior to the PFC under anaesthesia is contradictory to these previous results; however different anaesthesias (isoflurane vs urethane) or the comparison of spike output and EPSPs may underlie these differences. As this was not observed in the spike output of the awake animal, it may be an effect of the membrane potential of the cells while the animal is under anaesthesia.
Another finding presented here is the lack of interaction of the PFC and HC when the FF is stimulated prior to the PFC or vice versa. Based on previous data, we expected to see a large increase in the response to PFC when the HC was stimulated prior to the PFC. However, the results were identical to PFC alone when the HC was stimulated 50 or 150 ms prior to the PFC. However, at low stimulation intensity, we detected a significant difference when the HC was stimulated immediately prior to the PFC (10 ms), suggesting that there is a time and magnitude window during which these inputs will sublinearly integrate. These results suggest that if the HC input is necessary to maintain the depolarized state in order for the PFC to fire the cells, that this required input is consistently present in the awake animal for most cells, as only a few cells increased their responses when the HC was stimulated at longer intervals prior to the PFC.
Another surprising result was the comparison of the awake and isoflurane-anaesthetized responses. We expected that since anaesthesia has previously been demonstrated to induce hyperpolarized states in these cells, that isoflurane at deep levels would then reveal the gating phenomenon by having cells shift to the depolarized state by the FF stimulation. The results were the opposite of our prediction. Isoflurane anaesthesia actually reduced the mean response to the PFC when the HC was stimulated prior to the PFC. This response indicates that there was either a change in the state of the network induced by the HC stimulation, thus reducing the chance for PFC throughput, or that feedforward inhibition was now dominating the response and had temporarily rendered the cells non-responsive to PFC input (Tepper et al. 2004). Intracellular recordings may be able to dissociate the mechanisms underlying this phenomena.
Two possible explanations for the difference between our results and previous reports are the effect of the specific anaesthesia utilized, and a potential sampling bias. The Nacb has a very complicated overlay of inputs (Groenewegen et al. 1999), and it is possible that there is a bias towards a group of cells that received predominantly HC input in the previous work by choice of electrode locations, or away from them in our hands. Our histological recovery of recording sites does not allow us to specify the precise location of every cell recorded. Our histology indicated that we have covered the significant territory in which the HC is a dominant input, and the level of response to the HC stimulation also supported this. The anaesthesia issue is somewhat more complicated, in that there are very few data on the mechanism of action of dissociative anaesthetics. We chose isoflurane because at high levels of anaesthesia (2.5–3% isoflurane) there are regularly occurring epochs with no cortical activity, during which the electrical stimulation of the afferent structures becomes the primary inputs to the cell. Since we were not recording intracellularly, we could not time our stimulations according to the state of the cell during slow oscillations in the individual cell as was done previously under chloral hydrate anaesthesia (O’Donnell & Grace, 1995). Regardless, the discrepancy between the responses in the awake and the anaesthetized condition suggest that the responses in the anaesthetized preparation are not a good predictor of the integrative function of the MSP cells in the awake animal.
Another set of recent studies has elucidated aspects of the interaction of the HC and the PFC in the Nacb and their modulation by DA. It was demonstrated that the tonic dopaminergic modulation (D2 receptor-mediated) of PFC input to the Nacb can affect the behavioural responses of rats (Goto & Grace, 2005). However, the phasic DA modulation appears to modulate the HC inputs to a greater extent. Utilizing an LTP protocol in the anaesthetized animal, it was also demonstrated that tonic and phasic DA release function as mechanisms for attenuating plasticity associated with one afferent structure or another. These data suggest that the afferent inputs from the PFC and the HC to the Nacb are fine-tuned depending on tonic DA levels and phasic activity of ventral tegmental area neurons, as well as the previous history of afferent input. These mechanisms are subtle enough to suggest that the sublinear input integration would allow for a larger dynamic range than a simple binary gating response.
In summary, our data suggest that integration of afferent inputs from the PFC and HC in the Nacb is sublinear in the awake rat, with a non-uniform distribution of responses across cells. This is in accordance with the complex anatomical pattern of afferent inputs to the Nacb. Isoflurane anaesthesia drastically changes the response profile of all cells, and should not necessarily be utilized to infer the response of the cells to afferent input in the awake animal. We conclude from these results that while the Nacb may be integrating afferent streams of input, it does not appear to primarily gate PFC throughput via the HC. A revision of this hypothesis may include modulation of PFC throughput via theta entrainment of the Nacb cells (Berke et al. 2004; Wolf et al. 2005, 2007). Previous modelling studies suggest that an MSP cell's spike output can be entrained to an afferent theta oscillation with a relatively minor number of entrained inputs (∼20). In combination with our present data, this suggests that the HC may be modulating PFC input in a more subtle fashion than simply gating all throughput in an all-or-none fashion. We therefore propose that theta-entrained cells in the HC may be forming an ensemble of active cells in the Nacb that are then preferentially active when PFC inputs and theta-modulated input from the HC intersect (Pennartz et al. 1994, 2004; Siapas et al. 2005; Wolf et al. 2007).
Acknowledgments
This paper is dedicated to the memory of Leif H. Finkel, M.D., Ph.D., who tirelessly supported this project. The authors wish to thank Catharine Eeley and Esther Garcia de Yebenes for expert technical assistance. This work was supported by a Conte center award (NIH-MH064045) and two training grants (NIH-MH019112 and NIH-NS043126).
Glossary
Abbreviations
- DA
dopamine
- EPSP
excitatory post-synaptic potential
- FF
fimbria-fornix
- HC
hippocampus
- MSP
medium spiny neurons
- Nacb
nucleus accumbens
- PFC
pre-frontal cortex
Author contributions
J. A. Wolf designed and ran the experiments, designed the analysis and analysed the data, and wrote the manuscript. L. H. Finkel designed the experiments and wrote the manuscript. D. Contreras designed the experiments and analysis, and wrote the manuscript. These experiments were performed at the University of Pennsylvania in the laboratory of D. Contreras.
Supplemental material
Online supplemental material for this paper can be accessed at
http://jp.physoc.org/cgi/content/full/jphysiol.20010.1113/jphysiol.2008.168369/DC1
References
- Berke JD, Okatan M, Skurski J, Eichenbaum HB. Oscillatory entrainment of striatal neurons in freely moving rats. Neuron. 2004;43:883–896. doi: 10.1016/j.neuron.2004.08.035. [DOI] [PubMed] [Google Scholar]
- Boeijinga PH, Mulder AB, Pennartz CM, Manshanden I, Lopes da Silva FH. Responses of the nucleus accumbens following fornix/fimbria stimulation in the rat. Identification and long-term potentiation of mono-and polysynaptic pathways. Neuroscience. 1993;53:1049–1058. doi: 10.1016/0306-4522(93)90488-2. [DOI] [PubMed] [Google Scholar]
- Finch DM. Neurophysiology of converging synaptic inputs from the rat prefrontal cortex, amygdala, midline thalamus, and hippocampal formation onto single neurons of the caudate/putamen and nucleus accumbens. Hippocampus. 1996;6:495–512. doi: 10.1002/(SICI)1098-1063(1996)6:5<495::AID-HIPO3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Goto Y, Grace AA. Dopamine-dependent interactions between limbic and prefrontal cortical plasticity in the nucleus accumbens: disruption by cocaine sensitization. Neuron. 2005;47:255–266. doi: 10.1016/j.neuron.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Goto Y, O’Donnell P. Timing-dependent limbic-motor synaptic integration in the nucleus accumbens. Proc Natl Acad Sci U S A. 2002;99:13189–13193. doi: 10.1073/pnas.202303199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA. Gating of information flow within the limbic system and the pathophysiology of schizophrenia. Brain Res Brain Res Rev. 2000;31:330–341. doi: 10.1016/s0165-0173(99)00049-1. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Wright CI, Beijer AV, Voorn P. Convergence and segregation of ventral striatal inputs and outputs. Ann N Y Acad Sci. 1999;877:49–63. doi: 10.1111/j.1749-6632.1999.tb09260.x. [DOI] [PubMed] [Google Scholar]
- Gruber AJ, Powell EM, O’Donnell P. Cortically activated intemeurons shape spatial aspects of corticoaccumbens processing. J Neurophysiol. 2009 doi: 10.1152/jn.91002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jog MS, Connolly CI, Kubota Y, Iyengar DR, Garrido L, Harlan R, Graybiel AM. Tetrode technology: advances in implantable hardware, neuroimaging, and data analysis techniques. J Neurosci Methods. 2002;117:141–152. doi: 10.1016/s0165-0270(02)00092-4. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kasanetz F, Riquelme LA, Murer MG. Disruption of the two-state membrane potential of striatal neurones during cortical desynchronisation in anaesthetised rats. J Physiol. 2002;543:577–589. doi: 10.1113/jphysiol.2002.0024786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron. 2004;44:161–179. doi: 10.1016/j.neuron.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Kitano K, Cateau H, Kaneda K, Nambu A, Takada M, Fukai T. Two-state membrane potential transitions of striatal spiny neurons as evidenced by numerical simulations and electrophysiological recordings in awake monkeys. J Neurosci. 2002;22:RC230. doi: 10.1523/JNEUROSCI.22-12-j0003.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon S, Vautrelle N, Pezard L, Slaght SJ, Deniau JM, Chouvet G, Charpier S. Distinct patterns of striatal medium spiny neuron activity during the natural sleep-wake cycle. J Neurosci. 2006;26:12587–12595. doi: 10.1523/JNEUROSCI.3987-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell P, Grace AA. Synaptic interactions among excitatory afferents to nucleus accumbens neurons: hippocampal gating of prefrontal cortical input. J Neurosci. 1995;15:3622–3639. doi: 10.1523/JNEUROSCI.15-05-03622.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1998. [Google Scholar]
- Pennartz CM, Groenewegen HJ, Lopes da Silva FH. The nucleus accumbens as a complex of functionally distinct neuronal ensembles: an integration of behavioural, electrophysiological and anatomical data. Prog Neurobiol. 1994;42:719–761. doi: 10.1016/0301-0082(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Pennartz CM, Lee E, Verheul J, Lipa P, Barnes CA, McNaughton BL. The ventral striatum in off-line processing: ensemble reactivation during sleep and modulation by hippocampal ripples. J Neurosci. 2004;24:6446–6456. doi: 10.1523/JNEUROSCI.0575-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulet JF, Petersen CC. Internal brain state regulates membrane potential synchrony in barrel cortex of behaving mice. Nature. 2008;454:881–885. doi: 10.1038/nature07150. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M. Motivational views of reinforcement: implications for understanding the behavioral functions of nucleus accumbens dopamine. Behav Brain Res. 2002;137:3–25. doi: 10.1016/s0166-4328(02)00282-6. [DOI] [PubMed] [Google Scholar]
- Siapas AG, Lubenov EV, Wilson MA. Prefrontal phase locking to hippocampal theta oscillations. Neuron. 2005;46:141–151. doi: 10.1016/j.neuron.2005.02.028. [DOI] [PubMed] [Google Scholar]
- Steriade M. Impact of network activities on neuronal properties in corticothalamic systems. J Neurophysiol. 2001;86:1–39. doi: 10.1152/jn.2001.86.1.1. [DOI] [PubMed] [Google Scholar]
- Steriade M, Contreras D, Curro Dossi R, Nunez A. The slow (<1 Hz) oscillation in reticular thalamic and thalamocortical neurons: scenario of sleep rhythm generation in interacting thalamic and neocortical networks. J Neurosci. 1993;13:3284–3299. doi: 10.1523/JNEUROSCI.13-08-03284.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern EA, Kincaid AE, Wilson CJ. Spontaneous subthreshold membrane potential fluctuations and action potential variability of rat corticostriatal and striatal neurons in vivo. J Neurophysiol. 1997;77:1697–1715. doi: 10.1152/jn.1997.77.4.1697. [DOI] [PubMed] [Google Scholar]
- Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9:321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Tepper JM, Koós T, Wilson CJ. GABAergic microcircuits in the neostriatum. Trends Neurosci. 2004;27:662–669. doi: 10.1016/j.tins.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Vergara R, Rick C, Hernández-López S, Laville JA, Guzman JN, Galarraga E, Surmeier DJ, Bargas J. Spontaneous voltage oscillations in striatal projection neurons in a rat corticostriatal slice. J Physiol. 2003;553:169–182. doi: 10.1113/jphysiol.2003.050799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CJ, Groves PM. Spontaneous firing patterns of identified spiny neurons in the rat neostriatum. Brain Res. 1981;220:67–80. doi: 10.1016/0006-8993(81)90211-0. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Kawaguchi Y. The origins of two-state spontaneous membrane potential fluctuations of neostriatal spiny neurons. J Neurosci. 1996;16:2397–2410. doi: 10.1523/JNEUROSCI.16-07-02397.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf JA, Finkel LH, Contreras D. Entrainment of Nucleus Accumbens neurons to hippocampal theta and concurrent modulation of responsiveness to prefrontal cortical stimulation in the awake behaving rat. Abstr Soc Neurosci. 2007;622:21. [Google Scholar]
- Wolf JA, Moyer JT, Lazarewicz MT, Contreras D, Benoit-Marand M, O’Donnell P, Finkel LH. NMDA/AMPA ratio impacts state transitions and entrainment to oscillations in a computational model of the nucleus accumbens medium spiny projection neuron. J Neurosci. 2005;25:9080–9095. doi: 10.1523/JNEUROSCI.2220-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.