Abstract
The Q270K mutation of the skeletal muscle Na+ channel α subunit (Nav1.4) causes atypical paramyotonia with a striking sensitivity to cold. Attacks of paralysis and a drop in the compound muscle action potential (CMAP) are exclusively observed at cold. To understand the pathogenic process, we studied the consequences of this mutation on channel gating at different temperatures. WT or Q270K recombinant Nav1.4 channels fused at their C-terminal end to the enhanced green fluorescent protein (EGFP) were expressed in HEK-293 cells. Whole-cell Na+ currents were recorded using the patch clamp technique to examine channel gating at 30°C and after cooling the bathing solution to 20°C. Mutant channel fast inactivation was impaired at both temperatures. Cooling slowed the kinetics and enhanced steady-state fast inactivation of both mutant and WT channels. Mutant channel slow inactivation was fairly comparable to that of the WT at 30°C, but became clearly abnormal at 20°C. Cooling enhanced slow inactivation in the WT by shifting the voltage dependence toward hyperpolarization, but induced the opposite effect in the mutant. Destabilization of mutant channel slow inactivation in combination with defective fast inactivation is expected to increase the susceptibility to prolonged membrane depolarization, and can ultimately lead to membrane inexcitability and paralysis at cold. Thus, abnormal temperature sensitivity of slow inactivation can be a determinant pathogenic factor, and should therefore be more widely considered in thermosensitive Na+ channelopathies.
Mutations of the skeletal muscle Na+ channel are responsible for a wide spectrum of excitability disorders, classically divided into two broad clinical groups, i.e. periodic paralyses and myotonias (Cannon, 1996). A large fraction of Nav1.4 mutations identified to date are associated with paramyotonia congenita, a disease mainly characterized by episodes of myotonia induced by cold and aggravated by exercise. Muscle weakness may also occur in response to exertion or cold. We recently reported a mutation of the Nav1.4 (Q270K), which causes a peculiar phenotype of paramyotonia with unusual sensitivity to cold (Fournier et al. 2006). Cold weather or direct contact with cold water induced muscle stiffness followed by severe attacks of paralysis lasting from minutes to hours. At warm temperatures, most clinical symptoms disappeared. This was corroborated by the unique electromyographic pattern evoked by exercise trials performed at room temperature or after cooling the muscles. In patients with typical PC mutations (i.e. T1313M), exercise trials induced a decrease in CMAP amplitude that was further pronounced with cooling. By contrast, in patients carrying the Q270K mutation, a sustained drop in CMAP, concomitant with muscle paralysis, only occurred when exercise was performed at cold (Fournier et al. 2006).
The voltage-gated Na+ channel is critical to skeletal muscle excitability. It is made of a large α subunit (Nav1.4) that forms by itself a functional pore, and a small regulatory β-subunit (Barchi, 1983; Catterall, 2000). The Q270K mutation is located in the outermost part of the S5 segment of domain I (DI-S5) of Nav1.4, which consists of four homologous domains, each comprising six transmembrane segments (Noda et al. 1984; George et al. 1992). Only a few disease-causing mutations have been identified in this domain (Takahashi & Cannon, 1999; Wu et al. 2001), among which is L266V, a nearby mutation characterized by cold-aggravated myotonia but no weakness (Wu et al. 2001).
One of the common defects of mutant channels associated with paramyotonia is the slowing of entry to fast inactivation. Cold exacerbates this slowing to a degree that may represent the biophysical threshold for clinical myotonia (Bouhours et al. 2004, 2005). On the other hand, a number of studies on Na+ channelopathies with predominant muscle weakness point to a major pathogenic contribution of altered channel slow inactivation to paralysis (Ruff, 1994; Cummins & Sigworth, 1996; Hayward et al. 1999; Ruff & Cannon, 2000). To date, there are very few reports on temperature sensitivity of slow inactivation (Ruff & Cannon, 2000), although inherited Na+ channelopathies with various temperature-sensitive phenotypes are increasingly acknowledged (Fournier et al. 2006; Rossignol et al. 2007; Webb & Cannon, 2008). To elucidate the mechanism behind the peculiar phenotype caused by the Q270K mutation, we investigated both fast and slow inactivation at different temperatures (30 and 20°C). Our results show similar impairment of fast inactivation to that reported for the L266V mutation (Wu et al. 2001), but unique temperature-dependent alterations in slow inactivation that can explain the occurrence of muscle paralysis in a cold environment.
Methods
Plasmid construction, mutagenesis and cell transfection
The human SCN4A cDNA construct was subcloned into the pEGFP-N2 vector (Clontech) for expression in mammalian cells. The DNA sequence for EGFP was in frame to the 3′-terminal end of the SCN4A sequence immediately before the stop codon. In the resulting fusion protein, the C-terminus of Nav1.4 was fused to the N-terminus of EGFP. The Q270K mutation was inserted into the SCN4A-EGFP cDNA construct by overlap PCR. The final cDNA product was entirely sequenced to verify that the Q270K mutation was present, and that no other variant had been introduced by accident during DNA amplification.
Human embryonic kidney cells (HEK 293) were transfected with WT or Q270K SCN4A-EGFP constructs using the calcium phosphate precipitation method. Cells were routinely grown in a Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum (Bouhours et al. 2004, 2005). Na+ currents were recorded from both transiently and stably expressing cells (at least 2 clones) in order to ensure that no bias was introduced by cell selection. The basic gating properties of Nav1.4 WT channels fused to EGFP were first compared with those of the non-fused channels to ascertain than EGFP did not modify channel function (Supplemental Fig. 1).
In vitro electrophysiology
Na+ currents were recorded in the whole cell configuration of the patch-clamp technique (Hamill et al. 1981), using an EPC-7 amplifier (HEKA electronics). After breaking into the cell, 10–15 min was allowed for Na+ current stabilization. Standard pulse-evoked currents were filtered at 5 kHz through an 8-pole low-pass Bessel filter (M900, Frequency Devices, Corp., Sunnyvale, CA, USA), and usually digitized at a sampling rate of 20 μs using Digidata 1332 and the pCLAMP software (Axon Instruments, Union City, CA, USA). P/N protocols (Armstrong & Bezanilla, 1977) from a holding potential (HP) of –120 mV were applied to subtract residual leakage and capacity currents. Bath temperature was maintained at either 30 or 20°C by water flow through a Peltier-based thermal stage (HE-201, Dagan) connected to a temperature controller (HCC-100A). Pipettes were pulled from Drummond capillary glass using a Sutter P-97 puller. All pipettes were filled with a caesium-based solution (mm): 130 CsCl, 2 MgCl2, 10 glucose and 10 hemi-Na-Hepes (pH 7.4), and had a resistance of 1.1–1.8 MΩ. The bath solution contained (mm): 100 NaCl, 42 choline-Cl, 3 KCl, 2 MgCl2, 1 CaCl2, 10 glucose and 10 Hepes (pH 7.4). Data were analysed using Clampfit 9.2 (Axon Instruments), Microsoft Excel, CorelDraw 12 and Origin 7.5 (OriginLab Corp., Northampton, MA, USA) software. All values are expressed as means ± standard error of the mean (s.e.m.). Student's unpaired t-test was used for statistical analysis.
The peak conductance G(V) was computed from the peak current, and normalized conductance versus voltage curves were fitted to the Bolzmann equation (see Fig. 1 legend). Steady-state inactivation curves were fitted to a two-state Boltzmann distribution (see Figs 3 and 4 legends).
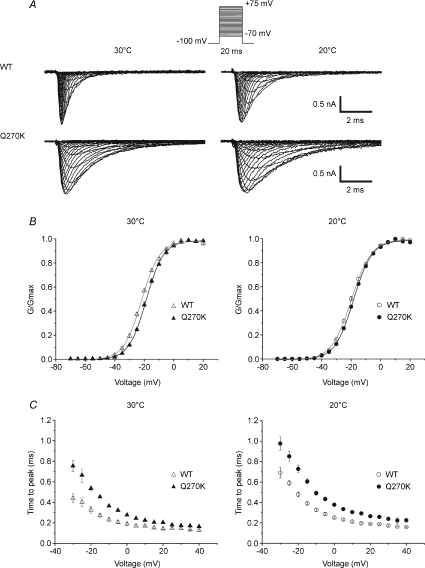
Figure 1. Voltage dependence of activation and effect of temperature.
A, representative tracings of whole-cell Na+ currents recorded from HEK cells expressing either Q270K or WT channels fused with EGFP. Recordings were performed at 30 or 20°C using the voltage-clamp protocol shown at the top (HP −100 mV, voltage steps from −70 to +75 mV in 5 mV increments). B, normalized conductance (G/Gmax) versus voltage curves obtained for Q270K (30°C (n= 8); 20°C (n= 8)) and WT channels (30°C (n= 7); 20°C (n= 9)). Na+ conductance was computed from the equation G(V) =Ipeak/(V–ENa). The reversal potential ENa was determined by extrapolation of the regression line to zero current (voltages > 0 mV). The continuous lines represent the best fits to the Boltzmann function G(V)/Gmax= 1/(1 + exp(−(V−V0.5)/k)), where Gmax is the maximum conductance, V0.5 the voltage for half-activation, and k the slope factor (mV/e-fold). C, kinetics of channel activation described by the time to peak (TTP). All symbols represent mean values ±s.e.m. Note the slight shift toward depolarization caused by the Q270K mutation. Cooling from 30 to 20°C barely changed the voltage dependence of activation, and moderately slowed the activation kinetics in both mutant and WT.
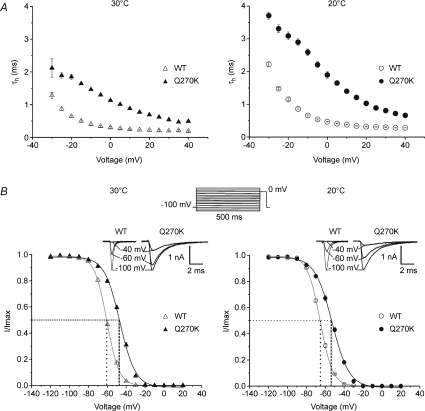
Figure 3. Fast inactivation and effect of temperature.
A, the kinetics of fast inactivation were assessed by the time constant (τh) of the exponential decay of whole-cell currents elicited at voltages of −30 to +40 mV. Recordings were carried out at 30 and 20°C in the Q270K mutant and WT. B, the voltage dependence of steady-state fast inactivation was studied at both temperatures using the protocol shown at the top: a 500 ms conditioning pulse to varying voltages (−120 to +20 mV) followed by a test pulse to 0 mV for 20 ms. Peak Na+ currents acquired during the test pulse were normalized to maximal peak value of the series, and plotted against voltage. Insets represent Na+ currents elicited by the test pulse following conditioning pulses at the indicated voltages. Steady-state fast inactivation curves were fitted to a two-state Boltzmann distribution: I(V)/Imax= 1/(1 + exp((V−V0.5)/k)) (continuous lines), where I(V) is the current elicited by the test pulse, Imax the maximum current, V the prepulse voltage, and k the slope factor (mV/e-fold). Broken lines are orthogonal projections of 50% current inactivation on the voltage axis to highlight V0.5. All symbols represent mean values ±s.e.m. Note that decreasing temperature shifted V0.5 toward hyperpolarization in both mutant and WT, leaving the difference between the two channels unchanged.
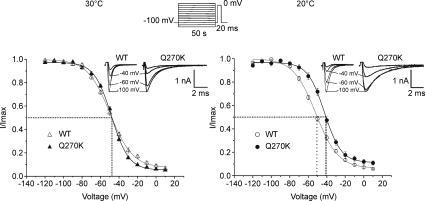
Figure 4. Voltage-dependent steady-state slow inactivation and effect of temperature.
Steady-sate slow inactivation curves obtained from the Q270K mutant and WT at 30 and 20°C. Slow inactivation was induced by the protocol shown at the top: 50 s prepulse potentials ranging from −120 to +10 mV were followed by a test pulse to 0 mV for 20 ms. A brief repolarizing step to −100 mV was applied for 20 ms prior to the test pulse to allow selective and full recovery from fast inactivation. Insets represent Na+ current tracings evoked during the test pulse for different conditioning pulses as indicated. Data were fitted with a two-state Boltzmann function with a non-zero pedestal I0: (I(V)/Imax= 1 −I0/1 + exp((V−V0.5)/k)) +I0 (continuous lines, see Fig. 3 legend for equation terms). Symbols are mean values ±s.e.m. Note that a difference of about 10 mV in V0.5 appears between mutant and WT when the temperature is decreased from 30 to 20°C.
Results
Kinetics and voltage dependence of activation
Na+ currents were recorded from HEK cells expressing WT or Q270K channels fused with the green fluorescent protein (EGFP) at 30 and 20°C. Normalized I–V and G–V curves were plotted for each cell and then averaged for statistical evaluation (Fig. 1). As can be seen from Fig. 1B, the Q270K mutation induced a very slight depolarizing shift in the voltage dependence of activation that was somewhat better resolved at 30°C (∼+3 mV) than at 20°C (∼+1.5 mV). The slope factor (k) remained comparable between WT and mutant at both temperatures. Cooling from 30 to 20°C did not significantly modify the voltage dependence of mutant or WT channel activation (see Table 1).
Table 1.
Boltzmann parameter estimates for Na+ channel activation and inactivation at 30 and 20°C
| 30°C | 20°C | ||||
|---|---|---|---|---|---|
| WT | Q270K | WT | Q270K | ||
| Activation | V0.5 | −21.8 ± 0.3 | −18.7 ± 0.5 | −19.7 ± 0.5 | −18.4 ± 0.2 |
| (mV) | (7) | (8)*** | (9) | (8)* | |
| k | 6.1 ± 0.1 | 5.8 ± 0.1 | 6.7 ± 0.2 | 6.3 ± 0.1 | |
| (mV/e-fold) | (7) | (8)* | (9) | (8)* | |
| ENa | (mV) | +70.4 ± 1.4 | +67.4 ± 0.7 | +68.1 ± 0.9 | +68.6 ± 0.6 |
| (7) | (8) | (9) | (8) | ||
| Fast | V0.5 | −60.6 ± 0.3 | −46.8 ± 0.5 | −65.3 ± 0.5 | −52.8 ± 0.7 |
| inactivation | (mV) | (10) | (8)*** | (11)* | (9)*** |
| k | 6.5 ± 0.1 | 8.2 ± 0.3 | 6.5 ± 0.1 | 8.9 ± 0.2 | |
| (mV/e-fold) | (10) | (8)*** | (11) | (9)*** | |
| Slow | V0.5 | −49.4 ± 0.9 | −47.8 ± 0.8 | −53.8 ± 0.5 | −42.7 ± 0.5 |
| (mV) | (8) | (12) | (8) | (8)*** | |
| k | 11.6 ± 0.3 | 8.7 ± 0.5 | 11.2 ± 0.5 | 7.7 ± 0.2 | |
| (mV/e-fold) | (8) | (12)*** | (8) | (8)*** | |
| Residual | 7 ± 1 | 6 ± 1 | 6 ± 1 | 12 ± 2 | |
| (%) | (8) | (12) | (8) | (8)** | |
Values are mean ±s.e.m. (n). Statistical significance of the difference between Q270K and WT for each temperature
P < 0.05
P < 0.01
P < 0.001
Time to peak Na+ current (TTP: 10 to 90% rise time) was measured as a simple estimation of the activation kinetics from the crude data. As shown in Fig 1C, TTP was slowed in Q270K mutant compared with WT, suggesting a decrease in the rate of channel activation. Note that the voltage dependence of TTP remained unchanged.
At 30°C, the mean values of TTP were 20 to 65% (average ∼45%) higher in Q270K mutant than in WT (voltage range −25 to +40 mV). Cooling from 30 to 20°C similarly increased (by 30–35%) the TTP in both mutant and WT.
Kinetics and voltage dependence of fast inactivation
Fast inactivation is a process by which Na+ channels become non-conducting following depolarization of the order of milliseconds. It contributes thereby to the repolarizing phase of the action potential. Recovery from fast inactivation terminates the refractory period, and enables the initiation of a new excitability cycle. Disruption of fast inactivation has been found in most Nav1.4 channelopathies in which myotonia predominates (Hayward et al. 1996).
To enable comparison between the Q270K and the neighboring L266V mutation associated with cold-aggravated myotonia, a detailed analysis of the kinetics of fast inactivation was first performed at 20°C (Fig. 2), a temperature at which the data for the L266V were obtained (Wu et al. 2001).
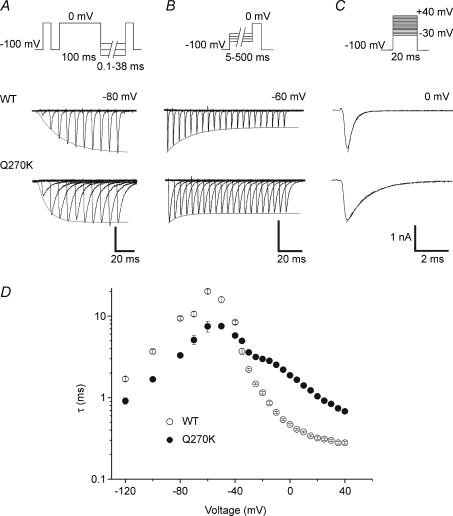
Figure 2. Kinetics of entry to and recovery from fast inactivation at 20°C.
A–C, full voltage-clamp protocols and representative tracings obtained at a given voltage from HEK cells expressing Q270K or WT channels to illustrate the following. A, the recovery from fast inactivation: a first pulse to 0 mV for 20 ms (HP −100 mV) was applied to obtain the reference current (Iref), followed by a voltage step to 0 mV for 100 ms to fully inactivate the channels, a recovery period of variable duration (0.1 to 38 ms) at various voltages from −120 to −80, and a last pulse to 0 mV to provide the test current (Itest). The fraction of recovered Na+ current (Itest/Iref) followed an exponential time course (continuous line on tracings) described by the time constant (τrecov). B, the entry to fast inactivation from the closed state: a conditioning pulse of increasing duration (5–500 ms) at voltages ranging from −40 mV to −70 mV followed by a test pulse to 0 mV (HP −100 mV). The relative peak current (Itest/Icond) decreased exponentially with time (continuous line). C, the entry to fast inactivation from the open state: same protocol as that used for the I–V relationship; τh is measured from the whole-cell current decay. D, mean values of the time constants for recovery (−120 to −80 mV), and entry to fast inactivation from the closed (−70 to −40 mV) or the open state (−30 to +40 mV) are plotted against voltage for Q270K and WT. All symbols represent mean values ±s.e.m.
Recovery from fast inactivation was assessed by a double-pulse protocol (see Fig. 2A). Q270K channels recovered faster from inactivation than the WT, as indicated by an average decrease of 46% in the time constant of recovery (τrecov) over the applied voltage range (Fig. 2D). The kinetics of entry to fast inactivation from the closed state (see Fig. 2B) were also accelerated by the Q270K mutation, as demonstrated by a 50% reduction in the time constant for the mutant compared with WT (Fig. 2D). The kinetics of entry to fast inactivation from the open state (Fig. 2C) were substantially slowed in the mutant, as can be seen from the higher mean values of the inactivation time constant (τh) (Fig. 2D). The Q270K mutation also reduced the voltage sensitivity of τh, especially between −20 and 0 mV (Fig. 2D). All these changes were similar to those reported for the L266V mutation (Wu et al. 2001). In particular, both mutations induced a crossover effect around −30 mV with faster closed state and slower open state inactivation kinetics as compared to the WT. It is worth noting that the accelerated closed-state inactivation was not associated with a hyperpolarizing shift in the voltage dependence of steady-state inactivation. Instead, as shown below (Fig. 3B), channel availability was increased for the Q270K mutant between −70 and −40 mV, suggesting a dominant effect of the accelerated rate of recovery from fast inactivation.
The effects of temperature were investigated on two main characteristics of channel fast inactivation: the time constant τh, and the voltage dependence of steady-sate inactivation (Fig. 3).
At 30°C, τh values obtained from the mutant were 142–330% (average 247%) higher than those of the WT (voltage range −25 to +40 mV). Decreasing temperature from 30 to 20°C induced an increase in τh by 28–63% (average 44%) in the mutant compared with 37–78% (average 55%) in the WT. The difference in τh between mutant and WT at 20°C ranged between 124 and 311% (average 224%), and was therefore slightly smaller than that found at 30°C. Taken together these data suggest that temperature sensitivity of the mutant is somewhat lower than that of the WT. Nevertheless, cooling enlarged the absolute difference between mutant and WT as can be seen from Fig. 3A, and mean values of τh reached up to 3.31 ms in the mutant as compared to 1.47 ms for the WT. Note that the τh/V relationship was not modified by cooling.
The voltage dependence of steady-sate fast inactivation was assessed by a two-pulse protocol (see Fig. 3). At 30°C, steady-state inactivation curves (h∞) obtained from the Q270K mutant showed a shift of 13–14 mV toward depolarization, and a reduced voltage sensitivity compared with WT (Fig. 3, see also Table 1). Decreasing temperature from 30 to 20°C caused a hyperpolarizing shift in h∞ to a similar degree for both channel types (mutant: ∼−6 mV; WT: ∼−5 mV). Thus, the difference in V0.5 between the two channels remained roughly the same upon cooling (Table 1). The slope factors obtained for mutant and WT were not affected by this temperature change. Altogether, these data demonstrate an impairment of the voltage dependence of steady-state fast inactivation by the Q270K mutation, but a normal response of this mutant channel characteristic to cooling.
Slow inactivation
Following prolonged depolarization Na+ channels shut off by a process that is distinct from fast inactivation, and is referred to as slow inactivation (Rudy, 1978). In the skeletal muscle, slow inactivation operates on a seconds time scale and is thought to regulate the availability of excitable Na+ channels as a function of the resting membrane potential (Simoncini & Stuhmer, 1987; Ruff et al. 1988). It is now well established that human Nav1.4 channelopathies with altered slow inactivation are associated with paralysis rather than myotonia (Hayward et al. 1997). In the present case, episodes of severe paralysis occur exclusively at cold. This led us to raise the hypothesis of an abnormal slow inactivation gating in Q270K mutant channels at low temperature. The sensitivity to temperature of Nav1.4 slow inactivation has been addressed in a few studies, with discrepancies between reports that remain unsolved (Ruff, 1999, 2008; Webb & Cannon, 2008). Thus, besides disclosing the effects of Q270K mutation, the present study provides further information on temperature-dependent changes of Nav1.4 slow inactivation.
Steady-state slow inactivation was induced by a double pulse protocol characterized by long prepulse potentials (50 s) (see Fig. 4). Steady-state slow inactivation curves (S∞) are depicted in Fig. 4. At 30°C, mutant channels showed a minor depolarizing shift in V0.5 (+1.6 mV) as well as a decrease in the slope factor (of ∼3 mV/e-fold), i.e. steeper voltage sensitivity than the WT (Table 1). The interesting finding was the occurrence of a significant difference of ∼11 mV between mutant and WT S∞ curves when temperature was decreased from 30 to 20°C (Fig. 4). This apparent difference in the voltage dependence of slow inactivation between mutant and WT was the result of a hyperpolarizing shift of ∼−4.5 mV for the WT, combined to a depolarizing shift of ∼+5 mV for the mutant. The steepness of both mutant and WT S∞ curves was not significantly altered by cooling (Table 1). In addition, the fraction of Na+ channels that did not slow-inactivate increased from 6 to 12% with cooling in the mutant, but did not significantly change in the WT (7 and 6% at 30 and 20°C, respectively) (Table 1).
Discussion
Among skeletal muscle Na+ channelopathies, paramyotonia congenita is known for its sensitivity to temperature. Indeed, episodes of myotonia can be induced or aggravated by cold. However, temperature-dependent symptoms of either myotonia or paralysis have also been observed in Nav1.4 channelopathies with atypical phenotypes (Sugiura et al. 2000, 2003; Wu et al. 2001). For most cases, the mechanisms behind thermosensitivity of the symptoms remain unclear. One of the reasons is that in vitro functional studies are usually performed at room temperature (20–22°C), and few data are available on the temperature sensitivity of mutant Na+ channel gating. This is particularly true for slow inactivation. Our study unveils Q270K mutant channel defects and provides evidence for specific responses of different channel gating characteristics to cold that can be correlated to the phenotype.
At 20°C, impairment of Q270K channel fast inactivation was strikingly similar to that reported for the neighboring mutation L266V. Both mutations slow down the kinetics of entry and accelerate the recovery with similar voltage dependence patterns. They also shift the voltage for half-inactivation toward depolarization by about 12 mV (Q270K: 12.5 mV, L266V: 12 mV) (Wu et al. 2001). For Q270K mutant, all such defects were also manifest at 30°C. Cooling from 30 to 20°C modified fast inactivation in a similar manner for WT and mutant channels, with about 50% slowing of the kinetics (τh), and 5–6 mV hyperpolarization shift in the voltage dependence of the steady-state. Overall, in healthy muscles, cooling exerts opposite effects on Na+ channel fast inactivation: it prolongs channel opening, while reducing the pool of excitable channels. This may represent a homeostatic process that prevents hyperexcitability of the skeletal muscle at cold. In patients carrying mutations that disrupt fast inactivation, such a process may no longer be effective, and cold would aggravate the tendency to repetitive firing and myotonia. In both L266V and Q270K, impairment of fast inactivation is not sufficient to cause marked symptoms at mild temperature, but becomes clearly pathogenic at low temperature. Changes of fast inactivation with temperature may explain the susceptibility to cold of myotonia in patients carrying the L266V mutation; however they are insufficient to explain attacks of paralysis induced by the Q270K mutation. Interestingly, a major difference between these two mutations is their effect on slow inactivation. Indeed, at 20°C, slow inactivation of L266V mutant channels was reported to be fairly normal, whereas that of Q270K channels is clearly impaired.
At 30°C, the voltage at which half of the Q270K mutant channels became slow inactivated was not significantly different from that of the WT. However, when temperature was decreased to 20°C, a difference of 11 mV in the voltage for half-inactivation became visible between the two channel types. Cooling induced a hyperpolarizing shift of the voltage dependence of slow inactivation in the WT but a depolarizing shift in the mutant.
Hyperpolarizing shift in S∞ curves of normal skeletal muscle Na+ channels with cooling was previously observed in rat muscle cells (Ruff, 1999). Similar response to temperature seems to be shared by other TTX-sensitive Na+ channels, such as those present in dorsal root ganglion (DRG) neurons (Zimmermann et al. 2007). However, in a recent study performed on HEK cells (Webb & Cannon, 2008), cooling was found to shift steady-state slow inactivation of normal Nav1.4 channel in the opposite direction. The discrepancy between these data and those obtained from muscle fibres remains to be clarified. Our data, which are in line with those of Ruff (1999), do not support the idea that this may be related to differences in expression systems (Ruff, 2008; Webb & Cannon, 2008). Other experimental factors could be involved, such as the type of solutions used to study slow inactivation, and in particular, the use of fluoride as a substitute for chloride in the internal pipette solution. Indeed, to improve seal stability during long protocols such as those required for Na+ channel slow inactivation, CsF is generally preferred to CsCl. Whether F− alters Na+ channel slow inactivation has been subject to debate (Coste et al. 2004). Fluoride is known as a phosphatase inhibitor (Pinkse et al. 1999) which, by modifying the phosphorylation status of Na+ channels, may alter their gating (Cantrell & Catterall, 2001; Chen et al. 2006). For these reasons, all experiments associated with the present study were performed using CsCl-based intra-pipette solution.
Enhancement of Nav1.4 channel slow inactivation at cold may represent a protective factor against alterations of muscle excitability in conditions that generate long-lasting sarcolemma depolarization. In this respect, cold shifts the resting membrane potential toward depolarization by reducing Na+/K+ ATPase activity, and lengthens the action potential by slowing down the gating kinetics of both the voltage-gated Na+ channels and the delayed rectifier K+ channels.
If slow inactivation operates normally in mutant channels with impaired fast inactivation, it will eventually shut off the defective channels and oppose the prolonged influx of Na+. This would prevent long-lasting membrane depolarization that may lead to loss of excitability and paralysis (Ruff, 1994). Such process is particularly important at low temperatures where the abnormal Na+ influx is exacerbated, and the resting membrane potential depolarized. Accordingly, the L266V mutation, which preserves slow inactivation at cold, will mainly cause myotonia, whereas the Q270K, which does not, will cause paralysis at low temperatures. So far, only one other mutation of the human Nav1.4 (P1158S) has been reported to destabilize slow inactivation upon cooling. Consistent with the above reasoning, the latter was also discerned by its unusual phenotype comprising cold-induced attacks of paralysis (Webb & Cannon, 2008).
The molecular mechanism of Na+ channel slow inactivation is still unclear. Current hypotheses propose that slow inactivation is linked to conformational changes of the pore region. Nonetheless, the contribution of the S5–S6 loops lining the outer pore (Balser et al. 1996; Vilin et al. 1999) versus the S6 transmembrane (TM) segments predicted to line the inner pore (Wang & Wang, 1997; O’Reilly et al. 2001) is still under debate (Struyk & Cannon, 2002). The emerging consensus is that structural determinants of slow inactivation involve both the outer (S5–S6 P-loops) and the inner vestibules (S5 and S6 TM segments) of the ion conducting pore (Chancey et al. 2007; Tikhonov & Zhorov, 2007).
The Q270K mutation is located at the extracellular end of the S5 transmembrane segment of domain I, close to the N-end of the S5–S6 pore loop. The functional role of the amino-acids in this region remains to be clarified (Lipkind & Fozzard, 1997). Our results indicate that the Q270 residue contributes to temperature sensitivity of slow inactivation. Its replacement with lysine may interfere with conformational changes that promote or stabilize Na+ channel slow inactivation at cold. Since glutamine and lysine have similar hydrophobicity, the observed alterations are likely to originate from the positive charge and/or the larger size (an additional methyl group) of lysine.
In conclusion, we have shown that the occurrence of cold-induced attacks of paralysis, a phenotypic peculiarity of the Q270K mutation of Nav1.4, can be explained by an abnormal decrease of slow inactivation upon exposure to cold. More generally, our data provide further evidence for an enhancement of slow inactivation at low temperature in normal Nav1.4 channels, and point out the need for exploring temperature sensitivity of slow inactivation in thermosensitive Nav1.4 channelopathies.
Acknowledgments
We thank Francine Moueza for her precious technical help, and members of Résocanaux network for their collaboration. This work was supported by INSERM institutional grants and by AFM (Association Française contre les Myopathies) grants to N.T.
Glossary
Abbreviations
- PC
paramyotonia congenita
- EMG
electromyography
- CMAP
compound muscle action potential
- EGFP
enhanced green fluorescence protein
- WT
wild type
- HEK
human embryonic kidney
- TTP
time to peak
Author contributions
T.C. performed experiments and N.T. contributed to some; T.C. and N.T. analysed data; E.F. provided valuable EMG data; B.F. and E.F. examined the patients; D.S. provided the molecular diagnosis; and N.T. conceived the study and wrote the manuscript.
Disclosure
The authors report no conflicts of interest.
Supplemental material
Online supplemental material for this paper can be accessed at
http://jp.physoc.org/cgi/content/full/jphysiol.2008.165787/DC1
References
- Armstrong CM, Bezanilla F. Inactivation of the sodium channel. II. Gating current experiments. J Gen Physiol. 1977;70:567–590. doi: 10.1085/jgp.70.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balser JR, Nuss HB, Chiamvimonvat N, Perez-Garcia MT, Marban E, Tomaselli GF. External pore residue mediates slow inactivation in mu 1 rat skeletal muscle sodium channels. J Physiol. 1996;494:431–442. doi: 10.1113/jphysiol.1996.sp021503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barchi RL. Protein components of the purified sodium channel from rat skeletal muscle sarcolemma. J Neurochem. 1983;40:1377–1385. doi: 10.1111/j.1471-4159.1983.tb13580.x. [DOI] [PubMed] [Google Scholar]
- Bouhours M, Luce S, Sternberg D, Willer JC, Fontaine B, Tabti N. A1152D mutation of the Na+ channel causes paramyotonia congenita and emphasizes the role of DIII/S4–S5 linker in fast inactivation. J Physiol. 2005;565:415–427. doi: 10.1113/jphysiol.2004.081018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouhours M, Sternberg D, Davoine CS, Ferrer X, Willer JC, Fontaine B, Tabti N. Functional characterization and cold sensitivity of T1313A, a new mutation of the skeletal muscle sodium channel causing paramyotonia congenita in humans. J Physiol. 2004;554:635–647. doi: 10.1113/jphysiol.2003.053082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon SC. Sodium channel defects in myotonia and periodic paralysis. Annu Rev Neurosci. 1996;19:141–164. doi: 10.1146/annurev.ne.19.030196.001041. [DOI] [PubMed] [Google Scholar]
- Cantrell AR, Catterall WA. Neuromodulation of Na+ channels: an unexpected form of cellular plasticity. Nat Rev Neurosci. 2001;2:397–407. doi: 10.1038/35077553. [DOI] [PubMed] [Google Scholar]
- Catterall WA. From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron. 2000;26:13–25. doi: 10.1016/s0896-6273(00)81133-2. [DOI] [PubMed] [Google Scholar]
- Chancey JH, Shockett PE, O’Reilly JP. Relative resistance to slow inactivation of human cardiac Na+ channel hNav1.5 is reversed by lysine or glutamine substitution at V930 in D2-S6. Am J Physiol Cell Physiol. 2007;293:C1895–1905. doi: 10.1152/ajpcell.00377.2007. [DOI] [PubMed] [Google Scholar]
- Chen Y, Yu FH, Surmeier DJ, Scheuer T, Catterall WA. Neuromodulation of Na+ channel slow inactivation via cAMP-dependent protein kinase and protein kinase C. Neuron. 2006;49:409–420. doi: 10.1016/j.neuron.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Coste B, Osorio N, Padilla F, Crest M, Delmas P. Gating and modulation of presumptive NaV1.9 channels in enteric and spinal sensory neurons. Mol Cell Neurosci. 2004;26:123–134. doi: 10.1016/j.mcn.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Cummins TR, Sigworth FJ. Impaired slow inactivation in mutant sodium channels. Biophys J. 1996;71:227–236. doi: 10.1016/S0006-3495(96)79219-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier E, Viala K, Gervais H, Sternberg D, Arzel-Hezode M, Laforet P, Eymard B, Tabti N, Willer JC, Vial C, Fontaine B. Cold extends electromyography distinction between ion channel mutations causing myotonia. Ann Neurol. 2006;60:356–365. doi: 10.1002/ana.20905. [DOI] [PubMed] [Google Scholar]
- George AL, Jr, Komisarof J, Kallen RG, Barchi RL. Primary structure of the adult human skeletal muscle voltage-dependent sodium channel. Ann Neurol. 1992;31:131–137. doi: 10.1002/ana.410310203. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hayward LJ, Brown RH, Jr, Cannon SC. Inactivation defects caused by myotonia-associated mutations in the sodium channel III-IV linker. J Gen Physiol. 1996;107:559–576. doi: 10.1085/jgp.107.5.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward LJ, Brown RH, Jr, Cannon SC. Slow inactivation differs among mutant Na channels associated with myotonia and periodic paralysis. Biophys J. 1997;72:1204–1219. doi: 10.1016/S0006-3495(97)78768-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward LJ, Sandoval GM, Cannon SC. Defective slow inactivation of sodium channels contributes to familial periodic paralysis. Neurology. 1999;52:1447–1453. doi: 10.1212/wnl.52.7.1447. [DOI] [PubMed] [Google Scholar]
- Lipkind GM, Fozzard HA. A model of scorpion toxin binding to voltage-gated K+ channels. J Membr Biol. 1997;158:187–196. doi: 10.1007/s002329900256. [DOI] [PubMed] [Google Scholar]
- Noda M, Shimizu S, Tanabe T, Takai T, Kayano T, Ikeda T, Takahashi H, Nakayama H, Kanaoka Y, Minamino N et al, et al. Primary structure of Electrophorus electricus sodium channel deduced from cDNA sequence. Nature. 1984;312:121–127. doi: 10.1038/312121a0. [DOI] [PubMed] [Google Scholar]
- O’Reilly JP, Wang SY, Wang GK. Residue-specific effects on slow inactivation at V787 in D2-S6 of Nav1.4 sodium channels. Biophys J. 2001;81:2100–2111. doi: 10.1016/S0006-3495(01)75858-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkse MW, Merkx M, Averill BA. Fluoride inhibition of bovine spleen purple acid phosphatase: characterization of a ternary enzyme-phosphate-fluoride complex as a model for the active enzyme-substrate-hydroxide complex. Biochemistry. 1999;38:9926–9936. doi: 10.1021/bi990446w. [DOI] [PubMed] [Google Scholar]
- Rossignol E, Mathieu J, Thiffault I, Tetreault M, Dicaire MJ, Chrestian N, Dupre N, Puymirat J, Brais B. A novel founder SCN4A mutation causes painful cold-induced myotonia in French-Canadians. Neurology. 2007;69:1937–1941. doi: 10.1212/01.wnl.0000290831.08585.2c. [DOI] [PubMed] [Google Scholar]
- Rudy B. Slow inactivation of the sodium conductance in squid giant axons. Pronase resistance. J Physiol. 1978;283:1–21. doi: 10.1113/jphysiol.1978.sp012485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff RL. Slow Na+ channel inactivation must be disrupted to evoke prolonged depolarization-induced paralysis. Biophys J. 1994;66:542. doi: 10.1016/s0006-3495(94)80807-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff RL. Effects of temperature on slow and fast inactivation of rat skeletal muscle Na+ channels. Am J Physiol Cell Physiol. 1999;277:C937–947. doi: 10.1152/ajpcell.1999.277.5.C937. [DOI] [PubMed] [Google Scholar]
- Ruff RL. Slow inactivation: slow but not dull. Neurology. 2008;70:746–747. doi: 10.1212/01.wnl.0000304253.88642.8d. [DOI] [PubMed] [Google Scholar]
- Ruff RL, Cannon SC. Defective slow inactivation of sodium channels contributes to familial periodic paralysis. Neurology. 2000;54:2190–2192. doi: 10.1212/wnl.54.11.2191. [DOI] [PubMed] [Google Scholar]
- Ruff RL, Simoncini L, Stuhmer W. Slow sodium channel inactivation in mammalian muscle: a possible role in regulating excitability. Muscle Nerve. 1988;11:502–510. doi: 10.1002/mus.880110514. [DOI] [PubMed] [Google Scholar]
- Simoncini L, Stuhmer W. Slow sodium channel inactivation in rat fast-twitch muscle. J Physiol. 1987;383:327–337. doi: 10.1113/jphysiol.1987.sp016411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struyk AF, Cannon SC. Slow inactivation does not block the aqueous accessibility to the outer pore of voltage-gated Na channels. J Gen Physiol. 2002;120:509–516. doi: 10.1085/jgp.20028672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura Y, Aoki T, Sugiyama Y, Hida C, Ogata M, Yamamoto T. Temperature-sensitive sodium channelopathy with heat-induced myotonia and cold-induced paralysis. Neurology. 2000;54:2179–2181. doi: 10.1212/wnl.54.11.2179. [DOI] [PubMed] [Google Scholar]
- Sugiura Y, Makita N, Li L, Noble PJ, Kimura J, Kumagai Y, Soeda T, Yamamoto T. Cold induces shifts of voltage dependence in mutant SCN4A, causing hypokalemic periodic paralysis. Neurology. 2003;61:914–918. doi: 10.1212/01.wnl.0000086820.54065.a0. [DOI] [PubMed] [Google Scholar]
- Takahashi MP, Cannon SC. Enhanced slow inactivation by V445M: a sodium channel mutation associated with myotonia. Biophys J. 1999;76:861–868. doi: 10.1016/S0006-3495(99)77249-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikhonov DB, Zhorov BS. Sodium channels: ionic model of slow inactivation and state-dependent drug binding. Biophys J. 2007;93:1557–1570. doi: 10.1529/biophysj.106.100248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilin YY, Makita N, George AL, Jr, Ruben PC. Structural determinants of slow inactivation in human cardiac and skeletal muscle sodium channels. Biophys J. 1999;77:1384–1393. doi: 10.1016/S0006-3495(99)76987-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SY, Wang GK. A mutation in segment I-S6 alters slow inactivation of sodium channels. Biophys J. 1997;72:1633–1640. doi: 10.1016/S0006-3495(97)78809-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb J, Cannon SC. Cold-induced defects of sodium channel gating in atypical periodic paralysis plus myotonia. Neurology. 2008;70:755–761. doi: 10.1212/01.wnl.0000265397.70057.d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu FF, Takahashi MP, Pegoraro E, Angelini C, Colleselli P, Cannon SC, Hoffman EP. A new mutation in a family with cold-aggravated myotonia disrupts Na+ channel inactivation. Neurology. 2001;56:878–884. doi: 10.1212/wnl.56.7.878. [DOI] [PubMed] [Google Scholar]
- Zimmermann K, Leffler A, Babes A, Cendan CM, Carr RW, Kobayashi J, Nau C, Wood JN, Reeh PW. Sensory neuron sodium channel Nav1.8 is essential for pain at low temperatures. Nature. 2007;447:855–858. doi: 10.1038/nature05880. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.