Abstract
Duchenne muscular dystrophy (DMD) results from null mutation of dystrophin, a membrane-associated structural protein that is expressed in skeletal muscle. Dystrophin deficiency causes muscle membrane lesions, muscle degeneration and eventually death in afflicted individuals. However, dystrophin deficiency also causes cognitive defects that are difficult to relate to the loss of dystrophin. We assayed neurogenesis in the dentate gyrus (DG) in the mdx mouse model of DMD, using bromodeoxyuridine incorporation as a marker of proliferation and NeuN expression as a marker of differentiation. Our findings show that dystrophin mutation disrupts adult neurogenesis by promoting cell proliferation in the DG and suppressing neuronal differentiation. Because loss of dystrophin from muscle results in the secondary loss of neuronal nitric oxide synthase (nNOS), and NO is able to modulate neurogenesis, we assayed whether the genetic restoration of nNOS to mdx muscles corrected defects in adult, hippocampal neurogenesis. Assays of NO in the sera of active mice showed significant reductions in NO caused by the dystrophin mutation. However, over-expression of nNOS in the muscles of mdx mice increased serum NO and normalized cell proliferation and neuronal differentiation in the DG. These findings indicate that muscle-derived NO regulates adult neurogenesis in the brain and loss of muscle nNOS may underlie defects in the central nervous system in DMD.
Cognitive defects were first identified as a feature of the pathology of Duchenne muscular dystrophy (DMD) in 1868 (Duchenne, 1868; Allen & Rodgin, 1960; Marsh & Munsat, 1974), although the relationship between the loss of dystrophin and the associated defects in learning and memory remain obscure. Cognitive defects in DMD are significant; DMD boys have IQs that are about 1 standard deviation below normal, and more than 30% of DMD boys score in the mentally retarded range (Karagan, 1979; Emery, 1993; Bresolin et al. 1994; Mehler, 2000; Anderson et al. 2002). Many early explanations for the cognitive impairments are inconsistent with current knowledge. For example, the expectation that lack of physical activity per se is a primary cause for the deficiencies in learning and memory in DMD was contradicted by the finding that boys with comparable motor impairments caused by spinal muscular atrophy show no significant defects in cognition (Whelan, 1987; Billard et al. 1998). Similarly, the possibility that the defects in cognition result from depression caused by the chronic, progressive disease state was not supported by findings that DMD boys show no increased frequency or severity of depressed mood state when compared to healthy boys (Hinton et al. 2001).
The specificity of cognitive defects in DMD suggests that specific brain regions may be selectively affected by dystrophin's absence. DMD boys show particular deficiencies in memory that appear most prominently as an impaired ability to recall recent verbal information, such as repeating stories told by others, recalling series of numbers, and following long, verbal instructions (Blake & Kroger, 2000; Hinton et al. 2000 2001). However, no significant deficiencies occur in more abstract cognitive skills (Hinton et al. 2001). Although no single region of the brain is solely responsible for the formulation or recall of memories, numerous observations implicate the hippocampus. For example, a human patient who received a bilateral hippocampectomy lost all ability to remember recent events, although long-distant memories and higher, abstract cognitive skills remained intact (Scoville & Milner, 1957). Similarly, rodents with hippocampal lesions show impaired memory (Morris et al. 1982). Together, these observations indicate that the hippocampus may be a site in the central nervous system in which functional defects result from the loss of dystrophin from muscle.
Current knowledge of the cellular basis of memory indicates that adult neurogenesis provides a mechanism through which synaptic plasticity and associated memory may be promoted in the hippocampus (van Praag et al. 1999; Shors et al. 2001, 2002; Kempermann & Gage, 2002; Drapeau et al. 2003). In the hippocampus, neuronal progenitor cells from the subgranular zone of the DG migrate into the granule cell layer where they differentiate into neuronal or glial cells. Newly generated neuronal cells can then extend their axons into the CA3 region of the hippocampus about 4–9 days following their mitosis; these axons can then form synapses with adjacent cells (Altman & Das, 1965; Gould et al. 2000; van Praag et al. 2002). Thus, the generation of new synaptic networks by recently differentiated neurons may possibly provide an adaptive outcome of adult neurogenesis that could influence memory formation. However, increased proliferation of neurons in the DG may also disrupt information processing within the hippocampus. Action potentials are more readily generated in newly born neurons in the DG, and the threshold for the induction of long-term potentiation (LTP) of presynaptic inputs to young granule cells is lower than in mature granule cells (Schmidt-Hieber et al. 2004). Thus, abnormally high or low levels of neurogenesis and the resulting abnormal patterns of synaptic connectivity or neural activity may contribute to cognitive impairments reported in DMD.
Although adult neurogenesis is subject to multiple regulatory factors, including growth factors, hormones and corticosteroids (Gould et al. 2000), recent studies have shown that nitric oxide (NO) can play important roles in regulating neurogenesis in vivo and in vitro. For example, in vitro observations show that NO donors promote the differentiation of embryonic hippocampal neurons (Hindley et al. 1997) while NOS inhibitors can reduce the expression of differentiation markers in PC12 cells (Poluha et al. 1997). Similar effects have been observed in vivo. Systemic administration of NOS inhibitors into adult mice increases cell proliferation in neurogenic regions of the brain but decreases neuronal differentiation (Cheng et al. 2003; Packer et al. 2003; Moreno-Lopez et al. 2004). NO may also affect adult neurogenesis by influencing survival of newly generated neurons. Although no in vivo data are yet available to address this possibility in the DG, NO can mediate NGF-promoted neuronal cell survival in vitro (Teng et al. 1999). These observations provide strong evidence that perturbations of NO levels can influence adult neurogenesis.
Loss of nNOS expression and activity in muscle is a physiologically important consequence of dystrophin deficiency. Muscles of DMD patients and mdx mice experience approximately an 80% loss of NOS activity in muscle (Chang et al. 1996) because dystrophin deficiency leads to a secondary loss of nNOS from muscle (Brenman et al. 1995; Chang et al. 1996). Loss of muscle-derived NO can disregulate the autonomic control of blood flow to muscle (Thomas et al. 1998; Sander et al. 2000), can perturb normal inflammatory processes in muscle (Wehling et al. 2001) and can disrupt the normal structure of neuromuscular junctions (Shiao et al. 2004). However, it is also possible that loss of muscle NO production leads to broader, systemic defects in NO-mediated processes that have not been identified. NO in expired gases and in the serum is significantly lower in DMD patients (Gucuyener et al. 2000; Straub et al. 2002), which suggests that much of systemic NO in healthy individuals may be derived from muscle nNOS. Thus, the reduced levels of systemic NO in dystrophinopathy could be associated with impaired neurogenesis, if NO were a regulator of adult neurogenesis in the DG.
In the present investigation, we tested the hypothesis that changes in nNOS expression and activity in muscle can influence neurogenesis in the DG. Specifically, we tested whether loss of nNOS in dystrophic muscle influences the proliferation of new neurons in the DG of active mice, and whether differentiation of DG neurons is affected by the dystrophin mutation. We then assessed whether the muscle specific expression of a nNOS transgene can affect levels of NO systemically and also influence proliferation of cells in the DG and the differentiation of DG neurons.
Methods
Mice
All experimental protocols involving the use of mice were conducted according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the University of California, Los Angeles Institutional Animal Care and Use Committee. Adult mdx (C57BL/10ScSn-Dmdmdx/J), nNOS null mutant (B6;129S4-Nos1tm1Plh/J) and wild-type (C57BL/6) mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). Muscle-specific nNOS transgenic mice (Tg+) and nNOS transgenic mdx mice (Tg+/mdx) were produced as we described previously (Wehling et al. 2001) using rat nNOS cDNA in pCMV5 (provided by Dr James T. Stull, University of Texas, SouthWestern, Dallas, TX, USA) that was cloned downstream of the 2.2 kb human skeletal actin promoter and the vp1 intron (provided by Dr Jeffrey Chamberlain, University of Washington, Seattle, WA, USA). Positive transgenic mice were identified by PCR analysis for human skeletal actin promoter. Overexpression of the transgene was confirmed by Western blotting for nNOS using goat anti-rat nNOS antibody (BD Biosciences, Franklin Lakes, NJ, USA). Transgenic mice were extensively back-crossed onto the C57BL/6 background. mdx mice that were used in the investigation were non-transgenic littermates of Tg+/mdx crossed onto the C57BL/6 background. Mice 4–6 months old were used for all experiments.
Mouse running
Mice used for treadmill running experiments were run for 30 min on the treadmill at 10 m min−1 and then immediately killed by intraperitoneal injection of pentobarbital sodium. In voluntary running assays, mice were housed individually in cages with running wheels for 14 days. The distance run by each mouse was recorded by automatic counter installed on its running wheel.
BrdU administration
Mice received one 10 mg ml−1 intraperitoneal injection of sterile 5-bromodeoxyuridine (BrdU; Sigma) in phosphate buffered saline (PBS) daily for 10 consecutive days at 50 μg (g body weight)−1.
Measurements of serum NO concentration
Blood was collected from the heart of ambulatory mice or from mice after the completion of running. Protein was precipitated to obtain plasma for NO analysis. NO concentrations in plasma were measured using NO analyser (NOA, Sievers, GE Analytical Instruments, Boulder, CO, USA). NOA measures the concentration of NO by first converting nitrate, nitrite and S-nitrosocompounds in the plasma to free NO with vanadium (III) chloride in hydrochloric acid. NO reacts with ozone in a gas-phase chemiluminescence reaction to form electrically excited nitrogen dioxide, the emission of which can be detected by a red-sensitive photomultiplier tube.
Immunohistochemistry
Brains were dissected and frozen in isopentane immediately after the mice were killed. Frozen brain sections 10 μm thick were cut serially throughout the rostrocaudal extent of the granule cell layer of the dentate gyrus. Immunohistochemistry staining was done in one out of 10 serial slides (100 μm apart) for labelling of BrdU, and in one of 40 serial slides (400 μm apart) for double labelling of BrdU and NeuN. The sections were then fixed with 2% paraformaldehyde and treated with 2 n HCl for denaturing DNA. The primary antibodies used were mouse anti-NeuN (1:100, Chemicon, Millipore, Billerica, MA, USA), rat anti-BrdU (1:200, Abcam Inc., Cambridge, UK). The secondary antibodies were anti-mouse FITC, anti-rat Texas Red, anti-rabbit FITC.
Stereology
BrdU+ cells in the left granule cell layer and subgranule cell layer were counted blindly in every tenth serial section along the rostrocaudal axis of the DG using fluorescence microscopy. The subgranule cell layer refers to the proliferative zone located at the border of granule cell layer facing hilus, which is approximately two granule cell diameters thick. The adjacent sections were stained with haematoxylin for measuring the volume of the granule cell layer by light microscopy. The granule cell volume was determined by summing the traced granule cell areas for each section multiplied by the distance between sections, and the total number of BrdU+ cells was determined in a similar way. Then the densities of BrdU+ cells in the DG were calculated by dividing the total BrdU+ cells by the volume of the DG (mm3).
Analysis of proliferative cell differentiation
Total cells labelled with BrdU and cells double-labelled with BrdU and NeuN were counted blindly in one-in-forty serial sections under the confocal microscope. The percentage of the double-labelled cells (BrdU and NeuN) out of the total number of BrdU+ cells was determined as an index of proliferative cell differentiation.
Apoptosis assay
The number of apoptotic cells in brain sections was quantified by performing the terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling (TUNEL) assay. This method identifies apoptotic cells in situ by using terminal deoxynucleotidyl transferase (TdT) to transfer biotin-dUTP to the free 3′OH of cleaved DNA. The biotin-labelled cleavage sites were then visualized by reaction with fluorescein-conjugated avidin. Briefly, the tissue slides were fixed, blocked and incubated with the TUNEL solution containing TdT and biotin-dUTP, fluorescein-conjugated avidin and then observed by fluorescence microscopy.
Western blot analysis
Western blot analysis was used to assay for nNOS, eNOS, or iNOS expression. Homogenized skeletal muscle, brain, kidney, liver and the ascending, arch, thoracic and abdominal aortae were tissue subjected to the SDS-polyacrylamide gel electrophoresis transferred onto nitrocellulose membrane, incubated with specific primary antibodies, goat anti-rat nNOS antibody (BD Biosciences), rabbit anti-endothelial NOS (anti-eNOS; BD Transduction Laboratories), or rabbit anti-inducible NOS (iNOS; Upstate Biotechnology, Inc., Lake Placid, NY, USA). The blots were then incubated with horseradish peroxidase-linked secondary antibodies (1:50 000, Amersham) and visualized with enhanced chemiluminescence.
Statistical analysis
Data are presented as mean ±s.e.m. One-way analysis of variance was used to test whether variation between groups was significant at P < 0.05. The Bonferonni's multiple comparisons test was then used to test for differences between pairs of experimental groups with P < 0.05.
Results
Muscle-derived NO is distributed systemically in physically active mice
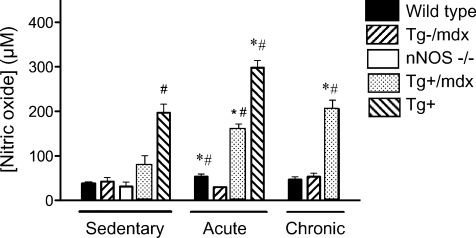
Nitric oxide concentration in the sera of sedentary animals does not differ in wild-type, mdx or nNOS null mice, showing that neither nNOS in skeletal muscle nor total systemic nNOS contributes detectably to serum NO in inactive animals (Fig. 1). Thus, serum NO is likely to reflect eNOS-derived NO under sedentary conditions in each of these mouse lines. The elevated NO in sera of wild-type mice that expressed the nNOS transgene (Tg+) (Fig. 1) reflects the 30-to 50-fold elevation in nNOS protein levels in this line (Wehling et al. 2001). NO concentration in sera collected immediately after a single bout of treadmill running shows that physical activity elevates serum [NO] in wild-type mice, but not in mdx mice that do not express a muscle-specific nNOS transgene (Tg–/mdx mice) (Fig. 1). This finding indicates that NO generated by muscle nNOS contributes significantly to systemic NO in physically active animals. Furthermore, expression of a muscle-specific nNOS transgene restores the activity-induced increase in serum [NO] in Tg–/mdx mice (Fig. 1), providing additional evidence that muscle-derived NO is distributed systemically in physically active animals. However, elevations of systemic NO in sera were not evident after prolonged periods of increased, voluntary activity in wild-type mice when there was a delay between increased physical activity and collection of serum for analysis (Fig. 1). This suggests that the elevation in muscle-derived NO in the serum is an acute effect of increased physical activity in wild-type mice. Although elevations in serum NO were not observed in wild-type mice after 14-days of voluntary treadmill running, NO levels in the serum of Tg+ mice were increased after a period of similar activity. This difference between wild-type and Tg+ mice is likely to reflect that the nNOS transgene is driven by the skeletal actin promoter, which may be influenced by chronic elevations in muscle activity in mice.
Figure 1. nNOS-derived NO contributes significantly to serum NO in active, but not sedentary, mice.
[NO] was measured in sera that were collected from sedentary mice, or mice immediately after completion of treadmill running (acute), or voluntary running for 14 days (chronic). The lack of difference in serum [NO] in sedentary wild-type (n= 14), Tg–/mdx (n= 6), nNOS null (n= 6) or Tg+/mdx (n= 6) mice shows that nNOS-derived NO does not contribute significantly to serum NO in sedentary mice. Ten mice were analysed in the Tg+ group. However, acute activity increased serum [NO] in wild-type (n= 20), Tg+/mdx (n= 6) and Tg+ (n= 10) mice, but not in Tg–/mdx (n= 6) mice, showing that muscle nNOS elevates serum [NO] in active mice. Voluntary running for 14 days increased serum [NO] in Tg+/mdx (n= 6) mice, but not in wild-type (n= 6) or mdx mice (n= 6). #Differs significantly from mdx under same treatment conditions. *Differs significantly from sedentary mice of same genotype.
Dystrophin-deficient mice show defects in hippocampal neurogenesis that are corrected by expression of a muscle-specific nNOS transgene
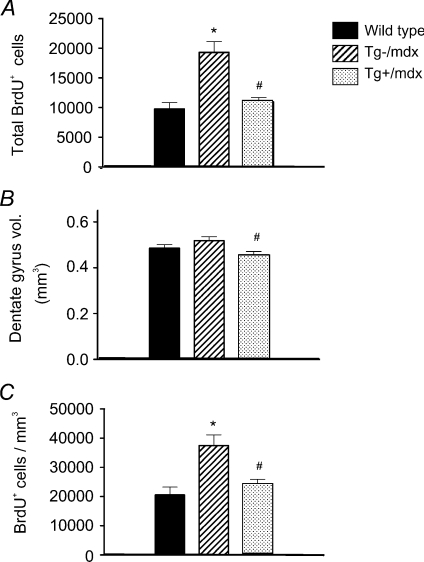
We tested whether adult, hippocampal neurogenesis differed in wild-type mice, mdx mice or mdx mice that expressed a muscle-specific nNOS transgene (Tg+/mdx mice) during voluntary running because this condition most resembles the natural activity level of mice. Although there was no significant difference in distance run in the strains (data not shown), the total number of BrdU+ cells in the DG of Tg–/mdx mice was over twofold greater than in wild-type mice (Fig. 2A), with similar differences in the densities of newly born cells (Fig. 2B, C). These increases in cell number and density were prevented by the expression of the nNOS transgene in mdx muscles, suggesting that the defect in neurogenesis in Tg–/mdx mice may be caused by loss of muscle-derived NO in the systemic circulation. Although measurements of serum NO concentration did not differ significantly between wild-type and Tg–/mdx mice, the delay between the completion of the most recent bout of voluntary activity and serum collection permitted serum NO levels to return to sedentary levels. NO levels in sera collected immediately after running (Fig. 1, ‘acute’ group) showed significant elevation of serum NO in wild-type mice, but not in Tg–/mdx mice.
Figure 2. The nNOS transgene significantly decreases cell proliferation in the DG of adult mdx mice during voluntary running.
The total BrdU+ cells in the DG (A), the total volume of the DG (B), and the densities of BrdU+ cells (C) are shown. *Differs significantly from wild-type. #Differs significantly from mdx (P < 0.05). Six mice were analysed in each group.
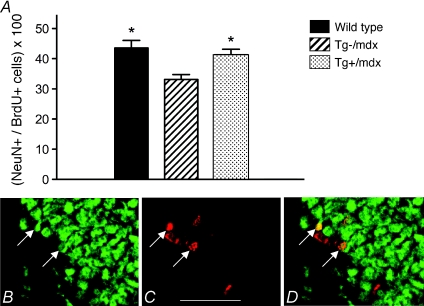
We also tested whether the differentiation of newly born cells in the hippocampus was influenced by dystrophin deficiency or the expression of a muscle-specific nNOS transgene in mdx mice. Hippocampal neuronal differentiation was measured after completion of 14 days of voluntary running by assaying the percentage of BrdU+ cells in the hippocampus that expressed NeuN, a marker of neuronal differentiation. Our results show that the large, significant decline in neuronal differentiation in Tg–/mdx mice is prevented by the expression of the nNOS transgene in muscle (Fig. 3A–D).
Figure 3. The nNos transgene normalizes the neuronal differentiation of proliferative cells in the DG of adult mdx mice. Differentiation is expressed as the percentage of BrdU+ cells that express NeuN in the DG (A).
*Differs significantly from mdx (P < 0.05). B–D, BrdU+ and NeuN+ cells in the DG. Sections were labelled with anti-NeuN (B) and anti-BrdU (C). Bar, 80 μm. The merged image is shown in D. Arrows indicate double-labelled cells. Six mice were analysed in each group.
Previous findings showing that DG cells can be lost by apoptosis in vivo (Bye et al. 2001) and that NO can affect the frequency of apoptosis in at least some cell types (Albina et al. 1993; Williams et al. 2008) suggested the possibility that the differences in the number of NeuN+/BrdU+ cells could be influenced by occurrence of apoptosis in wild-type or mdx DG cells. However, no TUNEL+ cells were observed in the hippocampi of wild-type or mdx mice sampled after the end of voluntary running (data not shown), showing that the net differences in proliferation or neuronal differentiation in the brains of wild-type and dystrophin-deficient mice were not a reflection of differences in neuronal apoptosis.
Expression of a nNOS transgene in muscle does not influence expression of NOS in the hippocampus
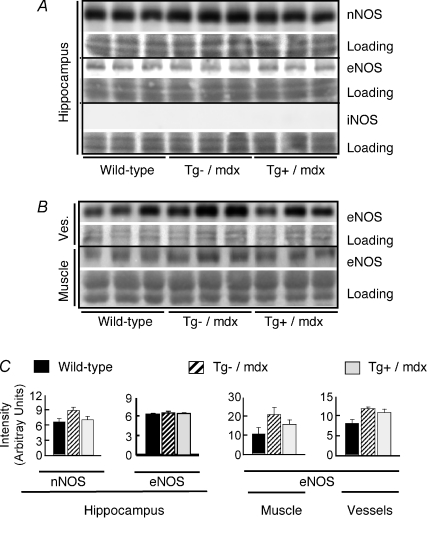
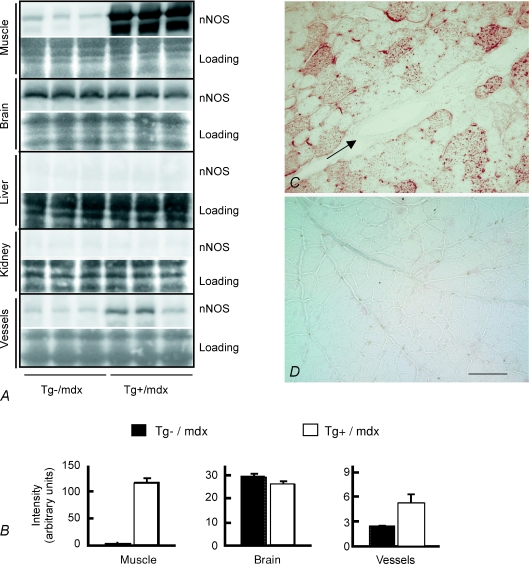
The finding that expression of the nNOS transgene driven by the skeletal muscle actin promoter affected hippocampal neurogenesis led us to test whether transgene expression influenced the expression of nNOS or eNOS in the hippocampus. If this were to occur, then the effects on neurogenesis could be attributable to local changes in NO production in the hippocampus, rather than increased NO production in muscle. However, the concentrations of nNOS and eNOS in the hippocampi of Tg+/mdx mice did not differ from their respective concentrations in either wild-type mice or Tg–/mdx mice (Fig. 4A). We also tested whether nNOS transgene expression influenced iNOS expression in the hippocampus because previous investigators have shown that elevated iNOS in the hippocampus could affect neurogenesis (Zhu et al. 2003). No iNOS was detected in the hippocampi of wild-type, Tg–/mdx or Tg+/mdx mice (Fig. 4A). We also assayed whether changes in NO in the sera of nNOS transgenic animals could be attributed to a secondary increase in eNOS in blood vessels or muscle of nNOS transgenic animals, but found no effect of transgene expression on the concentration of eNOS in those tissues (Fig. 4B). No nNOS was detectible in other major organs such as the kidneys or livers of Tg+/mdx or Tg–/mdx mice (Fig. 5). Although nNOS expression occurred in the brains of Tg+/mdx mice, Western blots showed no differences in nNOS expression in the brains of Tg–/mdx and Tg+/mdx mice (Fig. 5). Some Tg+/mdx mice showed a small increase in nNOS expression in large, muscular arteries, such as the thoracic and abdominal aortae (Fig. 5A). However, immunohistochemistry revealed no detectable nNOS expression in the walls of smaller arteries and arterioles in Tg+/mdx mice (Fig. 5C). Collectively, these data indicate that skeletal muscle is the major source of elevated, systemic NO in this Tg+/mdx line, although there may be a small contribution by smooth muscle in the walls of large, muscular arteries.
Figure 4. nNOS transgene expression does not elevate the expression of other NOS isoforms, indicating that increases in NO production in transgenic mice are attributable to nNOS transgene expression in muscle.
A, Western blots show no increase in the expression of nNOS, eNOS or iNOS in the hippocampi of Tg+/mdx mice compared to Tg–/mdx and wild-type controls. Specificity and reactivity of anti-iNOS to iNOS were confirmed in Western blots using peritoneal macrophages activated with lipopolysaccharide as a positive control (data not shown). Images of each Ponceau stained membrane (Loading) are shown beneath the image of the membrane after probing for NOS. B, Western blots show no increase in eNOS expression in muscles or blood vessels (Ves.) of Tg+/mdx mice compared to Tg–/mdx mice or wild-type controls. C, densitometric analysis of Western blots shown in A and B. Three mice were analysed in each group.
Figure 5. nNOS expression in tissues of transgenic mice.
A, Western blots for nNOS in selected tissues from Tg–/mdx and Tg+/mdx mice. Each blot was stained with Ponceau stain before incubating with anti-nNOS to confirm uniformity of sample loading between lanes. Images of each Ponceau stained membrane (Loading) are shown beneath the image of the membrane after probing for nNOS. B, densitometric analysis of Western blots shown in A. Three mice were analysed in each group. C and D, immunolabelling showing nNOS in Tg+/mdx (C) and Tg–/mdx muscles (D). No nNOS was detected in the vascular smooth muscle of arterioles in the muscle parenchyma (arrow). Bar, 100 μm.
Discussion
DMD is a monogenic disease that results from loss of dystrophin, a single, low-prevalence protein at the muscle cell membrane (Hoffman et al. 1987). Structural similarities between dystrophin and other cytoskeletal proteins (Koenig et al. 1998) and the weakening of the muscle cell membrane in the absence of dystrophin (Petroff et al. 1993; Pasternak et al. 1995) provided strong evidence for the mechanical role of dystrophin, and offered a clear explanation for why dystrophin-deficient muscle suffers injury and early death under physiological stresses. However, many features of the complex phenotype of DMD are inexplicable in terms of a mechanical defect at the muscle membrane. The discovery that dystrophin deficiency caused severe down-regulation of nNOS at the transcriptional level provided a basis for the hypothesis that defects in NO-mediated signalling could underlie some features of the complex pathophysiology of DMD (Chang et al. 1996). Subsequently, defects in the regulation of sympathetic control of blood supply to dystrophic muscle (Thomas et al. 1998; Sander et al. 2000), perturbations in neuromuscular junction structure (Shiao et al. 2004), disruptions in the normal regenerative capacity of dystrophic muscle (Wehling et al. 2001), cardiac fibrosis in dystrophinopathic hearts (Wehling-Henricks et al. 2005), and muscle inflammation (Wehling et al. 2001) were all shown to be attributable, at least in part, to the loss of muscle nNOS during muscular dystrophy.
The findings of the present investigation show that muscle-derived NO can significantly influence neurogenesis in the adult brain and that increasing levels of NO production in the muscle of dystrophic animals can correct defects in hippocampal neurogenesis that occur in muscular dystrophy. We believe that these findings provide novel insights into the regulatory interactions that occur between muscle and brain, and show for the first time that muscle pathology can disrupt brain differentiation. In the context of DMD, these observations suggest that these perturbations of NO-mediated interactions between muscle and brain may underlie cognitive defects that typically occur in DMD. However, whether the correction of defective hippocampal neurogenesis that occurred in the Tg+/mdx mice requires the supraphysiological levels of NO that occurred in the mouse line we examined or would also occur by normalizing NO levels to those that occur in wild-type mice remains unknown.
The influence of muscle-derived NO on hippocampal neurogenesis during muscular dystrophy emphasizes the systemic regulatory roles of NO. More traditionally, NO has been viewed as a local signalling molecule because it is a highly reactive molecule with a short half-life. However, several proteins including serum albumin and haemoglobin function as reservoirs of NO and effectively increase the half-life of NO to make its systemic, regulatory functions possible. Much of the systemic delivery of NO is attributable to its reversible reaction with sulfhydryl groups of amino acids, peptides and proteins, to form S-nitrosothiols. Concentrations of S-nitrosothiols in plasma are about 10 000 times higher than the concentration of free NO, and they have half-lives of many minutes (Stamler et al. 1992). In particular, S-nitroso-albumin comprises approximately 80% of the S-nitrosothiols in plasma, and provides an important delivery system for NO throughout the body (Stamler et al. 1992). The release of NO from S-nitroso-albumin and other S-nitrosoproteins is triggered by the mixed disulfide reaction with low-molecular-weight thiols (LMW-thiols), such as glutathione, or the non-enzymatic transfer of NO to LMW thiols (Stamler et al. 1992). The half-life of S-nitroso-albumin in plasma is about 40 min, which provides more than sufficient time for systemic delivery and function (Hallstrom et al. 2002). The in vivo release of NO from S-nitroso-albumin has been demonstrated experimentally when administration of exogenous S-nitroso-albumin was shown to elevate plasma NO (Hallstrom et al. 2002; Katsumi et al. 2005). In addition, this treatment prevented vasoconstriction after ischaemia–reperfusion (I/R) in recipients and functionally protected the animals from I/R injury (Hallstrom et al. 2002). Thus, the reversible conversion of NO to S-nitroso-albumin enables NO to function as a systemic, regulatory molecule.
The data presented here show a clear relationship between the levels of muscle-derived NO in circulation and the levels of neurogenesis in the DG, but the relationships between perturbations of neurogenesis and cognitive defects in muscular dystrophy are less clear. Although some reports found no disruptions of either hippocampal LTP or learning in a hippocampal-dependent task (spatial learning) in mdx mice (Sesay et al. 1996; Vaillend et al. 1998), other studies identified impaired spatial and recognition memory (Muntoni et al. 1991; Vaillend et al. 1995), as well as enhanced LTP in the CA1 region of the hippocampus (Vaillend et al. 2004). Although those investigators (Vaillend et al. 2004) did not examine LTP in the DG, LTP of synapses between perforant pathway axons and granule cells were also likely to have been exaggerated in mdx mice. Because LTP can be induced more easily in newly born cells, at least in the granule cells of the hippocampus (Schmidt-Hieber et al. 2004), the increase in neurogenesis in the mdx DG that is reported here may underlie the changes in LTP and the associated cognitive defects.
Evidence continues to accumulate to show that newly born cells in the hippocampus are functionally different from mature cells. For example, young granule cells differ from mature granule cells in both passive and active membrane properties that could profoundly affect their ability to establish and maintain synapses and to form functional networks (Wang et al. 2000; Ambrogini et al. 2004; Schmidt-Hieber et al. 2004). Furthermore, pathologically high levels of neurogenesis may cause disruption of normal neuronal circuitry, although physiological levels of incorporation of newly born neurons into existing circuitry would be expected to be adaptive to functional demands. Thus, a feasible but unexplored hypothesis is that cognitive defects in DMD result from the pathologically high proportion of immature neurons in the circuitry of the DG, which may disrupt established circuitry and shift the electrochemical function of DG neurons to a less mature phenotype. In addition, because newly born neurons in the adult DG are significantly more excitable than mature neurons (Schmidt-Hieber et al. 2004), abnormally high levels of neuronal activity within the mdx hippocampus may contribute to the observed cognitive deficits.
Although the findings of the present investigation show that manipulation of systemic NO levels can correct defects in neurogenesis in the brains of mdx mice, there may be numerous obstacles between these findings and the development of NO-based therapeutic treatments for cognitive defects in DMD. A constant challenge to developing NO-based therapies lies in the multiple functionality of NO as a regulatory molecule; manipulation of systemic levels may cause dysregulation of other physiological functions, even if they were able to correct cognitive defects in muscular dystrophy. Also, the tremendous variability of biological outcomes with changing dosages of NO presents challenges; NO may maintain homeostasis or be highly toxic, according to its concentration. Furthermore, the vast number of processes that may be modulated by NO suggests that systemic perturbations of NO concentration will have unpredictable consequences. For example, changes in NO levels can modify the activity of diverse signalling pathways in which protein kinase G is a component, or influence enzyme function by S-nitrosylation, or affect protein structure by ADP-ribosylation or modify cell function by affecting alpha-actin transcription (Susswein et al. 2004; Sunico et al. 2005; Sellman et al. 2006). Nevertheless, the potential value of NO-based therapy for the treatment of DMD supports its exploration as a treatment strategy.
Acknowledgments
This research was supported by a grant from the Muscular Dystrophy Association of America (MDA4042) and the National Institutes of Health (AR47721).
References
- Albina JE, Cui S, Mateo RB, Reichner JS. Nitric oxide-mediated apoptosis in murine peritoneal macrophages. J Immunol. 1993;150:5080–5085. [PubMed] [Google Scholar]
- Allen JE, Rodgin DW. Mental retardation in association with progressive dystrophy. Amer J Dis Child. 1960;100:208–211. doi: 10.1001/archpedi.1960.04020040210008. [DOI] [PubMed] [Google Scholar]
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Ambrogini P, Lattanzi D, Ciuffoli S, Agostini D, Bertini L, Stocchi V, Santi S, Cuppini R. Morpho-functional characterization of neuronal cells at different stages of maturation in granule cell layer of adult rat dentate gyrus. Brain Res. 2004;1017:21–31. doi: 10.1016/j.brainres.2004.05.039. [DOI] [PubMed] [Google Scholar]
- Anderson JL, Head SI, Rae C, Morley JW. Brain function in Duchenne muscular dystrophy. Brain. 2002;125:4–13. doi: 10.1093/brain/awf012. [DOI] [PubMed] [Google Scholar]
- Billard C, Gillet P, Barthez M, Hommet C, Bertrand P. Reading ability and processing in Duchenne muscular dystrophy and spinal muscular atrophy. Dev Med Child Neurol. 1998;40:12–20. doi: 10.1111/j.1469-8749.1998.tb15351.x. [DOI] [PubMed] [Google Scholar]
- Blake DJ, Kroger S. The neurobiology of Duchenne muscular dystrophy: learning lessons from muscle? Trends Neurosci. 2000;23:92–99. doi: 10.1016/s0166-2236(99)01510-6. [DOI] [PubMed] [Google Scholar]
- Brenman JE, Chao DS, Xia H, Aldape K, Bredt DS. Nitric oxide synthase complexed with dystrophin and absent from skeletal muscle sarcolemma in Duchenne muscular dystrophy. Cell. 1995;82:743–752. doi: 10.1016/0092-8674(95)90471-9. [DOI] [PubMed] [Google Scholar]
- Bresolin N, Castelli E, Comi GP, Felisari G, Bardoni A, Perani D, Grassi F, Turconi A, Mazzucchelli F, Gallotti D, Moggio M, Prelle A, Ausenda C, Fazio G. Cognitive impairment in Duchenne muscular dystrophy. Neuromuscul Disord. 1994;4:359–369. doi: 10.1016/0960-8966(94)90072-8. [DOI] [PubMed] [Google Scholar]
- Bye N, Zieba M, Wreford NG, Nichols NR. Resistance of the dentate gyrus to induced apoptosis during ageing is associated with increases in transforming growth factor-β1 messenger RNA. Neuroscience. 2001;105:853–862. doi: 10.1016/s0306-4522(01)00236-6. [DOI] [PubMed] [Google Scholar]
- Chang WJ, Iannaccone ST, Lau KS, Masters BS, McCabe TJ, McMillan K, Padre RC, Spencer MJ, Tidball JG, Stull JT. Neuronal nitric oxide synthase and dystrophin-deficient muscular dystrophy. Proc Natl Acad Sci U S A. 1996;93:9142–9147. doi: 10.1073/pnas.93.17.9142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng A, Wang S, Cai J, Rao MS, Mattson MP. Nitric oxide acts in a positive feedback loop with BDNF to regulate neural progenitor cell proliferation and differentiation in the mammalian brain. Dev Biol. 2003;258:319–333. doi: 10.1016/s0012-1606(03)00120-9. [DOI] [PubMed] [Google Scholar]
- Drapeau E, Mayo W, Aurousseau C, Le Moal M, Piazza PV, Abrous DN. Spatial memory performances of aged rats in the water maze predict levels of hippocampal neurogenesis. Proc Natl Acad Sci U S A. 2003;100:14385–14390. doi: 10.1073/pnas.2334169100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchenne GBA. Recherches sur la paralysie musculaire pseudohypertrophique, ou paralysie myo-sclerosique. Arch Gen Med. 1868;11:5–25. [Google Scholar]
- Emery AEH. Duchenne Muscular Dystrophy. Oxford University Press; 1993. [Google Scholar]
- Gould E, Tanapat P, Rydel T, Hastings N. Regulation of hippocampal neurogenesis in adulthood. Biol Psychiatry. 2000;48:715–720. doi: 10.1016/s0006-3223(00)01021-0. [DOI] [PubMed] [Google Scholar]
- Gucuyener K, Ergenekon E, Erbas D, Pinarli G, Serdaroglu A. The serum nitric oxide levels in patients with Duchenne muscular dystrophy. Brain Dev. 2000;22:181–183. doi: 10.1016/s0387-7604(00)00106-6. [DOI] [PubMed] [Google Scholar]
- Hallstrom S, Gasser H, Neumayer C, Fugl A, Nanobashvili J, Jakubowski A, Huk I, Schlag G, Malinski T. S-Nitroso human serum albumin treatment reduces ischemia/reperfusion injury in skeletal muscle via nitric oxide release. Circulation. 2002;105:3032–3038. doi: 10.1161/01.cir.0000018745.11739.9b. [DOI] [PubMed] [Google Scholar]
- Hindley S, Juurlink BH, Gysbers JW, Middlemiss PJ, Herman MA, Rathbone MP. Nitric oxide donors enhance neurotrophin-induced neurite outgrowth through a cGMP-dependent mechanism. J Neurosci Res. 1997;47:427–439. [PubMed] [Google Scholar]
- Hinton VJ, De Vivo DC, Nereo NE, Goldstein E, Stern Y. Poor verbal working memory across intellectual level in boys with Duchenne dystrophy. Neurology. 2000;54:2127–2132. doi: 10.1212/wnl.54.11.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton VJ, De Vivo DC, Nereo NE, Goldstein E, Stern Y. Selective deficits in verbal working memory associated with a known genetic etiology: the neuropsychological profile of Duchenne muscular dystrophy. J Int Neuropsychol Soc. 2001;7:45–54. doi: 10.1017/s1355617701711058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman EP, Brown RH, Jr, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- Karagan NJ. Intellectual functioning in Duchenne muscular dystrophy; a review. Psychol Bull. 1979;86:250–259. [PubMed] [Google Scholar]
- Katsumi H, Nishikawa M, Yamashita F, Hashida M. Development of PEGylated poly S-nitrosated serum albumin, a novel S-nitrosothiol for prolonged delivery of nitric oxide in the blood circulation in vivo. J Pharmacol Exp Ther. 2005;314:1117–1124. doi: 10.1124/jpet.105.087429. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gage FH. Genetic determinants of adult hippocampal neurogenesis correlate with acquisition, but not probe trial performance, in the water maze task. Eur J Neurosci. 2002;16:129–136. doi: 10.1046/j.1460-9568.2002.02042.x. [DOI] [PubMed] [Google Scholar]
- Koenig M, Monaco AP, Kunkel LM. The complete sequence of dystrophin predicts a rod-shaped cytoskeletal protein. Cell. 1998;53:219–228. doi: 10.1016/0092-8674(88)90383-2. [DOI] [PubMed] [Google Scholar]
- Marsh GG, Munsat TL. Evidence for early impairment of verbal intelligence in Duchenne muscular dystrophy. Arch Dis Child. 1974;49:118–122. doi: 10.1136/adc.49.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehler MF. Brain dystrophin, neurogenetics and mental retardation. Brain Res Brain Res Rev. 2000;32:277–307. doi: 10.1016/s0165-0173(99)00090-9. [DOI] [PubMed] [Google Scholar]
- Moreno-Lopez B, Romero-Grimaldi C, Noval JA, Murillo-Carretero M, Matarredona ER, Estrada C. Nitric oxide is a physiological inhibitor of neurogenesis in the adult mouse subventricular zone and olfactory bulb. J Neurosci. 2004;24:85–95. doi: 10.1523/JNEUROSCI.1574-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Muntoni F, Mateddu A, Serra G. Passive avoidance behaviour deficit in the mdx mouse. Neuromuscul Disord. 1991;1:121–123. doi: 10.1016/0960-8966(91)90059-2. [DOI] [PubMed] [Google Scholar]
- Packer MA, Stasiv Y, Benraiss A, Chmielnicki E, Grinberg A, Westphal H, Goldman SA, Enikolopov G. Nitric oxide negatively regulates mammalian adult neurogenesis. Proc Natl Acad Sci U S A. 2003;100:9566–9571. doi: 10.1073/pnas.1633579100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak C, Wong S, Elson EL. Mechanical function of dystrophin in muscle cells. J Cell Biol. 1995;128:355–361. doi: 10.1083/jcb.128.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petroff BJ, Shrager JB, Stedman HH, Kelly AM, Sweeney HL. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc Natl Acad Sci U S A. 1993;90:3710–3714. doi: 10.1073/pnas.90.8.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poluha W, Schonhoff CM, Harrington KS, Lachyankar MB, Crosbie NE, Bulseco DA, Ross AH. A novel, nerve growth factor-activated pathway involving nitric oxide, p53, and p21WAF1 regulates neuronal differentiation of PC12 cells. J Biol Chem. 1997;272:24002–24007. doi: 10.1074/jbc.272.38.24002. [DOI] [PubMed] [Google Scholar]
- Sander M, Chavoshan B, Harris SA, Iannaccone ST, Stull JT, Thomas GD, Victor RG. Functional muscle ischemia in neuronal nitric oxide synthase-deficient skeletal muscle of children with Duchenne muscular dystrophy. Proc Natl Acad Sci U S A. 2000;97:13818–13823. doi: 10.1073/pnas.250379497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellman J, DeRuisseau KC, Betters JL, Lira VA, Soltow QA, Selsby JT, Criswell DS. In vivo inhibitioin of nitric oxide synthase impairs upregulation of contractile protein mRNA in overloaded plantaris muscle. J Appl Physiol. 2006;100:258–265. doi: 10.1152/japplphysiol.00936.2005. [DOI] [PubMed] [Google Scholar]
- Shiao T, Fond A, Deng B, Wehling-Henricks M, Adams ME, Froehner SC, Tidball JG. Defects in neuromuscular junction structure in dystrophic muscle are corrected by expression of a NOS transgene in dystrophin-deficient muscles, but not in muscles lacking α-and β1-syntrophins. Hum Mol Genet. 2004;13:1873–1884. doi: 10.1093/hmg/ddh204. [DOI] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429:184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psych. 1957;21:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesay AK, Errington ML, Levita L, Bliss TV. Spatial learning and hippocampal long-term potentiation are not impaired in mdx mice. Neurosci Lett. 1996;211:207–210. doi: 10.1016/0304-3940(96)12747-6. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12:578–584. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamler JS, Simon DI, Osborne JA, Mullins ME, Jaraki O, Michel T, Singel DJ, Loscalzo J. S-Nitrosylation of proteins with nitric oxide: synthesis and characterization of biologically active compounds. Proc Natl Acad Sci U S A. 1992;89:444–448. doi: 10.1073/pnas.89.1.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub V, Ratjen F, Amthor H, Voit T, Grasemann H. Airway nitric oxide in Duchenne muscular dystrophy. J Pediatr. 2002;141:132–134. doi: 10.1067/mpd.2002.125226. [DOI] [PubMed] [Google Scholar]
- Sunico CR, Portillo G, Gonzalez-Forero D, Moreno-Lopez B. Nitric-oxide-directed synaptic remodeling in the adult mammal CNS. J Neurosci. 2005;25:1448–1458. doi: 10.1523/JNEUROSCI.4600-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susswein AJ, Miller N, Hurwitz I. Nitric oxide and memory. Neuroscientist. 2004;10:153–162. doi: 10.1177/1073858403261226. [DOI] [PubMed] [Google Scholar]
- Teng KK, Esposito DK, Schwartz GD, Lander HM, Hempstead BL. Activation of c-Ha-Ras by nitric oxide modulates survival responsiveness in neuronal PC12 cells. J Biol Chem. 1999;274:37315–37320. doi: 10.1074/jbc.274.52.37315. [DOI] [PubMed] [Google Scholar]
- Thomas GD, Sander M, Lau KS, Huang PL, Stull JT, Victor RG. Impaired metabolic modulation of α-adrenergic vasoconstriction in dystrophin-deficient skeletal muscle. Proc Natl Acad Sci U S A. 1998;95:15090–15095. doi: 10.1073/pnas.95.25.15090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillend C, Rendon A, Misslin R, Ungerer A. Influence of dystrophin-gene mutation on mdx mouse behavior. I. Retention deficits at long delays in spontaneous alternation and bar-pressing tasks. Behav Genet. 1995;25:569–579. doi: 10.1007/BF02327580. [DOI] [PubMed] [Google Scholar]
- Vaillend C, Billard JM, Claudepierre T, Rendon A, Dutar P, Ungerer A. Spatial discrimination learning and CA1 hippocampal synaptic plasticity in mdx and mdx3cv mice lacking dystrophin gene products. Neuroscience. 1998;86:53–66. doi: 10.1016/s0306-4522(98)00023-2. [DOI] [PubMed] [Google Scholar]
- Vaillend C, Billard JM, Laroche S. Impaired long-term spatial and recognition memory and enhanced CA1 hippocampal LTP in the dystrophin-deficient Dmd (mdx) mouse. Neurobiol Dis. 2004;17:10–20. doi: 10.1016/j.nbd.2004.05.004. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Scott BW, Wojtowicz JM. Heterogenous properties of dentate granule neurons in the adult rat. J Neurobiol. 2000;42:248–257. [PubMed] [Google Scholar]
- Wehling M, Spencer MJ, Tidball JG. A nitric oxide synthase transgene ameliorates muscular dystrophy in mdx mice. J Cell Biol. 2001;155:123–131. doi: 10.1083/jcb.200105110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehling-Henricks M, Jordan MC, Roos KP, Deng B, Tidball JG. Cardiomyopathy in dystrophin-deficient hearts is prevented by expression of a neuronal nitric oxide synthase transgene in the myocardium. Hum Mol Genet. 2005;14:1921–1933. doi: 10.1093/hmg/ddi197. [DOI] [PubMed] [Google Scholar]
- Whelan TB. Neuropsychological performance of children with Duchenne muscular dystrophy and spinal muscle atrophy. Dev Med Child Neurol. 1987;29:212–220. doi: 10.1111/j.1469-8749.1987.tb02138.x. [DOI] [PubMed] [Google Scholar]
- Williams HM, Lippok H, Doherty GH. Nitric oxide and peroxynitrite signalling triggers homocysteine-mediated apoptosis in trigeminal sensory neurons in vitro. Neurosci Res. 2008;60:380–388. doi: 10.1016/j.neures.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Zhu DY, Liu SH, Sun HS, Lu YM. Expression of inducible nitric oxide synthase after focal cerebral ischemia stimulates neurogenesis in the adult rodent dentate gyrus. J Neurosci. 2003;23:223–229. doi: 10.1523/JNEUROSCI.23-01-00223.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]