Abstract
Exercise studies have suggested that the presence of carbohydrate in the human mouth activates regions of the brain that can enhance exercise performance but direct evidence of such a mechanism is limited. The first aim of the present study was to observe how rinsing the mouth with solutions containing glucose and maltodextrin, disguised with artificial sweetener, would affect exercise performance. The second aim was to use functional magnetic resonance imaging (fMRI) to identify the brain regions activated by these substances. In Study 1A, eight endurance-trained cyclists ( 60.8 ± 4.1 ml kg−1 min−1) completed a cycle time trial (total work = 914 ± 29 kJ) significantly faster when rinsing their mouths with a 6.4% glucose solution compared with a placebo containing saccharin (60.4 ± 3.7 and 61.6 ± 3.8 min, respectively, P= 0.007). The corresponding fMRI study (Study 1B) revealed that oral exposure to glucose activated reward-related brain regions, including the anterior cingulate cortex and striatum, which were unresponsive to saccharin. In Study 2A, eight endurance-trained cyclists (
60.8 ± 4.1 ml kg−1 min−1) completed a cycle time trial (total work = 914 ± 29 kJ) significantly faster when rinsing their mouths with a 6.4% glucose solution compared with a placebo containing saccharin (60.4 ± 3.7 and 61.6 ± 3.8 min, respectively, P= 0.007). The corresponding fMRI study (Study 1B) revealed that oral exposure to glucose activated reward-related brain regions, including the anterior cingulate cortex and striatum, which were unresponsive to saccharin. In Study 2A, eight endurance-trained cyclists ( 57.8 ± 3.2 ml kg−1 min−1) tested the effect of rinsing with a 6.4% maltodextrin solution on exercise performance, showing it to significantly reduce the time to complete the cycle time trial (total work = 837 ± 68 kJ) compared to an artificially sweetened placebo (62.6 ± 4.7 and 64.6 ± 4.9 min, respectively, P= 0.012). The second neuroimaging study (Study 2B) compared the cortical response to oral maltodextrin and glucose, revealing a similar pattern of brain activation in response to the two carbohydrate solutions, including areas of the insula/frontal operculum, orbitofrontal cortex and striatum. The results suggest that the improvement in exercise performance that is observed when carbohydrate is present in the mouth may be due to the activation of brain regions believed to be involved in reward and motor control. The findings also suggest that there may be a class of so far unidentified oral receptors that respond to carbohydrate independently of those for sweetness.
57.8 ± 3.2 ml kg−1 min−1) tested the effect of rinsing with a 6.4% maltodextrin solution on exercise performance, showing it to significantly reduce the time to complete the cycle time trial (total work = 837 ± 68 kJ) compared to an artificially sweetened placebo (62.6 ± 4.7 and 64.6 ± 4.9 min, respectively, P= 0.012). The second neuroimaging study (Study 2B) compared the cortical response to oral maltodextrin and glucose, revealing a similar pattern of brain activation in response to the two carbohydrate solutions, including areas of the insula/frontal operculum, orbitofrontal cortex and striatum. The results suggest that the improvement in exercise performance that is observed when carbohydrate is present in the mouth may be due to the activation of brain regions believed to be involved in reward and motor control. The findings also suggest that there may be a class of so far unidentified oral receptors that respond to carbohydrate independently of those for sweetness.
Carbohydrate supplementation is widely used to improve or sustain athletic performance and the beneficial effects of high muscle glycogen content are well known in events where the body stores of glycogen become depleted (Bergstrom et al. 1967; Coyle et al. 1986; Coggan & Coyle, 1987). However, the value of exogenous carbohydrate is questionable during exercise lasting for around 1 h, such as a cycling time trial and in many team sports. Hawley and colleagues (1997) concluded that adequate glycogen remains in the working muscles after 1 h of all-out cycle exercise and others have suggested that the contribution of blood glucose to energy expenditure during intense exercise is minimal when compared to the high oxidation rates of muscle glycogen (Romijn et al, 1993; van Loon et al. 2001). Moreover, the amount of ingested carbohydrate that can be absorbed during 1 h of exercise is relatively small (∼22 g) and makes a minimal contribution to the total carbohydrate oxidation rate (McConell et al. 2000). Despite these theoretical reservations, the practical observation has been that carbohydrate feeding does improve performance during relatively short (∼1 h) and intense (>75% ) self-paced exercise tasks in thermoneutral (Neufer et al. 1987; Anantaraman et al. 1995; Jeukendrup et al. 1997) and hyperthermic conditions (Below et al. 1995; Millard-Stafford et al. 1997), although there are some reports to the contrary (Desbrow et al. 2004; Burke et al. 2005).
) self-paced exercise tasks in thermoneutral (Neufer et al. 1987; Anantaraman et al. 1995; Jeukendrup et al. 1997) and hyperthermic conditions (Below et al. 1995; Millard-Stafford et al. 1997), although there are some reports to the contrary (Desbrow et al. 2004; Burke et al. 2005).
A possible explanation of this paradox comes from the observations of (Carter et al. 2004a,b) who found that the route of administration of the exogenous carbohydrate was important for the enhancement of performance during exercise lasting about 1 h. They found that intravenous infusion of glucose, which made available large quantities of carbohydrate in the circulation, did not affect the time to complete a ∼1 h cycle time trial compared with a saline placebo. However, regularly rinsing the mouth with a non-sweet maltodextrin solution, which would have had no effect on circulating glucose levels, significantly reduced the time to complete the performance trial. The apparent absence of a peripheral metabolic action of exogenous carbohydrate in these circumstances raises the possibility of a centrally mediated effect. Carter and colleagues drew two conclusions from their observations. The first was that there are taste receptors in the mouth that can influence neural pathways, ultimately leading to improved exercise performance and, second, that there are receptors in the mouth sensitive to non-sweet carbohydrate.
Both suggestions might seem unlikely but there is accumulating evidence that there is a central neural response from an oral carbohydrate stimulus that may have behavioural consequences. Neuroimaging studies have shown that oral glucose produces activation of the primary taste cortex and the putative secondary taste cortex in the orbitofrontal cortex (O’Doherty et al. 2001; de Araujo et al. 2003a). The primary taste cortex and orbitofrontal cortex are believed to have projections to the dorsolateral prefrontal cortex, anterior cingulate cortex and ventral striatum, brain regions believed to mediate the behavioural and autonomic responses to rewarding stimuli, including taste (Rolls, 2007). It has therefore been suggested that activation of these taste-related brain regions can influence emotion and behaviour (Kringelbach, 2004) and this might, for instance, have an impact on exercise performance. Consequently we have explored the extent to which stimulation of oral receptors with glucose, maltodextrin and artificial sweetener affects exercise performance and how these behavioural responses may be related to the activation of different brain areas.
The first study (Study 1A) described here was designed to determine whether stimulation of oral receptors with glucose could improve performance during a ∼1 h cycle time trial compared to a placebo solution containing the artificial sweetener saccharin. Since performance was found to be improved with glucose compared to saccharin we hypothesised that there would be differences in the brain areas activated by these two sweet-tasting substances as revealed by functional magnetic resonance imaging (fMRI; Study 1B). The second study tested the effect of maltodextrin on exercise performance (Study 2A), showing it to be similar to that previously reported by (Carter et al. 2004a) and to the effects we observed with glucose. On the basis of these results we hypothesised that there would be similarities in the brain areas activated by oral glucose and maltodextrin despite the difference in perceived sweetness between the two carbohydrates (Study 2B).
Methods
Oral carbohydrate and exercise performance
Subjects
Eight male subjects, who had completed a general health questionnaire to exclude any history of diabetes, cardiovascular or respiratory disease, were recruited for Study 1A, the effects of oral glucose on performance. All participants were competitive or recreational cyclists involved in endurance training on a regular basis (≥2 sessions per week) and were familiar with the type of testing involved in these studies. Their mean age, weight, body mass index (BMI) and maximal oxygen uptake  were 29 ± 9 years, 77.1 ± 8.0 kg, 23.8 ± 2.5 kg m−2 and 60.8 ± 4.1 ml kg−1 min−1, respectively (mean ±s.d.). Six male and two female subjects volunteered for Study 2A, the effects of maltodextrin on performance. Their mean age, weight, BMI and
were 29 ± 9 years, 77.1 ± 8.0 kg, 23.8 ± 2.5 kg m−2 and 60.8 ± 4.1 ml kg−1 min−1, respectively (mean ±s.d.). Six male and two female subjects volunteered for Study 2A, the effects of maltodextrin on performance. Their mean age, weight, BMI and  were 22 ± 3 years, 69.7 ± 12.1 kg, 22.3 ± 2.7 kg m−2 and 57.8. ± 3.2 ml kg−1 min−1, respectively.
were 22 ± 3 years, 69.7 ± 12.1 kg, 22.3 ± 2.7 kg m−2 and 57.8. ± 3.2 ml kg−1 min−1, respectively.
Experimental design
The design and experimental conditions were essentially as described by (Carter et al. 2004a). Briefly, the subjects visited the laboratory on four occasions. Visit 1 was an incremental exercise test to exhaustion to determine  and maximum power output
and maximum power output  . Visits 2, 3 and 4 were simulated time trials in which the subjects had to complete a set amount of work in the shortest time possible. Visit 2 served to familiarize the subjects with the time trial procedure and ensure they could complete the required exercise. During visits 3 and 4, subjects performed trials in which they were given either the test solution containing glucose (GLU) in Study 1A, maltodextrin (MALT) containing artificial sweetener in Study 2A, or an artificially sweetened placebo solution (PLA) to rinse around their mouths at regular intervals. Trials with the test and placebo solutions were carried out in a randomised, counterbalanced and double-blind fashion, with each visit separated by a period of at least 3 days.
. Visits 2, 3 and 4 were simulated time trials in which the subjects had to complete a set amount of work in the shortest time possible. Visit 2 served to familiarize the subjects with the time trial procedure and ensure they could complete the required exercise. During visits 3 and 4, subjects performed trials in which they were given either the test solution containing glucose (GLU) in Study 1A, maltodextrin (MALT) containing artificial sweetener in Study 2A, or an artificially sweetened placebo solution (PLA) to rinse around their mouths at regular intervals. Trials with the test and placebo solutions were carried out in a randomised, counterbalanced and double-blind fashion, with each visit separated by a period of at least 3 days.
Visits 2–4
Subjects reported to the laboratory either in the morning (07:00–09:00) following an overnight fast or in the evening (17:00–18:00) following a 6 h fast. Each subject performed their consecutive trials at the same time of day to avoid circadian variation. The subjects were given a set amount of work, equivalent to cycling for 1 h at 75% (914 ± 29 kJ in Study 1A; 837 ± 68 kJ in Study 2A) to complete as fast as possible with the ergometer set so that 75%
(914 ± 29 kJ in Study 1A; 837 ± 68 kJ in Study 2A) to complete as fast as possible with the ergometer set so that 75% was obtained when pedalling at the subject's preferred cadence. The range of self-selected cadences was 80–100 r.p.m. Subjects exercised separately with no performance feedback other than the percentage of the trial completed and had minimal contact with the investigators. The time trial procedure is highly reproducible when performed in this way and with subjects experienced in this type of exercise (Jeukendrup et al. 1996).
was obtained when pedalling at the subject's preferred cadence. The range of self-selected cadences was 80–100 r.p.m. Subjects exercised separately with no performance feedback other than the percentage of the trial completed and had minimal contact with the investigators. The time trial procedure is highly reproducible when performed in this way and with subjects experienced in this type of exercise (Jeukendrup et al. 1996).
Mouth rinse solutions
The placebo (PLA) mouth rinse solution was made from 150 ml of a commercially available non-caloric concentrate sweetened with aspartame and saccharin (Robinsons Soft Drinks Ltd, UK) diluted to 1000 ml with water. The GLU mouth rinse contained 64 g glucose (Roquette, France) per 1000 ml of the same solution. The MALT mouth rinse contained 64 g maltodextrin (Roquette, France) per 1000 ml of the same solution. The strong artificial sweetness reduced any sensory clues that subjects might use to consciously differentiate between the GLU, MALT and PLA mouth rinses.
Mouth rinse protocol
At the start, and after every 12.5% of the time trial completed, subjects were given 25 ml of GLU, MALT or PLA. The subjects were instructed to rinse the fluid around their mouths for ∼10 s, and then spit the solution into a bowl held by the investigator. In Study 1A the subjects were asked to rate the GLU and PLA solutions, for sweetness and viscosity both pre-and post-exercise using 100 mm visual analogue scales with an anchor point at 0 mm labelled ‘Nil’ and at 100 mm labelled ‘Extreme’. In Study 2A the subjects were asked to rate the solutions only pre-exercise. At the end of the final trial subjects were asked whether they could distinguish between the rinse solutions tasted during visits 3 and 4.
Data and statistical analyses
All statistical analyses were carried out with an SPSS package, version 15.0 (SPSS Inc., USA). Paired Student's t tests were performed to study differences in time trial performance between PLA and either GLU or MALT in each study. Two-way (trial × time) repeated measures ANOVA was performed to determine differences in power output, heart rate and perceived exertion. Significant effects were followed up by post hoc comparisons (Tukey HSD). Data are reported as mean and standard deviation (mean ±s.d.), unless otherwise stated.
Brain responses to glucose, maltodextrin and saccharin
Two separate studies were performed to determine the central response to oral solutions containing glucose, maltodextrin and the artificial sweetener saccharin. The aim of the first study (Study 1B) was to compare the regions of the brain activated by caloric (glucose) and non-caloric sweetness (saccharin). The objective of the second study (Study 2B) was to reveal the brain areas activated by an oral maltodextrin solution and compare this with the pathways involved in glucose tasting.
Subjects
Seven right-handed subjects (of whom four were male) participated in Study 1B Their mean ages, weights and BMI were 23 ± 3 years, 72.6 ± 6.1 kg and 22.2 ± 1.0 kg m−2, respectively. For Study 2B, a further seven right-handed subjects (five male) were recruited. Their mean ages, weights and BMI were 24 ± 2 years, 67.9 ± 8.1 kg and 22.7 ± 0.7 kg m−2, respectively. The subjects completed the same general health questionnaire as for Studies 1A and 2A and were all healthy and recreationally active but not endurance-trained athletes used in the two exercise studies.
Experimental design
The subjects in both studies fasted, except for water, overnight from 22:00 and were scanned the next morning, starting between 09:30 and 11:00 This was to simulate the physiological condition of participants in Studies 1A and 2A and the investigation of (Carter et al. 2004a). Using functional magnetic resonance imaging (fMRI), the blood oxygenation level-dependent (BOLD) responses were determined in response to the introduction of different solutions into the mouth. In Study 1B, the caloric-sweetened stimulus was 90 g glucose (Roquette, France) in 1000 ml distilled water. The non-caloric sweetened stimulus consisted of 60 mg of sodium saccharin (Hermestas, Switerland) dissolved in 1000 ml of distilled water. The concentrations of the stimuli were chosen on the basis of a preliminary taste-testing session performed on a panel of subjects who matched the two solutions for sweetness from a range of concentrations presented. In Study 2B, the two isocaloric test stimuli were 180 g glucose (Roquette, France) and 180 g maltodextrin (Roquette, France) both made up in 1000 ml distilled water. The manufacturer's specifications state that the maltodextrin contained ∼2% glucose and ∼7% disaccharides and was predominantly composed of oligo-and polysaccharides of a variety of chain lengths. The control stimulus in both studies was a tasteless solution consisting of the main ionic components of saliva (25 mmol KCl and 2.5 mmol NaHCO3 in distilled water; O’Doherty et al. 2001). This control solution allowed neural representations of non-taste actions such as swallowing and tongue movements that were common to all tests, to be subtracted during subsequent analysis. An ‘artificial saliva’ solution was employed, instead of pure water, to minimize the activation of cortical taste areas which are sensitive to water in the mouth (de Araujo et al. 2003b). All solutions were delivered at room temperature (21–23°C).
The taste stimuli were delivered through separate plastic tubes to the subject's mouth. Each tube was connected to a reservoir via a syringe and a two-way tap that allowed 2.5 ml of the solution to be delivered in a similar way to that described by (de Araujo et al. 2003a,b). The experimental protocol was an event-related design. At the beginning of each trial (see Fig. 1) there was a 10 s rest period following which 2.5 ml of a test solution (either glucose or saccharin in Study 1B; glucose or maltodextrin in Study 2B), chosen at random, was delivered to the mouth. The subject was instructed to make one tongue movement to distribute the fluid in the oral cavity then 10 s after the delivery of the stimulus and cued by a visual stimulus on a screen in the scanner, the subject swallowed the solution within a 2 s period. This was followed by an 18 s rest period after which the control solution was administered in exactly the same way as the main stimulus. The subject was cued to swallow again after 10 s. Each full trial lasted 52 s and the next trial started immediately after the 2 s interval to swallow the control solution. In both studies the full trial was repeated 12 times for each test solution.
Figure 1. A single trial of stimulus and control delivery.
The stimulus was delivered at time 10 s and swallowing (SW) cued after 10 s and completed within a 2 s period. The control solution was delivered at 40 s and, 10 s later, swallowing was cued (SW). Each full trial lasted 52 s.
After completing the experiment, subjects were asked to rinse their mouths with 2.5 ml of the test and control solutions and rate each separately for sweetness, pleasantness and viscosity using 100 mm visual analogue scales. The anchor point at 0 mm was labelled ‘Very Unsweet/Very Unpleasant/Very Fluid’ and at 100 mm labelled ‘Very Sweet/Very Pleasant/Very Thick’. Participants were asked to make a mark on separate scales according to how sweet, pleasant or viscous they found each solution. Comparison of ratings between the test and control solutions was made using repeated measures ANOVA, with specific differences determined using paired Student's t tests performed with SPSS.
fMRI data acquisition
The experiments were conducted at the Birmingham University Imaging Centre (3T Achieva scanner; Philips, The Netherlands). Six hundred and twenty four T2*-weighted images were acquired from each subject using an 8-channel SENSE head coil and a 2D single-shot EPI sequence (34 axial slices, whole brain coverage, echo time = 35 ms, repetition time = 2 s, flip angle = 65°, field of view = 240 mm × 102 mm × 240 mm, 3 mm × 3 mm × 3 mm resolution). T1-weighted anatomical data were also collected (160 sagittal slices, 1 mm × 1 mm × 1 mm).
fMRI data analysis
Data processing was carried out using FEAT (FMRI Expert Analysis Tool) version 5.91, part of the FMRIB Software Library, (http://www.fmrib.ox.ac.uk/fsl). Prior to processing, slice timing was corrected and the volumes in each run were motion-corrected and realigned to the middle volume of the run using MCFLIRT (Jenkinson et al. 2002). The BOLD signals were then spatially filtered with a 5 mm full width at half-maximum (FWHM) Gaussian kernel and temporally high-and low-pass filtered. Statistical analysis at the level of the individual subject was carried out with FILM (Woolrich et al. 2001) that uses a general linear model approach. Explanatory variables associated with the oral delivery of each test solution were convolved with a gamma-derived haemodynamic response function (standard deviation of 3 s, mean lag of 6 s). The response to the control solution within the respective trials of each test solution was also entered into the model. The remaining time periods (i.e. the ‘rest’ intervals) were considered baseline and all activation levels in these individual contrasts were calculated relative to this unmodelled condition.
In Study 1B, the effects of oral glucose and saccharin were defined by the contrasts [Glucose – Control] and [Saccharin – Control]. For Study 2B, the effects of oral glucose and maltodextrin were defined by the contrasts [Glucose – Control] and [Maltodextrin – Control]. These differential contrasts identified areas of the brain with significantly greater activation with the oral test solution compared with their respective control. Inclusive masking was also performed with these differential contrasts and the individual test solutions contrast (i.e. [Glucose – Control] AND [Glucose]). This was to ensure that only positive responses to the test solution were retained. Registration to high resolution and standard space images (Montreal Neurological Institute (MNI)) was carried out using FLIRT (Jenkinson & Smith, 2001).
Higher level (group) statistical analysis was carried out using FMRIB's local analysis of mixed effects (FLAME) 1 + 2 (Beckmann et al. 2003; Woolrich et al. 2004). FLAME uses a hierarchical statistical model where subjects are treated as random variables to test for effects that could be generalised to the population (Beckmann et al. 2003; Woolrich et al. 2004). Z (Gaussianised T/F) statistic images were corrected for multiple comparisons using the methods described by Worsley (2001). Initially the Z-stat image for each contrast was thresholded at Z > 2.7 (equivalent to a one-tailed P of 0.003) to form contiguous clusters. Gaussian Random Field theory then gives the probability of finding each cluster, given its spatial extent and Z-threshold. Only significant clusters, at P < 0.05, were retained (Worsley, 2001). MRIcro (http://www.sph.sc.edu/comd/rorden/mricro) was utilized to display higher level statistical maps on a standard brain template and the WFU Pickatlas (Lancaster et al. 2000) and AAL atlas (Tzourio-Mazoyer et al. 2002) were used to identify the anatomical location of clusters of significant activation.
To reveal the common cortical areas that were activated by the glucose and saccharin solutions in Study 1B, a binary mask image was created from [Glucose – Control] AND [Saccharin – Control]. This descriptive method revealed cortical regions that were significantly activated by both glucose and saccharin solutions. Similarly, in Study 2B, a binary mask image from [Glucose – Control] AND [Maltodextrin – Control] was created to highlight areas that were significantly activated by both glucose and maltodextrin solutions. Within the regions of common activation highlighted by the conjunction overlay masks, the mean percentage change in BOLD signal produced by the test and control solutions was extracted using the FEATquery tool (FMRIB, Oxford). Separate mask images were created for each cluster of common activation revealed by the conjunction overlay masks. For every subject, FEATquery could then be used to calculate the mean percentage signal change associated with the modelled contrast within the brain area of each separate cluster mask. A comparison of the cluster average BOLD response produced by the test solutions and their respective control was subsequently made using paired Student's t tests performed with SPSS.
Ethical issues
All subjects gave their written consent and both studies were approved by the Local Ethics Committee and conformed to the Declaration of Helsinki.
Results
Study 1A: Oral glucose and exercise performance
Rinse solution detection
Subjects made no distinction between the GLU and PLA solutions for sweetness in their pre-or post-exercise ratings (P= 0.51, trial × time interaction). However, they rated the GLU solution more viscous than PLA post-exercise (54 ± 16 and 33 ± 11 mm, respectively, P= 0.02).
Performance time and power output
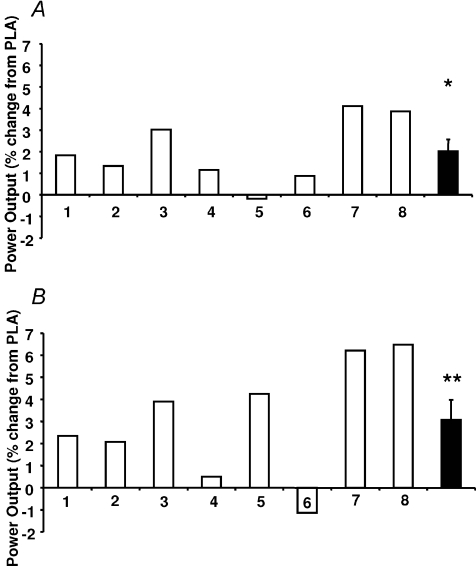
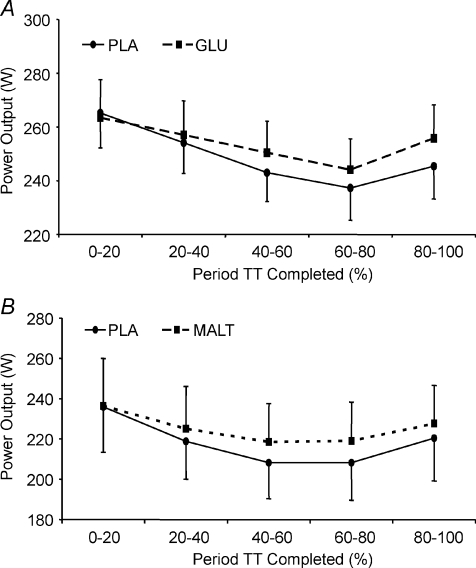
The subjects’ mean time to complete the familiarisation trial was 61.9 ± 3.6 min. Seven of the eight subjects completed the performance trial faster during the GLU trial; the average times were 60.4 ± 3.7 and 61.6 ± 3.8 min for the GLU and PLA trials, respectively, which were significantly different in a paired comparison (P= 0.007). In completing the same amount of work in a faster time, the subjects, with one exception, had a higher average power output. The percentage change for each subject is shown in Fig. 2A, the average improvement for the group being 2.0 ± 1.5%. When the average power output was calculated over successive 20% intervals of the time trial, there was a significant main effect of trial (Fig. 3A, P= 0.006). There was a tendency for power output to be somewhat higher in the middle and later stages of the GLU trial compared with PLA although this did not reach significance at any specific stage of the trial (P= 0.11, trial × time interaction).
Figure 2. Individual (open columns) and mean ±s.e.m. (filled columns) percentage change of power output compared to PLA during A, the GLU trial and B, the MALT trial.
*Significant difference from PLA (P= 0.007) and ** (P= 0.012).
Figure 3. Average power output calculated at intervals during the trials compared to PLA during A, the GLU trial and B, during the MALT trial.
Data are mean ±s.e.m.
Perception of exertion and heart rate
The subjects’ perception of exertion (RPE; 6 to 20 point scale; Borg, 1982) increased throughout the two trials (main effect of time; P < 0.01) but with no differences between conditions at any time (P= 0.95, trial × time interaction). The average RPE values were 16 ± 1.8 and 16 ± 1.6 for the GLU and PLA trials, respectively. Heart rate increased steadily throughout the performance trials, reaching values of 180 ± 3 and 177 ± 4 beats min−1 at the end of the GLU and PLA trials, respectively (main effect of time; P < 0.01). When average heart rate was calculated for every 10 min of exercise completed, a main effect of trial (P= 0.043) was found, consistent with the tendency for a higher power output with GLU although it was not significantly higher than PLA at any specific time (P= 0.36, trial × time interaction).
Study 1B: Representation in the human brain of oral glucose and saccharin
To be included in the statistical analysis, subjects had to have an average absolute voxel displacement (each time point with respect to the reference image) of < 1.5 mm. All subjects passed this data quality control procedure. The average absolute voxel displacement was 0.79 ± 0.14 mm (mean ±s.d.) and the maximum was 1.03 mm across the group.
Responses to the oral glucose solution
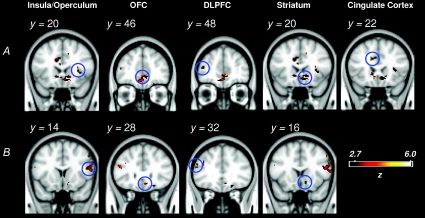
The cortical responses to oral glucose, revealed by the contrast [Glucose – Control], are presented in Fig. 4A. Activation was found in the insula/frontal operculum, which is the putative human primary taste cortex. Glucose taste also resulted in significant clusters of activation within left and right areas of the dorsolateral prefrontal cortex, a region of the right caudate which forms part of the striatum, and an anterior area of the cingulate cortex, The coordinates of the peak voxels of each cluster, and their respective Z-scores, are presented in Table 1.
Figure 4. Activations in the insula/frontal operculum, the dorsolateral prefrontal cortex (DLPFC), the striatum and the cingulate cortex by the contrasts A, [Glucose – Control] and B, [Saccharin – Control].
The blue circles indicate the region where the peak voxel of activation was found (see Table 1) in the group analysis. The colour bar indicates the Z-score significance level. Clusters were formed by thresholding the Z-stat image for each contrast at Z > 2.7 (equivalent to a one-tailed P of 0.003). The y values are with respect to the MNI co-ordinate system.
Table 1.
Peak voxels of the clusters activated in different brain regions by oral glucose and saccharin solutions as shown in Fig. 4
| Contrast | Brain region | Coordinates (MNI, peak) | Z-score |
|---|---|---|---|
| [Glucose – Control] | Insula/Operculum | 50, 14, 12 | 4.94 |
| −38, 18, −6 | 4.22 | ||
| DLPFC | −40. 44, 22 | 5.01 | |
| 26, 48, 14 | 4.83 | ||
| 38, 34, 34 | 4.75 | ||
| Striatum | 18, 14, 8 | 4.42 | |
| Cingulate Cortex | 4, 34, 26 | 5.01 | |
| [Saccharin – Control] | Insula/Operculum | 50, 12, 4 | 4.62 |
| DLPFC | −36, 36, 14 | 4.38 |
Clusters were formed by thresholding the Z-stat image for each contrast at Z > 2.7 (equivalent to a one-tailed P of 0.003). Dorsolateral prefrontal cortex (DLPFC)
Responses to the oral saccharin solution
The brain regions responding to an oral saccharin stimulus, revealed by the contrast [Saccharin – Control], are presented in Fig. 4B. Activation was found within the insula/frontal operculum. The only other significant cluster of activation was found in the left dorsolateral prefrontal cortex. The coordinates of the peak voxels of each cluster and Z-scores, are presented in Table 1. In contrast to the response of oral glucose, saccharin taste produced no activation within the anterior cingulate cortex or striatum.
Brain areas responding to both oral glucose and saccharin
To examine the cortical regions that were activated by both caloric and non-caloric sweetened solutions, a conjunction overlay mask of [Glucose − Control] AND [Saccharin − Control] was created. An area in the right insula/frontal operculum, extending from y= 10 to y= 14 and z= 8 to z= 18, was revealed (Fig. 5A). A region of the left dorsolateral prefrontal cortex was also shown to be responsive to both glucose and saccharin solutions (Fig. 5B). Figure 5C and D demonstrate the similar change in mean BOLD signal produced by the sweetened solutions and their respective control tastants within these clusters of common activation.
Figure 5. Areas of common activation from the glucose and saccharin contrasts ([Glucose – Control] AND [Saccharin – Control]).
A, the insula/frontal operculum (e.g. MNI coordinates: 54, 12, 14) and B, the dorsolateral prefrontal cortex (DLPFC) (e.g. MNI coordinates: −38, 38, 12). C and D demonstrate the average change in BOLD response produced by glucose, saccharin and their respective control solutions within the cluster of common activation highlighted in the insula/frontal operculum and the DLPFC, respectively. *Significant difference between solutions (P < 0.05); **(P < 0.001). Values are mean ±s.e.m.
Solution ratings
Glucose (55 ± 21 mm) and saccharin (39 ± 18 mm) solutions were rated significantly sweeter than the control solution (19 ± 8 mm; P= 0.003 and P= 0.005, respectively). A similar difference was also found between the solutions for subjective pleasantness as the glucose (64 ± 8 mm) and saccharin (54 ± 12 mm) were rated significantly more pleasant than the control solution (25 ± 6 mm; P= 0.002 and P= 0.024, respectively). There was no significant difference in subjective sweetness (P= 0.11) or pleasantness (P= 0.12) between the glucose and saccharin solutions. Subjects were also unable to distinguish any difference in viscosity between glucose (31 ± 22 mm), saccharin (27 ± 18 mm) and the control solution (21 ± 15 mm) (P= 0.17).
Study 2A: Oral maltodextrin and exercise performance
Rinse solution detection
Subjects made no distinction between the MALT and PLA solutions for sweetness (74 ± 14 and 130 ± 34 mm, respectively, P= 038) or viscosity (22 ± 18 and 21 ± 17 mm, respectively, P= 0.75) in the pre-exercise ratings. None of the subjects were able to distinguish between the rinse solutions after completing the final trial.
Performance time and power output
The subject's mean time to complete the familiarisation trial was 651 ± 4.4 min. Seven of the eight subjects completed the MALT faster than the PLA trial. The average times were 62.6 ± 4.7 min and 64.6 ± 4.9 min for the MALT and PLA trials, respectively (P= 0.012). The differences in average power output between the PLA and MALT trials for each subject are shown in Fig. 2B, the mean increase being 3.1 ± 1.7%. When the average power output was calculated for successive 20% intervals of the time trial, there was a significant main effect of trial (P= 0.013), although power during MALT was not significantly higher than PLA at any specific stage of the trial (Fig. 3B, P= 0.35, trial × time interaction).
Perception of exertion and heart rate
The average RPE values for the trials were 15 ± 18 and 15 ± 1.5 and at the end of exercise they were 17 ± 2.1 and 17 ± 1.8 for MALT and PLA trials, respectively (main effect of time; P < 0.01), with no differences between conditions at any time (P= 0.85, trial × trial interaction). Heart rate increased from 115 ± 13 and 111 ± 9 beats min−1 at the end of the warm up in the MALT and PLA trials, respectively, to 169 ± 4 and 167 ± 4 beats min−1 after 10 min of exercise. Thereafter, heart rate continued to increase reaching values of 181 ± 10 and 180 ± 10 beats min−1 at the end of the MALT and PLA trials, respectively (main effect of time; P < 0.01). There were no differences in heart rate responses between the two trials at any time (P= 0.68, trial × time interaction).
Study 2B: Representation in the human brain of oral glucose and maltodextrin
The mean absolute voxel displacement of all subjects was under the motion threshold, thus the data from all subjects were included in the subsequent statistical analysis. The mean absolute voxel displacement was 0.75 ± 0.15 mm and the maximum was 0.95 mm from the seven subjects.
Responses to the oral glucose solution
The brain regions responding to an oral glucose stimulus, revealed by the contrast [Glucose – Control], are shown in Fig 6A. Significant clusters of activation by glucose taste were found in the insula/frontal operculum, an area of the medial orbitofrontal cortex that extended into the bordering anterior cingulate cortex, the dorsolateral prefrontal cortex, a more dorsal region of the anterior cingulate cortex and the left and right caudate. The coordinates of the peak voxels of each cluster, and the Z-scores, are presented in Table 2.
Figure 6. Activations in the insula/frontal operculum, the orbitofrontal cortex (OFC), the dorsolateral prefrontal cortex (DLPFC), the striatum, and the cingulate cortex by the contrasts A, [Glucose – Control] and B, [Maltodextrin – Control].
The blue circles indicate the region where the peak voxel of activation was found (see Table 2) in the group analysis. The colour bar indicates the Z-score significance level. Clusters were formed by thresholding the Z-stat image for each contrast at Z > 2.7 (equivalent to a one-tailed P of 0.003). The y values are with respect to the MNI co-ordinate system.
Table 2.
Peak voxels of the clusters activated in different brain regions by oral glucose and maltodextrin solutions as shown in Fig. 6
| Contrast | Brain region | Coordinates (MNI, peak) | Z-score |
|---|---|---|---|
| [Glucose – Control] | Insula/Operculum | 30, 20, 10 | 4.99 |
| 56, 2, 12 | 4.78 | ||
| −52, 0, 32 | 4.18 | ||
| OFC | 2, 46, −6 | 5.07 | |
| DLPFC | −40, 48, 12 | 3.84 | |
| Striatum | 14, 20, −2 | 5.09 | |
| −12, 18, −6 | 4.94 | ||
| Cingulate Cortex | −14, 22, 30 | 5.16 | |
| 10, 20, 40 | 5.00 | ||
| [Maltodextrin – Control] | Insula/Operculum | 56, 14, 16 | 5.22 |
| −54, 12, −6 | 4.90 | ||
| OFC | 4, 28, −14 | 4.03 | |
| 12, 44, −2 | 3.84 | ||
| DLPFC | −48, 32, 22 | 5.04 | |
| Striatum | 10, 16, −12 | 4.56 |
Clusters were formed by thresholding the Z-stat image for each contrast at Z > 2.7 (equivalent to a one-tailed P of 0.003). Dorsolateral prefrontal cortex (DLPFC); Orbitofrontal cortex
Responses to the oral maltodextrin solution
The cortical activation from an oral maltodextrin stimulus, revealed by the contrast [Maltodextrin – Control], is shown in Fig. 6B. Regions of the insula/frontal operculum were activated by the taste of maltodextrin. Significant clusters of activation were also found in the medial orbitofrontal cortex that extended into an adjoining rostral part of the anterior cingulate cortex, the dorsolateral prefrontal cortex and the right caudate. In contrast to the response of the glucose solution, there was no significant activation of dorsal regions of the anterior cingulate cortex with maltodextrin. The coordinates of the peak voxels of each cluster, and the Z-scores, are presented in Table 2.
Brain areas responding to both oral glucose and maltodextrin
The conjunction overlay mask of [Glucose – Control] AND [Maltodextrin – Control] revealed activation in the right insula/frontal operculum in a region that extended from y=–4 to y= 14 and from z= 12 to z= 24 (Fig. 7A). A smaller area in the left frontal operculum (y= 4 to y= 10, z= 18 to z= 22) was also highlighted. There were also regions of the medial orbitofrontal cortex (Fig. 7B) and dorsolateral prefrontal cortex (Fig. 8A) activated by both glucose and maltodextrin taste. Responses in the right caudate (Fig. 8B) and rostral anterior cingulate cortex (Fig. 8C) were also revealed from both carbohydrate stimuli.
Figure 7. Areas of common activation from the glucose and maltodextrin contrasts ([Glucose – Control] AND [Maltodextrin – Control]).
A, the insula/frontal operculum (e.g. MNI coordinates: 58, 8, 20) and B the medial orbitofrontal cortex (OFC) (e.g. MNI coordinates: 18, 34, −16). C and D show the average change in BOLD response produced by glucose, maltodextrin and their respective control solutions within the cluster of common activation highlighted in the insula/frontal operculum and the medial OFC, respectively. *Significant difference between solutions (P < 0.05); **(P < 0.001). Values are mean ±s.e.m.
Figure 8. Areas of common activation from the glucose and maltodextrin contrasts ([Glucose – Control] AND [Maltodextrin – Control]).
A, the dorsolateral prefrontal cortex (DLPFC) (e.g. MNI coordinates: −28, 54, 20), B, the striatum (e.g. MNI coordinates: 10, 20, −10) and C, the anterior cingulate cortex (e.g. MNI coordinates: 8, 44, −4). D, E and F, show the average change in BOLD response produced by glucose, maltodextrin and their respective control solutions within the cluster of common activation highlighted in the dorsolateral prefrontal cortex, the striatum and the anterior cingulate cortex, respectively. *Significant difference between solutions (P < 0.05); **(P < 0.001). Values are mean ±s.e.m.
Solution ratings
The glucose solution (87 ± 3 mm, mean ±s.e.m.) was rated significantly sweeter than the maltodextrin (32 ± 6 mm) and the control (23 ± 7 mm) solutions (P < 0.001). There was no difference in perceived sweetness between the maltodextrin and control solutions (P= 0.41). The glucose solution (74 ± 6 mm) was rated significantly more pleasant than both the maltodextrin (34 ± 8 mm) and control (32 ± 5 mm) solutions (P= 0.005 and P= 0.008, respectively). Subjects were able to discriminate between the viscosities of the three taste stimuli. Both the maltodextrin (51 ± 12 mm) and glucose (54 ± 10 mm) solutions were rated ‘thicker’ than the control (18 ± 5 mm) stimuli (P= 0.021 and P= 0.013, respectively). There was no difference in perceived viscosity between the two carbohydrate solutions (P= 0.83).
Discussion
The present investigation has shown that a non-sweet carbohydrate in the human mouth produces a similar central neural response to that obtained with glucose, suggesting there may be a class of so far unidentified oral receptors that respond to the caloric property of carbohydrate independently of those for sweetness. Furthermore, there has been speculation that the brain responses to glucose in the mouth may mediate emotional and behavioural responses associated with rewarding stimuli and the present results demonstrate, for the first time, evidence of such a link with the improvements in exercise performance that were obtained with both glucose and maltodextrin in the mouth. For convenience the discussion will concentrate first on the exercise studies (Studies 1A and 2A) and then the brain imaging data (Studies 1B and 2B) rather than following the sequence in which they are presented in the Results section.
The effect of oral carbohydrate on exercise performance
A 1 h time trial is a demanding form of exercise during which power output typically declines steadily before a final sprint to the finish. Success in this event depends on minimising this loss of power and several studies have reported that oral carbohydrate feeding enhances performance compared to water or an artificially sweetened placebo (Anantaraman et al. 1995; Below et al. 1995; Jeukendrup et al. 1997). However, there is no clear metabolic explanation for the ergogenic action of the carbohydrate and several groups have speculated about a ‘non-metabolic’ (McConell et al. 2000) or ‘central’ (Jeukendrup et al. 1997) action. (Carter et al. 2004a) first produced evidence of such a central effect and proposed a mechanism involving the activation of higher brain centres by carbohydrate-sensitive receptors in the oral cavity. The two exercise studies (Studies 1A and 2A) have shown that repeated exposure of the oral cavity to a carbohydrate solution, containing either glucose or maltodextrin, improves performance of a simulated 1 h cycle time trial. The 2% reduction in time to complete the set work load and corresponding increase in mean power output in Study 1A, as a result of swilling the glucose solution, and the 3.1% increase in Study 2A, with the maltodextrin solution, were virtually the same as the 2.9% improvement reported by (Carter et al. 2004a) using non-sweet maltodextrin. Carter and colleagues used a plain water placebo and although the maltodextrin solution was non-sweet, some of their subjects reported a slight difference in texture of the two solutions. In the present studies care was taken to disguise the solutions with a strong artificial sweetener and while, post exercise in Study 1A, some subjects rated the glucose solution to be more viscous, they did not have any reason to associate this with the presence of glucose in the solution nor were they aware of the hypothesis behind the study. We conclude, therefore, that the results reported here confirm the suggestion of (Carter et al. 2004a) that carbohydrate in the oral cavity improves performance during simulated 1 h cycle time trials.
A very similar pattern of changing power output, or pacing strategy, was observed in the glucose and maltodextrin trials when compared to placebo (Fig. 3), with power declining during most of the exercise before increasing in the final phase as subjects completed a ‘sprint finish’. The trend in both the glucose and maltodextrin trials was for power to be better maintained compared with placebo, particularly in the latter stages of exercise, allowing subjects to complete the set workload in a significantly shorter time. Despite the higher power generated there were no differences in RPE in either glucose solution or maltodextrin trials compared to placebo, exactly as found by (Carter et al. 2004a). It has been noted that subjects tend to alter their power output during self-paced exercise so that their RPE remains relatively constant (Cole et al. 1996). The fact that the subjects in the glucose and maltodextrin trials were working at a higher power yet reporting the same RPE as in the placebo trials, suggests that oral exposure to carbohydrate evokes a central response that enables subjects to increase their power output by reducing the perception of a given workload.
A recent investigation failed to find a benefit from rinsing the mouth with a carbohydrate solution during a running time trial (Whitham & McKinney, 2007). As acknowledged by the authors, the lack of performance improvement in those studies may have been due to the study design as the participants had to make a conscious decision to alter the pace of the motorised treadmill and, as such, running speed was far more consistent throughout the performance test compared with the variable power outputs typically observed during a cycling time trial. An exercise performance test that requires conscious alteration of power output may therefore lack the sensitivity to observe the proposed unconscious central effect of a carbohydrate mouth rinse.
In the present investigation the glucose, maltodextrin and placebo solutions were artificially sweetened, demonstrating that the observed enhancement in exercise performance was independent of sweetness. This is consistent with previous studies which have reported an improvement in exercise performance with a carbohydrate solution when both the carbohydrate and placebo beverages were matched for sweetness, flavour and colour (Below et al. 1995; Jeukendrup et al. 1997). Furthermore, it has been shown that sweetness per se is not an important factor for carbohydrate supplementation to improve exercise performance in a hyperthermic environment (Carter et al. 2005).
The current studies and those of (Carter et al. 2004a) that have reported a positive effect of a carbohydrate mouth rinse on exercise performance used a design in which subjects began exercise after a prolonged fast (>6 h fast). For that reason, in both the exercise and fMRI studies we purposefully observed the brain responses to oral glucose, maltodextrin and saccharin with participants in a similar fasted state. Different pre-exercise nutritional practices could explain the discrepancy in some of the reports concerning carbohydrate ingestion during high-intensity exercise. The studies showing a performance enhancement have all entailed subjects commencing exercise following an overnight fast (Neufer et al. 1987; Below et al. 1995; Millard-Stafford et al. 1997) or in a post-absorptive state (> 4 h) (Anantaraman et al. 1995). Conversely, a common feature of investigations that fail to report an ergogenic action from carbohydrate feeding during high-intensity exercise is that subjects receive a meal designed to ‘top-up’ endogenous carbohydrate stores, ∼2 h prior to exercise (Desbrow et al. 2004; Burke et al. 2005). Similarly, the investigation of Whitham & McKinney (2007), which reported no improvement with a carbohydrate mouth rinse, had subjects commence exercise following a shorter period of fasting (4 h) than the present studies. Pre-exercise feeding may influence the brain responses to an oral carbohydrate stimulus during subsequent exercise as it is likely that the activation of brain regions associated with feeding and reward, such as the orbitofrontal cortex and striatum, are modulated by homeostatic regulation and the current physiological state of the body (Small et al. 2001). An interesting series of future studies would be to compare both the effect on exercise performance and brain responses of an oral carbohydrate stimulus in the fed and fasted states.
The effect of oral carbohydrate on brain responses
Studies 1B and 2B both used glucose as one of the tastants and while the brain responses were broadly similar, there were some differences. In Study 2B, glucose activated the orbitofrontal cortex and the adjoining rostral part of the anterior cingulate cortex, which was not seen in Study 1B. The difference may have been due to the proximity of the orbitofrontal cortex to the air-filled sinuses leading to signal dropout and artefacts (Wilson et al. 2002) and consequently negative findings from the orbitofrontal cortex should be treated with caution (Kringelbach, 2005). The use of a specific set of imaging parameters to minimize distortion artefacts in the orbitofrontal cortex, as used in other investigations (de Araujo et al. 2003a; de Araujo & Rolls, 2004; Frank et al. 2008), might have improved the consistency of data obtained in this area of the brain.
Previous functional neuroimaging studies of the anterior insula/frontal operculum, believed to be the human primary taste cortex, have shown it to be sensitive to various oral stimuli, including glucose, salt (O’Doherty et al. 2001) and umami (de Araujo et al. 2003a). The present study revealed activation in this region in response to both glucose and saccharin solutions (Fig. 5A). Comparable activation in response to glucose and saccharin was also found in the dorsolateral prefrontal cortex (Fig. 5B) which is suggested to have a role in the preparation and selection of cognitive responses (Rowe et al. 2000) and has previously been shown to be sensitive to a range of different taste stimuli (Kringelbach et al. 2004). Activation within this region is believed to reflect an engagement in cognitive and attentional processing induced by taste input. However, despite the inability of the subjects to distinguish between the glucose and saccharin solutions on a range of subjective measures (sweetness, pleasantness and viscosity), glucose activated a number of brain regions that were unresponsive to saccharin. These included the anterior cingulate cortex and the right caudate, that forms part of the striatum. These brain regions, in particular the dopaminergic pathways within the striatum, are believed to mediate the emotional and behavioural response to rewarding food stimuli (Berridge & Robinson, 1998; Kelley et al. 2002; Rolls, 2007). These observations are very similar to those of Frank et al. (2008) who found that, compared to a similar sweet-tasting sucralose solution, only sucrose activated the cingulate cortex, parts of the striatum and ventral tegmental area. The current study therefore supports the idea that it is not sweetness that is required for the activation of particular reward-related regions of the brain but rather some other property of natural sugars, possibly the caloric content.
The importance of the presumed caloric-content rather than the sweetness of natural carbohydrates is underlined by the similar cortical responses produced by oral glucose and maltodextrin (Figs 7 and 8), despite the obvious differences in perceived sweetness between the two solutions. Regions of common activation were found in the primary taste cortex (Fig. 7A) and a medial region of orbitofrontal cortex (Fig. 7B), which is the putative secondary taste cortex (Rolls, 2007). Previous functional neuroimaging studies have revealed activation in the orbitofrontal cortex from a variety of taste stimuli (O’Doherty et al. 2001; Small et al. 2001; de Araujo et al. 2003a; de Araujo & Rolls, 2004) and this is where the current hedonic value of an oral stimulus is thought to be represented since activation of this region can be suppressed by satiety (Small et al. 2001). The conjunction overlay mask also revealed clusters of common activation in response to glucose and maltodextrin in the dorsolateral prefrontal cortex (Fig. 8A) and a small area of the right caudate (Fig. 8B). Thus, despite not being rated as ‘pleasant’ as the glucose tastant, the complex carbohydrate solution still produced activation within a region of the ventral striatum, a crucial interface for many well-established motivational circuits in the brain (Kelley et al. 2002).
The orbitofrontal cortex is an important area of convergence for somatosensory inputs produced by the texture of food in the mouth (Rolls, 2007). Single-neuron recordings in the primate orbitofrontal cortex have revealed a population that responds to the oral texture of carboxymethylcellulose, a tasteless thickening agent used in the food industry (Verhagen et al. 2003). These oral texture responses have since been extended to the human brain with de Araujo & Rolls (2004) reporting activation in a lateral region of orbitofrontal cortex from both an oral fat (vegetable oil) stimulus and carboxymethylcellulose solution with a similar viscosity. Consequently, the central activation observed in Study 2B, particularly from the maltodextrin solution, might have been due to activation of oral somatosensory receptors (Simon et al. 2006) rather than specific taste receptors. There are, however, two reasons for thinking this is not the case. The first is the observation of de Araujo & Rolls (2004) that a rostral part of anterior cingulate cortex, where it borders the medial orbitofrontal cortex and the ventral striatum, was activated by oral fat independently of its viscosity. This led the authors to propose that activation in these brain regions may indicate the energy content of a food. The fact that maltodextrin in the present study produced a very similar response in this part of the brain would suggest that the complex carbohydrate solution was providing more than a simple somatosensory stimulus. A second reason for doubting that activation from the maltodextrin solution was a consequence of its viscosity is that although subjects were able to detect a difference, this solution would not normally be described as ‘viscous’. de Araujo & Rolls (2004) comment that the 18% sucrose solution they used had a viscosity of 2 centipoise (cP) compared with 50 cP for a carboxymethylcellulose solution which provided activation comparable to that of their sucrose solution in the primary taste cortex. The maltodextrin solution we used (18%) had a similar low viscosity of ∼2 cP and consequently is unlikely to have evoked a somatosensory response that was any greater than that provided by the control solution with a viscosity of 1–2 cP.
These observations raise the question of how the maltodextrin solution is sensed in the oral cavity. From the manufacturer's specifications (see Methods) and our measurements of free glucose, the maltodextrin solution would have contained ∼1.6% mono-and disaccharides, about 50 times less than the concentration of the comparison glucose solution. We are not aware of any dose–response studies regarding central activation by oral glucose, but it seems unlikely that the sweet mono-and disaccharides in the maltodextrin solution at this low concentration would generate the same activation as the pure glucose solution, especially as the maltodextrin solution was not perceived as ‘sweet’ by the subjects.
The mammalian sweet taste receptor combines two G-protein-coupled receptors, T1R2 and T1R3, which respond to both natural sugars and artificial sweeteners (Nelson et al. 2001). These taste receptor cells found primarily on the tongue, are innervated by afferent fibres that transmit information to taste regions in the cortex via the thalamus (Simon et al, 2006). Recent work using transgenic mice that lack the T1R3 protein suggests that natural caloric sugars activate taste afferents differently from non-caloric artificial sweeteners (Damak et al. 2003; Zhao et al. 2003). T1R3-knock-out (KO) mice showed no behavioural attraction to artificial sweeteners yet there was only a modest reduction in preference to caloric sugars (Damak et al. 2003) and T1R3-KO mice still had a detectable gustatory nerve response to natural sugar. More recently, Delay et al. (2006) reported that the detection threshold for sucrose was indistinguishable between T1R3-KO and wild-type mice. These results indicate that there are T1R3-independent taste receptors for natural carbohydrates in mice. The fact that in our experiments maltodextrin activated very similar brain areas compared to glucose and was not perceived as sweet suggests that there may also be human T1R3-independent taste receptors which have subtly different projections in the brain compared to the taste receptor cells that co-express T1R2 and T1R3 and convey sweetness.
The concentration of the glucose and maltodextrin solutions used in the fMRI experiments were nearly 3 times greater than those used in the exercise studies. This raises the obvious question of whether the lower concentrations used during the time trials would also activate the brain regions we have identified. The higher concentrations used in the fMRI studies were chosen to replicate and allow comparisons with earlier work on glucose tasting (e.g. O’Doherty et al. 2001). The intention was to determine whether it was possible that glucose and maltodextrin activate similar areas of the brain. This is clearly the case and lends strong support to the hypothesis that the oral carbohydrate in the exercise studies was acting via central neural pathways. However, definitive proof would require fMRI studies with lower carbohydrate concentrations. The current studies here, therefore demonstrate that the presence of either glucose or maltodextrin in the oral cavity can improve performance of a self-paced exercise task of ∼1 h duration while there is, at least, the potential for both carbohydrates to activate brain regions believed to mediate emotional and behavioural responses to a rewarding sensory stimulus (Kringelbach, 2004). For example, the dopaminergic system of the ventral striatum has been implicated in arousal, motivation and the control of motor behaviour (Berridge & Robinson, 1998). Prolonged exercise, such as a cycle time trial, generates a great deal of afferent information arising from muscles, joints, lungs, skin and core temperature receptors which may, over time, be perceived as unpleasant and consciously, or unconsciously, lead to an inhibition of motor output manifesting as ‘central’ fatigue. Individuals tend to regulate their physical activity to keep their levels of discomfort within acceptable limits and this has become known as the ‘Central Governor Model’ (Noakes, 2000; St Clair Gibson et al. 2001; Lambert et al. 2005). It is not clear which brain pathways are involved in this inhibitory activity but one possibility is a decrease in activity of dopaminergic pathways affecting either reward or the motor functions of the basal ganglia. Conversely, increased activity of these pathways might counteract the effects of fatigue and we suggest that this is the mode of action of oral carbohydrate. Central stimulants such as caffeine and amphetamines are well known to enhance performance during prolonged exercise (Gerald, 1978; Chandler & Blair, 1980) and administration of methamphetamine stimulates activity of reward circuitry in the human brain, including the medial orbitofrontal cortex and ventral striatum (Völlm et al. 2004), in a similar way to the oral carbohydrates used in the present investigation.
Probably the major limitation of the work presented here is that in the fMRI studies the subjects were all tested in the rested condition and it is possible that some of the consequences of exercise, such as hyperthermia, may alter the brain responses to oral carbohydrate. Our present speculations about mechanisms would therefore be strengthened if the response to a carbohydrate solution in reward-related brain regions was observed when individuals perform an intense exercise task. The possible interaction between this affective response and activation of brain regions that modify central motor drive during exercise could also be determined. Unfortunately it would be difficult to replicate the intense nature of a cycle time trial inside an fMRI scanner, as dynamic whole-body exercise is likely to cause substantial head movement that would create distortion artefacts in the BOLD signal. A further limitation of the present work is that the fMRI studies only observed the central neural responses to tasting saccharin while in the exercise studies the masking sweet taste was provided by a mixture of saccharin and aspartame. The only way in which this might invalidate our results would be if aspartame activated similar brain areas to the caloric carbohydrates. This seems unlikely and it is notable that the only other non-nutritive sweetener, sucralose, that has been tested in this way showed patterns of activation that were distinct from those of a natural carbohydrate (Frank et al. 2008).
In summary, we have shown that both sweet and non-sweet carbohydrate in the human mouth activate a variety of brain areas, some of which may be involved in reward and the regulation of motor activity. We suggest that activation of these regions of the brain may provide a mechanism to explain the improvement in exercise performance that is observed when carbohydrate is present in the mouth. The findings also support the existence of oral receptors sensitive to the caloric value of carbohydrate and which are independent of sweetness.
References
- Anantaraman R, Carmines AA, Gaesser GA, Weltman A. Effects of carbohydrate supplementation on performance during 1 hour of high-intensity exercise. Int J Sports Med. 1995;16:461–465. doi: 10.1055/s-2007-973038. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. Neuroimage. 2003;20:1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Below PR, Mora-Rodriguez R, Gonzalez-Alonso J, Coyle EF. Fluid and carbohydrate ingestion independently improve performance during 1 h of intense exercise. Med Sci Sports Exerc. 1995;27:200–210. [PubMed] [Google Scholar]
- Bergstrom J, Hermansen L, Hultman E, Saltin B. Diet, muscle glycogen and physical performance. Acta Physiol Scand. 1967;71:140–150. doi: 10.1111/j.1748-1716.1967.tb03720.x. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–381. [PubMed] [Google Scholar]
- Burke LM, Wood C, Pyne DB, Telford DR, Saunders PU. Effect of carbohydrate intake on half-marathon performance of well-trained runners. Int J Sport Nutr Exerc Metab. 2005;15:573–589. doi: 10.1123/ijsnem.15.6.573. [DOI] [PubMed] [Google Scholar]
- Carter J, Jeukendrup AE, Jones DA. The effect of sweetness on the efficacy of carbohydrate supplementation during exercise in the heat. Can J Appl Physiol. 2005;30:379–391. doi: 10.1139/h05-128. [DOI] [PubMed] [Google Scholar]
- Carter JM, Jeukendrup AE, Jones DA. The effect of carbohydrate mouth rinse on 1-h cycle time trial performance. Med Sci Sports Exerc. 2004a;36:2107–2111. doi: 10.1249/01.mss.0000147585.65709.6f. [DOI] [PubMed] [Google Scholar]
- Carter JM, Jeukendrup AE, Mann CH, Jones DA. The effect of glucose infusion on glucose kinetics during a 1-h time trial. Med Sci Sports Exerc. 2004b;36:1543–1550. doi: 10.1249/01.mss.0000139892.69410.d8. [DOI] [PubMed] [Google Scholar]
- Chandler JV, Blair SN. The effect of amphetamines on selected physiological components related to athletic success. Med Sci Sports Exerc. 1980;12:65–69. [PubMed] [Google Scholar]
- Coggan AR, Coyle EF. Reversal of fatigue during prolonged exercise by carbohydrate infusion or ingestion. J Appl Physiol. 1987;63:2388–2395. doi: 10.1152/jappl.1987.63.6.2388. [DOI] [PubMed] [Google Scholar]
- Cole KJ, Costill DL, Starling RD, Goodpaster BH, Trappe SW, Fink WJ. Effect of caffeine ingestion on perception of effort and subsequent work production. Int J Sport Nutr. 1996;6:14–23. doi: 10.1123/ijsn.6.1.14. [DOI] [PubMed] [Google Scholar]
- Coyle EF, Coggan AR, Hemmert MK, Ivy JL. Muscle glycogen utilization during prolonged strenuous exercise when fed carbohydrate. J Appl Physiol. 1986;61:165–172. doi: 10.1152/jappl.1986.61.1.165. [DOI] [PubMed] [Google Scholar]
- Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Varadarajan V, Zou S, Jiang P, Ninomiya Y, Margolskee RF. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science. 2003;301:850–853. doi: 10.1126/science.1087155. [DOI] [PubMed] [Google Scholar]
- de Araujo IE, Kringelbach ML, Rolls ET, Hobden P. Representation of umami taste in the human brain. J Neurophysiol. 2003a;90:313–319. doi: 10.1152/jn.00669.2002. [DOI] [PubMed] [Google Scholar]
- de Araujo IE, Kringelbach ML, Rolls ET, McGlone F. Human cortical responses to water in the mouth, and the effects of thirst. J Neurophysiol. 2003b;90:1865–1876. doi: 10.1152/jn.00297.2003. [DOI] [PubMed] [Google Scholar]
- de Araujo IE, Rolls ET. Representation in the human brain of food texture and oral fat. J Neurosci. 2004;24:3086–3093. doi: 10.1523/JNEUROSCI.0130-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delay ER, Hernandez NP, Bromley K, Margolskee RF. Sucrose and monosodium glutamate taste thresholds and discrimination ability of T1R3 knockout mice. Chem Senses. 2006;31:351–357. doi: 10.1093/chemse/bjj039. [DOI] [PubMed] [Google Scholar]
- Desbrow B, Anderson S, Barrett J, Rao E, Hargreaves M. Carbohydrate-electrolyte feedings and 1 h time trial cycling performance. Int J Sport Nutr Exerc Metab. 2004;14:541–549. doi: 10.1123/ijsnem.14.5.541. [DOI] [PubMed] [Google Scholar]
- Frank GK, Oberndorfer TA, Simmons AN, Paulus MP, Fudge JL, Yang TT, Kaye WH. Sucrose activates human taste pathways differently from artificial sweetener. Neuroimage. 2008;39:1559–1569. doi: 10.1016/j.neuroimage.2007.10.061. [DOI] [PubMed] [Google Scholar]
- Gerald MC. Effects of (+)-amphetamine on the treadmill endurance performance of rats. Neuropharmacology. 1978;17:703–704. doi: 10.1016/0028-3908(78)90083-7. [DOI] [PubMed] [Google Scholar]
- Hawley JA, Palmer GS, Noakes TD. Effects of 3 days of carbohydrate supplementation on muscle glycogen content and utilisation during a 1-h cycling performance. Eur J Appl Physiol Occup Physiol. 1997;75:407–412. doi: 10.1007/s004210050180. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jeukendrup A, Brouns F, Wagenmakers AJ, Saris WH. Carbohydrate-electrolyte feedings improve 1 h time trial cycling performance. Int J Sports Med. 1997;18:125–129. doi: 10.1055/s-2007-972607. [DOI] [PubMed] [Google Scholar]
- Jeukendrup A, Saris WH, Brouns F, Kester AD. A new validated endurance performance test. Med Sci Sports Exerc. 1996;28:266–270. doi: 10.1097/00005768-199602000-00017. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Bakshi VP, Haber SN, Steininger TL, Will MJ, Zhang M. Opioid modulation of taste hedonics within the ventral striatum. Physiol Behav. 2002;76:365–377. doi: 10.1016/s0031-9384(02)00751-5. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML. Food for thought: hedonic experience beyond homeostasis in the human brain. Neuroscience. 2004;126:807–819. doi: 10.1016/j.neuroscience.2004.04.035. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, de Araujo IE, Rolls ET. Taste-related activity in the human dorsolateral prefrontal cortex. Neuroimage. 2004;21:781–788. doi: 10.1016/j.neuroimage.2003.09.063. [DOI] [PubMed] [Google Scholar]
- Lambert EV, St Clair Gibson A, Noakes TD. Complex systems model of fatigue: integrative homoeostatic control of peripheral physiological systems during exercise in humans. Br J Sports Med. 2005;39:52–62. doi: 10.1136/bjsm.2003.011247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConell GK, Canny BJ, Daddo MC, Nance MJ, Snow RJ. Effect of carbohydrate ingestion on glucose kinetics and muscle metabolism during intense endurance exercise. J Appl Physiol. 2000;89:1690–1698. doi: 10.1152/jappl.2000.89.5.1690. [DOI] [PubMed] [Google Scholar]
- Millard-Stafford M, Rosskopf LB, Snow TK, Hinson BT. Water versus carbohydrate-electrolyte ingestion before and during a 15-km run in the heat. Int J Sport Nutr. 1997;7:26–38. doi: 10.1123/ijsn.7.1.26. [DOI] [PubMed] [Google Scholar]
- Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS. Mammalian sweet taste receptors. Cell. 2001;106:381–390. doi: 10.1016/s0092-8674(01)00451-2. [DOI] [PubMed] [Google Scholar]
- Neufer PD, Costill DL, Flynn MG, Kirwan JP, Mitchell JB, Houmard J. Improvements in exercise performance: effects of carbohydrate feedings and diet. J Appl Physiol. 1987;62:983–988. doi: 10.1152/jappl.1987.62.3.983. [DOI] [PubMed] [Google Scholar]
- Noakes TD. Physiological models to understand exercise fatigue and the adaptations that predict or enhance athletic performance. Scand J Med Sci Sports. 2000;10:123–145. doi: 10.1034/j.1600-0838.2000.010003123.x. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Rolls ET, Francis S, Bowtell R, McGlone F. Representation of pleasant and aversive taste in the human brain. J Neurophysiol. 2001;85:1315–1321. doi: 10.1152/jn.2001.85.3.1315. [DOI] [PubMed] [Google Scholar]
- Rolls ET. Sensory processing in the brain related to the control of food intake. Proc Nutr Soc. 2007;66:96–112. doi: 10.1017/S0029665107005332. [DOI] [PubMed] [Google Scholar]
- Romijn JA, Coyle EF, Sidossis LS, Gastaldelli A, Horowitz JF, Endert E, Wolfe RR. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am J PhysiolEndocrinol Metab. 1993;265:E380–E391. doi: 10.1152/ajpendo.1993.265.3.E380. [DOI] [PubMed] [Google Scholar]
- Rowe JB, Toni I, Josephs O, Frackowiak RS, Passingham RE. The prefrontal cortex: response selection or maintenance within working memory? Science. 2000;288:1656–1660. doi: 10.1126/science.288.5471.1656. [DOI] [PubMed] [Google Scholar]
- St Clair Gibson A, Lambert ML, Noakes TD. Neural control of force output during maximal and submaximal exercise. Sports Med. 2001;31:637–650. doi: 10.2165/00007256-200131090-00001. [DOI] [PubMed] [Google Scholar]
- Simon SA, de Araujo IE, Gutierrez R, Nicolelis MA. The neural mechanisms of gustation: a distributed processing code. Nat Rev Neurosci. 2006;7:890–901. doi: 10.1038/nrn2006. [DOI] [PubMed] [Google Scholar]
- Small DM, Zatorre RJ, Dagher A, Evans AC, Jones-Gotman M. Changes in brain activity related to eating chocolate: from pleasure to aversion. Brain. 2001;124:1720–1733. doi: 10.1093/brain/124.9.1720. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- van Loon LJ, Greenhaff PL, Constantin-Teodosiu D, Saris WH, Wagenmakers AJ. The effects of increasing exercise intensity on muscle fuel utilisation in humans. J Physiol. 2001;536:295–304. doi: 10.1111/j.1469-7793.2001.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhagen JV, Rolls ET, Kadohisa M. Neurons in the primate orbitofrontal cortex respond to fat texture independently of viscosity. J Neurophysiol. 2003;90:1514–1525. doi: 10.1152/jn.00320.2003. [DOI] [PubMed] [Google Scholar]
- Vollm BA, de Araujo IE, Cowen PJ, Rolls ET, Kringelbach ML, Smith KA, Jezzard P, Heal RJ, Matthews PM. Methamphetamine activates reward circuitry in drug naive human subjects. Neuropsychopharmacology. 2004;29:1715–1722. doi: 10.1038/sj.npp.1300481. [DOI] [PubMed] [Google Scholar]
- Whitham M, McKinney J. Effect of a carbohydrate mouthwash on running time-trial performance. J Sports Sci. 2007;25:1385–1392. doi: 10.1080/02640410601113676. [DOI] [PubMed] [Google Scholar]
- Wilson JL, Jenkinson M, de Araujo IE, Kringelbach ML, Rolls ET, Jezzard P. Fast, fully automated global and local magnetic field optimization for fMRI of the human brain. Neuroimage. 2002;17:967–976. [PubMed] [Google Scholar]
- Woolrich MW, Behrens TE, Beckmann CF, Jenkinson M, Smith SM. Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage. 2004;21:1732–1747. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14:1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Worsley KJ. Statistical analysis of activation images. In: Jezzard P, Matthews PM, Smith SM, editors. Functional MRI: An Introduction to Methods. Oxford: Oxford University Press; 2001. pp. 251–270. [Google Scholar]
- Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJ, Zuker CS. The receptors for mammalian sweet and umami taste. Cell. 2003;115:255–266. doi: 10.1016/s0092-8674(03)00844-4. [DOI] [PubMed] [Google Scholar]