Abstract
Deacetylation of PGC-1α by SIRT1 is thought to be an important step in increasing PGC-1α transcriptional activity, since in muscle cell lines SIRT1 induces PGC-1α protein expression and mitochondrial biogenesis. We examined the relationship between SIRT1 protein and activity, PGC-1α and markers of mitochondrial density, (a) across a range of metabolically heterogeneous skeletal muscles and the heart, and when mitochondrial biogenesis was stimulated by (b) chronic muscle stimulation (7 days) and (c) AICAR administration (5 days), and finally, (d) we also examined the effects of SIRT1 overexpression on mitochondrial biogenesis and PGC-1α. SIRT1 protein and activity were correlated (r= 0.97). There were negative correlations between SIRT1 protein and PGC-1α (r=−0.95), COX IV (r=−0.94) and citrate synthase (r=−0.97). Chronic muscle stimulation and AICAR upregulated PGC-1α protein (22–159%) and oxidative capacity (COX IV, 20–69%); in each instance SIRT1 protein was downregulated by 20–40%, while SIRT1 intrinsic activity was increased. SIRT1 overexpression in rodent muscle increased SIRT1 protein (+240%) and doubled SIRT1 activity, but PGC-1α (−25%), mtTFA (−14%) and COX IV (−10%) proteins were downregulated. Taken altogether these experiments are not consistent with the notion that SIRT1 protein plays an obligatory regulatory role in the process of PGC-1α-mediated mitochondrial biogenesis in mammalian muscle.
Peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α) is a transcriptional co-activator important for mitochondrial biogenesis through activation of both nuclear and mitochondrial transcription factors (Wu et al. 1999; Lin et al. 2002). While expression of PGC-1α is tightly coupled with oxidative capacity of mammalian skeletal muscle (Benton et al. 2008a), post-translational regulation also plays a role in PGC-1α-mediated mitochondrial biogenesis. PGC-1α is phosphorylated by 5-AMP-activated protein kinase (AMPK), calcium–calmodulin-dependent protein kinase CAMK, and/or p38 mitogen-activated protein kinase (MAPK) (Puigserver et al. 2001; Jager et al. 2007; Wright et al. 2007) and this phosphorylation can result in increased protein stability and expression (Puigserver et al. 2001) and/or induce translocation of PGC-1α from the cytosol to the nucleus (Wright et al. 2007). The increased expression and translocation of PGC-1α increases mitochondrial biogenesis (Puigserver & Spiegelman, 2003) and promotes transcription of genes involved in substrate transport, lipid oxidation and oxidative phosphorylation (Puigserver et al. 1998; Jager et al. 2007; Benton et al. 2008a,b). In addition to PGC-1α phosphorylation, SIRT1-mediated deacetylation has also been implicated as an important post-translational modification of PGC-1α activity.
SIRT1 (silent mating type information regulation 2 homolog 1) is a class III histone deacetylase that has been identified as a key enzyme involved in metabolic and mitochondrial regulation (Dali-Youcef et al. 2007). SIRT1 and PGC-1α co-immunoprecipitate in cell lines and in murine liver, suggesting that SIRT1, along with PGC-1α has an important role in regulating mitochondrial biogenesis (Nemoto et al. 2005; Rodgers et al. 2005). In addition, overexpression of SIRT1 protein content in Fao hepatocytes and C2C12 muscle cells promotes deacetylation of PGC-1α and increases the expression of selected mitochondrial genes (Rodgers et al. 2005; Gerhart-Hines et al. 2007). Conversely, in SIRT1 knockdown C2C12 muscle cells and in SIRT1-ablated murine embryonic fibroblasts (Gerhart-Hines et al. 2007) mitochondrial biogenesis and gene expression are downregulated. Collectively, these observations suggest an essential role for SIRT1 protein content in the regulation of PGC-1α-mediated mitochondrial biogenesis. There is some suggestion that changes in SIRT1 activity per se may also be important for the control of mitochondrial biogenesis (Baur et al. 2006; Lagouge et al. 2006; Milne et al. 2007). These activity changes correlate with SIRT1 protein expression (Rodgers et al. 2005; Gerhart-Hines et al. 2007).
While this apparently essential role of SIRT1 protein content in mitochondrial biogenesis has been demonstrated elegantly in cell line work, a number of studies have raised questions as to whether sirtuin proteins, such as SIRT1, have the same roles in mammalian cells as in lower eukaryotes (McBurney et al. 2003; Michishita et al. 2005; Kaeberlein, 2008). For example, in clonal PC12 cells a suppressive effect of SIRT1 has been observed on mitochondrial enzyme content (Nemoto et al. 2005), and a pro-aging effect of SIRT1 in mammalian neurons has been demonstrated (Li et al. 2008). Although studies with resveratrol suggest that SIRT1 could be a target for the treatment of metabolic disorders (Feige et al. 2008), it remains to be shown that these effects primarily involve skeletal muscle, or other metabolic tissues. Such caution is warranted in view of reports in which SIRT1 mRNA was inversely related to heart and muscle mitochondrial content (Michishita et al. 2005; Shi et al. 2005). Moreover, in transgenic mice SIRT1 overexpression demonstrated complex effects that were not easily generalizable (Banks et al. 2008). This suggests that there may well be tissue-specific SIRT1 effects, which prevented the identification of a clear role for SIRT1 in vivo. Hence, overexpression of SIRT1 in specific tissues may be important to elucidate its role in those tissues. Taken altogether, discrepancies in the literature suggest that caution may be warranted in attributing a role for SIRT1 in mitochondrial biogenesis. This underlies the importance of examining the function of SIRT1 in mammalian skeletal muscle and heart, tissues with highly variable mitochondrial content.
The relationship between SIRT1, PGC-1α and mitochondrial biogenesis in fully developed, mammalian skeletal muscle is uncertain. If the magnitude of SIRT1 protein expression has a regulatory role in the determination of skeletal muscle mitochondrial content and oxidative capacity, as has been suggested by studies in muscle cells (C2C12) and embryonic fibroblasts (Gerhart-Hines et al. 2007), it would be expected that SIRT1 protein expression would be correlated with the oxidative capacities of skeletal muscle, as has been shown for PGC-1α (Benton et al. 2008a). Indeed, when SIRT1 was transfected into 293T cells, PGC-1α expression was concomitantly upregulated, indicating that SIRT1 expression and PGC-1α expression are closely associated in these cells (Rodgers et al. 2005). Thus, if SIRT1 is critical to PGC-1α-mediated mitochondrial biogenesis in skeletal muscle, then chronic contraction-induced or 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR)-mediated stimulation of mitochondrial biogenesis in this tissue are presumably accompanied by an increased expression of SIRT1. In addition, muscle-specific SIRT1 protein overexpression should be accompanied by an increase in mitochondrial biogenesis.
In the present study we have examined these possibilities. Specifically, we compared the expression and activity of SIRT1 with PGC-1α and selected indices of mitochondrial densities in metabolically heterogeneous rat hindlimb muscles and the heart, tissues that are well known to differ with respect to mitochondrial density and PGC-1α protein expression. In addition, we examined the effects of increased mitochondrial biogenesis, induced by (a) chronic muscle contraction, and (b) AICAR administration on the expression of SIRT1 and PGC-1α. Finally, in order to examine the specific effects of SIRT1 on PGC-1α and mitochondrial biogenesis in skeletal muscle, we transfected SIRT1 into the tibialis anterior muscle of one limb. The advantage of this approach is that SIRT1 overexpression is confined to a single muscle, and therefore this does not result in the metabolic complexities observed in multi-organ SIRT1 overexpression, as has occurred in SIRT1 transgenic mice (Banks et al. 2008). Our study demonstrates that in mature mammalian skeletal muscle and heart (i) PGC-1α, but not SIRT1, correlates with the oxidative capacity of the tissue, (ii) among muscle and heart tissues PGC1α and SIRT1 protein expression are inversely related, and (iii) PGC-1α, but not SIRT1, is upregulated when mitochondrial biogenesis is induced by chronic muscle contraction and AICAR administration. Finally, (iv) the muscle-specific overexpression of SIRT1 protein was accompanied by a decrease in mitochondrial biogenesis, as shown by reductions in cytochrome oxidase IV (COX IV), mitochondrial transcription factor A (mtTFA) and PGC-1α. These observations suggest that in mature mammalian skeletal muscle and heart, SIRT1 protein expression does not play an obligatory role in mitochondrial biogenesis, and thus the role of SIRT1 in these tissues appears to differ from its action in cell line models.
Methods
All experiments were performed with mature Sprague–Dawley rats (female, 3–6 months, 250–300 g). Rats were bred on site, housed at 22.5°C, and maintained on a 12 h light (7:00–19:00) and 12 h dark cycle (19:00–7:00) in animal holding facilities. In total 32 rats were used in these experiments (numbers for each experiment are indicated in figure legends). At the end of each experiment animals were anaesthetized with an intraperitoneal injection (Somnotol, 60 mg kg−1) and the selected tissues were harvested, as described for each experiment below. The procedures for the experimental treatments, the harvesting of the muscle tissue and the killing of animals were approved by the animal care committee at the University of Guelph.
SIRT1 and oxidative capacity in heart and skeletal muscle
To compare the protein expression of SIRT1 with oxidative capacity, muscles tissues with different mitochondrial content were harvested from anaesthetized rats (n= 5). These included red gastrocnemius (RG), white gastrocnemius (WG), red tibialis anterior (RTA) and white tibialis anterior (WTA) muscles, as well as the heart (HRT). Separation of the red and white gastrocnemius and tibialis anterior (TA) was performed once the muscles were removed from the animal; the RG was cut below the tendon while the TA was cut along the tendon. Once separated, mixed tissue between red and white was removed until the tissue appeared free from contamination. Tissues were frozen in liquid nitrogen immediately after removal and stored at −80°C until analysed. Immediately after the harvesting of tissues the animals were killed with an overdose of Somnotol.
Chronic muscle stimulation
Mitochondrial biogenesis in muscle was induced with chronic muscle stimulation. For this purpose, red and white tibialis muscles in rats (n= 5) were chronically stimulated in one hindlimb as described previously (McCullagh et al. 1996; Campbell et al. 2004; Benton et al. 2008b). Briefly, stainless steel electrodes were sutured to muscles on either side of the peroneal nerve and passed subcutaneously from the thigh to the back of the neck where they were attached to an external electronic stimulator. This procedure was performed while animals were anaesthetized with isoflurane. Animals recovered from surgery for 7 days before stimulation (12 Hz, 50 μs duration) was initiated. Stimulation of the peroneal nerve was administered 24 h a day for 7 days. Twenty-four hours following cessation of the stimulation, chronically contracting muscles were removed, flash frozen (liquid nitrogen) and stored at −80°C for later analysis. Muscles from the sham-operated contralateral limb were also removed and used as control.
AICAR treatment
Mitochondrial biogenesis was also induced via chronic AICAR administration. Rats (n= 6) were injected subcutaneously with either 1 mg g−1 AICAR dissolved in saline solution (4.5% saline) or saline solution alone for 5 days. Twenty-four hours after the final AICAR or saline injection, hindlimb and heart muscles were removed, flash frozen (liquid nitrogen) and stored at −80°C for later analysis.
Transfection of SIRT1 into skeletal muscle in vivo
SIRT1 cDNA in pcDNA 3.0 (gift from Dr P. Puigserver, Dana Farber Cancer Institute, Harvard University, Boston, MA, USA) was produced by large-scale plasmid isolation from transformed Escherichia coli cells (One-Shot Invitrogen, San Diego, CA, USA) using commercially available kits (GIGA-prep kits, Invitrogen, Burlington, ON, Canada) as described previously (Benton et al. 2008a).
Electrotransfection of DNA into rat hindlimbs (n= 10) was performed as described previously (Clarke et al. 2004; Holloway et al. 2007), with some modifications (Benton et al. 2008a,b). Briefly, animals were anaesthetized with isoflurane (Aerrane, Baxter Corporation, Mississauga, ON, Canada). Once sedated, the animals' hindlimbs were shaved, sterilized (iodine and 70% ethanol) and the TA muscles were injected with 100 μl of hyaluronidase (0.15 U μl−1 in 50% v/v saline). Following 2 h of recovery TA muscles were electrotransfected with SIRT1-pcDNA plasmid (500 μg SIRT1 in 0.45% saline). Immediately following the injection of DNA, a pair of 0.8 cm diameter plate electrodes, attached to a set of ruled callipers (BTX, San Diego, CA, USA), were applied onto the skin that overlies the TA muscle. Electroporation of the intact TA muscles was then performed by administering eight electric pulses (200 V cm−1, 1 Hz, 20 ms duration) with anode and cathode electrodes switching between the lateral and medial aspects of the hindlimb after four pulses (ECM 830 Square Wave Electroporator; BTX, San Diego, CA, USA). Muscle tissue was harvested 2 weeks after transfection and used for measurement of selected proteins. Transfection with empty vector did not alter the parameters under consideration.
Western blotting
Proteins were isolated from the tissues as previously described (Bonen et al. 1998, 2000; Luiken et al. 2001). Proteins were separated by SDS-PAGE using a 7.5% (SIRT1, PGC-1α, mtTFA) or 12.5% (COX IV) polyacrylamide gel (α-Tubulin was used as a loading control), and were subsequently transferred to a polyvinylidene difluoride membrane. For the detection of proteins, commercially available antibodies were used for SIRT1 (Upstate Biotechnology, Temeculas, CA, USA), PGC-1α (Calbiochem, San Diego, CA, USA), mtTFA (Santa Cruz Biotechnology, Santa Cruz, CA, USA), COX IV (Molecular Probes, Carlsbad, CA, USA) and α Tubulin (Abcam, Cambridge, MA, USA). Proteins were visualized by chemiluminescence detection, according to the manufacturer's instructions (Perkin Elmer Life Sciences, Boston, MA, USA). Blots were quantified using the ChemiGenius 2 Bioimaging system (Syngene, Cambridge, UK).
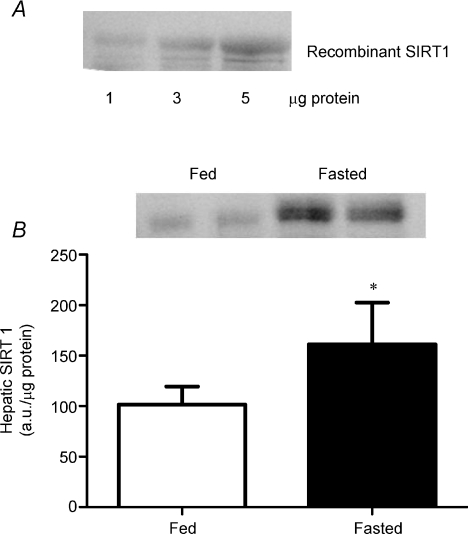
To confirm the specificity of the SIRT1 antibody for rat tissues we first detected known quantities (1–5 μg) of recombinant SIRT1 protein (Upstate Biotechnology) with the SIRT1 antibody. In addition, as a further positive control, we compared the hepatic SIRT1 response to a 24 h fast in rats (n= 3 control, n= 3 fasted), as has been done elsewhere (Rodgers & Puigserver, 2007). We have also observed a greater concentration of SIRT1 (virtually all) in the cytosolic extraction than in nuclear extractions from red and white TA muscles (data not shown), a finding in agreement with previous reports (Li et al. 2008).
SIRT1 activity
SIRT1 activity was measured using a SIRT1 fluorometric assay kit (BIOMOL, Plymouth Meeting, PA, USA) as described by the manufacturer. Briefly, frozen tissue (∼50 mg) was homogenized as previously described (Bonen et al. 1998, 2000; Luiken et al. 2001). A 25 μl volume of homogenate was incubated with 15 μl of Fluor de Lys-SIRT1 substrate (100 μm) and NAD+ (100 μm) for 30 min at 37°C. The reaction was stopped by the addition of 50 ml of developer reagent and nicotinamide (2 mm) and the fluorescence was subsequently monitored for 30 min at 360 nm (excitation) and 460 nm (emission). The change in fluorescence (arbitrary fluorescence units (AFU)) per minute was normalized to the amount of total protein in the sample.
Citrate synthase activity
A small portion of muscle (∼10 mg) from HRT, RG, WG, RTA and WTA muscle was used for determination of citrate synthase (CS) activity. Total CS activity was measured in Tris-HCl buffer (50 mm Tris-HCl, 2 mm EDTA, and 250 μm NADH pH 7.0) and 0.04% Triton X. The reaction was started by the addition of 10 mm oxaloacetate and activity was measured spectrophotometrically at 37°C by measuring the disappearance of NADH at 412 nm (Bergmeyer, 1974).
Statistics
Analyses of variance were used to compare the effects of muscle type, chronic stimulation, AICAR treatment, and SIRT1 transfection on protein expression. Post hoc tests were conducted using the Bonferroni post test. Correlations between protein expressions were determined using least squares linear regression.
Results
We have confirmed that the SIRT1 antibody is specific for SIRT1 protein (Fig. 1A). Previously, we have reported that PGC-1α is also detected appropriately, as the transfection of PGC-1α cDNA into muscle increases PGC-1α protein (Benton et al. 2008a,b; Holloway et al. 2008). We also confirm that a 24 h fast increases SIRT1 protein expression in liver, as has been reported in mice by others (Rodgers & Puigserver, 2007). These control experiments indicate that SIRT1 and PGC-1α proteins are appropriately detected in rat tissues, and that hepatic SIRT1 responds normally to fasting.
Figure 1. Specificity of the SIRT1 antibody for rat tissue.
The SIRT1 antibody detects recombinant SIRT1 protein (A) and the SIRT1 hepatic response to 24 h fasting (B) replicates previous reports. (n= 3 fed, n= 3 fasted). *Significantly different (P < 0.05) from fed. (Mean ±s.e.m., a.u., arbitrary units).
Protein expression and SIRT1 activity in heart, and in red and white skeletal muscle
The rat heart and hindlimb muscles examined in the present study are well known to exhibit a wide range of mitochondrial content and oxidative capacities. This is evidenced by the wide range of CS activity and COX IV protein expression in these tissues (Fig. 2A, B and C). Similarly, PGC-1α protein expression followed the same trend (Fig. 2D). In contrast, SIRT1 protein expression was lowest in the heart followed by the RG and RTA, and WG and WTA, respectively (Fig. 2E). SIRT1 activity was consistent with protein expression with the lowest activity observed in the heart followed by the RG, RTA, WG and WTA respectively (Fig. 2F) and did not differ among tissues when expressed relative to SIRT1 protein (Fig. 2G).
Figure 2. The relationship between SIRT1, PGC-1α, COX IV and CS activity in rat heart and metabolically heterogeneous hindlimb muscles.
A, representative blots for SIRT1, PGC-1α, COX IV and α-tubulin across the range of muscles studied. The citrate synthase activity (B), the protein expressions for COX IV (C), PGC-1α (D) and SIRT1 (E), SIRT1 activity (F) and SIRT1 activity relative to SIRT1 protein (G), in heart and skeletal muscles are also shown (n= 5). *Significantly different (P < 0.05) from heart. †Significantly different (P < 0.05) from RG. ‡Significantly different (P < 0.05) from RTA. (Mean ±s.e.m.; AFU, arbitrary fluorescence units; ww, wet weight)
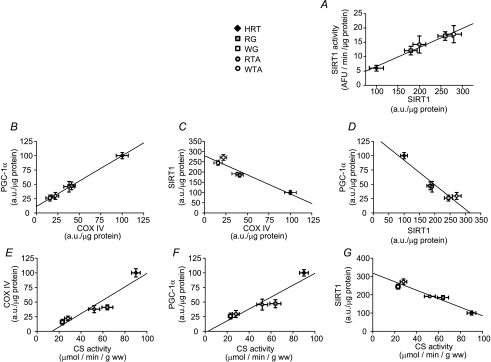
Across the range of muscle tissues examined, SIRT1 protein was positively correlated with SIRT1 activity (Fig. 3A) (r= 0.97). As expected, PGC-1α was positively correlated with COX IV (Fig. 3B) (r= 0.99) and CS activity (Fig. 3F) (r= 0.94). COX IV and CS activity were also positively correlated (Fig. 3E) (r= 0.94). In contrast, SIRT1 protein was negatively related to COX IV expression (Fig. 3C) (r=−0.94), CS activity (Fig. 3G) (r=−0.97) and PGC-1α (Fig. 3D) (r=−0.95).
Figure 3. Comparison of SIRT1, PGC-1α and COX IV in heart and skeletal muscle.
There was a positive relationship between SIRT1 protein and SIRT1 activity (A) (r= 0.97; P < 0.05) and PGC-1α and COX IV (B) (r= 0.99; P < 0.05), a negative relationship between SIRT1 and COX IV (C) (r=−0.94; P < 0.05), and PGC-1α and SIRT1 (D), (r=−0.95; P < 0.05), a positive relationship between COX IV and CS activity (E), (r= 0.94; P < 0.05) and PGC-1α and CS activity (F) (r= 0.94; P < 0.05), and a negative relationship between SIRT1 and CS activity (G) (r=−0.97; P < 0.05). (Mean ±s.e.m.)
Effects of chronic muscle stimulation
Chronic muscle stimulation was employed to increase mitochondrial biogenesis (Fig. 4A). MCT4 was employed as a control (Fig. 4B), since this is not altered with chronic muscle stimulation (Benton et al. 2008b). Chronic muscle stimulation (7 days), increased COX IV in both RTA (+22%) and WTA muscles (+69%) (Fig. 4C). In addition, there was also an increase in PGC-1α protein in RTA (+25%) and WTA muscles (+159%) (Fig. 4D). Consistent with an increase in PGC-1α content we also observed an increase in mtTFA in WTA (+59%, P < 0.05) but not in RTA muscle (+13%, P > 0.05) (Fig. 4E). There was a marked decrease (P < 0.05) in SIRT1 protein expression in both the RTA (−48%) and WTA (−39%) (Fig. 4F). There was, however, an increase in SIRT1 activity in the WTA (+65%, P < 0.05), as well as an increase (P < 0.05) in SIRT1 intrinsic activity (i.e. SIRT1 activity relative to SIRT1 protein expression) in both the WTA (+95%) and RTA (+140%) (Fig. 4G).
Figure 4. Effects of 7 days of chronic muscle stimulation on SIRT1, PGC-1α, COX IV and mtTFA.
Representative blots (A), the protein expression of MCT4 (B), COX IV (C), PGC-1α (D), mtTFA (E) and SIRT1 (F), SIRT1 activity (G), and SIRT1 activity relative to SIRT1 protein (H) in RTA and WTA following 7 days of chronic stimulation (n= 5). MCT4 was used as a positive control, as it does not respond to chronic muscle stimulation (Benton et al. 2008b). *Significant difference (P < 0.05) between control limb (open bars) and stimulated limb (filled bars). (Mean ±s.e.m.)
Effects of chronic AICAR administration
In an attempt to discern a mechanism responsible for the observed changes in SIRT1 expression and activity following chronic muscle stimulation, we administered AICAR for 5 days. AICAR treatment resulted in significant (P < 0.05) increases in COX IV (HRT +8%; RG +20%; WG +14%; RTA +10%; WTA +12%) (Fig. 5A and B), PGC-1α (HRT +6%; RG +25%; WG +31%; RTA +13%; WTA +25%) (Fig. 5A and C) and mtTFA (HRT +23%; RG +11%; WG +7%; RTA +3%; WTA +18%) (Fig. 5A and D) and a significant (P < 0.05) decrease in SIRT1 (HRT–26%; RG–7%; WG–19%; RTA–45%; WTA–15%) (Fig. 5A and E). While SIRT1 activity in HRT, and RTA and WTA muscles was not altered (Fig. 5F), there was an increase in SIRT1 intrinsic activity in the HRT (+16%) and RTA muscle (+80%) (Fig. 5G).
Figure 5. Effects of 5 days of AICAR administration on protein content of SIRT1, PGC-1α, COX IV and mtTFA in rat heart and skeletal muscle.
Representative blots (A), and protein expression of COX IV (B), PGC-1α (C), mtTFA (D) and SIRT1 (E), SIRT1 activity (F), and SIRT1 activity relative to SIRT1 protein (G) following saline (open bars) and AICAR (filled bars) injection (n= 6). There was a main effect (P < 0.05) of AICAR treatment for COX IV, PGC-1α, SIRT1 and mtTFA across the muscles studied. *Significant difference (P < 0.05) between saline and AICAR treatments for individual muscle groups. (Mean ±s.e.m.)
Effect of SIRT1 transfection in skeletal muscle in vivo
To examine the direct effect of SIRT1 on mitochondrial biogenesis, we overexpressed SIRT1 in skeletal muscle. Following transfection there was an increase in SIRT1 protein (Fig. 6A and E). This was accompanied by concomitant reductions in COX IV (−10%), PGC-1α (−25%) and mtTFA (−14%) (P < 0.05, Fig. 6B, C and D, respectively). The increase in SIRT1 protein (+240%, P < 0.05, Fig. 6E) was accompanied by a doubling in SIRT1 activity (P < 0.05, Fig. 6F), but there was a decrease in SIRT1 activity relative to SIRT1 protein (Fig. 6G).
Figure 6. Effects of SIRT1 electrotransfection on SIRT1, PGC-1α, COX IV and mtTFA in skeletal muscle.
Representative blots (A), protein expression of COX IV (B), PGC-1α (C), mtTFA (D), SIRT1 (E), SIRT1 activity (F) and SIRT1 activity relative to SIRT1 protein (G) following transfection of SIRT1 (n= 10). Control (open bars) and transfected muscles (filled bars). *Significant difference (P < 0.05) between control and transfected muscles. (Mean ±s.e.m.)
Discussion
SIRT1 has been proposed as an important regulator of PGC-1α transcriptional activity and as such may play an important role in the induction of mitochondrial biogenesis. This effect on PGC-1α appears to be achieved via increases in SIRT1 protein content permitting a greater degree of deacetylation and increased PGC-1α transcriptional activity in a variety of cells (Nemoto et al. 2005, PC12 cells; Rodgers et al. 2005, mouse liver; Gerhart-Hines et al. 2007, C2C12 cells). This proposed relationship led us to hypothesize that there were likely to be positive relationships (a) between SIRT1 protein expression, SIRT1 activity, and the oxidative capacities of heart and skeletal muscle, and (b) between changes in oxidative capacity of muscle in response to contractile-and AICAR-mediated mitochondrial biogenesis.
Unexpectedly, these hypotheses were not fully supported by the experimental evidence. Indeed, there are a number of novel observations in the present study. First, SIRT1 protein content and activity are inversely related to mitochondrial densities and to PGC-1α protein expression in heart and skeletal muscle. Second, in experimental treatments that are known to increase mitochondrial biogenesis, namely AICAR treatment and chronic electrical stimulation of skeletal muscle, SIRT1 protein expression was downregulated, while mitochondrial biogenesis was increased, as shown by the upregulation of PGC-1α, mtTFA and COX IV protein expressions. Interestingly, with these interventions, designed to alter mitochondrial biogenesis, there was a concomitant increase in SIRT1 intrinsic activity (i.e. SIRT1 activity relative to SIRT1 protein) in most of the muscles studied. Finally, overexpression of SIRT1, which increased whole-muscle SIRT1 activity, reduced the expression of COX IV, mtTFA and PGC-1α. Taken together these experiments demonstrate, unexpectedly, that in mammalian muscle tissues, SIRT1 protein expression and activity are unrelated to mitochondrial content and appear to repress mitochondrial biogenesis (Figs 2 3 and 6). However, when muscle is presented with stimuli designed to increase mitochondrial biogenesis rapidly (Figs 4 and 5) SIRT1 activation may have an as yet undefined role.
SIRT1 and oxidative capacity of heart and skeletal muscle
The negative correlation between SIRT1 protein expression and PGC-1α and COX IV in mature mammalian skeletal muscle is not consistent with the model of SIRT1-mediated activation of PGC-1α that has been proposed based on studies from selected cell lines (Rodgers et al. 2005; Gerhart-Hines et al. 2007). Although a recent study has demonstrated an increase in muscle
SIRT1 protein following exercise training and a positive correlation between SIRT1 protein and some, but not all, markers of muscle oxidative capacity (Suwa et al. 2008), we have demonstrated an inverse relationship between SIRT1 protein expression and mitochondrial density using a number of different experimental approaches, all of which were congruent. Among these the SIRT1 transfection into muscle provides proof that SIRT1 overexpression downregulates mitochondrial content in muscle. While ours appears to be the first report of this negative relationship between SIRT1 and mitochondrial biogenesis in mammalian muscle, there are other studies that are also indirectly suggestive of such a relationship. Specifically, in human (Michishita et al. 2005) and murine (Shi et al. 2005) heart and skeletal muscles, SIRT1 mRNA abundance varied inversely with the known differences in the mitochondrial content of these tissues. Thus, these studies support the similar inverse relationship between SIRT1 and mitochondrial content in the present study. Also in support of our findings, Nemoto et al. (2005) demonstrated that increased SIRT1 expression resulted in a depression of PGC-1α transcriptional activity, oxygen consumption and oxidative protein expression in neuronal PC12 cells. Thus together, several studies and our results raise the possibility of an inhibitory relationship between SIRT1 protein expression and the oxidative capacity of mammalian skeletal muscle.
The difference between our findings and the model of SIRT1-mediated control of mitochondrial biogenesis proposed previously (Gerhart-Hines et al. 2007) is difficult to explain. However, these seemingly incongruous results between cell line work and intact mammalian muscle are not without precedent. Others have also questioned whether the function of SIRT1 in higher eukaryotic cells is similar to that in lower eukaryotes (McBurney et al. 2003; Michishita et al. 2005). Indeed, the role of SIRT1 in mammalian tissue in general remains largely controversial with evidence for differing and opposing roles of SIRT1 being reported across a range of tissues and models (Banks et al. 2008; Kaeberlein, 2008; Li et al. 2008). Thus, while the role of SIRT1 in intact mammalian muscle remains controversial, our study and others (Michishita et al. 2005; Nemoto et al. 2005; Shi et al. 2005) lend support to the possibility that increases in SIRT1 protein are not obligatory for increases in mitochondrial biogenesis in intact mammalian skeletal muscle.
Chronic stimulation-and AICAR-induced mitochondrial proliferation and SIRT1
We examined the relationship between SIRT1 and mitochondrial proliferation by utilizing models that are accompanied by mitochondrial biogenesis, specifically chronic muscle stimulation (McCullagh et al. 1996; Benton et al. 2008b), and AICAR treatment (Holmes et al. 1999; Suwa et al. 2003; Winder et al. 2006). Consistent with previous reports we have demonstrated increases in both PGC-1α and COX IV protein in both the red and white tibialis anterior muscles, following chronic muscle stimulation (Fig. 4) and following AICAR treatment (Fig. 5). We have used mtTFA as an index of PGC-1α activation and following both chronic stimulation and AICAR treatments, there were increases in mtTFA, consistent with the observed increase in COX IV and PGC-1α protein expression. While these increases in COX IV, PGC-1α and mtTFA were observed in the face of decreasing SIRT1 protein content, a concomitant reduction in SIRT1 activity was not observed in any of the muscles studied. Hence, there was an increase in the activation of SIRT1 (SIRT1 activity relative to SIRT1 protein) in four of five muscles. These results, observed during a period of increased mitochondrial biogenesis, demonstrate that SIRT1 activity can be altered independently of its expression. The role of an increased intrinsic SIRT1 activity is unclear, as on a per whole-muscle basis SIRT1 activity was generally not altered.
Previously, a discordant relationship between SIRT1 protein expression and SIRT1 activity has also been observed in a model of muscle wasting, in which there was an increase in muscle fibre SIRT1 expression while both SIRT1 activity and nuclear SIRT1 expression were decreased (Nogalska et al. 2008). It was proposed that this increase in whole-fibre SIRT1 expression might reflect an attempt by the cell to protect itself against decreased SIRT1 activity and nuclear SIRT1 by increasing SIRT1 synthesis (Nogalska et al. 2008). Our results following chronic stimulation and AICAR seem to be consistent with a regulatory loop in muscle that attempts to downregulate SIRT1 in response to an increase in SIRT1 activity. It is possible that this observed increase in the activation status of SIRT1 might also be accompanied by a translocation of SIRT1 from the cytosol to the nucleus, as has been observed to occur with PGC-1α (Wright et al. 2007).
SIRT1 overexpression
In addition to these models of increased mitochondrial biogenesis we also overexpressed SIRT1 protein in skeletal muscle in vivo, in an attempt to further elucidate the role of SIRT1 in mitochondrial biogenesis. In these experiments SIRT1 protein and SIRT1 activity were increased. These effects were accompanied by a concomitant decrease in mitochondrial biogenesis, as evidenced by reductions in COX IV, PGC-1α and mtTFA protein (Fig. 6). Interestingly, there was also a decrease in the activation status of SIRT1 (SIRT1 activity relative to SIRT1 protein). The effect of this observed decrease in activation status of SIRT1 is currently unknown, although this appears to repress mitochondrial biogenesis, when other stimuli are absent. Nevertheless, our results in skeletal muscle are in marked contrast to work in cell lines (C2C12 cells, 293T cells and embryonic fibroblasts), where overexpression of SIRT1 protein increased selected mitochondrial genes (Rodgers et al. 2005; Gerhart-Hines et al. 2007) and knockdown of SIRT1 downregulated mitochondrial biogenesis and gene expression (Gerhart-Hines et al. 2007). These apparently conflicting results add to the controversy surrounding the function of SIRT1 in mammalian cells compared to lower eukaryotes (McBurney et al. 2003; Michishita et al. 2005; Kaeberlein, 2008) and once again suggest that caution is warranted when applying findings from cell line work to physiologically important tissues, such as fully mature skeletal muscle in vivo.
Control of SIRT1 protein expression and activity
At present little is known about how SIRT1 protein content is regulated or how the activity state of SIRT1 protein is controlled in vivo. In vitro experiments have implicated the NAD+/NADH ratio (Blander & Guarente 2004; Kanfi et al. 2008) and AMPK (Greer et al. 2007) in control of SIRT1 transcriptional activity and protein expression. The extent to which NAD+ impacts SIRT1 has been questioned (Anderson et al. 2003) and it is not known whether there is an increase in SIRT1 protein as a result of elevated NAD+. The reduction in SIRT1 induced by chronic muscle stimulation, a treatment that is expected to increase intramuscular NAD+, suggests that the NAD+/NADH ratio does not contribute to upregulating SIRT1 protein expression in mammalian muscle, in vivo. However, the increase in relative SIRT1 activity following chronic stimulation does suggest that an increase in the NAD+/NADH ratio may mediate an increase in the intrinsic activity of SIRT1.
AMPK may contribute to regulating SIRT1 protein expression but probably not its activity. In our study, AMPK appears to inhibit SIRT1 protein expression in mammalian muscle, as this was reduced during treatments known to activate AMPK, namely chronic AICAR treatment and chronic muscle stimulation. While these treatments increased the intrinsic activity of SIRT1, this may not be attributable to AMPK, as AMPK activation does not phosphorylate SIRT1 (Greer et al. 2007). Taken altogether, the factors controlling SIRT1 expression and activity in mammalian muscle in vivo remain to be identified.
Summary
In summary, we have demonstrated a dissociation between SIRT1 protein content and mitochondrial biogenesis of intact skeletal muscle and heart tissue. SIRT1 protein content and activity were inversely related to COX IV and PGC-1α protein expression and citrate synthase activity in heart and skeletal muscle. In addition, increases in mitochondrial biogenesis following chronic electrical stimulation and AICAR treatment were accompanied by a marked reduction in SIRT1 protein content. Finally, SIRT1 overexpression in muscle demonstrated that this repressed mitochondrial biogenesis. Taken altogether, the present experiments are not consistent with the notion that SIRT1 protein expression plays an obligatory regulatory role in PGC-1α-mediated mitochondrial biogenesis in mammalian skeletal muscle.
Acknowledgments
These studies were supported by the Natural Sciences and Engineering Research Council of Canada, the Canadian Institutes of Health, and the Canada Research Chair program. A. Bonen is the Canada Research Chair in Metabolism and Health.
Glossary
Abbreviations
- AICAR
5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside
- AMPK
AMP-activated protein kinase
- COX IV
cytochrome oxidase IV
- CS
citrate synthase
- HRT
heart
- mtTFA
mitochondrial transcription factor A
- PGC-1α
peroxisome proliferator-activated receptor γ coactivator-1α
- RG
red gastrocnemius
- RTA
red tibialis anterior
- SIRT1
silent mating type information regulation 2 homolog 1
- WG
white gastrocnemius
- WTA
white tibialis anterior
Author contributions
Conception and design, or analysis and interpretation of data (B.J.G., Y.Y., J.L., G.P.H., A.B.). Drafting the article or revising it critically for important intellectual content (B.J.G., Y.Y., J.L., G.P.H., A.B.). Final approval of the version to be published (B.J.G., Y.Y., J.L., G.P.H., A.B.)
References
- Anderson RM, Latorre-Esteves M, Neves AR, Lavu S, Medvedik O, Taylor C, Howitz KT, Santos H, Sinclair DA. Yeast life-span extension by calorie restriction is independent of NAD fluctuation. Science. 2003;302:2124–2126. doi: 10.1126/science.1088697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks AS, Kon N, Knight C, Matsumoto M, Gutiérrez-Juárez R, Gu W, Accili D. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab. 2008;8:333–341. doi: 10.1016/j.cmet.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy M, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabor, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton CR, Nickerson JG, Lally J, Han XX, Holloway GP, Glatz JF, Luiken JJ, Graham TE, Heikkila JJ, Bonen A. Modest PGC-1α overexpression in muscle in vivo is sufficient to increase insulin sensitivity and palmitate oxidation in subsarcolemmal, not intermyofibrillar, mitochondria. J Biol Chem. 2008a;283:4228–4240. doi: 10.1074/jbc.M704332200. [DOI] [PubMed] [Google Scholar]
- Benton CR, Yoshida Y, Lally J, Han XX, Hatta H, Bonen A. PGC-1α increases skeletal muscle lactate uptake by increasing the expression of MCT1 but not MCT2 or MCT4. Physiol Genomics. 2008b;35:45–54. doi: 10.1152/physiolgenomics.90217.2008. [DOI] [PubMed] [Google Scholar]
- Bergmeyer HU. Methods of Enzymatic Analysis. New York: Verlag Chemie Weinheim; 1974. [Google Scholar]
- Blander G, Guarente L. The SIR2 family of protein deacetylases. Annu Rev Biochem. 2004;73:417–435. doi: 10.1146/annurev.biochem.73.011303.073651. [DOI] [PubMed] [Google Scholar]
- Bonen A, Luiken JJFP, Liu S, Dyck DJ, Kiens B, Kristiansen S, Turcotte LP, Van Der Vusse J, Glatz JFC. Palmitate transport and fatty acid tansporters in red and white muscles. Am J Physiol Endocrinol Metab. 1998;275:E471–478. doi: 10.1152/ajpendo.1998.275.3.E471. [DOI] [PubMed] [Google Scholar]
- Bonen A, Luiken JJFP, Arumugam Y, Glatz JFC, Tandon NN. Acute regulation of fatty acid uptake involves the cellular redistribution of fatty acid translocase. J Biol Chem. 2000;275:14501–14508. doi: 10.1074/jbc.275.19.14501. [DOI] [PubMed] [Google Scholar]
- Campbell SE, Tandon NN, Woldegiorgis G, Luiken JJFP, Glatz JFC, Bonen A. A novel function for fatty acid translocase (FAT)/CD36. J Biol Chem. 2004;279:36235–36241. doi: 10.1074/jbc.M400566200. [DOI] [PubMed] [Google Scholar]
- Clarke DC, Miskovic D, Han XX, Calles-Escandon J, Glatz JF, Luiken JJ, Heikkila JJ, Bonen A. Overexpression of membrane-associated fatty acid binding protein (FABPpm) in vivo increases fatty acid sarcolemmal transport and metabolism. Physiol Genomics. 2004;17:31–37. doi: 10.1152/physiolgenomics.00190.2003. [DOI] [PubMed] [Google Scholar]
- Dali-Youcef N, Lagouge M, Froelich S, Koehl C, Schoonjans K, Auwerx J. Sirtuins: The ‘magnificent seven’, function, metabolism and longevity. Ann Med. 2007;39:335–345. doi: 10.1080/07853890701408194. [DOI] [PubMed] [Google Scholar]
- Feige JN, Lagouge M, Canto C, Strehle A, Houten SM, Milne JC, Lambert PD, Mataki C, Elliot PJ, Auwerx J. Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab. 2008;8:347–358. doi: 10.1016/j.cmet.2008.08.017. [DOI] [PubMed] [Google Scholar]
- Gerhart-Hines Z, Rodgers JT, Bare O, Lerin C, Kim SK, Mostoslavsky R, Alt FW, Wu Z, Puiserver Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1α. EMBO J. 2007;26:1913–1923. doi: 10.1038/sj.emboj.7601633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer EL, Oskour PR, Banko MR, Maniar JM, Gygi MP, Gygi SP, Brunet A. The energy sensor AMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J Biol Chem. 2007;282:30107–30119. doi: 10.1074/jbc.M705325200. [DOI] [PubMed] [Google Scholar]
- Holloway GP, Lally J, Nickerson JG, Alkhateeb H, Snook LA, Heigenhauser GJ, Calles-Escandon J, Glatz JF, Luiken JJ, Spriet LL, Bonen A. Fatty acid binding protein facilitates sarcolemmal fatty acid transport but not mitochondrial oxidation in rat and human skeletal muscle. J Physiol. 2007;582:393–405. doi: 10.1113/jphysiol.2007.135301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway GP, Perry CGR, Thrush BA, Heigenhauser GJF, Dyck DJ, Bonen A, Spriet LL. PGC-1α's relationship with skeletal muscle palmitate oxidation is not present with obesity despite maintained PCG-1α and PGC-1β protein. Am J Physiol Endocrinol Metab. 2008;294:E1060–E1069. doi: 10.1152/ajpendo.00726.2007. [DOI] [PubMed] [Google Scholar]
- Holmes BF, Kurth-Kraczek EJ, Winder WW. Chronic activation of 5′-AMP-activated protein kinase increases GLUT-4, hexokinase, and glycogen in muscle. J Appl Physiol. 1999;87:1990–1995. doi: 10.1152/jappl.1999.87.5.1990. [DOI] [PubMed] [Google Scholar]
- Jäger S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1α. Proc Natl Acad Sci U S A. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M. The ongoing saga of sirtuins and aging. Cell Metab. 2008;8:4–5. doi: 10.1016/j.cmet.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Kanfi Y, Peshti V, Gozlan YM, Rathaus M, Gil R, Cohen HY. Regulation of SIRT1 protein levels by nutrient availability. FEBS Lett. 2008;582:2417–2423. doi: 10.1016/j.febslet.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Li Y, Xu W, McBurney MW, Longo VD. SirT1 inhibition reduces IGF-1/IRS-2/RAS/ERK1/2 signalling and protects neurons. Cell Metab. 2008;8:36–48. doi: 10.1016/j.cmet.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Wu H, Tarr PT, Zhang C, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Dubyk R, Spiegelman BM. Transcriptional co-activator PGC-1α drives the formation of slow-twitch muscle fibres. Nature. 2002;418:797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- Luiken JJFP, Willems J, Van Der Vusse GJ, Glatz JFC. Electrostimulation enhances FAT/CD36-mediated long-chain fatty acid uptake by isolated rat cardiac myocytes. Am J Physiol Endocrinol Metab. 2001;281:E704–712. doi: 10.1152/ajpendo.2001.281.4.E704. [DOI] [PubMed] [Google Scholar]
- McBurney MW, Yang X, Jardine K, Hixon M, Boekelheide K, Webb JR, Lansdrop PM, Lemieux M. The mammalian SIR2a protein has a role in embryogenesis and gametogenesis. Mol Cell Biol. 2003;23:38–54. doi: 10.1128/MCB.23.1.38-54.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullagh KJ, Juel C, O'Brien M, Bonen A. Chronic muscle stimulation increases lactate transport in rat skeletal muscle. Mol Cell Biochem. 1996;156:51–57. doi: 10.1007/BF00239319. [DOI] [PubMed] [Google Scholar]
- Michishita E, Park JY, Burneskis JM, Barrett JC, Horikawa I. Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Mol Biol Cell. 2005;16:4623–4635. doi: 10.1091/mbc.E05-01-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, Bemis JE, Xie R, Disch JS, Ng PY, Nunes JJ, Lynch AV, Yang H, Galonek H, Israelian K, Choy W, Iffland A, Lavu S, Medvedik O, Sinclair DA, Olefsky JM, Jirousek MR, Elliott PJ, Westphal CH. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemoto S, Fergusson MM, Finkel T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1α. J Biol Chem. 2005;280:16456–16460. doi: 10.1074/jbc.M501485200. [DOI] [PubMed] [Google Scholar]
- Nogalska A, D'Agostino C, Engel WK, Davies KJA, Askanas V. Decreased SIRT1 deacetylase activity in sporadic inclusion-body myositis muscle fibers. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.08.021. DOI 10.1016/j.neurobiolaging.2008.08.021. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Wu Z, Park CW, Graves R, Wright M, Speigelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:929–939. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Rhee J, Lin J, Wu Z, Yoon JC, Zhang CY, Krauss S, Mootha VK, Lowell BB, Spiegelman BM. Cytokine stimulation of energy expenditure through p38 MAP kinase activation of PPARγ coactivator-1. Mol Cell. 2001;8:971–982. doi: 10.1016/s1097-2765(01)00390-2. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Spiegelman BM. Peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α): transcriptional coactivator and metabolic regulator. Endocr Rev. 2003;24:78–90. doi: 10.1210/er.2002-0012. [DOI] [PubMed] [Google Scholar]
- Rodgers JT, Lerin C, Hass W, Gygl SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- Rodgers JT, Puigserver P. Fasting-dependent glucose and lipid metabolic response though hepatic sirtuin 1. Proc Natl Acad Sci U S A. 2007;104:12861–12866. doi: 10.1073/pnas.0702509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi T, Wang F, Stieren E, Tong Q. SIRT3, a mitochondrial sirtuin deacetylase, regulates mitochondrial function and thermogenesis in brown adipocytes. J Biol Chem. 2005;280:13560–13567. doi: 10.1074/jbc.M414670200. [DOI] [PubMed] [Google Scholar]
- Suwa M, Nakano H, Kumagai S. Effects of chronic AICAR treatment on fibre composition, enzyme activity, UCP3, and PGC-1 in rat muscle. J Appl Physiol. 2003;95:960–968. doi: 10.1152/japplphysiol.00349.2003. [DOI] [PubMed] [Google Scholar]
- Suwa M, Nakano H, Radak Z, Kumagai S. Endurance exercise increases the SIRT1 and peroxisome proliferator-activated receptor. γ coactivation-1α protein expressions in rat skeletal muscle. Metabolism. 2008;57:986–998. doi: 10.1016/j.metabol.2008.02.017. [DOI] [PubMed] [Google Scholar]
- Winder WW, Taylor EB, Thomson DM. Role of AMP-activated protein kinase in the molecular adaptation to endurance exercise. Med Sci Sports Exerc. 2006;38:1945–1949. doi: 10.1249/01.mss.0000233798.62153.50. [DOI] [PubMed] [Google Scholar]
- Wright DC, Han DH, Garcia-Roves PM, Geiger PC, Jones TE, Holloszy JO. Exercise-induced mitochondrial biogenesis begins before the increase in muscle PGC-1α expression. J Biol Chem. 2007;282:194–199. doi: 10.1074/jbc.M606116200. [DOI] [PubMed] [Google Scholar]
- Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1α. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]