Abstract
Expression and activity of CC motif ligand 2 (CCL2) is down-regulated by curcumin, the active phytochemical ingredient of turmeric (Curcuma longa), a dietary supplement often self-prescribed to promote prostate health. CCL2 is a potent chemotactic factor of prostate cancer (PCa) with important roles in development of bone metastasis. The relationship between CCL2 and curcumin, however, has not been studied in PCa. Adhesion, invasion and motility of PC-3 cells were measured in response to exposure to curcumin (30 μM; 18hr), CCL2 (100 ng/ml; 18 hr) or PMA (100 ng/ml; 18hr). CCL2 mRNA expression and protein secretion levels were measured by Real-time PCR and ELISA respectively. Curcumin significantly blocked CCL2 induced adhesion, invasion and motility. Curcumin also significantly suppressed the mRNA expression and secreted CCL2 protein levels. The addition of PMA, a protein kinas C (PKC) activator, blocked the effects of curcumin, leading to an increase in CCL2 expression as well as an increase in PC-3 cell adhesion, invasion and motility. The introduction of a PKC inhibitor, however, blocked the effects of CCL2. We also found that curcumin, CCL2 and PMA, in part, function through the differential regulation of the proteolytic protein matrix metalloproteinase (MMP)-9. These data indicate a potential mechanism; by which curcumin can block the chemotactic effects of CCL2 on PCa. Curcumin exerts potential anti-metastatic effects in bone-derived PCa cells by blocking CCL2 mediated actions on invasion, adhesion and motility, in part through differential regulation of PKC and MMP-9 signaling.
Keywords: prostate cancer, MCP-1, CCL2, PKC, nutrition, diet
Introduction
The most common site of prostate cancer (PCa) metastasis is the bone, with skeletal metastases identified at autopsy in up to 90% of patients dying from PCa (1). Skeletal metastases in PCa patient result in significant complications which diminish the patient's quality of life. These include bone pain, diminished mobility, pathologic fracture, spinal cord compression, and symptomatic hypocalcaemia. Current therapeutic approaches to the treatment of prostatic bone metastases have largely remained ineffective (2,3). The underlying mechanism of bone metastasis is generally poorly understood, involving complex yet aberrant crosstalk signaling between the primary tumor and the bone (2,3).
CC motif ligand 2 (CCL2; aka monocyte chemoattractant protein-1 or MCP-1) is a potent chemotactic factor vital in the development and progression of PCa bone metastasis (4-6). CCL2 is a member of the CC subfamily of low molecular weight chemokines, a class of proteins that are essential in immune regulation, inflammation, and the healing response (7). Under aberrant pathological conditions, CCL2 has essential roles in the infiltration of tumor associated macrophages, which play a key role in increased tumorigenicity of PCa and other cancers (8). CCL2 acts as an autocrine and paracrine mitogenic and motogenic factor of the malignant human androgen-independent prostatic PC-3 cell line (6). Additionally, increased expression of CCR2, the G-protein coupled receptor of CCL2, correlates with increased PCa progression (9).
CCL2 expression and activity can be inhibited by curcumin, the active polyphenolic compound of the powdered turmeric (Curcuma longa) root (10), although this relationship has never been studied in connection to PCa. Curcumin is a commonly used spice of India and Southeast Asia and is a popular dietary supplement due to its potent anti-inflammatory and anti-oxidant properties (10-12). Curcumin has shown potential as a natural low-toxicity self-treatment for benign prostatic hyperplasia (BPH) and PCa (13).
In the present study, we tested the hypothesis that curcumin inhibits CCL2 induced adhesion, invasion, and motility of PC-3 cells, a human bone-derived androgen-independent PCa cell line. We found that cells treated with curcumin exhibited decreased expression of CCL2 protein and mRNA levels, which was caused, in part, through the inhibition of the protein kinase C (PKC) signaling pathway. The PKC pathway is a central intracellular signaling mediator, with important roles in invasion and is known to up-regulate CCL2 expression (14-16). We also found, that curcumin, through inhibition of PKC blocks CCL2-induced invasion, adhesion and motility, leading to important changes in the activity of matrix-metalloproteinase protein (MMP)-9, an important invasion related protein in PCa.
Materials and Methods
Cell Culture
Human derived PC-3 prostate cancer cells were maintained and grown in RPMI-1640 (Invitrogen; Carlsbad, CA) cell culture media supplemented with 10% filter sterilized fetal bovine serum (FBS) and incubated at 37 °C at 5% CO2. In some experiments cells were cultured for 18 hr in cell media that also contained various reagents purchased from Sigma (St. Louis, MO) including curcumin (cur; 30 μm), 1′-[2-[4-(trifluoromethyl)phenyl]ethyl]-spiro[4H-3,1-benzoxazine-4,4′-peperidin]-2(1H)-one (CCR2i; 10 μg/mL), phorbol 12-myristate 13-acetate (PMA; 100 ng/mL), Go6976, (GO; 2 μM). Some cells were also cultured with CCL2 (100 ng/ML) purchased from R&D Biosystems (Minneapolis, MN). Control cultures were treated with vehicle only. Cells were harvested at 70%-80% confluency.
Quantitative Real-Time PCR
Total RNA was extracted using Tri-reagent from Applied Biosystems Inc. (ABI, Foster City, CA). First strand cDNA synthesis was performed using 500 ng of total RNA using random hexamer primers and Superscript III (Invitrogen). Inventoried Taqman Real-Time PCR primers for CCL2 and glyceraldehyde 3-phosphate (GAPDH) were obtained from ABI. PCR was performed on the 7500 FAST Real-time PCR system (ABI) using Taqman Universal PCR master mix (ABI). The cycling conditions for the real-time PCR: 1× [50 °C for 2 min], 40× [95 °C for 15 sec, 60.0 °C for 1 min]. Using the relative ΔΔCT method as described by ABI, CT levels for CCL2 were normalized to GAPDH to obtain the ΔCT value. The ΔCT value was normalized to the control treatment to obtain the ΔΔCT. ΔΔCT value, in turn, was used to determine the relative expression of CCL2. Experiments were performed in triplicate wells and averaged. Results are an average of at least three independent experiments. Results are presented as a percentage of cells grown in the presence of curcumin, PMA, and GO compared to cells grown with vehicle controls. Statistical analysis was performed using ΔCT values.
ELISA assay
CCL2 protein levels were measured from the PC-3 cell culture media by using the human CCL2 ELISA Ready-SET-Go! kit (Ebioscience, San Diego, Ca) according to manufacturer's specifications. Briefly cells were added to a 96-well plate and allowed to reach 70% confluency, the media was removed, and fresh media supplemented with either curcumin, PMA, GO or vehicle control were added to the cells for 18 hr. The media was used in the ELISA reaction and the concentration was determined in a colorimetric reaction, in relation to a standard curve, derived from known levels of human recombinant CCL2. Experiments were performed in triplicate wells and averaged. Results are an average of at least three separate experiments and presented as a percentage of cells grown in the presence of curcumin, PMA, GO compared to cells grown with vehicle controls.
Adhesion Assay
Cellular adhesion was determined as described previously with a few modifications (17). Fifty-thousand cells were suspended in serum-free media supplemented with 0.1% bovine serum albumin (BSA) and added to the wells of a 48 well plate that were coated with 20 μg/mL fibronectin, collagen, or laminin. The plates were incubated for 30 and 60 min after which the plates were washed twice with PBS to remove unattached cells. Attached cells were fixed with methanol and stained with Eosin Y (Sigma). Attached cells were counted at five random optical fields (40×) as determined by light microscopy. Experiments were performed in triplicate wells and the results were averaged. Results are presented as a percentage of cellular adhesion in the presence of curcumin, CCL2, CCR2i, PMA, GO compared to cells grown with vehicle controls. The number of cells adhering under control conditions was assigned the threshold value of 100%.
Chemo-motility and chemo-invasion Assays
Chemo-motility and chemo-invasion assays were performed using the BD Falcon 24-well plate transwell system (BD Biosciences; Franklin Lakes, NJ) with an 8-μm porous polyethane membrane. One hundred thousand cells suspended in serum-free medium supplemented with 0.1% BSA were transferred into the upper wells of the chamber. The lower wells contained medium with 5 % FBS, which served as a chemoattractant to facilitate motility or invasion. Curcumin, CCL2, CCR2i, PMA, GO, vehicle control or a combination thereof were added to the lower wells as appropriate. For the invasion assay, the transwell was coated with 40 μg/mL of matrigel (BD Biosciences), while the motility assay did not have any membrane coating. The transwell plate was incubated for 18 h in a tissue incubator at 37°C with 5% CO2. Cells were fixed in 100% methanol and stained with Eosin Y. Cells that did not cross the polyethane membrane were removed from the upper well by scraping. Cells that crossed to the underside of the membrane were measured by counting cells in five random optical fields of duplicate wells under 40× magnification using an inverted light microscope. The results were averaged and expressed as a percentage relative to loading controls.
Gelatin Zymography
The proteolytic enzyme activity of pro-MMP-9 was measured by gelatin zymography (18). Briefly, protein concentrations were determined with the BioRad DC (BioRad Laboratories; Hercules, CA) protein assay kit from conditioned media and cell-lysate of PC-3 cells cultured under growing conditions as described previously (18). Fifteen μg of total protein was mixed with non-denaturing buffer (125 mM Tris-HCl, 4% SDS, 20% glycerol, 0.004% bromophenol blue) and the protein samples were subjected to electrophoresis through a 10% Zymogram Ready Gel (BioRad Laboratories). After electrophoresis, the gels were rinsed with wash buffer (50 mM Tris-HCl; 5 mM CaCl2, 2.5% Triton X-100) for an hour, in six 10 min intervals. After the washes, the gels were incubated over night in incubation buffer (mM Tris-HCl; 5 mM CaCl2) at 37°C. The gels were then stained with 0.2% coomassie brilliant blue for 30 min and then destained with (30% Methanol, 1% Glacial Acetic acid) with 3-5 washes.
Statistical Analysis
Adhesion data was analyzed with Two-way ANOVA followed by Bonferonni's post-hoc comparison. Comparisons for select data groups were graphically presented. Motility, migration, CCL2 expression and zymography data were analyzed with One-way ANOVA followed by Tukey's post hoc comparisons. Statistical comparisons were performed using GraphPad Prism Version 4.00 for Windows (GraphPad Software, San Diego California).
Results
Curcumin blocks the effects of CCL2 induced PC-3 adhesion, motility and invasion
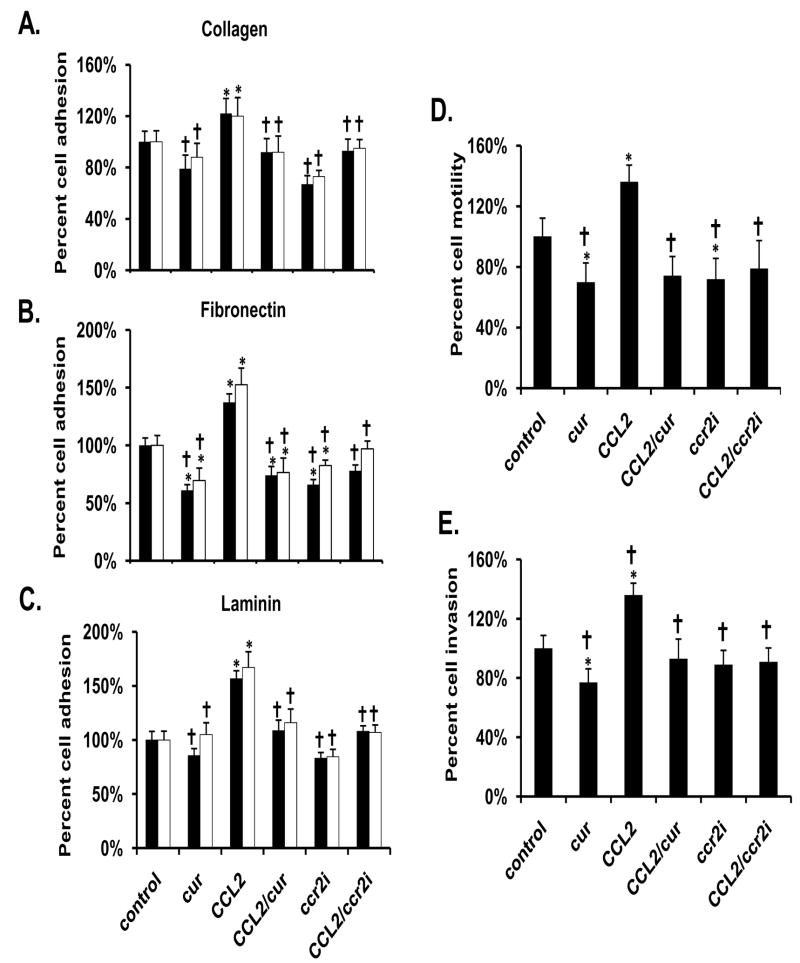
PC-3 cells exposed to CCL2 exhibited a significantly higher level of adhesion to collagen, laminin, and fibronectin, when compared to controls. Curcumin blocked this effect (Fig 1A-1C). Exposure to curcumin alone, significantly decreased adhesion of PC-3 cells to fibronectin when compared to vehicle-treated controls, but not to collagen or laminin (Fig 1A-1C). Curcumin alone also inhibited the pro-motile and pro-invasive characteristics of PC-3 cells, and blocked the increased motility and invasion induced by exogenous CCL2 (Fig 1D-1E). Furthermore, the addition of the CCL2 receptor (CCR2) antagonist, CCR2i, closely mimicked the effects that were observed with curcumin; inhibiting the pro-adhesive, pro-motile, and pro-invasive effects of CCL2.
Figure 1.
Curcumin (30 μM) blocks the adhesive, motile and invasive characteristics of PC-3 cells exposed to CCL2. Adhesion assays were performed on fibronectin (A), collagen (B), or laminin (C) using either 30 min (black bars) or 60 min (white bars) incubations. The motility (D) and invasion (E) assays were performed using a transwell system incubated for 18 hr in the absence and presence of matrigel, respectively. The results are presented as the percentage of vehicle-treated control. Each bar represents the mean ± SEM of 2-3 independent experiments. See Materials and methods for statistical analysis. *, p< 0.05; compared to vehicle-treated control cells. †, p< 0.05 compared to CCL2-treated cells. Abbreviations: cur: curcumin, CCL2: CC motif ligand 2, CCR2i: a CCL2 receptor inhibitor.
Curcumin blocks the effects of PKC induced PC-3 adhesion, motility and invasion
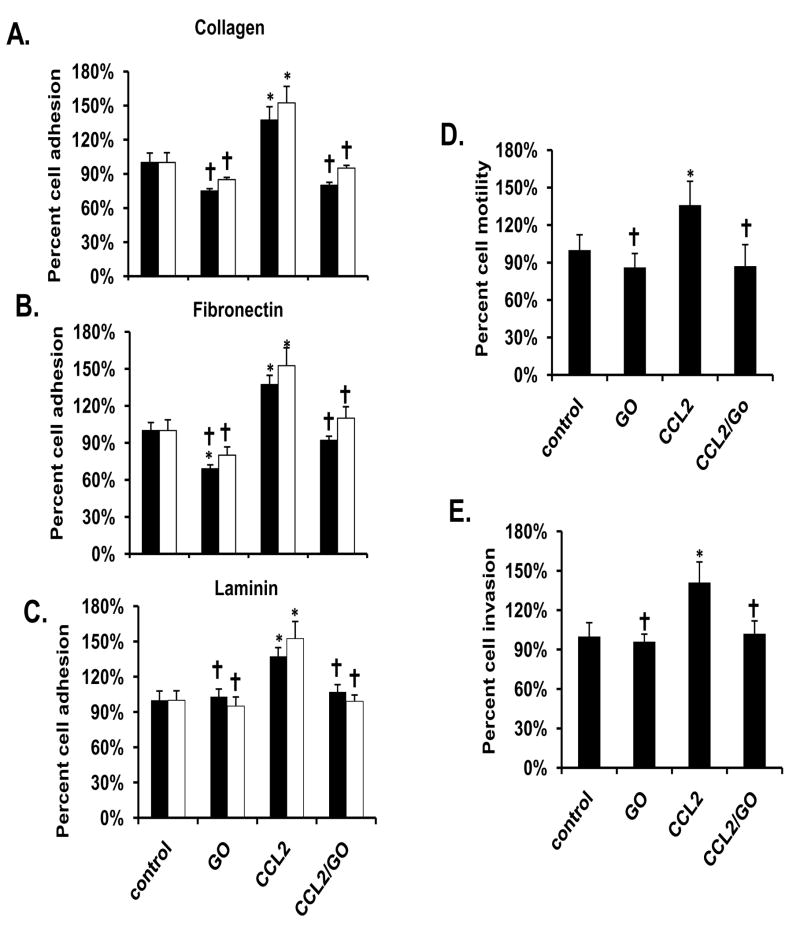
Cells exposed to the PKC activator, PMA exhibited a significant increase of adhesion, motility and invasion compared to vehicle-treated controls, in a pattern similar to CCL2 (Fig 2A-2E). Curcumin significantly blocked these effects. Treatment with the PKC inhibitor GO blocked CCL2 induced adhesion, invasion, and motility similar to the effects of curcumin, but unlike curcumin did not alter adhesion, motility, or invasion in vehicle-treated cells (Fig 3A-3E).
Figure 2.
Curcumin (30 μM) blocks the effects of PMA on PC-3 cell adhesion, motility and invasion. Adhesion assays were performed fibronectin (A), collagen (B), or laminin (C) using either 30 min (black bars) or 60 min (white bars) incubations. The motility (D) and invasion (E) assays were performed using a transwell system incubated for 18 hr in the absence and presence of matrigel, respectively. The results are presented as the percentage of vehicle-treated control. Each bar represents the mean ± SEM of 2-3 independent experiments. See Materials and methods for statistical analysis. *, p< 0.05 compared to vehicle-treated control cells. †, p< 0.05 compared to PMA-treated cells. Abbreviations: cur: curcumin, PMA: phorbol 12-myristate 13-acetate.
Figure 3.
GO (2 μM) inhibits the adhesive, motile and invasive effects of 30 μM CCL2 on PC-3 cells. Adhesion assays were performed fibronectin (A), collagen (B), or laminin (C) using either 30 min (black bars) or 60 min (white bars) incubations. The motility (D) and invasion (E) assays were performed using a transwell system incubated for 18 hr in the absence and presence of matrigel, respectively. The results are presented as the percentage of vehicle-treated control. Each bar represents the mean ± SEM of 2-3 independent experiments. See Materials and methods for statistical analysis. *, p< 0.05 compared to vehicle-treated control cells. †, p< 0.05 compared to CCL2-treated cells. Abbreviations: GO: GO6976, CCL2: CC motif ligand 2.
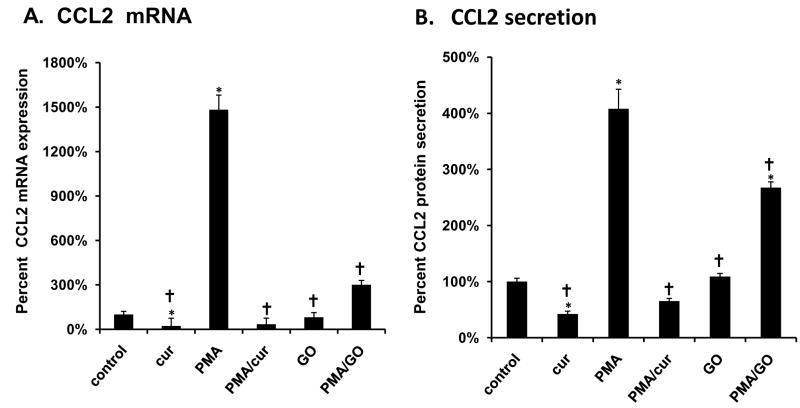
Curcumin decreases CCL2 expression and secretion
PC-3 cells that were exposed to curcumin, exhibited significantly lower CCL2 mRNA levels as well as significantly lower levels of CCL2 protein secretion into the media compared to vehicle-treated cells grown in the absence of curcumin (FIG. 4A-4B). We also found that PC-3 cells grown in the presence of PMA exhibited increased CCL2 mRNA expression and secretion. Curcumin treatment blocked both of these effects. The addition of GO alone did not have a significant effect on CCL2 mRNA expression and secretion, but did significantly block the increases induced by PMA (Fig 4A-4B). Go was more effective in blocking PMA-induced mRNA expression than PMA-induced CCL2 secretion.
Figure 4.
Curcumin decreases control and PMA-induced CCL2 mRNA expression and protein secretion. PC-3 cells were exposed to curcumin (30 μm), PMA (100 ng/mL), and/or GO (2 μm) for 18 hrs. (A) Changes in mRNA levels of CCL2 were measured by relative real-time PCR with TaqMan Universal PCR Master Mix (Applied Biosystems). Expression of target gene mRNA levels was normalized to GAPDH mRNA. (B) Changes in CCL2 protein expression expression/secretion was measured by a human CCL2 ELISA Ready-Set-Go! kit from Ebiosciences. The results are presented as the percentage of vehicle-treated control. Each bar represents the mean ± SEM of 2-3 independent experiments. See Materials and methods for statistical analysis. *, p< 0.05 compared to vehicle-treated control cells. †, p< 0.001compared to CCL2-treated cells. Abbreviations: cur: curcumin, CCL2: CC motif ligand 2, PMA: phorbol 12-myristate 13-acetate, GO: GO6976.
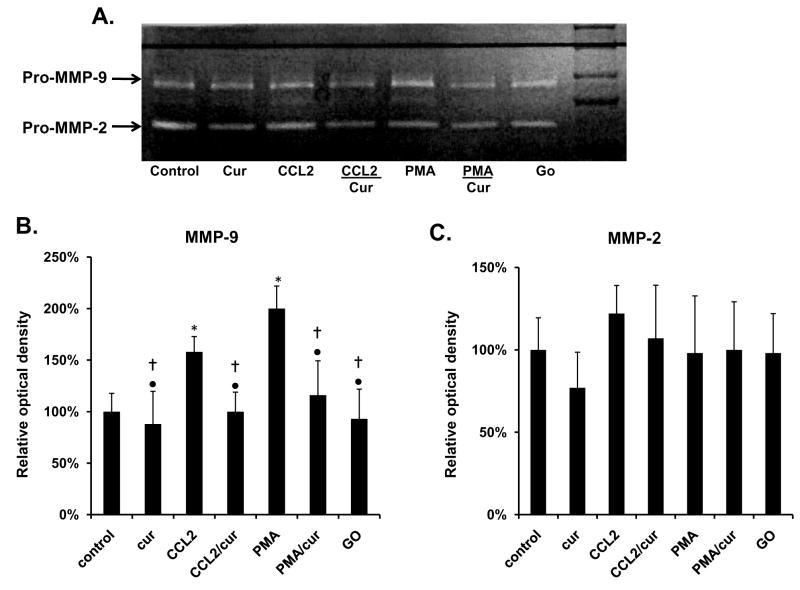
Curcumin blocks CCL2 induced MMP-9 expression
MMP-9 activity was measured in cell media of PC-3 cells by a gelatin zymography (Fig 5A). Cells incubated with CCL2, exhibited significant 64% increase in MMP-9 activity when compared to vehicle-treated controls. Curcumin blocked these effects, but had no effect on vehicle-treated control cells (Fig 5B). Curcumin also significantly inhibited the 75% increase of MMP-9, observed in cells incubated with PMA. There was no significant change among the different groups in relationship to MMP-2 levels (Fig 5C).
Figure 5.
Curcumin (30 μM for 18 h) decreases CCL2 and PMA induced MMP-9. Secretion and activity of pro-MMP-9 and pro-MMP-2 activity was measured by gelatin zymography. The zymography gel is representative of three separate experiments. The results were determined by measuring optical density differences of the gel using the UVP and are presented as the percentage of vehicle-treated control. See Materials and methods for statistical analysis. *Different from control cells at p≤0.05. †Different from CCL2 at p≤0.05. •Different from PMA exposed cells at p≤0.05. Abbreviations: cur: curcumin, CCL2: CC motif ligand 2, PMA: phorbol 12-myristate 13-acetate, GO: GO6976.
Discussion
PC-3 cells exposed to curcumin (30 μM) alone for 18 hrs; a concentration and timepoint that were less than necessary to induce cytotoxicity (data not shown), exhibited a significant decrease in adhesion to fibronectin and collagen, but these cells exhibited only a moderate non-significant inhibitory trend of adhesion to laminin.
Cells exposed to curcumin also exhibited a modest, yet significant effect on invasion and motility. This finding is supported by other published reports. Hong etal for example, showed that curcumin decreased PCa tumor and metastasis burden of DU145 xenografts implanted into SCID mice, in part through the inhibition of MMP proteins after 24 hr exposure (19). Additionally, they found that at increasing concentrations of curcumin (1, 5, 10 μg/mL), the invasive characteristics of cultured DU145 cells were blocked in a dose dependent manner, with highest concentration inhibiting invasion by 71%. While, the 30 μM curcumin concentration used in our study was similar to the highest and most effective dose used in the Hong study (10 μg/mL or ∼27 μM), we observed the effect of curcumin in our study was far more subtle (19). We found that curcumin (30 μM; 18 hr) blocked the invasive characteristics of PC-3 cells by 23% of control cells. Furthermore, we did not observe any significant effects on invasion at lower concentrations of curcumin (1, 3, or 10 μM) (Data not shown).
There are several possible reasons that might explain the differences between these studies. Curcumin, for example can have very different effects in different cell types. We found that PC-3 cells exposed to higher concentrations of curcumin (≥ 50 μM) exhibited a significant decrease in cell viability after 24 hr exposure (unpublished data). However, at equivalent curcumin concentrations and exposure times, LNCaP, a malignant PCa cell line and RWPE1, an immortalized non-tumorigenic prostate epithelial cell line, were resistant to the effects of curcumin, exhibiting no significant changes in cell viability (unpublished data). These differences in effects of curcumin can also be observed in other cell types as well (20,21).
Additionally, unlike the Hong study we opted to supplement curcumin in the lower well of the transwell plates rather than the upperwell of our invasion and motility assays. In doing so, we decreased the risk undue stress on the cells in the assay, which are incubated in serum free media in the upperwells of the transwell plates. This approach was supported by our preliminary data that showed curcumin was more cytotoxic in cells grown without FBS. Our experimental approach may have provided a more moderate curcumin effect, but the risk of cytotoxicity skewing the results was also decreased.
In this study, we also found that curcumin effectively blocked CCL2 mediated adhesion to collagen, laminin, and fibronectin as well as invasion and motility of PC-3 cells. While, CCL2 is an essential regulator of the inflammatory response, it is also a potent growth and chemotactic factor of PCa. CCL2 is an essential player in the invasive and metastatic characteristics of cancer, playing a major role in the development and progression of PCa tumor growth (22). In SCID mice, Loberg etal., showed that by using a CCL2 specific neutralizing antibody, the progression of PCa can be retarded (22). Additionally, we were able to mimic the effects observed with curcumin alone or in combination with CCL2 by treating the PC-3 cells with a CCR2 antagonist (CCR2i). CCR2 is a potential biomarker of PCa progression; increased expression of CCR2 correlates with increased malignancy of the disease (22).
PC-3 cells exposed to curcumin (30 μM, 18hr) exhibited significantly decreased levels of CCL2 mRNA and secreted protein. In preliminary experiments, we did not observe significant changes in CCL2 expression at lower concentrations of curcumin in PC-3 cells, nor did we observe significant changes in CCL2 mRNA expression in LNCaP or RWPE1 cells (data not shown). Although published reports indicate that curcumin effectively inhibits CCL2 expression and secretion in various model systems including human umbilical vein endothelial cells and ex-vivo murine pancreatic islets (23,24), the relationship between CCL2 and curcumin has not been studied previously in PCa.
We also found that PC-3 cells incubated with PMA, a pharmacological activator of PKC, exhibited a significant increase in both CCL2 mRNA and protein expression. Curcumin significantly blocked this effect. It has been reported previously that increased PKC signaling can lead to increased CCL2 expression and activity (16). It has also been reported that curcumin can inhibit PKC activity (25). This is further supported by our observation that PC-3 cells incubated with PMA, exhibited increased adhesion, invasion, and motility at levels comparable to what was previously observed with CCL2 incubation. Curcumin effectively blocked the effects of PMA, similarly to the inhibitory effects observed with curcumin on CCL2 signaling. Taken together, our data suggests that curcumin acts, in part, to inhibit the activation of the PKC pathway leading to a down-regulation of CCL2 expression, as well as a decrease in adhesion, invasion and motility.
While, PKC can act as an upstream regulator of CCL2 expression (26), it also may be a downstream signaling mediator of CCL2 activity (27,28). This is supported in part, by the fact that GO, a pharmacological inhibitor of PKC, successfully blocked the pro-adhesive, motile and invasive effects of exogenous CCL2 (Fig 3). We also found that CCR2i blocked the effects of PMA (unpublished data), suggesting that the CCR2 receptor could be upstream of PKC. These data suggest that CCL2 could potentially exist in a positive feedback loop.
Although we did not observe any effects of exogenous CCL2 on mRNA expression or protein secretion of CCL2 (data not shown), it has been reported that several various isotypes can play differential roles in the regulation of CCL2 expression and signaling. For example, the PKC-β isoform is necessary for CCL2 induced chemotaxis, while PKC-ε plays a key role in CCL2 expression, for example (29). PMA is known to activate numerous PKC isotypes including α,β,δ,ε,η,θ,ι and μ (30,31) and it is likely that CCL2 regulates PKC levels and various isotypes more selectively than PMA, which could indicate a way CCL2 might utilize PKC to induce chemotaxis, but not to up-regulate CCL2 expression. Interestingly we found that GO significantly down-regulated the effects of PMA, however, the inhibition was not complete, which was particularly noticeable when measuring the changes in CCL2 expression (Fig 4). GO is largely a PKC α/β inhibitor (32) and it is likely that PMA is activating the pathway to a degree that GO cannot completely block. However, since we observe that GO blocks CCL2 induced invasion, adhesion, and motility; this indicates that CCL2 may largely act through the PKC α/β pathways (26,27). Additionally this data suggests that curcumin also likely inhibits the PKC α/β pathways, as well as other PKC isotypes, particularly since the effects of curcumin on PC-3 were greater than observed with GO.
These effects of curcumin may also be in part due to the differential regulation of MMP-9. As a class of proteins, MMP proteases have essential roles in the regulation of invasion, as well as important roles in motility and adhesion, through their ability to degrade extracellular matrix (ECM) proteins (33). While important in the healing response, MMP proteins are often de-regulated in cancers and can lead to increased invasion and metastasis (33). We observed, through zymography, that CCL2 increased MMP-9 activity, but not MMP-2 in PC-3 cells and this up-regulation was blocked by curcumin (Fig 5). Although the exact effects of CCL2 on MMP-9 are not well understood and have not been studied extensively, it has been noted that CCL2 up-regulates MMP-9 in cells of the blood-brain barrier, increasing the chance of HIV infected cells to cross into the brain (34). Additionally, macrophages from CCR2-/- mice expressed less MMP-9 compared to macrophages from a CCR2 +/+ mouse (35). MMP-9 also was up regulated by PMA, which was blocked by curcumin. It has been reported that PMA/PKC signaling is an important regulator of MMP-9 expression (36).
The exact mechanism of curcumin and its relationship with PKC and MMP-9 in CCL2 induced PCa chemotaxis is not entirely understood, however it is thought that transcription factors can play an important role (Fig 6). For example, in human umbilical vein endothelial cells (HUVEC), CCL2 mRNA and protein levels can be regulated by NFκB and AP-1, through PKC signaling (37). NFκB and AP-1 can also be inhibited by curcumin (38,39). NFκB and AP-1 are also important transcription regulators for MMP-9 expression (36,40). Other transcription factors such as hypoxia inducible factor 1(HIF-1) a, aryl hydrocarbon nuclear transport (ARNT aka HIF-1b), CREB, etc have roles in the regulation of CCL2 and MMP-9 expression are also regulated by curcumin and PKC as well (38,39,41,42,42)
Figure 6.
Possible pathways by which curcumin regulates CCL2 invasion and adhesion of PC-3 cells. Through an inhibition of PKC, curcumin can lead to a down-regulation of CCL2 expression and MMP-9 expression, which may be due in part to differential activation of transcription factors. Also since GO blocks CCL2 induced invasion, adhesion and motility, and curcumin also blocks the up-regulation of MMP-9 by CCL2, and CCR2i blocks the effects of PMA, a potential feedback loop could be in play.
In summary, this study demonstrates, that curcumin blocks adhesion, invasion, and motility of the human PC-3 cell line, in part through the down-regulation of CCL2 activity via the inhibition of PKC and MMP proteins. Further research must be performed to elucidate the importance of differential PKC isotype regulation in CCL2 signaling, as well as to achieve a better understanding of this novel mechanism by which curcumin can induce chemopreventative effects against PCa.
Acknowledgments
The research was supported by NIH grants R21 AT003397 and T32 HD07133.
References
- 1.Bubendorf L, Schopfer A, Wagner U, et al. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31:578–83. doi: 10.1053/hp.2000.6698. [DOI] [PubMed] [Google Scholar]
- 2.Bussard KM, Gay CV, Mastro AM. The bone microenvironment in metastasis; what is special about bone? Cancer Metastasis Rev. 2008;27:41–55. doi: 10.1007/s10555-007-9109-4. [DOI] [PubMed] [Google Scholar]
- 3.Ye L, Kynaston HG, Jiang WG. Bone metastasis in prostate cancer: molecular and cellular mechanisms (Review) Int J Mol Med. 2007;20:103–11. [PubMed] [Google Scholar]
- 4.Craig MJ, Loberg RD. CCL2 (Monocyte Chemoattractant Protein-1) in cancer bone metastases. Cancer Metastasis Rev. 2006;25:611–9. doi: 10.1007/s10555-006-9027-x. [DOI] [PubMed] [Google Scholar]
- 5.Lu Y, Xiao G, Galson DL, et al. PTHrP-induced MCP-1 production by human bone marrow endothelial cells and osteoblasts promotes osteoclast differentiation and prostate cancer cell proliferation and invasion in vitro. Int J Cancer. 2007;121:724–33. doi: 10.1002/ijc.22704. [DOI] [PubMed] [Google Scholar]
- 6.Lu Y, Cai Z, Galson DL, et al. Monocyte chemotactic protein-1 (MCP-1) acts as a paracrine and autocrine factor for prostate cancer growth and invasion. Prostate. 2006;66:1311–8. doi: 10.1002/pros.20464. [DOI] [PubMed] [Google Scholar]
- 7.Luster AD. Chemokines--chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–45. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 8.Loberg RD, Tantivejkul K, Craig M, Neeley CK, Pienta KJ. PAR1-mediated RhoA activation facilitates CCL2-induced chemotaxis in PC-3 cells. J Cell Biochem. 2007;101:1292–300. doi: 10.1002/jcb.21252. [DOI] [PubMed] [Google Scholar]
- 9.Lu Y, Cai Z, Xiao G, Liu Y, Keller ET, Yao Z, Zhang J. CCR2 expression correlates with prostate cancer progression. J Cell Biochem. 2007;101:676–85. doi: 10.1002/jcb.21220. [DOI] [PubMed] [Google Scholar]
- 10.Sharma RA, Gescher AJ, Steward WP. Curcumin: the story so far. Eur J Cancer. 2005;41:1955–68. doi: 10.1016/j.ejca.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 11.Hsing AW, Tsao L, Devesa SS. International trends and patterns of prostate cancer incidence and mortality. Int J Cancer. 2000;85:60–7. doi: 10.1002/(sici)1097-0215(20000101)85:1<60::aid-ijc11>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 12.Quinn M, Babb P. Patterns and trends in prostate cancer incidence, survival, prevalence and mortality. Part I: international comparisons. BJU Int. 2002;90:162–73. doi: 10.1046/j.1464-410x.2002.2822.x. [DOI] [PubMed] [Google Scholar]
- 13.Dorai T, Gehani N, Katz A. Therapeutic potential of curcumin in human prostate cancer-I. curcumin induces apoptosis in both androgen-dependent and androgen-independent prostate cancer cells. Prostate Cancer Prostatic Dis. 2000;3:84–93. doi: 10.1038/sj.pcan.4500399. [DOI] [PubMed] [Google Scholar]
- 14.Takahara N, Kashiwagi A, Maegawa H, Shigeta Y. Lysophosphatidylcholine stimulates the expression and production of MCP-1 by human vascular endothelial cells. Metabolism. 1996;45:559–64. doi: 10.1016/s0026-0495(96)90024-4. [DOI] [PubMed] [Google Scholar]
- 15.Wang G, Siow YL, O K. Homocysteine stimulates nuclear factor kappaB activity and monocyte chemoattractant protein-1 expression in vascular smooth-muscle cells: a possible role for protein kinase C. Biochem J. 2000;352(Pt 3):817–26. [PMC free article] [PubMed] [Google Scholar]
- 16.Wu Y, Wu G, Qi X, Lin H, Qian H, Shen J, Lin S. Protein kinase C beta inhibitor LY333531 attenuates intercellular adhesion molecule-1 and monocyte chemotactic protein-1 expression in the kidney in diabetic rats. J Pharmacol Sci. 2006;101:335–43. doi: 10.1254/jphs.fp0050896. [DOI] [PubMed] [Google Scholar]
- 17.Herman JG, Meadows GG. Increased class 3 semaphorin expression modulates the invasive and adhesive properties of prostate cancer cells. Int J Oncol. 2007;30:1231–8. [PubMed] [Google Scholar]
- 18.Chua PK, Melish ME, Yu Q, Yanagihara R, Yamamoto KS, Nerurkar VR. Elevated levels of matrix metalloproteinase 9 and tissue inhibitor of metalloproteinase 1 during the acute phase of Kawasaki disease. Clin Diagn Lab Immunol. 2003;10:308–14. doi: 10.1128/CDLI.10.2.308-314.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hong JH, Ahn KS, Bae E, Jeon SS, Choi HY. The effects of curcumin on the invasiveness of prostate cancer in vitro and in vivo. Prostate Cancer Prostatic Dis. 2006;9:147–52. doi: 10.1038/sj.pcan.4500856. [DOI] [PubMed] [Google Scholar]
- 20.Khar A, Ali AM, Pardhasaradhi BV, Varalakshmi CH, Anjum R, Kumari AL. Induction of stress response renders human tumor cell lines resistant to curcumin-mediated apoptosis: role of reactive oxygen intermediates. Cell Stress Chaperones. 2001;6:368–76. doi: 10.1379/1466-1268(2001)006<0368:iosrrh>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim SJ, Hellerstein MK. Pharmacological doses of dietary curcumin increase colon epithelial cell proliferation in vivo in rats. Phytother Res. 2007;21:995–8. doi: 10.1002/ptr.2053. [DOI] [PubMed] [Google Scholar]
- 22.Loberg RD, Ying C, Craig M, et al. Targeting CCL2 with systemic delivery of neutralizing antibodies induces prostate cancer tumor regression in vivo. Cancer Research. 2007;67:9417–24. doi: 10.1158/0008-5472.CAN-07-1286. [DOI] [PubMed] [Google Scholar]
- 23.Kim YS, Ahn Y, Hong MH, et al. Curcumin attenuates inflammatory responses of TNF-alpha-stimulated human endothelial cells. J Cardiovasc Pharmacol. 2007;50:41–9. doi: 10.1097/FJC.0b013e31805559b9. [DOI] [PubMed] [Google Scholar]
- 24.Amoli MM, Mousavizadeh R, Sorouri R, Rahmani M, Larijani B. Curcumin inhibits in vitro MCP-1 release from mouse pancreatic islets. Transplant Proc. 2006;38:3035–8. doi: 10.1016/j.transproceed.2006.08.172. [DOI] [PubMed] [Google Scholar]
- 25.Kumar MA, Krishna M. In vivo modulation of signaling factors involved in cell survival. J Radiat Res (Tokyo) 2004;45:491–5. doi: 10.1269/jrr.45.491. [DOI] [PubMed] [Google Scholar]
- 26.Zen K, Masuda J, Sasaguri T, Kosaka C, Ogata J. Gene expression of monocyte chemoattractant protein-1 in human monocytes is regulated by cell density through protein tyrosine kinase and protein kinase C. Exp Cell Res. 1994;215:172–9. doi: 10.1006/excr.1994.1329. [DOI] [PubMed] [Google Scholar]
- 27.Porreca E, Di Febbo C, Reale M, et al. Monocyte chemotactic protein 1 (MCP-1) is a mitogen for cultured rat vascular smooth muscle cells. J Vasc Res. 1997;34:58–65. doi: 10.1159/000159202. [DOI] [PubMed] [Google Scholar]
- 28.Carnevale KA, Cathcart MK. Protein kinase C beta is required for human monocyte chemotaxis to MCP-1. Journal of Biological Chemistry. 2003;278:25317–22. doi: 10.1074/jbc.M304182200. [DOI] [PubMed] [Google Scholar]
- 29.Pai R, Ha H, Kirschenbaum MA, Kamanna VS. Role of tumor necrosis factor-alpha on mesangial cell MCP-1 expression and monocyte migration: mechanisms mediated by signal transduction. J Am Soc Nephrol. 1996;7:914–23. doi: 10.1681/ASN.V76914. [DOI] [PubMed] [Google Scholar]
- 30.Lin WW, Chen BC. Distinct PKC isoforms mediate the activation of cPLA2 and adenylyl cyclase by phorbol ester in RAW264.7 macrophages. Br J Pharmacol. 1998;125:1601–9. doi: 10.1038/sj.bjp.0702219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Szijgyarto Z, Szucs K, Kovacs I, Zakany R, Sipka S, Gergely P. The role of protein kinase C isoenzymes in the regulation of calcineurin activity in human peripheral blood mononuclear cells. Int J Mol Med. 2007;20:359–64. [PubMed] [Google Scholar]
- 32.Koivunen J, Aaltonen V, Koskela S, Lehenkari P, Laato M, Peltonen J. Protein kinase C alpha/beta inhibitor Go6976 promotes formation of cell junctions and inhibits invasion of urinary bladder carcinoma cells. Cancer Res. 2004;64:5693–701. doi: 10.1158/0008-5472.CAN-03-3511. [DOI] [PubMed] [Google Scholar]
- 33.Orlichenko LS, Radisky DC. Matrix metalloproteinases stimulate epithelial-mesenchymal transition during tumor development. Clin Exp Metastasis. 2008;25:593–600. doi: 10.1007/s10585-008-9143-9. [DOI] [PubMed] [Google Scholar]
- 34.Eugenin EA, Osiecki K, Lopez L, Goldstein H, Calderon TM, Berman JW. CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood-brain barrier: a potential mechanism of HIV-CNS invasion and NeuroAIDS. J Neurosci. 2006;26:1098–106. doi: 10.1523/JNEUROSCI.3863-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okuma T, Terasaki Y, Kaikita K, Kobayashi H, Kuziel WA, Kawasuji M, Takeya M. C-C chemokine receptor 2 (CCR2) deficiency improves bleomycin-induced pulmonary fibrosis by attenuation of both macrophage infiltration and production of macrophage-derived matrix metalloproteinases. J Pathol. 2004;204:594–604. doi: 10.1002/path.1667. [DOI] [PubMed] [Google Scholar]
- 36.Woo MS, Jung SH, Kim SY, Hyun JW, Ko KH, Kim WK, Kim HS. Curcumin suppresses phorbol ester-induced matrix metalloproteinase-9 expression by inhibiting the PKC to MAPK signaling pathways in human astroglioma cells. Biochemical and Biophysical Research Communications. 2005;335:1017–25. doi: 10.1016/j.bbrc.2005.07.174. [DOI] [PubMed] [Google Scholar]
- 37.Ishizuka T, Sawada S, Sugama K, Kurita A. Thromboxane A2 (TXA2) receptor blockade suppresses monocyte chemoattractant protein-1 (MCP-1) expression by stimulated vascular endothelial cells. Clin Exp Immunol. 2000;120:71–8. doi: 10.1046/j.1365-2249.2000.01169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shankar S, Ganapathy S, Chen Q, Srivastava RK. Curcumin sensitizes TRAIL-resistant xenografts: molecular mechanisms of apoptosis, metastasis and angiogenesis. Mol Cancer. 2008;7:16. doi: 10.1186/1476-4598-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin CW, Hou WC, Shen SC, Juan SH, Ko CH, Wang LM, Chen YC. Quercetin inhibition of tumor invasion via suppressing PKC delta/ERK/AP-1-dependent matrix metalloproteinase-9 activation in breast carcinoma cells. Carcinogenesis. 2008;29:1807–15. doi: 10.1093/carcin/bgn162. [DOI] [PubMed] [Google Scholar]
- 40.Himelstein BP, Lee EJ, Sato H, Seiki M, Muschel RJ. Transcriptional activation of the matrix metalloproteinase-9 gene in an H-ras and v-myc transformed rat embryo cell line. Oncogene. 1997;14:1995–8. doi: 10.1038/sj.onc.1201012. [DOI] [PubMed] [Google Scholar]
- 41.Di Giulio C, Rapino M, Zingariello M, Antonucci A, Cataldi A. PKC alpha-mediated CREB activation is oxygen and age-dependent in rat myocardial tissue. Histochem Cell Biol. 2007;127:327–33. doi: 10.1007/s00418-006-0245-7. [DOI] [PubMed] [Google Scholar]
- 42.Chen Y, Shu W, Chen W, Wu Q, Liu H, Cui G. Curcumin, both histone deacetylase and p300/CBP-specific inhibitor, represses the activity of nuclear factor kappa B and Notch 1 in Raji cells. Basic Clin Pharmacol Toxicol. 2007;101:427–33. doi: 10.1111/j.1742-7843.2007.00142.x. [DOI] [PubMed] [Google Scholar]