Abstract
Genome amplification methods such as polymerase chain reaction (PCR) have revolutionized our ability to detect viruses in spinal fluids of patients with neurologic diseases. It is not as well appreciated among clinicians that PCR protocols, quality assurance, and technical expertise vary significantly among laboratories. In a multi-laboratory blinded study of herpes simplex virus PCR, the most widely used and best validated CSF PCR assay, low-level positives were often missed and false positives were not uncommon [Schloss L, van Loon AM, Cinque P, Cleator G, Echevarria JM, Falk KI, et al. An international external quality assessment of nucleic acid amplification of herpes simplex virus. J Clin Virol 2003;28(2):175–85]. In addition, genome variability and mutations, which are increasingly recognized for a number of different viruses, can lead to falsely low or negative results. Both clinicians and laboratories must recognize the limitations of PCR, since misleading results may have serious consequences.
We present here a case of a rapidly progressive, fatal neurologic illness in a young mother, whose CSF JCV DNA PCR at a reference laboratory was falsely negative. Ultimately, brain biopsy established the diagnosis of progressive multifocal leukoencephalopathy (PML). Repeat PCR testing of the same CSF targeting a different region of the genome yielded a high positive result.
Keywords: JC virus, PML, Job’s syndrome, Hyper IgE, PCR, Polyomavirus
1. Case report
A 31-year-old woman was admitted to Yale New Haven Hospital for high-dose corticosteroid therapy and therapeutic plasma exchange after being diagnosed with rapidly progressive multiple sclerosis. Her illness began 8 weeks prior to admission, when she was 2 weeks post-partum, and presented with clumsiness of her left hand and difficulty walking. Brain MRI prior to admission had been interpreted as compatible with multiple sclerosis. CSF tests for Cryptococcus, AFB, Lyme and syphilis were negative. Her private neurologist treated her with intravenous prednisolone, but she continued to deteriorate. She had a history of hyperimmunoglobulin E or Job’s syndrome and asthma.
On admission, she was afebrile, tearful, oriented to person and place, but not date. She had left sided facial droop and left sided weakness, and could not ambulate. On the third hospital day, a repeat brain MRI was read as “consistent with the given diagnosis of multiple sclerosis, but the pattern of white matter changes was not the most typical”. Acute disseminated encephalomyelitis, PML, and vasculitis were considered and intravenous immunoglobulin therapy was initiated. On hospital day 7, spinal fluid analysis revealed oligoclonal bands, 13 nucleated cells/μL (92% lymphocytes), and normal protein and glucose. Cytology was negative for malignant cells. PCR for VZV, CMV, EBV, HSV (in-house assays), and JCV (reference laboratory) were all negative. Tests for AFB, mycology, and Cryptococcus were also negative.
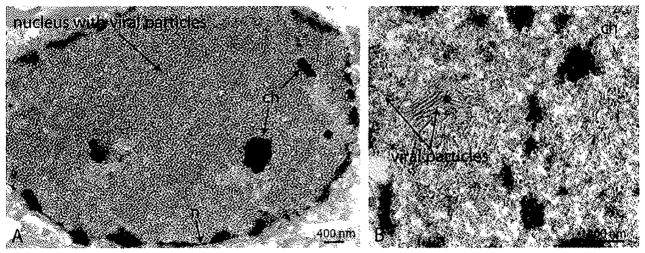
She was given seven sessions of therapeutic plasma exchange; however, treatment was terminated due to disease progression. The patient developed respiratory distress due to aspiration, was intubated and transferred to the ICU. She subsequently developed a pulmonary embolus and upper GI bleed. On hospital day 46 she developed decerebrate posturing, loss of gag and corneal reflexes and seizures. On day 49, a brain biopsy was performed. H&E staining showed demyelination, myelin-debris-laden foamy macrophages, enlarged nuclei but no definitive intranuclear inclusions in oligodendroglial cells and no bizarre astrocytes. Polyomavirus particles were observed on EM (Fig. 1). On hospital day 58, the patient was transferred to the Medical Service for comfort care. CD4 cells which were 303 μL−1 on hospital day 23, had declined to 113 μL−1 on hospital day 60 (normal range 479–1210 μL−1). The patient expired on hospital day 61, approximately 5 months after the onset of symptoms.
Fig. 1.
Electron micrograph of frontal brain biopsy, (A) Nucleus of an oligodendrocyte packed with viral particles, with only few fragments of nuclear chromatin (ch) remaining. (B) Higher magnification reveals “spaghetti and meatball” arrangement, which is characteristic of polyomavirus nuclear assembly. Abbreviations: n, Nuclear membrane, (scale bars 400 nm).
After the patient’s death, the physician contacted the laboratory to obtain HIV testing. Both HIV-1/2 antibody and HIV-1 p24 antigen tests performed on pre-mortem serum samples were negative. Once the laboratory became aware of the discrepancy between the pathologic findings and the negative CSF PCR, a second aliquot of the spinal fluid from hospital day 7 and slides from the brain biopsy were sent to Dr. Eugene Major’s laboratory at the NIH to test for polyomaviruses JC, BK and SV40, It was hypothesized that a different polyomaviras might explain the negative JCV PCR. Surprisingly, the spinal fluid was found to contain high amounts of JCV DNA by PCR targeting the large T antigen gene (70,000 copies/mL CSF). In situ hybridization at the NINDS laboratory confirmed JCV DNA in the brain tissue. The original reference laboratory was notified and asked to retest the sample, which they again found to be negative with their standard assay, which targeted VPI gene. However, after setting up a new assay targeting JCV large T antigen gene, they too confirmed high titers of JCV DNA in the same CSF sample that they had previously tested twice and reported as negative.
The case presented here is only the second case of PML reported in Job’s syndrome, or hyperimmunoglobulin E syndrome, a primary immunodeficiency characterized by the clinical triad of recurrent staphylococcal abscesses, recurrent pneumonia, and an elevated serum IgE level.1 The etiology is unresolved but recent research implicates a skewed T helper 1 (Th1) cell/Th2 cell ratio and alterations in chemokines. Patients often succumb to pulmonary infections or lymphoma.
The first case reported of PML in Job’s syndrome was of an 8-year-old boy who developed progressive hemiparesis, hyporeflexia and apathy in the face of declining CD4 counts.2 He was treated with methylprednisolone for presumed para-infectious encephalitis, but continued to deteriorate and developed seizures. He was treated with cidofovir with no effect and died 5 months after onset.
In our patient, neurologic symptoms developed post-partum and high does of steroids were administered. One case of PML in a presumed normal host in pregnancy has been reported.3 However, evidence of prior immune dysfunction in our patient was plentiful. In addition to typical recurrent bacterial pulmonary and skin infections, she had developed recurrent in situ carcinoma of both vulva and cervix due to human papillomavirus (HPV), and squamous cell carcinoma of the finger. Similar to the other case of PML in Job’s syndrome, her CD4 count declined from 303 to 113 μL−1 over the hospital admission for unclear reasons. PML is most commonly seen in HIV-1 infected patients with low CD4 counts and, unless immunosuppression can be reversed, deterioration is progressive with death in 6 months.4 PML in patients with idiopathic CD4 lymphocytopenia have also been reported.5
Despite the initial diagnosis of multiple sclerosis, PML was considered and CSF PCR was ordered on the 7th hospital day, but the test was negative. A negative PCR early in the course of PML can occur due to low levels of DNA in the CSF. Therefore, testing of a subsequent CSF should be considered if suspicion is high. In addition, CSF PCR assays are not standardized, vary tremendously in sensitivity between laboratories, and may miss low levels of virus.6 In this case the levels of JCV DNA were very high in the initial sample.
When PCR assays are developed for clinical use, conserved areas of the genome are chosen. However, this information is based on a limited number of sequenced genomes and is continually changing. The initial reference laboratory used the VP1 gene as target.7 This test had performed satisfactorily for years to their knowledge, detecting several positive samples each week. The NIH laboratory used a TaqMan PCR assay with the large T antigen as the gene target, since the amino-terminal end of large T antigen is now known to be more highly conserved than VPI.8 However, the potential for missing viral variants is present for any assay using one set of primers and probe, especially if the viral genome demonstrates variability in some genomic locations.
A related polyomavirus, BK virus, causes nephropathy and graft loss in renal transplant recipients and has been shown to have greater genome variability than previously recognized.9 When seven published quantitative BKV PCR assays were compared, significant discrepancies were found in 20% of the clinical samples tested. Consequently, a multiplex PCR with multiple primers and probes targeting both VPI and large T antigen regions has been developed to compensate for genome variability.10 It is possible that a multiplex assay targeting both VP1 and large T antigen may also be indicated for JCV in CSF, however, this has not yet been studied.
In summary, we have presented a case of a young woman with Job’s syndrome and PML to highlight the potential for false negative JCV PCR results despite high levels of virus, due to genome variability. As molecular testing is more widely used, more problems can be expected to emerge. Laboratories should be alert to this possibility and encourage clinicians to provide feedback on missed diagnoses to laboratories. If suspicion remains high, clinicians should communicate with the laboratory and a PCR assay targeting a different area of the genome performed, or a tissue diagnosis obtained if appropriate. Even when the ultimate outcome is unchanged, delayed or missed diagnoses can lead to unnecessary tests, potentially harmful therapies, delayed comfort care, and undue anguish for families, the patient, and the healthcare team.
Footnotes
Conflict of interest
The authors have no conflicts of interest.
References
- 1.Grimbacher B, Holland SM, Puck JM. Hyper-IgE syndromes. Immunol Rev. 2005;203:244–50. doi: 10.1111/j.0105-2896.2005.00228.x. [DOI] [PubMed] [Google Scholar]
- 2.Angelini L, Pietrogrande MC, Delle Piane MR, Zibordi F, Cinque P, Maccagnano C, et al. Progressive multifocal leukoencephalopathy in a child with hyperimmunoglobulin E recurrent infection syndrome and review of the literature. Neuropediatrics. 2001;32(5):250–5. doi: 10.1055/s-2001-19119. [DOI] [PubMed] [Google Scholar]
- 3.Rosas MJ, Simoes-Ribeiro F, An SF, Sousa N. Progressive multifocal leukoen-cephalopathy: unusual MRI findings and prolonged survival in a pregnant woman. Neurology. 1999;52(3):657–9. doi: 10.1212/wnl.52.3.657. [DOI] [PubMed] [Google Scholar]
- 4.Marzocchetti A, Sanguinetti M, Giambenedetto SD, Cingolani A, Fadda G, Cauda R, et al. Characterization of JC virus in cerebrospinal fluid from HIV-1 infected patients with progressive multifocal leukoencephalopathy: insights into viral pathogenesis and disease prognosis. J Neurovirol. 2007;13(4):338–46. doi: 10.1080/13550280701381324. [DOI] [PubMed] [Google Scholar]
- 5.Haider S, Nafziger D, Gutierrez JA, Brar I, Mateo N, Fogle J. Progressive multifocal leukoencephalopathy and idiopathicCD4+lymphocytopenia: a case report and review of reported cases. Clin Infect Dis. 2000;31(4):E20–2. doi: 10.1086/318120. [DOI] [PubMed] [Google Scholar]
- 6.Schloss L, van Loon AM, Cinque P, Cleator G, Echevarria JM, Falk Kl, et al. An international external quality assessment of nucleic acid amplification of herpes simplex virus. J Clin Virol. 2003;28(2):175–85. doi: 10.1016/s1386-6532(03)00003-9. [DOI] [PubMed] [Google Scholar]
- 7.McGuire D, Barhite S, Hollander H, Miles M. JC virus DNA in cerebrospinal fluid of human immunodeficiency virus-infected patients: predictive value for progressive multifocal leukoencephalopathy. Ann Neurol. 1995;37(3):395–9. doi: 10.1002/ana.410370316. [DOI] [PubMed] [Google Scholar]
- 8.Ryschkewitsch C, Jensen P, Hou J, Fahle G, Fischer S, Major EO. Comparison of PCR-southern hybridization and quantitative real-time PCR for the detection of JC and BK viral nucleotide sequences in urine and cerebrospinal fluid. J Virol Methods. 2004;121(2):217–21. doi: 10.1016/j.jviromet.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 9.Atienza E, Cook L, Hoffman NG, Jerome KR. Evaluation of seven real-time PCR assays for urine BK virus quantitation. 23rd Annual Clinical Virology Symposium; 2007. [Google Scholar]
- 10.Cook LEA, Hoffman N, Jerome KR. A new BK PCR assay with improved detection of all recently described variant genotypes. 24th Annual Clinical Virology Symposium; 2008. [Google Scholar]