Abstract
Objective
1) To determine whether JC virus (JCV) DNA was present in the cerebrospinal fluid (CSF) and blood from patients with multiple sclerosis (MS) in comparison with controls and 2) to find out if our clinical material, based on presence of JCV DNA, included any patient at risk for progressive multifocal leukoencephalopathy (PML).
Methods
The prevalence of JCV DNA was analyzed in CSF and plasma from 217 patients with MS, 86 patients with clinically isolated syndrome (CIS), and 212 patients with other neurological diseases (OND). In addition, we analyzed CSF cells, the first report of JCV DNA in CSF cells in a single sample, and peripheral blood cells in a subgroup of MS (n = 49), CIS (n = 14) and OND (n = 53).
Results
A low copy number of JCV DNA was detected in one MS cell free CSF sample and in one MS CSF cell samples. None of these had any signs of PML or developed this disease during follow-up. In addition, two OND plasma samples were JCV DNA positive, whereas all the other samples had no detectable virus.
Conclusion
A low copy number of JCV DNA may occasionally be observed both in MS and other diseases and may occur as part of the normal biology of JC virus in humans. This study does not support the hypothesis that patients with MS would be at increased risk to develop PML, and consequently screening of CSF as a measurable risk for PML is not useful.
Keywords: cerebrospinal fluid, JC virus DNA, multiple sclerosis, progressive multifocal, leukoencephalopathy
Introduction
The interest in progressive multifocal leukoencephalopathy (PML) in non-immune suppressed patients has increased because the report of this disease in three of over 3000 patients treated with natalizumab (anti-VLA4; Tysabri, Biogenidec, Cambridge, Massachusetts, USA) [1]. Natalizumab is regarded as an effective multiple sclerosis (MS) treatment [2] with more than 30,000 patients with MS currently treated (http://www.biogenidec.com). PML is a fatal demyelinating disease caused by the polyomavirus JC virus (JCV) [3,4], Histopathdogically, PML is characterized by a triad of pathologic features: demyelination, giant bizarre astrocytes, and oligodendrocytes with nuclear inclusion bodies [4,5]. In the normal population, infection with JCV usually occurs asymptomatically early in childhood resulting in a seroprevalence of 50–90% in the adult population [6,7]. After infection, the virus establishes latency in kidneys, lymphoid tissues, and bone marrow [8,9]. The control of JCV infection is disrupted under conditions of suppression of cell-mediated immunity and the virus can get reactivated and emerge from latency and cause PML in the CNS. It is not fully understood how the virus penetrates the blood brain barrier (BBB) but it is believed to transit through B-lymphocytes or as free virus [5,10,11]- JCV has a unique capability for targeting macroglial cells. Because of its capacity for latency, persistence, and tropism for oligodendrocytes, JCV has been questioned for a role in the pathogenesis of MS [12], In view of the aberrant immune functions in MS, higher rates of reactivated JCV in persons with MS compared with healthy individuals can be hypothesized. Moreover, if subgroups of treatment naive persons with MS have JCV DNA in cerebrospinal fluid (CSF) or blood, such subgroups would be considered to have a higher risk of developing PML and therefore should be excluded from treatment with natalizumab. Furthermore, and in general, because not only natalizumab but also a series of upcoming other treatments for MS potently intervene in immune functions, it is of major importance to increase our knowledge about the reactivation and mechanisms of JCV infection. As a primary goal, we should get knowledge on JCV expression patterns in MS compared with controls. Detection of JCV DNA by PCR analysis of the CSF is proposed to be diagnostic for PML [13,14]. Previous reports on detection of JCV DNA in CSF among patients with MS are discordant. In 1998, Ferrante, et al [15] analyzed 121 patients with MS and detected JCV DNA in CSF in 9% compared with 0% in the control group. However, the same year, Bogdanovic, et al, [16] did not find any positive sample of JCV DNA in CSF from 45 patients with MS. In a recent study, a frequency of 4.7% JCV DNA in CSF among 43 patients with MS was detected at their first demyelinating event [17]. Franciotta, et al. [18] investigated 54 patients with MS but failed to detect JCV DNA in CSF in any of them. Detection of JCV DNA was also attempted in natalizumab-treated patients with MS; 329 CSF samples were analyzed and all of them where negative but five of 214 plasma samples were positive [1]. Concerning detection of JCV DNA in blood, several studies report no detection of JCV DNA from healthy controls [18–20], whereas other groups found detection of JCV DNA in both healthy controls and immunoimpaired patients [21,22]. In patients with MS, some studies report JCV in peripheral blood mononuclear cells (PBMCs) without any difference in frequency from control groups [15,23,24], whereas others [18] did not find any JCV DNA in blood from patients with MS or controls. Recent investigations have also been performed on the potential effect of treatment with interferon-β on JCV DNA detection in PBMC: S Delbue [23] found a significantly lower detection (13.6%) of JCV DNA in interferon-β-treated patients compared with untreated patients (46.1%). These results were not confirmed in another study [24] where the JCV DNA detection was 6.8% in both interferon-β-treated and -untreated patients with MS. Certainly, there remain significant differences in findings on the presence of JCV in clinical samples. Consequently, we evaluated a series of CSF and blood samples and report results from a large number of untreated patients with MS and controls including 217 patients with MS, 86 patients with clinically isolated syndrome (CIS), and 212 patients with other neurological diseases (OND). In addition, we also tested for the presence of JCV DNA in CSF cells from individuals with MS (n = 42), CIS (n = 14), and OND (n = 53).
Material and methods
Samples
In total, 505 cell free CSF samples, 458 plasma samples, 109 CSF cells samples, and 116 PBMCs samples (Table 1) were obtained from a biobank at the Department of Neurology, Karolinska University Hospital, Stockholm, The CSF samples were collected from patients having undergone diagnostic lumbar punctures from year 2001 and 2006. These samples have been aliquot and stored at −80 °C, coded, and made anonymous in accordance with the Swedish research council guidelines on the ethical use of biological specimen collections in clinical research. A total of 446 paired CSF and plasma samples were available. An additional 109 CSF cells and 116 PBMCs samples were included in the analysis.
Table 1.
Samples analyzed in different groups
| MS (n = 217) |
OND (n = 212) |
||||||
|---|---|---|---|---|---|---|---|
| CIS | RRMS | SPMS | PPMS | Devic | Non.INF | INF | |
| Total no. of patients | 86 | 175 | 34 | 8 | 1 | 116 | 96 |
| Paired CSF and plasma | 69 | 150 | 27 | 7 | 1 | 100 | 92 |
| Paired CSF, CSF cells, plasma and PBMCs | 14 | 20 | 19 | 2 | 0 | 23 | 30 |
| Cell-free CSF | 86 | 173 | 29 | 8 | 1 | 115 | 93 |
| Plasma | 69 | 152 | 33 | 7 | 1 | 101 | 95 |
| CSF cells | 14 | 21 | 19 | 2 | 0 | 23 | 30 |
| PBMCs | 14 | 22 | 25 | 2 | 0 | 23 | 30 |
MS, multiple sclerosis; n, number of patients; CIS, clinically isolated syndrome suggestive of MS; RRMS, relapsing–remitting MS; SPMS, secondary progressive MS; PPMS, primary progressive MS; OND, other neurological disease with (INF) or without (Non.INF) signs of inflammation; PBMCs, peripheral blood mononudear cells.
Patients
The MS cohorts who were defined clinically definite MS according to the revised McDonald criteria [25], consisted of 175 relapsing-remitting MS (RRMS), 34 secondary progressive MS (SPMS), and 8 primary progressive (PPMS). Eighty-six patients had CIS, that is, patients who had their first clinical relapse with one or more magnetic resonance imaging (MRI) lesions characteristic to MS [25], and one patient had Devics disease (neuromyelitis optica). The control group consisted of 212 OND (116 without any sign of inflammation and 96 with signs of inflammation [OND.INF]). More detailed characteristics of the clinical material are given in Table 2. The MS disease activity was characterized based on clinical evaluation (remission or relapse) and by the Kurtzke Expanded Disability Status Scale (EDSS) at the time of sampling. Eighteen percent of the patients with RRMS were sampled during relapse. EDSS varied highly but the median EDSS was 2.0 in RRMS, 5.5 in SPMS, 4.0 in PPMS, and 1.5 in the CIS group.
Table 2.
Patients’ characteristics
| MS (n = 217) |
OND (n = 212) |
||||||
|---|---|---|---|---|---|---|---|
| CIS | RRMS | SPMS | PPMS | Devic | Non.INF | INF | |
| Total no. of patients | 86 | 175 | 34 | 8 | 1 | 116 | 96 |
| Female/male | 63/23 | 127/48 | 22/12 | 4/4 | 1/0 | 79/37 | 64/32 |
| Age, median (IQR) | 35 (29–42) | 38 (30–46) | 53 (45–62) | 50 (38–56) | 32 | 44 (33–53) | 52 (40–61) |
| EDSS, median (IQR) | 1.5 (1.0–2.0) | 2 (1.0–2.5) | 5.5 (4.5–6.0) | 4 (3.0–4.0) | 2.0 | n.a. | n.a. |
| Disease duration, years, median (IQR) | 2 (1–2) | 1 (1–2) | 8 (2–17.3) | 3 (2–7.5) | 1 | - | - |
| % sampled during relapse | 22 | 18 | n.a. | n.a. | n.a. | n.a. | n.a. |
| % OCB positive | 68 | 85 | 81 | 100 | 100 | 0 | 8.3 |
| CSF mononuclear cells (cells/μL), median(IQR) | 3 (1–8) | 5 (2–10) | 3(1–5) | 3(0.8–4.3) | 4 | 1 (1–2.3) | 2 (1–4) |
| CSF protein (g/L), median (IQR) | 0.4 (0.3–0.5) | 0.5 (0.4–0.6) | 0.5 (0.5–0.7) | 0.4 (0.4–0.6) | 0.2 | 0.4 (0.3–0.5) | 0.5 (0.4–0.6) |
MS, multiple sclerosis; n, number of patients; CIS, clinically isolated syndrome suggestive of MS; RRMS, relapsing–remitting MS; SPMS, secondary progressive MS; PPMS, primary progressive MS; OND, other neurological disease; OND.INF, other neurological disease with signs of inflammation; EDSS, Expanded Disability Scale; IQR, Inter Quartile Range; OCB, oligoclonal bands; n.a., not applicable.
Patients defined as OND had a wide range of different diagnoses; unspecific sensory symptoms (n = 33), psychosis (n = 14), vertigo (n = 10), tension headache (n = 7), unspecific white matter lesions (n = 6), migraine (n = 4), dissociate paralysis (n = 4), spinal stenosis (n = 3), ALS (n = 3), neuralgia (n = 3), polyneuropathy (n = 2), neurasthenia (n = 2), neuromuscular bladder disturbance (n = 2), communicating hydrocephalus (n = 2), postcommotio syndrome (n = 2), cerebral vascular disease (n = 2), epilepsy (n = 1), muscle pain (n = 1), pseudo tumor cerebri (n = 1), Parkinson’s disease (n = 1), vestibular neuritis (n = 1), hereditary spastic paraparesis (n = 1), benign intracranial hypertension (n = 1), Arnold chiari 1 anomaly (n = 1), Horners syndrome (n = 1), diplopia/striabism (n = 1), dischernia (n = 1), B12-deficiency (n = 1), meningeoma (n = 1), cervical ependymoma (n = 1), N, Pudendus damage (n = 1). The OND.INF group consisted of postpolio syndrome (n = 42), SLE (n = 23), herpes encephalitis (n = 10), cerebral vasculitis (n = 2), Lyme disease (n = 3), transverse myelitis (n = 3), infliximab-treated RA with neurological symptoms (n = 3), sclerodermia (n = 1), unspecific encephalomyelitis (n = 1), neurosarcoidosis (n = 1), encephalitis post-vaccination (n = 1), bacterial meningitis (n = 1), mild viral meningitis (n = 1), genital herpes and lumbago (n = 1), sensory neural deafness and ulcerative colitis (n = 1). At the time of sampling, 26 patients with MS were on immunomodulating or immunosupressive treatments; IFN-β1b (n = 14), IFN-β1a (n = 3), cyclosphosphamide (n = 2), methotrexate (n = 2), azathioprine (n = 2), immune globulin (n = 1), prednisolone (n = 1), mitoxantrone (n = 1). In the OND.INF group, 30 patients were on immunosuppressive treatments; prednisolone (n = 14), azathioprine (n = 5), chloroquine phosphate (n = 2), methotrexate (n = 2), etanercept (n = 2), cyclosphosphamide (n = 2), hydroxychloroquine (n = 2), and infliximab (n = 1).
Routine CSF examinations
The mean numbers of mononuclear cells in the CSF samples were 5 cells/μL in patients with MS, 3 cells/μL in patients with CIS, 1 cell/μL in patients with OND, and 2 cells/μL in patients with OND.INF. Oligoclonal bands in the CSF were recorded in 87% of the patients with MS, 0.0% of the patients with OND, and 8.3% of the patients with OND.INF (Table 2).
Preparation of CSF, CSF cells, plasma, and PBMCs
Blood and CSF samples were collected and processed within 1 h. The peripheral blood was collected in sodium citrate-containing cell preparation tubes (Vacutainer CPT; BD Biosciences, San Jose, California, USA) and EDTA tubes. The CSF was collected in siliconized glass tubes. CSF samples were immediately centrifuged and the cell pellet was recovered. The CSF supernatants were batched and stored at −80 °C. PBMCs were separated by density gradient centrifugation. Cells from the interphase were collected and washed twice with Dulbeccos’s PBS. The plasma was recovered by centrifugation at 1500 × g for 15 min. The samples were stored at −80 °C until use.
PCR analysis
Cell free CSF, CSF cells, plasma, and PBMCs samples were tested for JCV DNA at the NIH laboratory with extensive experience in the use of assays described previously [1,26]. The nucleic-acid extraction was performed with QIAamp viral RNA mini-kit (Ojagen Inc., Valencia, California, USA). A starting volume of 200 μL of the sample was extracted and eluted to 50 μL. All samples were assayed in duplicate with the use of 10 μL of DNA template by polymerase chain reaction (PCR) using an ABI 7500 sequence-detection system (Applied Biosystems, Foster City, California, USA). The viral genomic locations of the primer and probe, the cycling conditions, and the standard curve design have been described previously [26]. In general, the assay uses the Taq-Man polymerase, a total of 40 cycles, and a read out of copy number determined from a set cross-over of a threshold based on control standards for each assay. The level of sensitivity of the assay, validated and certified through independent review under United States CLIA regulations, is adjusted to volume dilution at 25 copies/mL.
EIA titer (JCV)
The presence of IgG antibodies to JCV was tested using a hybrid JCV construct, JCV-SVE (Δ) (a JCV-simian virus 40 (SV40) strain that has a ligated regulatory sequence from SV40 into the regulatory region of JCV [27]), as antigen. The optimum concentrations of JCV antigen and the respective reagents used to perform enzyme immunoassay (EIA) techniques were determined by block titration as described previously [27]. EIA was performed in 96-well, flat-bottom, Immulon-4 microtiter plates (Dynatech Laboratories, Chantilly, Virginia, USA). Coating solution, washing solution, blocking buffer, sample diluent solution, and other reagents specified were purchased from Kirkegaard and Perry Laboratories, Gaithersburg, Maryland, USA, and used in accordance with the manufacturer’s instructions. Sample absorbance was measured at 450 nm with a SpectraMAX Plus spectrophotometer (Molecular Devices Corp., Sunnyvale, California, USA). Serum samples with an absorbance of 0.05 greater than that of serum controls were considered positive. EIA titers of ≥640 were considered positive, titers of 640–2560 were counted as low, >2560 to 10,240 as moderate, and ≥10,240 as high titers. Positive controls were derived from individuals known to be infected with JCV. Negative controls were derived from pediatric patients <3 years old. All EIA positive controls tested positive and all the negative controls tested negative.
Results
Detection and quantification of JCV DNA
Detection of JCV DNA in duplicates of each sample was defined as “detectable” samples. The results are shown in Tables 3 and 4.
Table 3.
Patients with detectable copy numbers of JCV DNA in their samples
| Patient no. | Diagnosis | Specific diagnosis | Plasma | PBMCs | Cell-free CSF | CSF cells |
|---|---|---|---|---|---|---|
| 1 | MS | SPMS | Undet. | Undet. | 103 | Undet. |
| 2 | MS | RRMS remission | Undet. | Undet. | Undet. | 25 |
| 3 | OND.INF | SLE | 1123 | Undet. | Undet. | Undet. |
| 4 | OND.INF | SLE | 409 | Undet. | Undet. | Undet. |
Number of copies/mL in duplicate samples.
Undet, undetectable; PBMCs, peripheral blood mononuclear cells; RRMS, relapsing-remitting MS; SPMS, secondary progressive MS; OND, other neurological disease; OND.INF, other neurological disease with sign of inflammation; SLE, systemic lupus erythematosus.
Table 4.
Number of samples with detectable JCV copies in duplicates
| Samples | MS patients | CIS patients | Control patients | Total |
|---|---|---|---|---|
| Plasma | 0/192 (0.0%) | 0/69 (0.0%) | 2/196 (1.0%) | 2/458 (0.4%) |
| Cell-free CSF | 1/210 (0.5%) | 0/86 (0.0%) | 0/208 (0.0%) | 1/505 (0.2%) |
| CSF cells | 1/42 (2.4%) | 0/14 (0.0%) | 0/53 (0.0%) | 1/109 (0.9%) |
| PBMCs | 0/49 (0.0%) | 0/14 (0.0%) | 0/53 (0.0%) | 0/116 (0.0%) |
Patients with MS include relapsing–remitting MS, secondary progressive MS, and primary progressive MS.
CIS, clinically isolated syndrome suggestive of MS; control patients, patients with other neurological diseases with or without sign of inflammation.
JCV DNA in cell-free CSF
1/210 (0.5%) of the patients with MS had detectable JCV with a viral load of 103 copies/mL. This patient was a 39-year-old male and had been diagnosed with MS 4 years before the CSF analysis was performed. At the time of sampling, he had developed secondary progressive MS and had an EDSS of 4.0. Routine laboratory data of the CSF showed: six mononuclear cells/μL, 182 mg/L albumin, presence of oligoclonal bands, and elevated IgG index. Brain MRI, 1 day before sampling, showed typical MS lesions but no signs of PML. He had no disease modifying treatment at sample time but had the same year discontinued injections with INF-β1a after 4 years of treatment. After sampling, he has been followed clinically for 24 months with a stable neurological condition and no signs of PML development.
None of the cell-free CSF samples from the OND (n = 115) or the OND.INF (n = 93) group had detectable JCV DNA in the CSF.
JCV DNA in CSF cells
1/42 (2.4%) of the CSF cell samples from patients with MS was positive for JCV DNA with a viral load of 25 copies/mL. This patient was a 44-year-old female diagnosed with relapsing-remitting MS the same year as sampling was performed. Routine laboratory data of the CSF showed: six mononuclear cells/μL, 217 mg/L albumin, presence of oligoclonal bands but normal IgG index. Her EDSS was 1.5. Cranial MRI, 1 month after CSF sampling, showed typical MS lesions but no signs of PML. She was treatment naive at sample time but has now started treatment with INF-β1b and has been followed clinically for 30 months without developing any signs of PML. Both MS patients with detectable JCV DNA, (in cell-free CSF and CSF cells, respectively), have not been retested for JCV DNA. None of the CSF cell samples from the control group, including OND (n = 23) and OND.INF (n = 30), had detectable JCV DNA.
JCV DNA in plasma
None of the 192 patients with MS tested for JCV in their plasma had detectable viral loads. In addition, all 101 samples from the OND group had non-detectable JCV DNA in their plasma but 2/95 patients in the OND.INF group, both diagnosed with SLE, had detectable JCV DNA. The first patient, a 57-year-old female with 6 years history of SLE had a viral load of 409 copies/mL. Her routine CSF laboratory work up was within normal ranges. She was examined with an MRI of her brain 1 month before the sampling, which showed no signs of PML but eight unspecific, 1–3 mm hyperintense supratentorial white matter lesions. At the time of sampling, she was treated with chloroquine phosphate. She had earlier been treated only with prednisolone. The other patient with positive JCV was a 21-year-old female with 5 years history of SLE. She had a viral load of 1123 copies/mL in her plasma. Her routine CSF laboratory work up was normal. She had ongoing treatment with prednisolone, but, 8 months before the JCV analysis was performed, had treatment with six doses of rituximab combined with cyclosphosphamide. She had also, 1 year before, been treated with azathioprine. Her neuroimaging with MRI, 2 weeks after sampling, was normal. Both patients with SLE have, after JCV analysis, been followed clinically for 31 and 25 months, respectively, without any signs of PML. They have not been retested for JCV.
All four patients listed above, with detectable JCV DNA, had detectable virus in only one body fluid compartment.
JCV DNA in PBMCs
JCV DNA was not detected in PBMCs from patients with MS (n = 49), patients with CIS (n = 14), or control patients (n = 53).
Detection of JCV antibodies in plasma and CSF
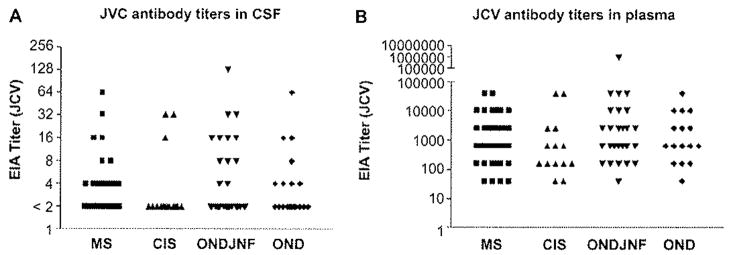
Screening for presence of JCV antibodies in CSF and plasma was performed in 49 patients with MS, 14 patients with CIS, and 53 patients with OND, None of the CSF samples showed a positive antibody titer. In plasma, 69% (80/110) of all samples showed a range of positive titer from modest to high, as usually seen. EIA titers of 640–2560 were counted as low, >2560 to 10,240 as moderate and ≥10,240 as high titers (Figure 1). The prevalence found here was in the same rate as it has been described previously in the general population [5–7]. There were no significant difference in prevalence of positive JCV antibody titer in patients with MS compared with the control group.
Figure 1.
CSF (A) and plasma (B) samples were screened for the presence of JCV antibodies from 49 patients with MS, 14 patients with CIS, and 53 patients with OND. EIA titers of 640–2560 were counted as low, >2560 to 10,240 as moderate and ≥10,240 as high titers. Detection of JCV antibodies in CSF and Plasma.
Discussion
In the large cohorts studied here, we found only occasional samples with low copy number of JCV DNA among both persons with MS, most of which were treatment naive, and OND. We thereby conclude that these groups do not differ in their JCV expression patterns in the compartments studied. Thus, these data provide no evidence in favor for persons with MS being at higher risk for PML, a result that is consistent with the clinical findings of the incidence of confirmed diagnosis of PML. Our findings are in contrast to the study from Ferrante, et al. [15], who reported positive JCV DNA in CSF from 11/121 patients with MS, but in agreement with the results from Alvarez-Lafuente, et al. [17], Bogdanovic, et al, [16], and Franciotta, et al, [18]. In the cases with very low but detectable JCV copy numbers, JCV was only found in one compartment, that is, either blood or CSF. Methodologically, we feel secure with our method using a Taq-Man real-time PCR protocol earlier validated as highly sensitive and reproducible for detecting JCV DNA [26]. We found 103 copies/ml of JCV DNA in cell-free CSF from one patient with SPMS and 25 copies/mL in CSF cells from one patient with RRMS compared with none in the control group. The two patients with MS, who developed PML after natalizumab-treatment, had detectable JCV DNA in CSF. One of the patients had a viral load of 2500 copies/mL in the CSF, 225 copies/mL in PBMCs, and 6050 copies/mL in the serum about 3 months after onset of PML symptoms and 4 months after the first PML lesion appeared on MRI scan |28], The positive CSF analysis from the other patient (the study report does not give the exact viral load of the CSF or plasma [29]) was also performed about 3 months after symptom onset and 2 months after MRI findings of PML. It would be very valuable to know at what time point JCV DNA starts to get detectable in blood and CSF before development of PML and what factors that may allow its entry into CNS resident cells and any ensuing development of PML. However, because PML is such a rare disease, longitudinal evaluation of individuals who may be at risk because of immune compromised status may not be feasible. A review of the data from the patients with PML suggests that clinical PML may be preceded by JC viremia. Our patients with positive JCV DNA in CSF did not have any virus in their blood. In addition, they had a much lower virus load than previously described for JCV DNA in the CSF of patients with confirmed PML, ranging from 3.65 × 104 to 1.2 × 107 copies/mL CSF [30,31]. Our two patients with positive JCV in the CSF have been followed for 24 and 30 months, respectively, without developing any signs of PML. Because the clinical picture has remained stable, no tests in additional CSF or plasma samples for JCV DNA have been performed. However, it would be informative if future samples were tested to assess any persistence or trafficking of virus through the nervous system. We also found two SLE patients with positive JCV DNA in their plasma but without any detectable JCV in their CSF. It is known that patients with rheumatic diseases, including SLE, have an increased risk for developing PML [32]. Calabrese, et al. [33] found 24 SLE patients with PML when reviewing the literature. Ten of ten of them had positive JCV PCR in their CSF. PML has also been reported In two of 10,000 patients with SLE treated with rituximab [34]. Both these patients displayed JCV DNA in their CSF. Our SLE cases with detectable JCV DNA had virus in plasma alone and have been followed clinically for more than 2 years without developing signs of PML.
Although it is expected that occasional patients display JCV DNA in the plasma as part of the normally innocuous JCV infection in healthy persons, the detection of the virus in the CSF in certain individuals without PML is more surprising. We think that it is less likely that it represents viral persistence in the CNS. Instead, the finding could be consistent with the fact that blood-derived lymphocytes physiologically and normally traffic through the CSF. Then, if carrying JCV DNA, they may be detected. This means that JC viral DNA might be found in the CSF in rare cases of individuals in the normal population. This may also be the case with other ubiquitous DNA containing viruses that have tropism for the brain or circulate in the peripheral blood and traffic through the brain. However, the extremely minute observations preclude any firm conclusions on this. From a clinical perspective, however, the data show that with even very well validated methods and highly sensitive techniques, JCV DNA can be detected in CSF samples of persons without PML, and thus a diagnosis of PML must rely on a combined judgment of clinical picture, MRI and JCV DNA determination.
Further studies on the mechanism of JCV reactivation and factors leading to PML are important.
Acknowledgments
This study was supported through the NINDS Division of Intramural Research, by Torsten and Ragnar Soderberg Foundation, the Swedish research council, and NeuroProMise (LSHM-CT-2005-018637).
References
- 1.Yousry TA, Major EO, Ryschkewitsch C, et al. Evaluation of patients treated with nalalizumab for progressive multifocal leukoencephalopathy. N Engl J Med. 2006;354:924–933. doi: 10.1056/NEJMoa054693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bosier A, Edan G, Frohman E, et al. Intense immunosuppression in patients with rapidly worsening multiple sclerosis: treatment guidelines for the clinician. Lancet Neurol. 2008;7:173–183. doi: 10.1016/S1474-4422(08)70020-6. [DOI] [PubMed] [Google Scholar]
- 3.Astrom KE, Mancall EL, Richardsson EP. Progressive multifocal leukoencephalopathy; a hitherto unrecognized complication of chronic lymphatic leukemia and Hodgkin’s disease. Brain. 1958;81:93–111. doi: 10.1093/brain/81.1.93. [DOI] [PubMed] [Google Scholar]
- 4.Padgett BL, Walker DL, ZuRhein GM, Eckroade RJ, Dessel BH. Cultivation of papova-like virus from human brain with progresive multifocal leukoencephalopathy. Lancet. 1971;1:1257–1260. doi: 10.1016/s0140-6736(71)91777-6. [DOI] [PubMed] [Google Scholar]
- 5.Sabath BF, Major EO. Traffic of JC virus from sites of initial infection to the brain: the path to progressive multifocal leukoencephalopathy. J Infect Dis. 2002;186:180–186. doi: 10.1086/344280. [DOI] [PubMed] [Google Scholar]
- 6.Knowles WA, Pipkin P, Andrews N, et al. Population-based study of antibody to the human polyomaviruses BKV and JCV and the simian polyomavirus SV40. J Med Virol. 2003;71:115–123. doi: 10.1002/jmv.10450. [DOI] [PubMed] [Google Scholar]
- 7.Stolt A, Sasnauskas K, Koskela P, Lehinen M, Dillner J. Seroepidemiology of the human polyomaviruses. J Gen Virol. 2003;84:1499–1504. doi: 10.1099/vir.0.18842-0. [DOI] [PubMed] [Google Scholar]
- 8.Dörries K, ter Meulen V. Progressive multifocal leukoencephalopathy: detection of papovavirus JC in kidney tissue. J Med Virol. 1983;11:307–317. doi: 10.1002/jmv.1890110406. [DOI] [PubMed] [Google Scholar]
- 9.Houff SA, Major EO, Katz DA, et al. Involvement of JC virus-infected mononuclear cells from the bone marrow and spleen in the pathogenesis of progressive multifocal leukoencephalopathy. N Engl J Med. 1988;318:301–305. doi: 10.1056/NEJM198802043180507. [DOI] [PubMed] [Google Scholar]
- 10.Monaco MC, Atwood WJ, Gravell M, Tornatore CS, Major EO. JC virus infection of hematopoietic progenitor cells, primary B lymphocytes, and tonsillar stromal cells: implications for viral latency. J Virol. 1996;70:7004–7012. doi: 10.1128/jvi.70.10.7004-7012.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jensen PN, Major EO. Viral variant nucleotide sequences help expose leukocytic positioning in the JC virus pathway to the CNS. J Leukoc Biol. 1999;65:428–438. doi: 10.1002/jlb.65.4.428. [DOI] [PubMed] [Google Scholar]
- 12.Khalili K, White MK. Human demyelinating disease and the polyomavirus JCV. Mult Scler. 2006;12:133–142. doi: 10.1191/135248506ms1264oa. [DOI] [PubMed] [Google Scholar]
- 13.Fong IW, Britton CB, Luinstra KE, Toma E, Mahony JB. Diagnostic value of delecting JC virus DNA in cerebrospinal fluid of patients with progressive multifocal leukoencephalopathy. J Clin Microbiol. 1995;33:484–486. doi: 10.1128/jcm.33.2.484-486.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hammarin AL, Bogdanovic G, Svedhem V, Pirskanen R, Morfeldt L, Grandien M. Analysis of PCR as a tool for detection of JC virus DNA in cerebrospinal fluid for diagnosis of progressive multifocal leukoencephalopathy. J Clin Microbiol. 1996;34:2929–2932. doi: 10.1128/jcm.34.12.2929-2932.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrante P, Omodeo-Zorini E, Caldarelli-Stefano R, et al. Detection of JC virus DNA in cerebrospinal fluid from multiple sclerosis patients. Mult Scler. 1998;4:49–54. doi: 10.1177/135245859800400202. [DOI] [PubMed] [Google Scholar]
- 16.Bogdanovic G, Priftakis P, Hammarin AL, et al. Detection of JC virus in cerebrospinal fluid (CSF) samples from patients with progressive multifocal leukoencephalopathy but not in CSF samples from patients with herpes simplex encephalitis, enteroviral meningitis, or multiple sclerosis. J Clin Microbiol. 1998;36:1137–1138. doi: 10.1128/jcm.36.4.1137-1138.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alvarez-Lafuente R, García-Montojo M, De Las Heras V, Bartolomé M, Arroyo R. JC virus in cerebrospinal fluid samples of multiple sclerosis patients at the first demyelinating event. Mult Scler. 2007;13:590–595. doi: 10.1177/1352458506073116. [DOI] [PubMed] [Google Scholar]
- 18.Franciotta D, Bestetti A, Bergamaschi R, Piccolo G, Persico A, Cinque P. Failure to detect JC virus DNA in cerebrospinal fluid of multiple sclerosis patients. Mult Scler. 2006;12:674–675. doi: 10.1177/1352458506070635. [DOI] [PubMed] [Google Scholar]
- 19.Tornatore C, Berger JR, Houff SA, et al. Detection of JC virus DNA in peripheral lymphocytes from patients with and without progressive multifocal leukoencephalopathy. Ann Neural. 1992;31:454–462. doi: 10.1002/ana.410310426. [DOI] [PubMed] [Google Scholar]
- 20.Randhawa P, Uhrmacher J, Pasculle W, et al. A comparative study of BK and JC virus infections in organ transplant recipients. J Med Virol. 2005;77:238–243. doi: 10.1002/jmv.20442. [DOI] [PubMed] [Google Scholar]
- 21.Dörries K, Sbiera S, Drews K, Arendt G, Eggers C, Dörries R. Association of human polyomavirus JC with peripheral blood of immunoimpaircd and healthy individuals. J Neurovirol. 2003;9:81–87. doi: 10.1080/13550280390195379. [DOI] [PubMed] [Google Scholar]
- 22.Lafon ME, Dutronic H, Dubois V, et al. JC virus remains latent in peripheral blood B lymphocytes but replicates actively in urine from AIDS patients. J Infect Dis. 1998;177:152–155. doi: 10.1086/515305. [DOI] [PubMed] [Google Scholar]
- 23.Delbue S, Guerini FR, Mancuso R, et al. JC virus viremia in interferon-beta-treated and untreated Italian multiple sclerosis patients and healthy controls. J Neurovirol. 2007;13:73–77. doi: 10.1080/13550280601094563. [DOI] [PubMed] [Google Scholar]
- 24.Alvarez-Lafuente R, Garcia-Montojo M, De Las Heras V, Bartolome M, Arroyo R. Interferon-beta treatment and active replication of the JC virus in relapsing-remitting multiple sclerosis patients. Eur J Neurol. 2007;13:233–236. doi: 10.1111/j.1468-1331.2006.01638.x. [DOI] [PubMed] [Google Scholar]
- 25.Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol. 2005;58:840–846. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- 26.Ryschkewitsch C, Jensen P, Hou J, Fahle G, Fisher S, Major EO. Comparison of PCR-southern hybridization and quantitative real-time PCR for the detection of JC and BK viral nucleotide sequences in urine and cerebrospinal fluid. J Virol Methods. 2004;121:217–221. doi: 10.1016/j.jviromet.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 27.Hamilton RS, Gravell M, Major EO. Comparison of antibody titers determined by hemagglutination inhibition and enzyme immunoassay for JC virus and BK virus. J Clin Microbiol. 2000;38:105–109. doi: 10.1128/jcm.38.1.105-109.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Langer-Gould A, Atlas SW, Green AJ, Bollen AW, Pellelier D. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N Engl J Med. 2005;353:375–381. doi: 10.1056/NEJMoa051847. [DOI] [PubMed] [Google Scholar]
- 29.Kleinschmidt-DeMasters BK, Tyler KL. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and intcrferon beta-1a for multiple sclerosis. N Engl J Med. 2005;353:369–374. doi: 10.1056/NEJMoa051782. [DOI] [PubMed] [Google Scholar]
- 30.Eggers C, Stellbrink HJ, Buhk T, Dörries K. Quantification of JC virus DNA in the cerebrospinal fluid of patients with human immunodeficiency virus-associated progressive mutifocal leukoencephalopathy – a longitudinal study. J Infect Dis. 1999;180:1690–1694. doi: 10.1086/315087. [DOI] [PubMed] [Google Scholar]
- 31.Koralnik IJ, Boden D, Mai VX, Lord CI, Letvin NL. JC virus DNA load in the cerebrospinal fluid in patients with and without progressive mutifocal leukoencephalopathy. Neurology. 1999;52:253–260. doi: 10.1212/wnl.52.2.253. [DOI] [PubMed] [Google Scholar]
- 32.Boren EJ, Cheema GS, Naguwa SM, Ansari AA, Gershwin ME. The emergence of progressive multifocal leukoencephalopathy (PML) in rheumatic diseases. J Autoimmun. 2008;30:90–98. doi: 10.1016/j.jaut.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 33.Calabrese LH, Molloy ES, Huang DR, Ransohoff RM. Progressive multifocal leukoencephalopathy in rheumatic diseases: evolving clinical and pathological patterns of disease. Arthritis Rheum. 2007;7:2116–2128. doi: 10.1002/art.22657. [DOI] [PubMed] [Google Scholar]
- 34.US Food and Drug Administration. FDA Alert: rituximab (marketed as Rituxan) 2006. [Accessed 18 December 2006]. http://www.fda.gov/cder/drug/Infopage/rituximab/default.htm.