Abstract
Activation of reduced nicotinamide-adenine dinucleotide phosphate (NADPH) oxidase by angiotensin II is integral to the formation of oxidative stress in the vasculature and the kidney. 3-Hydroxy-3-methylglutaryl-coenzyme A reductase inhibition is associated with reductions of oxidative stress in the vasculature and kidney and associated decreases in albuminuria. Effects of 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibition on oxidative stress in the kidney and filtration barrier integrity are poorly understood. To investigate, we used transgenic TG(mRen2)27 (Ren2) rats, which harbor the mouse renin transgene and renin-angiotensin system activation, and an immortalized murine podocyte cell line. We treated young, male Ren2 and Sprague-Dawley rats with rosuvastatin (20 mg/kg IP) or placebo for 21 days. Compared with controls, we observed increases in systolic blood pressure, albuminuria, renal NADPH oxidase activity, and 3-nitrotryosine staining, with reductions in the rosuvastatin-treated Ren2. Structural changes on light and transmission electron microscopy, consistent with periarteriolar fibrosis and podocyte foot-process effacement, were attenuated with statin treatment. Nephrin expression was diminished in the Ren2 kidney and trended to normalize with statin treatment. Angiotensin II–dependent increases in podocyte NADPH oxidase activity and subunit expression (NOX2, NOX4, Rac, and p22phox) and reactive oxygen species generation were decreased after in vitro statin treatment. These data support a role for increased NADPH oxidase activity and subunit expression with resultant reactive oxygen species formation in the kidney and podocyte. Furthermore, statin attenuation of NADPH oxidase activation and reactive oxygen species formation in the kidney/podocyte seems to play roles in the abrogation of oxidative stress-induced filtration barrier injury and consequent albuminuria.
Keywords: angiotensin II, albuminuria, glomerular filtration barrier, transgenic Ren2 rat, rosuvastatin
Renin-angiotensin system (RAS) activation and subsequently elevated angiotensin II (Ang II) exert the pressor, proliferative, profibrotic, and proinflammatory actions.1–3 Activation of tissue reduced nicotinamide-adenine dinucleotide phosphate (NADPH) oxidase seems to contribute to deleterious actions, such as oxidative stress and endothelial dysfunction manifesting as hypertension, albuminuria, and progressive glomerular dysfunction, that may ultimately lead to chronic kidney disease.1,4 There is accumulating evidence that tissue-based RAS further modulates cell growth, metabolism, and tissue remodeling.5,6
Evidence for a local RAS in the glomerulus raises the prospect of NADPH oxidase–induced podocyte and filtration barrier injury.7,8 Furthermore, in vitro protein exposure, mechanical stretch, and glomerular hypertension enhance tissue Ang II production, which may potentiate the impact of elevated blood pressure on glomerular injury manifesting as albuminuria.9,10 Previous work related to the pathogenesis of albuminuria delineated abnormalities such as basement membrane thickening, loss of the slit-pore diaphragm integrity, and widening of the podocyte foot process base width.11 Recent evidence characterized foot process effacement and loss of the slit-pore diaphragm as critical in decreasing filtration barrier integrity.12
3-Hydroxy-3-methylglutaryl (HMG)-coenzyme A (CoA) reductase inhibitors (statins) exert beneficial actions on oxidative stress and endothelial dysfunction independent of their cholesterol-lowering properties. Many of the actions of statins are thought to be mediated by decreasing reactive oxygen species (ROS) formation in various tissues.13 ROS derived from NADPH oxidase activation have been shown to play a critical role in hypertrophy, fibrosis, and remodeling in the heart and vasculature.14–16 NADPH oxidase is a multi-component enzyme complex that is composed of the membrane-bound heterodimer gp91phox (phox indicates phagocytic oxidase; NOX2) and its homologue NOX4; p22phox; the cytosolic regulatory subunits p40phox, p47phox, and p67phox; and the small GTP-binding protein, Rac1.14,15 Statins reduce cellular levels of isoprenoids and inhibit the subsequent isoprenylation of small G proteins such as Rac.17,18
Accordingly, we evaluated the impact of statin treatment on increases in NADPH oxidase activity and contemporaneously on the glomerular filtration barrier and podocyte integrity. We used the transgenic TG(mRen2)27 (Ren2) rat with the mouse renin transgene and RAS activation, as well as an immortalized murine podocyte cell line. We hypothesized that Ang II stimulation of NADPH oxidase would contribute to glomerular filtration barrier remodeling and the podocyte generation of ROS. We further hypothesized that the beneficial effects of statin therapy would be mediated in part through reductions in NADPH oxidase activation and generation of ROS.
Materials and Methods
Animals and Treatments
All of the animal procedures were approved by the institutional animal care and use committees at the University of Missouri Harry S. Truman Veterans’ Affairs Medical Center and housed in accordance with National Institutes of Health guidelines. Ren2 (5- to 6-week–old) and age-matched Sprague-Dawley (SD) male rats were randomly assigned to placebo-treated control (Ren2-C and SD-C, respectively; n=6 each) or rosuvastatin (AstraZeneca) treatment groups (Ren2-RSV and SD-RSV, respectively; n=4 each). Rosuvastatin (20 mg/kg per day) in saline or an equal volume of saline was administered intraperitoneally to the rosuvastatin or control groups, respectively, for 21 days.
Systolic Blood Pressure and Albuminuria
Details of systolic blood pressure (SBP) and albuminuria are available in a data supplement available online at http://hyper.ahajournals.org. Briefly, SBP was determined at the end of rosuvastatin treatment on days 19 or 20 using the tail cuff after acclimatization and restraint conditioning for 48 hours on each group of rats (SDC, n=6; SD-RSV, n=4; Ren2-C, n=6; Ren2-RSV, n=4).12–16 In addition, albuminuria from each group of rats (SDC, n=6; SD-RSV, n=4; Ren2-C, N=6; Ren2-RSV, n=4) was determined at the beginning, middle, and end of treatment.12
Transmission Electron Microscopy Methods
Details of the transmission electron microscopy (TEM) in this study are available in the data supplement. Briefly, kidney cortical tissue from each group of rats (SDC, n=6; SD-RSV, n=4; Ren2-C, n=6; Ren2-RSV, n=4) was prepared as described previously,12,14,19 and 3 glomeruli per rat were evaluated with five 10-k and 60-k images per glomeruli. Five measurements were performed for each variable per image, including basement membrane thickness, slit pore diameter and number per 100 μm, and foot-process base width.
Immunofluorescent Studies
Details of the immunofluorescent studies are available in the data supplement. Briefly, harvested kidney cortical tissue from each group of rats (SDC, n=4; SD-RSV, n=4; Ren2-C, n=4; Ren2-RSV, n=4) was prepared13–16 and evaluated for nephrin C-17, gp91phox (NOX2), and Rac1 antibody, as well as to assess 3-nitrotyrosine content, a marker for peroxynitrite formation.13–15
Western Blot Analysis
Details of the Western blot analysis are available in the data supplement. Briefly, kidney cortical tissue from each group of rats (SDC, n=4; SD-RSV, n=4; Ren2-C, n=4; Ren2-RSV, n=4) was used to evaluate nephrin with 2 antibodies (N-20 and C-17); total protein was stained with amido-black staining to normalize the data.
Measurement of NADPH Oxidase Activity
NADPH oxidase activity was determined in plasma membrane fractions from each group of rats (SDC, n=6; SD-RSV, n=4; Ren2-C, n=6; Ren2-RSV, n=4) and podocyte cell lysates.15–17
Light Microscopy
Harvested kidney cortical tissue from each group of rats (SDC, n=4; SD-RSV, n=4; Ren2-C, n=4; Ren2-RSV, n=4) were stained with Verhoeff-van Gieson, which is specific for fibrosis and stains elastin (black), nuclei (blue black), collagen (red), and connective tissue (yellow).14,15,19
Podocyte Cell Culture
Details of cell culture are available in the data supplement. Briefly, a dose-response curve was established for Ang II stimulation of NADPH oxidase activity in podocyte cell lysates using a time-dependency assay, and rosuvastatin inhibition was based on previous experiments.17 In addition, superoxide generation in podocyte cell lysates was evaluated using oxidative fluorescent dihydroethidium staining.17
RNA Extraction and Subunit Expression by Real-Time PCR
Details of the determination of RNA extraction from podocyte cell lysates are available in the data supplement. RNA was extracted from podocytes using RNeasy Mini kits (Qiagen), and 100 ng of starting cDNA was used for Real-Time PCR with the following primers: p22phox and NOX4, gp91phox (NOX2; 5′-CCA ACT GGG ATA ACG AGT trichloroacetic acid (TCA)-3′) and reverse (5′-GAG AGT TTC AGC CAA GGC TTC-3′) and Rac1.20 Expression levels were normalized using 18S as the control.
Statistical Analysis
All of the values are expressed as mean±SE. Statistical analyses were performed in SPSS 13.0 (SPSS Inc) using ANOVA with Fisher’s least significant difference as appropriate and Student’s t test for paired analysis. Significance was accepted as P<0.05.
Results
Effect of Rosuvastatin on SBP and Albuminuria
As measured at the end of the treatment period, there were increases in SBP in Ren2 (207.4±1.3 mm Hg) when compared with SD controls (156.0±9.8 mm Hg; P<0.05) without reductions in rosuvastatin-treated animals (202±4.5 mm Hg and 159.7±9.7 mm Hg, respectively; Figure 1A). Albuminuria was increased in the Ren2 rats at 5 weeks (0.15±0.05 mg/mg), 7 to 8 weeks (0.33±0.05 mg/mg), and significantly at 9 weeks (0.47±0.08 mg/mg; P<0.05) when compared with SD controls at 5 weeks (0.12±0.02 mg/mg), 7 to 8 weeks (0.14±0.04 mg/mg), and 9 weeks (0.11±0.01 mg/mg; Figure 1B). There were improvements in the rosuvastatin-treated Ren2 rats at 5 weeks (0.17±0.03 mg/mg), 7 to 8 weeks (0.18±0.04 mg/mg), and significantly at 9 weeks (0.26±0.04 mg/mg; P<0.05).
Figure 1.

SBP and albuminuria in transgenic Ren2 rats. A, SBP in Ren2 rats at the end of treatment period. B, Albuminuria in the Ren2 rat as measured at the beginning of treatment (6 weeks of age or 0 weeks of treatment), middle of treatment period (7 to 8 weeks of age or 1.5 weeks of treatment), and end of treatment period (9 weeks of age or 3 weeks of treatment). *P<0.05 vs age-matched SD-C (n=6); #P=0.05 when Ren2-C (n=6) 3 week is compared with 0 weeks; **P<0.05 when Ren2-RSVs (n=4) are compared with age-matched SD-C rats.
Effect of Rosuvastatin on Glomerular Remodeling
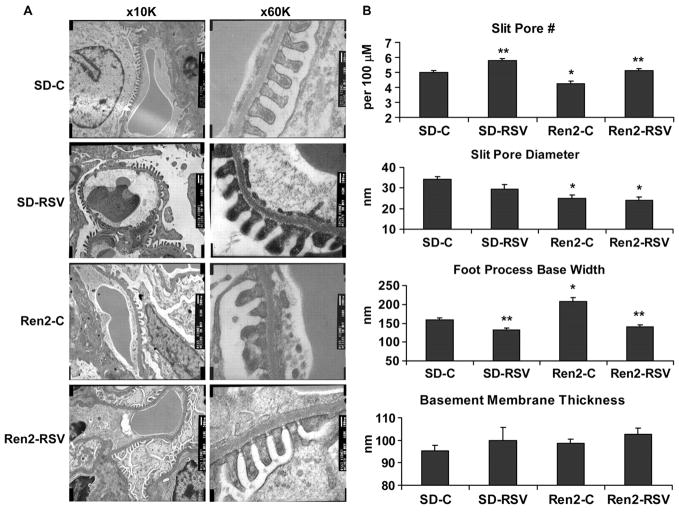
Glomerular filtration barrier structural integrity was measured by TEM and light microscopy with Verhoeff-van Gieson and nephrin immunostaining. TEM images at 10 000 and 60 000 (Figure 2A) were used to evaluate 4 criteria for filtration barrier integrity as described above. Significant changes in all 4 of the variables were observed when comparing Ren2 to SD glomeruli, and these changes were attenuated with rosuvastatin treatment (Figure 2B).
Figure 2.
Rosuvastatin improves indices of podocyte foot-process effacement on TEM. A, Representative TEM images at ×10 000 (left panel) and ×60 000 (right panel). B, Indices for glomerular filtration barrier integrity. *P<0.05 when Ren2-Cs (n=6) are compared with age-matched SD-Cs (n=6); **P<0.05 when Ren2-RSVs (n=4) or SD-RSVs (n=4) are compared with age-matched controls.
There were fewer slit-pores in Ren2 rats (4.3±0.2 slit pores/100 μm; P±0.05) versus SD control rats (5.0±0.1 slit pores/100 μm), improved with rosuvastatin treatment in Ren2 and SD rats (5.1±0.1 and 5.8±0.1 slit pores per 100 μm, respectively; each P<0.05; Figure 2B). Similarly, slit-pore diameter was less in Ren2 glomeruli (25.0±1.5 nm) than in SD (34.3±1.3 nm; P<0.05). However, rosuvastatin treatment did not improve slit-pore diameter in the Ren2 or SD rats (23.9±1.9 nm and 29.3±2.5 nm, respectively; each P>0.05). Increases in podocyte foot process base width paralleled the loss of the slit-pore number and diameter in the Ren2 rats (208.2±9.7 nm) when compared with SD controls (159.6±5.5 nm) that improved with rosuvastatin treatment in both Ren2 and SD rats (140.6±4.9 nm and 131.8±5.7 nm, respectively; each P<0.05). Interestingly, basement membrane thickness was not greater in the Ren2 (98.7±1.9 nm) when compared with SD (95.3±2.6 nm) glomeruli (P>0.05), with no rosuvastatin treatment effect in either Ren2 or SD rats (102.6±2.9 nm and 99.9±5.9 nm; each P>0.05).
There were attendant decreases in nephrin by immunostaining and Western analysis in the Ren2 rats (19.5±1.6 average grayscale intensities; 0.24±0.07 arbitrary units) when compared with SD controls (27.9±4.5 average grayscale intensities; 0.431±0.090 arbitrary units; each P<0.05) with a trend to improvement in the rosuvastatin-treated Ren2 rats (21.7±3.6 average grayscale intensities and 0.32±0.04; each P>0.05; Figure S1A through S1E).
To evaluate the effects of rosuvastatin on glomerular remodeling, we morphometrically evaluated periarteriolar fibrosis in Verhoeff-van Gieson–stained sections of the kidney. There was substantial periarteriolar fibrosis in the adventitia of Ren2 rats (0.65±0.01% area fibrosis) when compared with SD controls (0.39±0.01% area fibrosis; P<0.05) that was improved in the rosuvastatin-treated Ren2 rats (0.41±0.01% area fibrosis; P<0.05; Figure S2A and S2B). Interestingly, there were decreases in the percent area in the media of the Ren2 rats (0.28±0.01% area) compared with SD controls (0.45±0.01% area; P<0.05), which improved in the rosuvastatin-treated Ren2 rats (0.47±0.01% area; P<0.05).
Effect of Ang II and Rosuvastatin on NADPH Oxidase Activity and Subunits
There were expected increases in NADPH oxidase activity in the Ren2 controls (80.9±0.1 mOD/mg per minute) when compared with SD controls (67.9±0.2 mOD/mg per minute; P<0.05) that improved with rosuvastatin treatment in the Ren2 rats (57.1±0.1 mOD/mg per minute; P<0.05; Figure 3A). Similarly, there were dose-dependent increases in Ang II stimulation of NADPH oxidase activity in podocytes with a maximum occurring at 10 nm (Figure 4A). The increase in NADPH oxidase activity induced with 10 nM of Ang II (1.15±0.03 mOD/mg per minute; P<0.05) was completely prevented with rosuvastatin treatment (0.94±0.04 mOD/mg per minute; P<0.05; Figure 4B).
Figure 3.
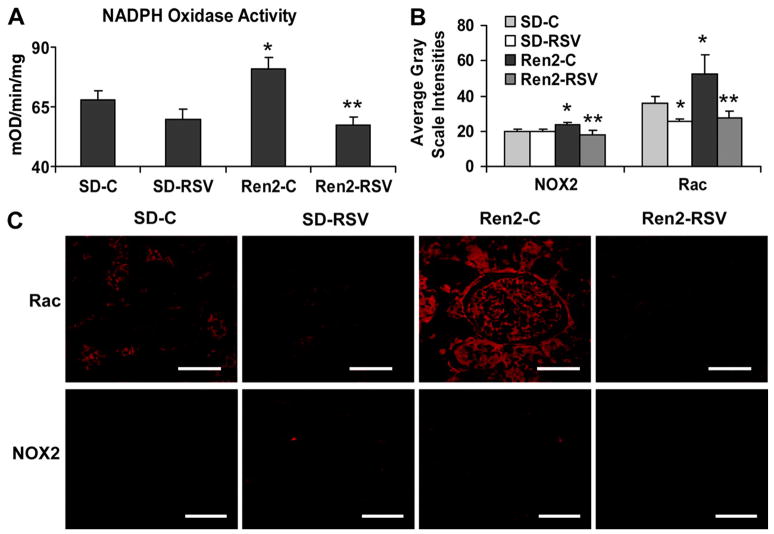
Rosuvastatin attenuation of NADPH oxidase in the transgenic Ren2 rat. A, Kidney cortical tissue NADPH oxidase activity in the Ren2 rat. B, Grayscale intensity measures of NADPH oxidase subunit expression for C. C, Representative images of glomerular sections immunostained for NADPH oxidase subunits NOX2 and Rac. (scale bar=50 μmol/L). *P<0.05 when Ren2-Cs (n=6) are compared with age-matched SD-Cs (n=6); **P<0.05 when Ren2-RSVs (n=4) or SD-RSVs (n=4) are compared with age-matched controls.
Figure 4.
Rosuvastatin attenuation of Ang II stimulation of NADPH oxidase in podocytes. A, Dose response for Ang II stimulation of NADPH oxidase activity (n=6). B, Rosuvastatin reversal of Ang II stimulation of NADPH oxidase activity (n=7). C, Representative immunostaining of NADPH oxidase subunits in podocyte cell culture. D, mRNA expression of NADPH oxidase subunits in podocyte cell culture. *P<0.05 when Ang II was compared with control; for NOX4, Rac1 and p22phox (n=14) and NOX2 (n=12). **P<0.05 when compared with Ang II stimulation alone; each (n=8).
Similar to enzyme activity, there were increases in NADPH oxidase subunits in the kidney cortex of the Ren2 rats and in podocytes. There were increases in NOX2 and Rac in the Ren2 rats (23.5±1.4 and 54.9±10.9 average grayscale intensities, respectively; each P<0.05) compared with SD controls (19.6±1.5 and 31.7±3.6 average grayscale intensities, respectively; each P<0.05) improved with rosuvastatin treatment (18.1±2.1 and 27.2±4.0 average grayscale intensities, respectively; each P<0.05; Figure 3B and 3C). Similarly, increases were seen in podocytes with Ang II stimulation of NOX2 and Rac mRNA expression (3.75±1.30 and 3.34±0.76 average grayscale intensities, respectively; each P<0.05) relative to controls that were reversed with rosuvastatin (0.71±0.08 and 1.16±0.30 average grayscale intensities, respectively; Figure 4D). There were similar increases in mRNA expression of NOX4 and p22 in podocytes after Ang II stimulation (3.09±0.61 and 2.79±0.68 average grayscale intensities relative to control, respectively; each P<0.05) reversed with rosuvastatin treatment (1.17±0.30 and 0.99±0.23 average grayscale intensities, respectively; each P<0.05; Figure 4D).
Effect of Ang II and Rosuvastatin on Oxidative Stress Markers
To ascertain whether rosuvastatin affects oxidative stress, we measured 3-nitrotyrosine as a marker of peroxynitrite formation in the Ren2 and dihydroethidium in podocytes as a marker of superoxide anion generation. Kidney tissue nitrotyrosine content was increased in the Ren2 (26.3±1.7 average grayscale intensities) when compared with SD rats (12.9±1.4 average grayscale intensities; P<0.05) reversed with rosuvastatin treatment in the Ren2 rats (15.4±1.1 average grayscale intensities; P<0.05; Figure S3A and S3B). Similarly, there were increases in superoxide anion generation after Ang II stimulation (24.5±4.6 average grayscale intensities; P<0.05) that were reversed with rosuvastatin (35.4±4.8 average grayscale intensities; P<0.05; Figure S3C and S3D).
Discussion
This investigation addressed the impact of HMG-CoA reductase inhibitors (statins) on NADPH oxidase activation and subunit expression, oxidative stress, and resultant glomerular filtration barrier and podocyte injury. Filtration barrier/podocyte integrity was evaluated in the transgenic Ren2 rat, an animal model with the mouse renin transgene and RAS activation, shown previously to demonstrate enhanced renal tissue oxidative stress and albuminuria.12,19 Data from the current investigation indicate that statin therapy has renoprotective effects, as demonstrated by reductions in albuminuria, oxidative stress, and improvements in filtration barrier indices, as well as basement membrane thickening and podocyte foot process effacement. Findings from this study are consistent with our previous findings in the heart and other reports that statins decrease NADPH oxidase–related ROS species generation through inhibition of cytosolic small-molecular-weight G proteins, such as Rac, and the membrane subunits of NADPH oxidase.14,18,21
The filtration barrier is composed of endothelium, basement membrane, and visceral epithelial cells called podocytes. Increasing evidence supports podocyte regulation of various glomerular functions, including basement membrane turnover, maintenance of the filtration barrier, regulation of the ultrafiltration coefficient, and mechanical support of the glomerular tuft.22 Intraglomerular capillaries are continuously exposed to elevated hydrostatic pressure gradients and are susceptible to the effects of NADPH oxidase activation and oxidative stress.21,23 Thus, the findings of periarteriolar and glomerular fibrosis, in addition to podocyte foot-process effacement, independent of changes in the basement membrane, in an animal model of NADPH oxidase activation support a role for ROS-mediated podocyte injury and albuminuria. Furthermore, the reductions in fibrosis and foot-process effacement with in vivo statin therapy suggest a role for statin reductions in NADPH oxidase and ROS. This supports the notion that statins may exert a renoprotective effect in maintaining the integrity of the podocyte and filtration barrier.
In the present study, the Ren2 rat demonstrated increases in NADPH oxidase activity, subunit expression, and oxidative stress in conjunction with podocyte remodeling/effacement and loss of the slit-pore diaphragm integrity. NADPH oxidase activation was accompanied by downregulation of nephrin, an important protein necessary for maintenance of podocyte slit-pore diaphragm integrity.22–24 The loss of nephrin, in turn, contributed to effacement of podocytes and loss of integrity of the slit-pore diaphragm, both requisites for progression of albuminuria.23–25 Indeed, these changes occurred in the absence of basement membrane thickening, suggesting the paramount importance of changes in slit-pore integrity in the pathogenesis of albuminuria.
In parallel with increased NADPH oxidase activity, there was an increase in peroxynitrite formation, as measured by nitrotyrosine staining in Ren2 kidneys, significantly reduced by statin therapy. NADPH oxidase catalyzes the 1-electron reduction of molecular oxygen to superoxide anion, which can react to form short-lived peroxynitrite. Peroxynitrite then forms stable 3-nitrotyrosine–conjugated molecules.14 Indeed, nitrotyrosine staining and accompanying fibrosis were particularly pronounced in the glomerulus. Oxidative stress is a known stimulus for fibrosis; ROS formation can activate redox-sensitive transcription factors, such as nuclear factor-κβ, which promotes collagen and other connective tissue deposition. The current observation that in vivo statin treatment significantly decreases NADPH oxidase activity and ROS levels in concert with decreases in albuminuria and periarteriolar and glomerular fibrosis is the first such report. Moreover, benefits of rosuvastatin treatment were independent of any effects on basement membrane thickness or blood pressure.
Data in cultured podocyte cells complement our ex vivo data demonstrating that Ang II stimulates NADPH oxidase with resultant superoxide anion generation and reversed with statin treatment. Previous work suggested a role for ATP-dependent NADPH subunits p22phox, p47phox, p67phox, and NOX2 in human podocytes.26 Our data further define a role for NOX4 in the rodent podocyte. NOX4 is a homologue of NOX2 and has been defined previously in the endothelium,27 and recent immunohistochemical data support a role for NOX4 in renal mesangial cell injury in db/db diabetic mice.28 Current data further indicate that the NOX4 homologue is a critical component of the functional membrane NADPH oxidase enzyme in rodent podocytes and that statin treatment reduces the expression of this subunit in concert with attenuation of podocyte injury.
Perspectives
In summary, data from this investigation support a role for activation of NADPH oxidase with resultant oxidative stress and associated loss of glomerular filtration barrier/podocyte integrity, processes that are reversed with statin treatment, suggesting a potential renoprotective effect. These salutary effects of statin treatment in this investigation were mediated independent of effects on glomerular basement membrane thickening. However, in interpreting the results of this or any study that investigates blood pressure–mediated effects, measurement of SBP can be susceptible to large variability without a time course determination.
Acknowledgments
The immortalized murine podocyte cell line was kindly provided by Dr Peter Mundel at Mount Sinai School of Medicine. Special thanks go to Charles Wiedmeyer, DVM, PhD, and Matthew Morris for technical assistance. We also acknowledge support from the electron microscope core facility and Cheryl Jensen (electron microscopy specialist) for help with preparation of the transmission electron micrographs.
Sources of Funding
This research was supported by National Institutes of Health grants R01 HL73101-01A1 (to J.R.S.) and P01 HL-51952 (to C.F.), the Veterans Affairs Merit System (0018; to J.R.S.), and an investigator-initiated grant from AstraZeneca.
Footnotes
Disclosures
None.
References
- 1.Sowers JR. Hypertension, angiotensin II, and oxidative stress. N Engl J Med. 2002;346:1999–2001. doi: 10.1056/NEJMe020054. [DOI] [PubMed] [Google Scholar]
- 2.Griendling KK, Ushio-Fukai M. Reactive oxygen species as mediators of angiotensin II signaling. Regul Pept. 2000;91:21–27. doi: 10.1016/s0167-0115(00)00136-1. [DOI] [PubMed] [Google Scholar]
- 3.Wolf G. Role of reactive oxygen species in angiotensin II-mediated renal growth, differentiation, and apoptosis. Antioxid Redox Signal. 2005;7:1337–1345. doi: 10.1089/ars.2005.7.1337. [DOI] [PubMed] [Google Scholar]
- 4.Hayden MR, Whaley-Connell A, Chowdhury N, Sowers JR. Role of angiotensin II in diabetic cardiovascular and renal disease. Curr Opin Endocrinol Diabetes Obes. 2006;13:135–140. [Google Scholar]
- 5.Sadoshima J, Izumo S. Molecular characterization of angiotensin II-induced hypertrophy of cardiac myocytes and hyperplasia of cardiac fibroblasts. Critical role of the AT1 receptor subtype. Circ Res. 1993;73:413–423. doi: 10.1161/01.res.73.3.413. [DOI] [PubMed] [Google Scholar]
- 6.Crabos M, Roth M, Hahn AW, Erne P. Characterization of angiotensin II receptors in cultured adult rat cardiac fibroblasts. Coupling to signaling systems and gene expression. J Clin Invest. 1994;93:2372–2378. doi: 10.1172/JCI117243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blantz RC, Konnen KS, Tucker BJ. Angiotensin II effects upon the glomerular microcirculation and ultrafiltration coefficient of the rat. J Clin Invest. 1976;57:419–434. doi: 10.1172/JCI108293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Velez JC, Bland AM, Arthur JM, Raymond JR, Janech MG. Characterization of renin-angiotensin system enzyme activities in cultured mouse podocytes. Am J Physiol Renal Physiol. 2007;293:F398–F407. doi: 10.1152/ajprenal.00050.2007. [DOI] [PubMed] [Google Scholar]
- 9.Wolf G, Ziyadeh FN. Cellular and molecular mechanisms of proteinuria in diabetic nephropathy. Nephron Physiol. 2007;106:26–31. doi: 10.1159/000101797. [DOI] [PubMed] [Google Scholar]
- 10.Gloy J, Henger A, Fischer KG, Nitschke R, Mundel P, Bleich M, Schollmeyer P, Greger R, Pavenstadt H. Angiotensin II depolarizes podocytes in the intact glomerulus of the rat. J Clin Invest. 1997;99:2772–2781. doi: 10.1172/JCI119467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White KE, Bilous RW. Estimation of podocyte number: a comparison of methods. Kidney Int. 2004;66:663–667. doi: 10.1111/j.1523-1755.2004.00787.x. [DOI] [PubMed] [Google Scholar]
- 12.Whaley-Connell AT, Chowdhury NA, Hayden MR, Stump CS, Habibi J, Wiedmeyer CE, Gallagher PE, Tallant EA, Cooper SA, Link CD, Ferrario C, Sowers JR. Oxidative stress and glomerular filtration barrier injury: role of the renin-angiotensin system in the Ren2 transgenic rat. Am J Physiol Renal Physiol. 2006;291:F1308–F1314. doi: 10.1152/ajprenal.00167.2006. [DOI] [PubMed] [Google Scholar]
- 13.Sowers J. Effects of statins on the vasculature: Implications for aggressive lipid management in the cardiovascular metabolic syndrome. Am J Cardiol. 2003;20:91:14B–22B. doi: 10.1016/s0002-9149(02)03269-1. [DOI] [PubMed] [Google Scholar]
- 14.Habibi J, Whaley-Connell A, Qazi MA, Hayden MR, Cooper SA, Stump C, Ferrario C, Muniyappa R, Sowers J. Rosuvastatin, a 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor, decreases cardiac oxidative stress and remodeling in Ren2 transgenic rats. Endocrinology. 2007;148:2181–2188. doi: 10.1210/en.2006-1355. [DOI] [PubMed] [Google Scholar]
- 15.Whaley-Connell A, Govindarajan G, Habibi J, Hayden MR, Cooper SA, Wei Y, Ma L, Qazi M, Link D, Karuparthi PR, Stump CS, Ferrario C, Sowers JR. Angiotensin-II mediated oxidative stress promotes myo-cardial tissue remodeling in the transgenic TG (mRen2) 27 Ren2 Rat. Am J Physiol Endocrinol Metab. 2007;293:E355–E363. doi: 10.1152/ajpendo.00632.2006. [DOI] [PubMed] [Google Scholar]
- 16.Wei Y, Whaley-Connell A, Chen K, Habibi J, Uptergrove G, Clark SE, Stump CS, Ferrario C, Sowers JR. Angiotensin II-induced NADPH oxidase activation contribute to vascular inflammation, apoptosis and remodeling in hypertensive transgenic Ren2 rats. Hypertension. 2007;50:384–391. doi: 10.1161/HYPERTENSIONAHA.107.089284. [DOI] [PubMed] [Google Scholar]
- 17.Whaley-Connell A, Morris EM, Rehmer N, Yaghoubian JC, Hayden MR, Habibi J, Stump CS, Sowers JR. Attenuation of albumin activation of NAD(P)H oxidase is mediated via Rac1 inhibition in proximal tubule cells. Am J Nephrol. 2007;27:15–23. doi: 10.1159/000098432. [DOI] [PubMed] [Google Scholar]
- 18.Maach C, Kartes T, Killer H. Oxygen free radical release in human failing myocardium is associated with increased activity of Rac1-GTPase and represents a target for statin treatment. Circulation. 2003;108:1567–1574. doi: 10.1161/01.CIR.0000091084.46500.BB. [DOI] [PubMed] [Google Scholar]
- 19.Hayden MR, Chowdhury N, Cooper S, Whaley-Connell A, Witte L, Wiedmeyer C, Stump CS, Ferrario C, Sowers JR. Mitigation of proximal tubuler actin cytoskeleton microvilli remodeling in the TG(mRen2)27 (Ren2) transgenic rat with AT1 receptor blockade. Am J Physiol Renal Physiol. 2007;292:F861–F867. doi: 10.1152/ajprenal.00252.2006. [DOI] [PubMed] [Google Scholar]
- 20.Szöcs K, Lassègue B, Sorescu D, Hilenski LL, Valppu L, Couse TL, Wilcox JN, Quinn MT, Lambeth JD, Griendling KK. Upregulation of Nox-based NAD(P)H oxidases in restenosis after carotid injury. Arterioscler Thromb Vasc Biol. 2002;22:21–27. doi: 10.1161/hq0102.102189. [DOI] [PubMed] [Google Scholar]
- 21.Wei Y, Sowers JR, Nistala R, Gong H, Uptergrove GM, Clark SE, Morris EM, Szary N, Manrique C, Stump CS. Angiotensin II-induced NADPH oxidase activation impairs insulin signaling in skeletal muscle cells. J Biol Chem. 2006;17:281:35137–35146. doi: 10.1074/jbc.M601320200. [DOI] [PubMed] [Google Scholar]
- 22.Lapinski R, Perico N, Remuzzi A, Sangalli F, Benigni A, Remuzzi G. Angiotensin II modulates glomerular capillary permselectivity in rat isolated perfused kidney. J Am Soc Nephrol. 1996;7:653–660. doi: 10.1681/ASN.V75653. [DOI] [PubMed] [Google Scholar]
- 23.Davis BJ, Cao Z, de Gasparo M, Kawachi H, Cooper ME, Allen TJ. Disparate effects of angiotensin II antagonists and calcium channel blockers on albuminuria in experimental diabetes and hypertension: potential role of nephrin. J Hypertens. 2003;21:209–216. doi: 10.1097/00004872-200301000-00031. [DOI] [PubMed] [Google Scholar]
- 24.Doublier S, Salvidio G, Lupia E, Ruotsalainen V, Verzola D, Deferrari G, Camussi G. Nephrin expression is reduced in human diabetic nephropathy: evidence for a distinct role for glycated albumin and angiotensin II. Diabetes. 2003;52:1023–1030. doi: 10.2337/diabetes.52.4.1023. [DOI] [PubMed] [Google Scholar]
- 25.Hoffmann S, Podlich D, Hahnel B, Kriz W, Gretz N. Angiotensin II type 1 receptor overexpression in podocytes induces glomerulosclerosis in transgenic rats. J Am Soc Nephrol. 2004;15:1475–1487. doi: 10.1097/01.asn.0000127988.42710.a7. [DOI] [PubMed] [Google Scholar]
- 26.Greiber S, Münzel T, Kästner S, Müller B, Schollmeyer P, Pavenstädt H. NAD(P)H oxidase activity in cultured human podocytes: effects of adenosine triphosphate. Kidney Int. 1998;53:654–663. doi: 10.1046/j.1523-1755.1998.00796.x. [DOI] [PubMed] [Google Scholar]
- 27.Ago T, Kitazono T, Ooboshi H, Iyama T, Han YH, Takada J, Wakisaka M, Ibayashi S, Utsumi H, Iida M. Nox4 as the major catalytic component of an endothelial NAD(P)H oxidase. Circulation. 2004;109:227–233. doi: 10.1161/01.CIR.0000105680.92873.70. [DOI] [PubMed] [Google Scholar]
- 28.Fujii M, Inoguchi T, Maeda Y, Sasaki S, Sawada F, Saito R, Kobayashi K, Sumimoto H, Takayanagi R. Pitavastatin ameliorates albuminuria and renal mesangial expansion by downregulating NOX4 in db/db mice. Kidney Int. 2007;72:473–480. doi: 10.1038/sj.ki.5002366. [DOI] [PubMed] [Google Scholar]