Abstract
Approximately 10% of patients with gastrointestinal stromal tumors (GIST) develop other neoplasms, either synchronously or metachronously. In this report we describe coexistence of a gastrointestinal stromal tumor and a hepatic perivascular epithelioid cell tumor (PEComa) in a 51-year-old woman with no evidence of tuberous sclerosis. A subcapsular hepatic nodule (0.8 cm in diameter) was found during surgery for symptomatic gastric neoplasm (15 cm in diameter) arising from the lesser curvature. Both tumors revealed histomorphological and immunohistochemical features confirming a diagnosis of a small incidental hepatic PEComa and a high risky extramural gastric GIST, respectively. The patient remained disease-free 25 mo after surgery with no evidence of tumor recurrence or new neoplasms. To our knowledge, this is the first report of PEComa in a patient with GIST. Hepatic lesions detected synchronously or metachronously in patients with GISTs may represent histogenetically distinct lesions and should be sampled to confirm or exclude metastatic GISTs.
Keywords: Gastrointestinal stromal tumors, PEComa, Coexistence, Liver, Synchronous
INTRODUCTION
Gastrointestinal stromal tumors (GISTs) represent the most common mesenchymal neoplasms of the gastrointestinal (GI) tract with an estimated incidence of 14.5 cases/million inhabitants[1]. Histologically, GISTs may show pure spindle cells, epithelioid, mixed and rarely pleomorphic morphology and are characteristically strongly immunoreactive for c-kit (CD117). GISTs occur more frequently in the stomach (60%-70%), small intestine (20%-30%), colorectum and esophagus (together ≤ 10%). Metastases predominantly occur in the peritoneum and liver[2].
Perivascular epithelioid cell tumors (PEComas) belong to a family of mesenchymal neoplasms including angiomyolipoma, clear-cell “sugar” tumors of the lung and other organs, lymphangiomyomatosis (LAM) and a rare group of morphologically and immunophenotypically similar lesions that may affect soft tissues, viscera and bones[3–5]. The term PEC (perivascular epithelioid cell) was coined by Bonetti et al[6] to describe the epithelioid cells found in these neoplasms. Primary hepatic PEComas are extremely rare[7], the majority of them were reported as monotypic or epithelioid angiomyolipoma.
Synchronous or metachronous malignant neoplasms are detected in almost 10% of GIST patients. In this regard, the majority of GISTs associated with other neoplasms are incidentally detected during surgery for intra-abdominal cancers. Hepatic neoplasm during GIST surgery is exceptionally rare. In this report, we describe the first case of GIST with a synchronously diagnosed hepatic PEComa, both of them were completely surgically resected.
CASE REPORT
A 51-year-old woman sought medical care in November 2004 due to pain in the left hypochondrium for 2 mo. She reported a weight loss of 7 kg during that period. Physical examination revealed no signs of the tuberous sclerosis complex (TSC). A computerized tomography (CT) of the abdomen showed a 15 cm well circumscribed solid expansile epigastric mass in contact with the left hepatic lobe and the gastric wall (Figure 1). CT of the thorax and pelvis did not show metastasis. At surgery, the mass was found to be attached to and infiltrating the gastric wall near the small curvature and could be successfully resected together with the large omentum and adjacent gastric wall involving the tumor. During surgery, a small subcapsular nodule located in the left hepatic lobe near the large gastric tumor was detected and resected. No tumor was found in the kidneys. The patient had a total abdominal hysterectomy for uterine leiomyomatosis in December 2006. At present, 25 mo after the surgery, she is under regular clinical follow-up with no evidence of tumor recurrence or metastasis.
Figure 1.
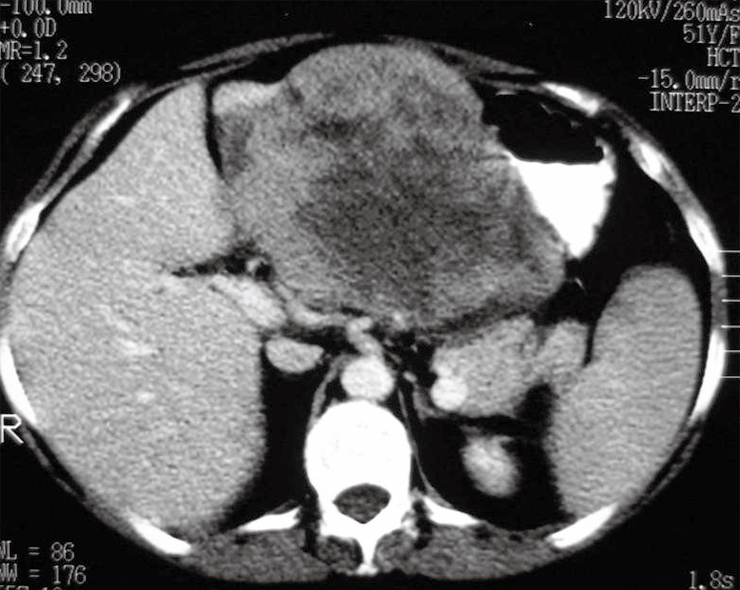
Computed tomography of the abdomen showing a 15-cm heterogeneous, solid expansile, regularly-shaped lesion located in the epigastrium and in contact with the left hepatic lobe and gastric wall.
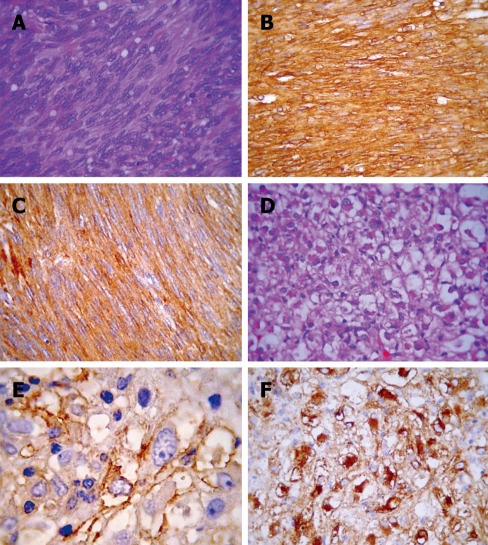
The resected gastric tumor measured 15.5 cm × 13.0 cm × 11.5 cm and was highly cellular, consisting of fascicles of spindled cells with elongated nuclei and finely granulated chromatin. Mitotic activity was brisk (3 mitoses/10 high-power fields). Cellular atypia was minimal and necrosis was absent (Figure 2A). Immunohistochemistry showed a strong expression of CD117 and CD34 (Figure 2B and C). The hepatic nodule measured 0.8 cm × 0.6 cm and was located near the hepatic surface, partly covered by its capsule. Histology showed sheets of large epithelioid cells with abundant eosinophilic, sometimes clear cytoplasm and mildly pleomorphic nuclei with evident nucleoli. A prominent feature was the presence of numerous dilated vascular spaces with a hemangiopericytic pattern. Necrosis or mitotic figures were not observed (Figure 2D). Immunohistochemistry showed a strong expression of HMB-45 and muscle specific actin (HHF-35), establishing a diagnosis of hepatic PEComa (Figure 2E and F). Table 1 summarizes the immunohistochemical data for both lesions.
Figure 2.
Morphological and immunohistochemical features of gastric GIST (A-C) and hepatic PEComa (D-F). A: GIST with characteristic spindled bipolar cells with occasional paranuclear cytoplasmic vacuoles; B and C: Strong staining for CD117 and CD34, respectively; D: Hepatic PEComa with a monotonous epithelioid morphology with cytoplasmic clearing that is hardly distinguishable from epithelioid GIST (note scattered cells with rhabdoid cytoplasm); E: A coarsely granular HMB45 reactivity; F: HH35 showing a strong paranuclear cytoplasmic reactivity.
Table 1.
Summary of immunohistochemical features of the two tumors surgically excised
| Antigen (Antibody clone)1 | Dilution | PEComa | GIST |
| CD117 | 1:600 | - | + |
| CD34 | 1:50 | - | + |
| Vimentin | 1:350 | + | + |
| Cytokeratins (AE1/AE3) | 1:250 | - | - |
| HHF-35 | 1:2000 | + | - |
| HMB-45 | 1:40 | + | - |
| S100 protein | 1:200 | - | + |
DakoCytomation, Carpinteria, California, USA.
DISCUSSION
The diagnosis of synchronous or metachronous neoplasias is not uncommon in clinical practice. Genetic predisposition and exposure to specific etiological or risk factors may be responsible for the association of histogenetically distinct tumors[8]. In such a circumstance, a second neoplasm developing in a patient with a known history of cancer is at a special risk of being misinterpreted as a metastatic disease, in particular, if this metachronous neoplasm presented at a site typically involving metastasis from the previously treated cancer, underscoring the need for sampling and a more careful interpretation of clinical, radiological and histopathological findings.
It was reported that the occurrence of synchronous and metachronous neoplasms is approximately 10% in patients diagnosed with GISTs and half of the cases can be diagnosed synchronously[9]. Gastrointestinal tract carcinomas are most frequently associated with GIST[9,10].
The presence of hepatic nodules in patients with a GIST must be carefully evaluated using appropriate immunohistochemical marker panel in order to distinguish metastatic GIST from other histogenetically distinct lesions (PEComa in the current case). Synchronous or metachronous primary hepatic neoplasms found incidentally in GIST patients are exceptionally rare with ≤ 5 cases reported so far, including four hepatocellular carcinomas and one inflammatory pseudotumor[9,11,12].
GISTs may enter the differential diagnosis of PEComas, since both can affect intra-abdominal structures. Due to their heterogeneous morphologic spectrum, GIST and PEComa may show largely overlapping features, thus representing a great differential diagnostic challenge for histopathologists. PEComas can be erroneously mistaken for GIST due to the monotonous epithelioid morphology and cytoplasmic clearing seen in a subset of both, combined with positive CD117 observed in some PEComas[5,7,13] and lacking or weak CD117 expression observed in a subset of epithelioid GISTs harboring mutations in platelet-derived growth factor receptor A (PDGFRA)[14]. Evert et al[13] reported a case of a malignant PEComa of the rectovaginal space mimicking a GIST and advocated the use of melanocytic markers in cases of suspected GIST with less than 50% of tumor cells expressing CD117. However, the characteristic morphology of PEComas may facilitate diagnosis in most of the cases. In difficult cases, immunohistochemistry would confirm the diagnosis of PEComa, as PEComas commonly show a characteristic myomelanocytic marker profile, occasionally with a characteristic perivascular distribution of HMB45 positive cells. PEComas commonly consist of epithelioid to elongated cells with abundant clear to granular eosinophilic cytoplasm with a perivascular organization of PECs in some of the cases[15]. While expression of HMB45 is a regular feature in most PEComas, smooth muscle markers and S100 show a rather heterogeneous and inconsistent reactivity pattern. CD117 expression in PEComas shows variable results, however, this finding is probably rare (5%) when appropriate immunohistochemical techniques are applied[5,16]. CD34 expression is generally lacking in PEComas.
Renal angiomyolipomas are frequently associated with TSC and even the sporadic cases present loss of heterozigosity (LOH) in chromosome 16p (harboring the TSC2 locus)[3]. The pathogenesis of PEComas other than angiomyolipoma and LAM is not well understood. GISTs have not been reported to be associated with the TSC. TSC2 LOH, as detected in some PEComas, have not been analysed in GISTs. Based on this and the finding that lesions of the PEComa family are KIT-negative in the majority of cases[5], it is likely that the coexistence of these two lesions in the current case represents a coincidence.
Hepatic PEComas, previously referred to as epithelioid or monotypic angiomyolipoma, are extremely rare[7,17–20]. To our knowledge, this is the first case reported in a patient with GIST.
In summary, we documented the first case of a GIST coexisting with a PEComa. Careful histopathological evaluation is mandatory for correct interpretation of the findings and, consequently, for appropriate patient treatment. A wide immunohistochemical panel is necessary to approach this differential diagnostic dilemma.
Supported by Fundação para o Desenvolvimento da UNESP - FUNDUNESP, São Paulo, Brazil
Peer reviewer: Paolo Del Poggio, Dr, Hepatology Unit, Department of Internal Medicine, Treviglio Hospital, Piazza Ospedale 1, Treviglio Bg 24047, Italy
S- Editor Yang RH L- Editor Wang XL E- Editor Wang HF
References
- 1.Nilsson B, Bumming P, Meis-Kindblom JM, Oden A, Dortok A, Gustavsson B, Sablinska K, Kindblom LG. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era--a population-based study in western Sweden. Cancer. 2005;103:821–829. doi: 10.1002/cncr.20862. [DOI] [PubMed] [Google Scholar]
- 2.Trent JC, Benjamin RS. New developments in gastrointestinal stromal tumor. Curr Opin Oncol. 2006;18:386–395. doi: 10.1097/01.cco.0000228747.02660.e2. [DOI] [PubMed] [Google Scholar]
- 3.Hornick JL, Fletcher CD. PEComa: what do we know so far? Histopathology. 2006;48:75–82. doi: 10.1111/j.1365-2559.2005.02316.x. [DOI] [PubMed] [Google Scholar]
- 4.Fukunaga M. Perivascular epithelioid cell tumor (PEComa) of soft tissue: case report with ultrastructural study. APMIS. 2004;112:98–104. doi: 10.1111/j.1600-0463.2004.apm1120203.x. [DOI] [PubMed] [Google Scholar]
- 5.Folpe AL, Mentzel T, Lehr HA, Fisher C, Balzer BL, Weiss SW. Perivascular epithelioid cell neoplasms of soft tissue and gynecologic origin: a clinicopathologic study of 26 cases and review of the literature. Am J Surg Pathol. 2005;29:1558–1575. doi: 10.1097/01.pas.0000173232.22117.37. [DOI] [PubMed] [Google Scholar]
- 6.Bonetti F, Pea M, Martignoni G, Zamboni G. PEC and sugar. Am J Surg Pathol. 1992;16:307–308. doi: 10.1097/00000478-199203000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Parfitt JR, Bella AJ, Izawa JI, Wehrli BM. Malignant neoplasm of perivascular epithelioid cells of the liver. Arch Pathol Lab Med. 2006;130:1219–1222. doi: 10.5858/2006-130-1219-MNOPEC. [DOI] [PubMed] [Google Scholar]
- 8.Maiorana A, Fante R, Maria Cesinaro A, Adriana Fano R. Synchronous occurrence of epithelial and stromal tumors in the stomach: a report of 6 cases. Arch Pathol Lab Med. 2000;124:682–686. doi: 10.5858/2000-124-0682-SOOEAS. [DOI] [PubMed] [Google Scholar]
- 9.Agaimy A, Wunsch PH, Sobin LH, Lasota J, Miettinen M. Occurrence of other malignancies in patients with gastrointestinal stromal tumors. Semin Diagn Pathol. 2006;23:120–129. doi: 10.1053/j.semdp.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Wronski M, Ziarkiewicz-Wroblewska B, Gornicka B, Cebulski W, Slodkowski M, Wasiutynski A, Krasnodebski IW. Synchronous occurrence of gastrointestinal stromal tumors and other primary gastrointestinal neoplasms. World J Gastroenterol. 2006;12:5360–5362. doi: 10.3748/wjg.v12.i33.5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lo OS, Poon RT, Lam CM, Fan ST. Inflammatory pseudotumor of the liver in association with a gastrointestinal stromal tumor: a case report. World J Gastroenterol. 2004;10:1841–1843. doi: 10.3748/wjg.v10.i12.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaworski R, Jastrzebski T, Swierblewski M, Drucis K, Kobierska-Gulida G. Coexistence of hepatocellular carcinoma and gastrointestinal stromal tumor: a case report. World J Gastroenterol. 2006;12:665–667. doi: 10.3748/wjg.v12.i4.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evert M, Wardelmann E, Nestler G, Schulz HU, Roessner A, Rocken C. Abdominopelvic perivascular epithelioid cell sarcoma (malignant PEComa) mimicking gastrointestinal stromal tumour of the rectum. Histopathology. 2005;46:115–117. doi: 10.1111/j.1365-2559.2005.01991.x. [DOI] [PubMed] [Google Scholar]
- 14.Medeiros F, Corless CL, Duensing A, Hornick JL, Oliveira AM, Heinrich MC, Fletcher JA, Fletcher CD. KIT-negative gastrointestinal stromal tumors: proof of concept and therapeutic implications. Am J Surg Pathol. 2004;28:889–894. doi: 10.1097/00000478-200407000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Zamboni G, Pea M, Martignoni G, Zancanaro C, Faccioli G, Gilioli E, Pederzoli P, Bonetti F. Clear cell "sugar" tumor of the pancreas. A novel member of the family of lesions characterized by the presence of perivascular epithelioid cells. Am J Surg Pathol. 1996;20:722–730. doi: 10.1097/00000478-199606000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Makhlouf HR, Remotti HE, Ishak KG. Expression of KIT (CD117) in angiomyolipoma. Am J Surg Pathol. 2002;26:493–497. doi: 10.1097/00000478-200204000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Goodman ZD, Ishak KG. Angiomyolipomas of the liver. Am J Surg Pathol. 1984;8:745–750. doi: 10.1097/00000478-198410000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Tsui WM, Colombari R, Portmann BC, Bonetti F, Thung SN, Ferrell LD, Nakanuma Y, Snover DC, Bioulac-Sage P, Dhillon AP. Hepatic angiomyolipoma: a clinicopathologic study of 30 cases and delineation of unusual morphologic variants. Am J Surg Pathol. 1999;23:34–48. doi: 10.1097/00000478-199901000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Yamasaki S, Tanaka S, Fujii H, Matsumoto T, Okuda C, Watanabe G, Suda K. Monotypic epithelioid angiomyolipoma of the liver. Histopathology. 2000;36:451–456. doi: 10.1046/j.1365-2559.2000.00848.x. [DOI] [PubMed] [Google Scholar]
- 20.Dalle I, Sciot R, de Vos R, Aerts R, van Damme B, Desmet V, Roskams T. Malignant angiomyolipoma of the liver: a hitherto unreported variant. Histopathology. 2000;36:443–450. doi: 10.1046/j.1365-2559.2000.00891.x. [DOI] [PubMed] [Google Scholar]