Abstract
Rationale: Recruited leukocytes play an important role in ventilator-induced lung injury, although studies have focused predominantly on neutrophils. Inflammatory subset Gr-1high monocytes are recruited to sites of inflammation and have been implicated in acute lung injury induced by systemic endotoxin.
Objectives: To investigate the recruitment and role of Gr-1high monocytes in an in vivo mouse model of ventilator-induced lung injury.
Methods: Anesthetized mice were ventilated with low or high stretch. Flow cytometry was used to quantify monocyte subset margination to the lungs, and to assess their in situ cellular activation in response to mechanical stretch. To investigate monocyte involvement in lung injury progression, a two-hit model was used, with a subclinical dose of lipopolysaccharide (intraperitoneal) given 2 hours prior to high-stretch ventilation. In some animals, monocytes were depleted using intravenous clodronate liposomes. Development of lung injury was assessed in ventilated animals by peak inspiratory pressure and respiratory system mechanics.
Measurements and Main Results: High-stretch ventilation induced significant pulmonary margination of Gr-1high but not Gr-1low monocytes compared with nonventilated mice. These monocytes displayed increased activation status, with higher CD11b (vs. nonventilated mice) and lower L-selectin expression (vs. low-stretch ventilation). Lipopolysaccharide challenge led to enhanced lung margination of Gr-1high monocytes and neutrophils, and sensitized the lungs to high stretch–induced pulmonary edema. Clodronate-liposome pretreatment depleted lung monocytes (but not neutrophils) and significantly attenuated lung injury.
Conclusions: High-stretch mechanical ventilation promotes pulmonary margination of activated Gr-1high monocytes, which play a role in the progression of ventilator-induced lung injury.
Keywords: Gr-1high monocytes, acute lung injury, stretch, inflammation, mechanical ventilation
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Recruited leukocytes are known to play an important role in ventilator-induced lung injury (VILI), although studies have focused on neutrophils. Recent studies implicate lung-marginated monocytes in the development of other forms of acute lung injury.
What This Study Adds to the Field
This study demonstrates that inflammatory subset Gr-1high monocytes are recruited to the lung during high-stretch mechanical ventilation and contribute to the progression of pulmonary edema during VILI.
The development of ventilator-induced lung injury (VILI), produced by excessive lung stretch during mechanical ventilation, is known to have a significant impact on the outcome of clinical acute lung injury (ALI)/acute respiratory distress syndrome (ARDS) (1). VILI, both clinically and experimentally, is characterized by pulmonary edema and inflammation (2–4). Historically, analysis of the roles of leukocytes in VILI has focused predominantly on alveolar macrophages (5, 6) and recruited neutrophils (7–11), at least in part because of the ease of recovery and visual identification of these cells.
Recent evidence has suggested, however, that lung-recruited monocytes may also play a significant role in the pathogenesis of ALI (12, 13). Monocytes are a pluripotent and heterogeneous population of cells of the mononuclear phagocytic system, capable of releasing a variety of inflammatory mediators. Our understanding of these cells has dramatically increased over the last 5 years with the identification of phenotypically distinct monocyte subsets (14, 15). Immature monocytes (defined in mice as Gr-1high/CCR2+/CX3CR1low) enter the circulation from the bone marrow and have been shown to migrate to local sites of inflammation and injury (15–17). Hence, these Gr-1high cells have been termed inflammatory subset monocytes. During maturation of monocytes there is a change to a less inflammatory (Gr-1low/CCR2−/CX3CR1high) phenotype (18), cells of which are committed to differentiate into tissue macrophages and dendritic cells (15, 19). We have previously demonstrated using mouse models that monocytes are rapidly recruited to the lung microvasculature during systemic endotoxemia and that these marginated cells contribute to the development of ALI by promoting the activation of pulmonary endothelial cells (12). Furthermore, we have shown a clear role for lung-marginated inflammatory Gr-1high monocytes in the development of pulmonary edema in a lipopolysaccharide (LPS)/zymosan model of ALI (13).
Based on these findings, in the current study we investigated the potential role of monocytes in the development of VILI using an in vivo mouse model. We demonstrated that monocytes, specifically the inflammatory Gr-1high subset, were recruited to the lungs and activated during high-stretch mechanical ventilation. Depletion of Gr-1high monocytes in a clinically relevant, two-hit model of VILI attenuated the progression of VILI, suggesting a substantial role for lung-marginated monocytes in VILI development. Some of the results of these studies have been previously reported in the form of an abstract (20).
METHODS
Monocyte Recruitment/Activation during Mechanical Ventilation
All protocols were approved by the UK Home Office in accordance with the Animals (Scientific Procedures) Act 1986, UK. The in vivo mouse model of VILI has been described previously (11, 21), and additional details are provided in an online data supplement. In brief, anesthetized male C57BL6 mice (Charles River, Margale, MA) aged 8 to 12 weeks were tracheostomized and ventilated using a custom-made jet ventilator (21, 22). Animals were ventilated with either high-stretch (VT 34–36 ml/kg, 0 positive end-expiratory pressure) or low-stretch (VT 7–8 ml/kg, 2–3 cm H2O positive end-expiratory pressure) protocols. High-stretch ventilation with the same constant VT was continued until peak inspiratory pressure (PIP) increased by approximately 40%, indicating substantial pulmonary edema, which took between 140 and 200 minutes (mean 160 min). Low-stretch ventilation was continued for 180 minutes. A carotid artery cannula was used for blood pressure monitoring and blood gas analysis. Respiratory system compliance (Crs) and resistance (Rrs) were assessed periodically by end-inflation occlusion (22).
At termination, lung cell suspensions were prepared from excised lungs by mechanical disruption for flow cytometry analysis, as described in detail previously (11–13). Samples were stained with fluorophore-conjugated anti-mouse antibodies for CD11b, Gr-1 (Ly6C/G), F4/80, L-selectin, or appropriate isotype-matched controls, and then analyzed using a FACSCalibur cytometer with CellQuest (Becton Dickinson, Oxford, UK) and FlowJo (Tree Star, Ashland, OR) software. Full details of the leukocyte identification procedure are provided in the online data supplement. In brief, monocytes were identified as CD11b+, F4/80+ events and subsets defined as expressing either low or high Gr-1, and were differentiated from F4/80−, Gr-1 very high neutrophils. Cells were quantified using microsphere counting beads (Caltag Medsystems, Buckingham, UK) and activation assessed based on L-selectin and CD11b adhesion molecule expression.
Involvement of Monocytes in Pulmonary Edema Formation during Ventilator-induced Lung Injury
The involvement of recruited monocytes in the progression of VILI was assessed in a clinically relevant two-hit model. LPS challenge (20 ng/mouse; Ultrapure LPS, InVivoGen, San Diego, CA) was given intraperitoneally to induce a subclinical inflammation. In some animals, intravascular monocytes and macrophages were depleted by pretreatment with intravenous clodronate-loaded liposomes (200 μl, gift from Roche Diagnostics GmbH, Mannheim, Germany) given 24 or 48 hours prior to LPS challenge (13, 18, 23). Two hours after LPS challenge (with/without clodronate-liposome pretreatment), some animals were killed and neutrophil and Gr-1high monocyte numbers within the lungs determined, while other animals were anesthetized, instrumented, and ventilated with low or high stretch for 2 hours (see Figure 1 and online supplement for full details of treatment groups). In this two-hit model ventilation parameters were similar to those described above, except that for high stretch, a slightly lower VT (28–30 ml/kg) was used, because LPS challenge was expected to significantly exacerbate the degree of VILI. To further delineate the potential role of systemic monocyte activation by LPS, depletion experiments were also performed in the one-hit, pure VILI model using the ventilation parameters described above. VILI progression was evaluated by changes in PIP, Crs, and Rrs.
Figure 1.
Design of experimental groups for study of involvement of monocytes in pulmonary edema formation during ventilator-induced lung injury (VILI). (A) Two-hit VILI model. Mice were treated with/without clodronate-loaded liposomes (200 μl, tail vein) 24 or 48 hours before challenge with/without lipopolysaccharide (LPS) (20 ng, intraperitoneal [i.p.]). Two hours post LPS, some animals were killed to determine lung leukocyte margination and other mice were then instrumented and ventilated for 2 hours using high- or low-stretch ventilation. A slightly lower VT was used for this two-hit model with LPS than the one-hit model. (B) One-hit, pure VILI model. Mice were treated with/without clodronate-loaded liposomes 24 hours before either killing to determine lung leukocyte margination or 2 hours of high-stretch ventilation.
Statistical Analysis
Data are expressed as mean ± SD. Statistical comparisons were made by t tests or analysis of variance with Bonferroni tests using Prism software (version 4.0). Statistical significance was defined as P < 0.05.
RESULTS
Monocyte Recruitment/Activation during Mechanical Ventilation
High-stretch mechanical ventilation induced a substantial deterioration in lung function consistent with pulmonary edema formation, compared with animals exposed to low-stretch noninjurious ventilation (Table 1). Low-stretch ventilation for 180 minutes did not induce significant changes in PIP or blood gases, and only very small changes in Crs and Rrs. In contrast, high-stretch ventilation induced a substantial increase in PIP, associated with large changes in respiratory mechanics and deterioration in oxygenation.
TABLE 1.
PHYSIOLOGICAL VARIABLES OF ANIMALS VENTILATED WITH LOW- OR HIGH-STRETCH VENTILATION
| Low Stretch
|
High Stretch
|
|||
|---|---|---|---|---|
| 30 Minutes | End | 30 Minutes | End | |
| PIP, cm H2O | 11.2 ± 0.9 | 11.5 ± 0.8 | 29.2 ± 1.0 | 43.0 ± 1.0* |
| ΔCrs, % | — | −2.0 ± 4.4 | — | −30.4 ± 3.6† |
| ΔRrs, % | — | −6.7 ± 8.5 | — | 175 ± 30.7† |
| Po2, mm Hg | 105 ± 7.3 | 111 ± 6.8 | 139 ± 6.9 | 54.8 ± 15.6* |
| Pco2, mm Hg | 40.0 ± 3.9 | 35.0 ± 3.0 | 41.1 ± 3.9 | 46.9 ± 4.6* |
Definition of abbreviations: Crs = respiratory system compliance; PIP = peak inspiratory pressure; Rrs = respiratory system resistance.
Initial measurements were taken after 30 minutes of ventilation. Change in mechanics (ΔCrs, ΔRrs) was defined as the percentage of increase/decrease in Crs or Rrs at the end of protocol versus the 30-minute value. Note that air containing 4% CO2 was used for animals ventilated with high stretch to avoid excessive hypocapnia. N = 6–8.
P < 0.01 versus 30-minute value (paired t test).
P < 0.01 versus low stretch (unpaired t test).
After the ventilation protocols, margination of monocytes and neutrophils to the lungs was determined using previously validated flow cytometry methods (11–13). High-stretch ventilation promoted a significant increase in the number of Gr-1high monocytes within the lungs compared with nonventilated animals (Figure 2A). There was no change in the number of Gr-1low monocytes (Figure 2B), leading to a dramatic increase in the ratio of Gr-1high to Gr-1low monocytes within the lungs after high-stretch ventilation (Figure 2C). As anticipated, high-stretch ventilation also promoted a significant increase in the number of neutrophils within the lungs, compared with both nonventilated and low-stretch–ventilated mice (Figure 2D). As the recruitment of monocytes due to high-stretch ventilation was effectively limited to the Gr-1high subset, subsequent experiments focused on these cells.
Figure 2.
Numbers of (A) Gr-1high and (B) Gr-1low monocytes, (C) Gr-1high:Gr-1low ratio, and (D) number of neutrophils within the lung tissue of nonventilated mice, or mice ventilated with low- or high-stretch ventilation; n = 6–9. Cell counts were square-root transformed to ensure Gaussian distribution before analysis of variance with Bonferroni tests. NS = not significant. *P < 0.05, **P < 0.01, ***P < 0.001.
The activation status of lung-marginated Gr-1high monocytes was evaluated by assessing cell surface expression of L-selectin and CD11b. Lung-marginated Gr-1high monocytes displayed increased expression of CD11b compared with nonventilated mice, indicating increased activation (Figure 3A). Surface L-selectin expression was higher on lung-marginated Gr-1high monocytes after either ventilation protocol (high or low stretch) compared with nonventilated animals, suggesting an increase in the proportion of less mature (i.e., bone marrow–derived, high L-selectin–expressing) cells within the gated population. L-selectin expression was much lower on cells from high-stretch than low-stretch ventilated mice (Figure 3B), consistent with L-selectin shedding and hence greater activation of these monocytes after high-stretch compared with low-stretch ventilation.
Figure 3.
Expression of adhesion molecules (A) CD11b and (B) L-selectin on the surface of lung-marginated Gr-1high monocytes. Data are expressed as mean fluorescence intensity (MFI). n = 7–14 for CD11b and 5–10 for L-selectin. *P < 0.05, **P < 0.01, ***P < 0.001, by analysis of variance with Bonferroni tests.
Involvement of Monocytes in Pulmonary Edema Formation during Ventilator-induced Lung Injury
Based on these findings of increased Gr-1high monocyte margination and activation within the lungs during high-stretch ventilation, we investigated whether these cells may play a role in the development of high stretch–induced pulmonary edema. To investigate this, we used a two-hit model of VILI consisting of an intraperitoneal LPS challenge (subclinical dose) followed by mechanical ventilation. We considered this model to have a strong clinical relevance, as many intensive care patients are likely to have underlying systemic inflammation and consequently preexisting lung monocyte margination when they first receive ventilatory support. LPS challenge for 2 hours induced a substantial increase in the numbers of both Gr-1high monocytes and neutrophils within the lungs (i.e., increased margination prior to the start of ventilation) (Figure 4). The specific involvement of monocytes in this model was assessed through the use of intravenous injection of clodronate liposomes, a standard method used for depletion of monocytes and resident intravascular macrophages (24). A 24-hour clodronate-liposome treatment substantially reduced the numbers of marginated Gr-1high monocytes within the lungs in LPS-challenged mice, but had no effect on neutrophil numbers. In addition, alveolar macrophages (determined in lavage fluid by hemacytometer and differential cytology) were not affected at this time point by the intravenous clodronate-liposome treatment (1.8 ± 0.2 × 104 vs. 1.9 ± 0.5 × 104 alveolar macrophages in LPS-challenged mice, in the presence and absence of clodronate-liposome treatment, respectively). At 48 hours after clodronate-liposome treatment, marginated Gr-1high monocyte numbers were returned to the normal levels seen in LPS-challenged mice (Figure 4). These effects of clodronate liposomes on lung monocytes over time (i.e., effective ablation of LPS-induced monocyte margination at 24 hours but its restoration at 48 hours) are consistent with our previous findings with this method in C57BL6 mice (13).
Figure 4.
Numbers of (A) Gr-1high monocytes and (B) neutrophils in lung tissue of nonventilated mice. Animals were either untreated or challenged with lipopolysaccharide (LPS) for 2 hours. Of the LPS-challenged animals, a number were pretreated for 24 or 48 hours with intravenous clodronate liposomes (clod) before LPS. n = 3–10. Cell counts were square-root transformed to ensure Gaussian distribution before analysis of variance with Bonferroni tests. **P < 0.01, ***P < 0.001.
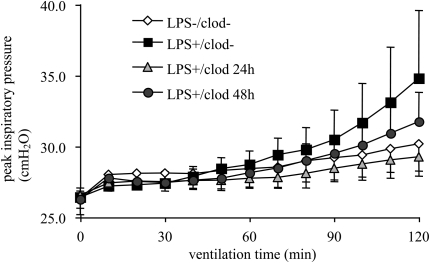
Having confirmed that LPS challenge substantially enhanced leukocyte margination to the lungs and that clodronate-liposome treatment had the desired effects, separate groups of treated animals were subjected to mechanical ventilation. Immediately after surgical instrumentation, baseline physiological parameters were assessed in these mice (Table 2). Neither LPS challenge nor clodronate-liposome pretreatment had any effect on the parameters measured, despite the differences in lung-marginated leukocyte numbers at this point. We then mechanically ventilated animals for 2 hours with high-stretch or low-stretch ventilation. Because a slightly smaller VT (28–30 ml/kg) was used in this model, the progression of VILI due solely to high-stretch ventilation (in the absence of LPS) was slower than in the previously described one-hit experiments (i.e., producing only mild deterioration in respiratory function with small changes in PIP) (Figure 5) and respiratory mechanics (Figure 6) within the 2-hour protocol. LPS challenge, however, caused a sensitization of the lung to high-stretch ventilation, as shown by greater PIP increase over time and larger mechanics changes (Figures 5 and 6). LPS-challenged animals ventilated with low-stretch ventilation, on the other hand, showed no substantial increase in PIP with negligible changes in respiratory mechanics (ΔCrs 1.1 ± 6.3%, ΔRrs −10.4 ± 6.8%; n = 4), indicating that LPS challenge (and the consequent systemic inflammation) per se did not affect lung function in this model. Animals pretreated with clodronate liposomes for 24 hours prior to LPS demonstrated significantly attenuated VILI in response to high-stretch ventilation (compared with mice without clodronate-liposome pretreatment) in terms of final PIP, ΔCrs, and ΔRrs (Figures 5 and 6). In contrast, animals pretreated with clodronate liposomes for 48 hours before LPS challenge showed no significant attenuation of injury (Figures 5 and 6).
TABLE 2.
BASELINE PHYSIOLOGICAL VARIABLES OF HIGH-STRETCH VENTILATED ANIMALS WITH/WITHOUT TREATMENTS
| No Pretreatment | LPS | LPS + clod 24 h | LPS + clod 48 h | |
|---|---|---|---|---|
| BP, mm Hg | 83 ± 14 | 80 ± 10 | 85 ± 14 | 91 ± 21 |
| Crs, ml/kg/cm H2O | 1.66 ± 0.09 | 1.57 ± 0.15 | 1.56 ± 0.11 | 1.62 ± 0.19 |
| Rrs, cm H2O/ml/s | 1.62 ± 0.10 | 1.62 ± 0.16 | 1.74 ± 0.31 | 1.70 ± 0.20 |
| Po2, mm Hg | 125 ± 10 | 128 ± 8.5 | 125 ± 11 | 119 ± 11 |
| Pco2, mm Hg | 36.3 ± 2.2 | 34.3 ± 2.5 | 33.0 ± 4.0 | 33.6 ± 2.9 |
| pH | 7.372 ± 0.04 | 7.377 ± 0.04 | 7.437 ± 0.02* | 7.385 ± 0.04 |
Definition of abbreviations: BP = mean arterial blood pressure; clod = clodronate liposomes; Crs = respiratory system compliance; LPS = lipopolysaccharide; Rrs = respiratory system resistance.
Baseline physiological variables (i.e., before change to high-stretch protocol) of either untreated animals, LPS-challenged animals, or animals treated with clodronate liposomes for 24 or 48 hours and then challenged with LPS.
* P < 0.05 versus LPS challenged mice by analysis of variance with Bonferroni tests.
Figure 5.
Peak inspiratory pressure changes during high-stretch mechanical ventilation of either untreated animals, lipopolysaccharide (LPS)–challenged animals, or animals pretreated with clodronate liposomes (clod) for 24 or 48 hours and then challenged with LPS. Pressure changes over time during ventilation were different between the treatment groups as indicated by a significant interaction P value by 2-way analysis of variance (P < 0.001).
Figure 6.
(A) Final peak inspiratory pressure (PIP) and (B) change in respiratory system compliance and (C) resistance during high-stretch ventilation of either untreated animals, lipopolysaccharide (LPS)–challenged animals, or animals pretreated with clodronate liposomes (clod) for 24 or 48 hours and then challenged with LPS. Mechanics changes are expressed as percentage of increase in respiratory system compliance (Crs) or respiratory system resistance (Rrs) between 30 minutes after start of protocol and the end of protocol. n = 4–6. NS = not significant. *P < 0.05, **P < 0.01, by analysis of variance with Bonferroni tests.
Finally, to address the probability that lung margination, rather than systemic activation of monocytes by LPS, was primarily responsible for the exacerbation of VILI in the two-hit model, the impact of monocyte depletion was also assessed in the one-hit, pure VILI model. At 24 hours after clodronate-liposome treatment, Gr-1high monocyte numbers within the lung (in the absence of LPS) were substantially lower (60–70%) than those in untreated mice, again with no effect on lung neutrophil number (Figure 7). Separate groups of untreated and monocyte-depleted animals were then subjected to high-stretch ventilation. PIP increased in response to high stretch in both groups of animals, but the increase in PIP over time showed a small but significant attenuation in animals pretreated with clodronate liposomes compared with untreated mice (Figure 8, P < 0.05 for interaction between treatment and ventilation time). Changes in Rrs at the end of the protocol, although not Crs, were also significantly attenuated by clodronate-liposome pretreatment.
Figure 7.
Numbers of (A) Gr-1high monocytes and (B) neutrophils in lung tissue of nonventilated mice. Animals were either untreated or pretreated for 24 hours with intravenous clodronate liposomes (clod) prior to culling. n = 3–4. Cell counts were square-root transformed to ensure Gaussian distribution before analysis by unpaired t tests. **P < 0.01.
Figure 8.
(A) Peak inspiratory pressure changes during high-stretch mechanical ventilation of either untreated animals or animals pretreated with clodronate liposomes for 24 hours before ventilation. Pressure changes over time during ventilation were different between the treatment groups as indicated by a significant interaction P value by two-way analysis of variance (P < 0.05). (B) Change in respiratory system compliance and (C) resistance, during high-stretch ventilation of either untreated animals or animals pretreated with clodronate liposomes for 24 hours before ventilation. Mechanics changes are expressed as percentage of increase in respiratory system compliance (Crs) or respiratory system resistance (Rrs) between 30 minutes after start of protocol and the end of protocol. n = 5. *P < 0.05, by unpaired t tests. clod = clodronate liposomes; VILI = ventilator-induced lung injury.
DISCUSSION
The purpose of the current study was to investigate the role of lung-marginated monocytes in the development of VILI. The potential involvement of monocytes in ALI in general has been largely overlooked, partly because they are less easy to identify in biological tissues than neutrophils for example, and partly because it is only in the last few years that the importance of monocyte subset functional heterogeneity has started to become fully appreciated (14, 25). The flow cytometric method used in the current study enables quantification of lung-marginated monocytes, and their categorization into inflammatory and resident subsets (13, 15, 18), thus allowing the involvement of these cells in VILI to be evaluated for the first time.
We found that mechanical ventilation, in particular high-stretch ventilation associated with substantial deterioration in pulmonary function, induced the margination of significant numbers of monocytes to the lung. The newly recruited monocytes were of the Gr-1high subset and there was no change in the numbers of Gr-1low monocytes within the lungs. These findings are consistent with our previous observation that specifically Gr-1high monocytes marginate to the pulmonary microcirculation in response to systemic endotoxin (13). The number of monocytes recruited to the lungs after 2 to 3 hours of high-stretch ventilation was comparable to that of recruited neutrophils, although the Gr-1high monocyte:neutrophil proportion in the lungs was smaller than that observed in response to systemic endotoxin, wherein the number of Gr-1high monocytes equals or even exceeds the number of neutrophils (13). The mechanisms by which Gr-1high monocytes are recruited to the lung by stretch are not addressed in this study, although high-stretch ventilation has been shown to increase lavage fluid concentrations of the chemokine CCL2/monocyte chemoattractant protein-1 (26). The receptor for CCL2 (CCR2) is preferentially expressed on Gr-1high (and not Gr-1low) monocytes (15), and CCL2 is considered to be central to the recruitment of monocytes to inflammatory sites (17, 27). Importantly, Gr-1high monocytes were recruited to the lungs within the same time frame as were neutrophils, although the current data do not allow us to determine which, if either, cell is recruited into the lung first, and precisely when the process begins. As only Gr-1high monocytes were recruited by high-stretch ventilation we chose to focus our attention on this subset. However, it has recently been proposed that Gr-1low resident monocytes may play a patrolling role in certain microvascular beds, such as mesenteric postcapillary venules (28), so their potential involvement within the lung cannot be entirely discounted.
The activation status of lung-marginated Gr-1high monocytes was assessed through the surface expression levels of the adhesion molecules CD11b and L-selectin, both of which are expressed on Gr-1high monocytes as well as neutrophils. CD11b levels were significantly increased with high-stretch ventilation versus nonventilated animals, whereas L-selectin levels were significantly reduced with high-stretch versus low-stretch ventilation, consistent with increased proteolytic shedding of L-selectin (i.e., increased activation after high-stretch ventilation). Interestingly, Gr-1high monocytes from the lungs of both high and low stretch–ventilated animals had higher levels of surface L-selectin than untreated control animals. High levels of surface L-selectin are considered to be a marker of immature monocytes (29), suggesting that both ventilation strategies recruit newly mobilized monocytes (as opposed to cells demarginated from other organs) to the lungs. Indeed, we have shown that a major proportion of lung-marginated Gr-1high monocytes during systemic endotoxemia come directly from bone marrow, rather than from the circulating pool (13). Overall, the data indicate that both high- and low-stretch ventilation (or some other associated factor, such as anesthesia or surgery) may induce some degree of Gr-1high monocyte margination to the lungs, but that high-stretch ventilation is associated with both greater recruitment and greater monocyte activation.
Having found that high-stretch mechanical ventilation promotes both the recruitment and activation of Gr-1high monocytes within the lung, we investigated whether these lung-marginated monocytes may play any role in the development of high stretch–induced pulmonary edema. To investigate this we used two complementary approaches, first promoting the recruitment of additional monocytes to the lungs using systemic LPS administration, and second depleting circulating and lung-marginated monocytes using intravenous clodronate-loaded liposomes. Consistent with our previous study with intravenous LPS (13), the use of a low, subclinical dose of intraperitoneal LPS caused substantial margination of both Gr-1high monocytes and neutrophils to the lungs, without producing any manifestations of sickness or changes in lung function prior to the start of ventilation. Subsequent low-stretch ventilation did not induce any change in respiratory parameters, indicating that LPS treatment per se did not induce lung dysfunction. However, high-stretch ventilation in LPS-challenged mice induced a substantial worsening of VILI compared with animals that did not receive LPS, indicating that systemic inflammation sensitizes the lungs to the effects of high-stretch ventilation. It is well known that preinjured or prestimulated lungs are more sensitive to the effects of mechanical ventilation (30–35). Such sensitization has previously been ascribed to synergistic increases in soluble mediators (26, 31–33) and/or increased neutrophil load within the lungs (30, 32, 35), but the involvement of monocytes has never been described.
To dissect out the contribution of enhanced margination of Gr-1high monocytes versus neutrophils in this sensitization, we performed monocyte depletion experiments using clodronate liposomes. This substantially depleted Gr-1high monocytes from the lungs after 24 hours and substantially attenuated the development of VILI, in terms of lower PIP after 2 hours of high stretch and smaller changes in both resistance and compliance. Importantly, this treatment had no impact on the numbers of neutrophils or alveolar macrophages within the lungs, depletion of either of which would be expected to affect VILI progression. To exclude the possibility that these effects may be related to off-target effects of clodronate liposomes (e.g., effects on splenic macrophages or Kupffer cells, which would also be depleted at this 24-h time point [36, 37]), a separate group of mice was left for 48 hours after clodronate-liposome pretreatment before experimentation. At this time point the number of Gr-1high monocytes within the lung returned to normal (i.e., levels without clodronate-liposome pretreatment), whereas liver and splenic macrophages are not expected to start to reappear until 5 to 6 days post depletion (36, 37). In contrast to the findings at 24 hours, there was no attenuation of VILI at 48 hours after clodronate-liposome pretreatment. Together these data strongly indicate a role for lung-marginated monocytes in VILI progression.
It is possible in this two-hit model that circulating monocytes may be activated after the LPS injection, thus producing various cytokines and exacerbating the development of VILI. Although we are not able to completely exclude this possibility, we speculate that this is of lesser importance than lung-marginated cells, because (1) mice treated with LPS and low-stretch ventilation did not develop lung dysfunction, and (2) monocyte depletion tended to reduce injury in pure VILI experiments, in the absence of systemic activation. In these pure VILI experiments the high stretch–induced change in PIP was marginally attenuated by monocyte depletion, and this was associated with a reduction in the deterioration of Rrs but not Crs. We suggest that the more obvious attenuation of injury in the two-hit versus the one-hit model of VILI relates to the degree of premargination of monocytes caused by LPS challenge, such that monocytes are already present (and perhaps primed) when injurious ventilation begins. The two-hit scenario is more likely to reflect the clinical situations, wherein many patients who require ventilator support would already have underlying inflammation and preexisting monocyte margination within the lung. The precise mechanisms by which monocytes play a role in the progression of lung injury induced by high-stretch ventilation are not yet clear. We have previously demonstrated that monocytes recruited to the lung during systemic endotoxemia express increased levels of membrane-associated TNF, and that these monocytes activate pulmonary endothelial cells in a cell contact–dependent, TNF-mediated manner (12), although whether such a mechanism occurs in VILI is not known.
Historically, the involvement of recruited leukocytes in VILI has focused on neutrophils for a number of reasons: (1) neutrophils are easily identifiable and accumulate within a readily accessible compartment (alveolar space) during clinical ALI/ARDS and experimental VILI (7, 38), and (2) attenuation of lung neutrophil recruitment, by depletion of circulating neutrophils (39), interference with leukocyte adhesion processes (40), or inhibition of leukocyte chemoattractants (8) can reduce injury. However, the supposedly critical role of neutrophils in ALI progression may need to be reevaluated in light of emerging evidence. For example, it is known that simple recruitment of neutrophils into the lungs does not necessarily induce edema (41), and that ALI can develop in neutropenic patients (42). In addition, it has been reported that Gfi-deficient mice (lacking a zinc finger protein that acts as a transcriptional repressor) are highly susceptible to LPS-induced pulmonary inflammation (43). These animals are neutropenic but display increased cytokine production and substantial monocyte infiltration to the lungs, which could play a substantial role in the enhanced sensitivity of these mice to inflammatory stimuli. Furthermore, we have recently demonstrated in a mouse model of VILI that pulmonary edema formation is modulated by TNF signaling in a manner that is independent of the degree of pulmonary neutrophil recruitment (44). The current data strongly support a role for recruited monocytes in the development of VILI, although whether this is independent of, or intertwined with, neutrophil biology is unclear. The role of monocytes in all forms of ALI may have been substantially underestimated (and potentially, the role of neutrophils overestimated) in the past, because a number of the methods used to quantify and interfere with neutrophil biology, such as anti Gr-1 antibodies, myelosuppressing pharmacological agents, and adhesion molecule antagonists, are not neutrophil specific and are known (or likely) to affect Gr-1high monocytes also (13, 45, 46). Even blockade of neutrophil chemoattractants, such as CXCL2 (MIP-2/KC), is likely to alter monocyte biology, as monocyte trafficking is substantially influenced by CXCL2 (47). Overall, although the involvement of neutrophils is not heavily disputed, the important roles of other inflammatory cell types in ALI are becoming more greatly appreciated.
There are a number of potential limitations regarding the clinical relevance of the methods used in the current study, which warrant some discussion. First, the VT used to produce VILI was greater than would be used in the clinical setting in humans. However, the results should still give important insights into the pathophysiology of VILI, as the lung stretch induced in healthy lungs by such VT may not be dissimilar from that experienced clinically (48), due to the loss of aerated lung capacity (“baby lung”) with ARDS (49). In addition, directly comparing the absolute values of either VT or inspiratory pressure between mice and humans is likely to be misleading, as the mouse respiratory system mechanics are very different from other species, such that intact mouse lungs can be temporarily inflated to pressures above 60 cm H2O (relating to a VT of 60–70 ml/kg) without reaching a traditionally defined total lung capacity or producing morphological damage (50). Second, although we have not assessed the role of monocytes on indices of lung injury (e.g., morphology, wet:dry weight) in addition to respiratory mechanics, we (21, 44) and others (51) have previously demonstrated that in the case of VILI, changes in respiratory mechanics correlate very closely with these other lung injury markers. Finally, the flow cytometry–based quantification of lung-marginated monocytes used in this study may underestimate the precise number of cells within the tissue, as the recovery of cells may not be complete during the preparation of lung cell suspensions due to their tendency to adhere to the glassware. However, the observation that the number of Gr-1high monocytes increases during VILI, whereas that of Gr-1low monocytes does not, indicates that the relative changes in marginated Gr-1high monocytes during the different ventilation strategies are likely to be realistic. In addition, we have previously shown that for neutrophils, flow cytometric quantification agrees very closely with quantification by myeloperoxidase activity assay (11). Currently there is no established alternative to flow cytometry for the detection and quantification of monocyte subsets within tissue samples, although we have demonstrated using immunohistochemistry that the density of Gr-1 positive cells within the lungs (Gr-1high monocytes plus neutrophils) is increased in response to systemic LPS challenge, consistent with changes determined by flow cytometry (13).
In conclusion, we have shown for the first time that high-stretch mechanical ventilation promotes the margination of activated Gr-1high inflammatory monocytes within the lung, and that these monocytes are involved in the development of stretch-induced pulmonary edema. The current data support an important, novel role for lung-marginated Gr-1high monocytes in the pathophysiology of VILI.
Supplementary Material
Acknowledgments
The authors thank Mr. M.E. Goddard for his invaluable contribution in development of the animal model.
Supported by grants from Biotechnology and Biological Sciences Research Council UK (#D01820X), Medical Research Council UK (#G0000101), and Wellcome Trust (#081208).
This article has an online supplement, which is available from the issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200806-877OC on February 12, 2009
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000;342:1301–1308. [DOI] [PubMed] [Google Scholar]
- 2.Dreyfuss D, Saumon G. Ventilator-induced lung injury: lessons from experimental studies. Am J Respir Crit Care Med 1998;157:294–323. [DOI] [PubMed] [Google Scholar]
- 3.Ranieri VM, Suter PM, Tortorella C, De Tullio R, Dayer JM, Brienza A, Bruno F, Slutsky AS. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. JAMA 1999;282:54–61. [DOI] [PubMed] [Google Scholar]
- 4.Tremblay L, Valenza F, Ribeiro SP, Li J, Slutsky AS. Injurious ventilatory strategies increase cytokines and c-fos m-RNA expression in an isolated rat lung model. J Clin Invest 1997;99:944–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frank JA, Wray CM, McAuley DF, Schwendener R, Matthay MA. Alveolar macrophages contribute to alveolar barrier dysfunction in ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol 2006;291:L1191–L1198. [DOI] [PubMed] [Google Scholar]
- 6.Takata M, Abe J, Tanaka H, Kitano Y, Doi S, Kohsaka T, Miyasaka K. Intraalveolar expression of tumor necrosis factor-alpha gene during conventional and high-frequency ventilation. Am J Respir Crit Care Med 1997;156:272–279. [DOI] [PubMed] [Google Scholar]
- 7.Imanaka H, Shimaoka M, Matsuura N, Nishimura M, Ohta N, Kiyono H. Ventilator-induced lung injury is associated with neutrophil infiltration, macrophage activation, and TGF-beta 1 mRNA upregulation in rat lungs. Anesth Analg 2001;92:428–436. [DOI] [PubMed] [Google Scholar]
- 8.Belperio JA, Keane MP, Burdick MD, Londhe V, Xue YY, Li K, Phillips RJ, Strieter RM. Critical role for CXCR2 and CXCR2 ligands during the pathogenesis of ventilator-induced lung injury. J Clin Invest 2002;110:1703–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson MR, Choudhury S, Takata M. Pulmonary inflammation induced by high-stretch ventilation is mediated by tumor necrosis factor signaling in mice. Am J Physiol Lung Cell Mol Physiol 2005;288:L599–L607. [DOI] [PubMed] [Google Scholar]
- 10.Quinn DA, Moufarrej RK, Volokhov A, Hales CA. Interactions of lung stretch, hyperoxia, and MIP-2 production in ventilator-induced lung injury. J Appl Physiol 2002;93:517–525. [DOI] [PubMed] [Google Scholar]
- 11.Choudhury S, Wilson MR, Goddard ME, O'Dea KP, Takata M. Mechanisms of early pulmonary neutrophil sequestration in ventilator-induced lung injury in mice. Am J Physiol Lung Cell Mol Physiol 2004;287:L902–L910. [DOI] [PubMed] [Google Scholar]
- 12.O'Dea KP, Young AJ, Yamamoto H, Robotham JL, Brennan FM, Takata M. Lung-marginated monocytes modulate pulmonary microvascular injury during early endotoxemia. Am J Respir Crit Care Med 2005;172:1119–1127. [DOI] [PubMed] [Google Scholar]
- 13.O'Dea KP, Wilson MR, Dokpesi JO, Wakabayashi K, Tatton L, van Rooijen N, Takata M. Mobilization and margination of bone marrow Gr-1high monocytes during sub-clinical endotoxemia predisposes the lungs towards acute injury. J Immunol 2009;182:1155–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol 2005;5:953–964. [DOI] [PubMed] [Google Scholar]
- 15.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 2003;19:71–82. [DOI] [PubMed] [Google Scholar]
- 16.Henderson RB, Hobbs JA, Mathies M, Hogg N. Rapid recruitment of inflammatory monocytes is independent of neutrophil migration. Blood 2003;102:328–335. [DOI] [PubMed] [Google Scholar]
- 17.Tsou CL, Peters W, Si Y, Slaymaker S, Aslanian AM, Weisberg SP, Mack M, Charo IF. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest 2007;117:902–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sunderkotter C, Nikolic T, Dillon MJ, Van Rooijen N, Stehling M, Drevets DA, Leenen PJ. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol 2004;172:4410–4417. [DOI] [PubMed] [Google Scholar]
- 19.Landsman L, Varol C, Jung S. Distinct differentiation potential of blood monocyte subsets in the lung. J Immunol 2007;178:2000–2007. [DOI] [PubMed] [Google Scholar]
- 20.Wilson MR, O'Dea KP, Shearman AD, Goddard ME, Takata M. Margination of activated monocytes to the lung in response to high stretch ventilation in mice. Am J Respir Crit Care Med 2007;175:A223. [Google Scholar]
- 21.Wilson MR, Choudhury S, Goddard ME, O'Dea KP, Nicholson AG, Takata M. High tidal volume upregulates intrapulmonary cytokines in an in vivo mouse model of ventilator-induced lung injury. J Appl Physiol 2003;95:1385–1393. [DOI] [PubMed] [Google Scholar]
- 22.Ewart S, Levitt R, Mitzner W. Respiratory system mechanics in mice measured by end-inflation occlusion. J Appl Physiol 1995;79:560–566. [DOI] [PubMed] [Google Scholar]
- 23.van Rooijen N, Sanders A. Elimination, blocking, and activation of macrophages: three of a kind? J Leukoc Biol 1997;62:702–709. [DOI] [PubMed] [Google Scholar]
- 24.Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods 1994;174:83–93. [DOI] [PubMed] [Google Scholar]
- 25.Tacke F, Randolph GJ. Migratory fate and differentiation of blood monocyte subsets. Immunobiology 2006;211:609–618. [DOI] [PubMed] [Google Scholar]
- 26.Altemeier WA, Matute-Bello G, Frevert CW, Kawata Y, Kajikawa O, Martin TR, Glenny RW. Mechanical ventilation with moderate tidal volumes synergistically increases lung cytokine response to systemic endotoxin. Am J Physiol Lung Cell Mol Physiol 2004;287:L533–L542. [DOI] [PubMed] [Google Scholar]
- 27.Maus U, von Grote K, Kuziel WA, Mack M, Miller EJ, Cihak J, Stangassinger M, Maus R, Schlondorff D, Seeger W, et al. The role of CC chemokine receptor 2 in alveolar monocyte and neutrophil immigration in intact mice. Am J Respir Crit Care Med 2002;166:268–273. [DOI] [PubMed] [Google Scholar]
- 28.Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 2007;317:666–670. [DOI] [PubMed] [Google Scholar]
- 29.Goto Y, Hogg JC, Whalen B, Shih CH, Ishii H, Van Eeden SF. Monocyte recruitment into the lungs in pneumococcal pneumonia. Am J Respir Cell Mol Biol 2004;30:620–626. [DOI] [PubMed] [Google Scholar]
- 30.Schreiber T, Hueter L, Schwarzkopf K, Hohlstein S, Schmidt B, Karzai W. Increased susceptibility to ventilator-associated lung injury persists after clinical recovery from experimental endotoxemia. Anesthesiology 2006;104:133–141. [DOI] [PubMed] [Google Scholar]
- 31.Lin SM, Lin HC, Lee KY, Huang CD, Liu CY, Wang CH, Kuo HP. Ventilator-induced injury augments interleukin-1beta production and neutrophil sequestration in lipopolysaccharide-treated lungs. Shock 2007;28:453–460. [DOI] [PubMed] [Google Scholar]
- 32.Altemeier WA, Matute-Bello G, Gharib SA, Glenny RW, Martin TR, Liles WC. Modulation of lipopolysaccharide-induced gene transcription and promotion of lung injury by mechanical ventilation. J Immunol 2005;175:3369–3376. [DOI] [PubMed] [Google Scholar]
- 33.Nakamura T, Malloy J, McCaig L, Yao LJ, Joseph M, Lewis J, Veldhuizen R. Mechanical ventilation of isolated septic rat lungs: effects on surfactant and inflammatory cytokines. J Appl Physiol 2001;91:811–820. [DOI] [PubMed] [Google Scholar]
- 34.Rasaiah VP, Malloy JL, Lewis JF, Veldhuizen RA. Early surfactant administration protects against lung dysfunction in a mouse model of ARDS. Am J Physiol Lung Cell Mol Physiol 2003;284:L783–L790. [DOI] [PubMed] [Google Scholar]
- 35.Bregeon F, Delpierre S, Chetaille B, Kajikawa O, Martin TR, Autillo-Touati A, Jammes Y, Pugin J. Mechanical ventilation affects lung function and cytokine production in an experimental model of endotoxemia. Anesthesiology 2005;102:331–339. [DOI] [PubMed] [Google Scholar]
- 36.Yamamoto T, Naito M, Moriyama H, Umezu H, Matsuo H, Kiwada H, Arakawa M. Repopulation of murine Kupffer cells after intravenous administration of liposome-encapsulated dichloromethylene diphosphonate. Am J Pathol 1996;149:1271–1286. [PMC free article] [PubMed] [Google Scholar]
- 37.van Rooijen N, Kors N, Kraal G. Macrophage subset repopulation in the spleen: differential kinetics after liposome-mediated elimination. J Leukoc Biol 1989;45:97–104. [DOI] [PubMed] [Google Scholar]
- 38.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000;342:1334–1349. [DOI] [PubMed] [Google Scholar]
- 39.Kawano T, Mori S, Cybulsky M, Burger R, Ballin A, Cutz E, Bryan AC. Effect of granulocyte depletion in a ventilated surfactant-depleted lung. J Appl Physiol 1987;62:27–33. [DOI] [PubMed] [Google Scholar]
- 40.Rimensberger PC, Fedorko L, Cutz E, Bohn DJ. Attenuation of ventilator-induced acute lung injury in an animal model by inhibition of neutrophil adhesion by leumedins (NPC 15669). Crit Care Med 1998;26:548–555. [DOI] [PubMed] [Google Scholar]
- 41.Martin TR. Neutrophils and lung injury: getting it right. J Clin Invest 2002;110:1603–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ognibene FP, Martin SE, Parker MM, Schlesinger T, Roach P, Burch C, Shelhamer JH, Parrillo JE. Adult respiratory distress syndrome in patients with severe neutropenia. N Engl J Med 1986;315:547–551. [DOI] [PubMed] [Google Scholar]
- 43.Jin J, Zeng H, Schmid KW, Toetsch M, Uhlig S, Moroy T. The zinc finger protein Gfi1 acts upstream of TNF to attenuate endotoxin-mediated inflammatory responses in the lung. Eur J Immunol 2006;36:421–430. [DOI] [PubMed] [Google Scholar]
- 44.Wilson MR, Goddard ME, O'Dea KP, Choudhury S, Takata M. Differential roles of p55 and p75 tumor necrosis factor receptors on stretch-induced pulmonary edema in mice. Am J Physiol Lung Cell Mol Physiol 2007;293:L60–L68. [DOI] [PubMed] [Google Scholar]
- 45.Rydstrom A, Wick MJ. Monocyte recruitment, activation, and function in the gut-associated lymphoid tissue during oral salmonella infection. J Immunol 2007;178:5789–5801. [DOI] [PubMed] [Google Scholar]
- 46.Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol 2008;83:64–70. [DOI] [PubMed] [Google Scholar]
- 47.Smith DF, Galkina E, Ley K, Huo Y. Gro family chemokines are specialized for monocyte arrest from flow. Am J Physiol Heart Circ Physiol 2005;289:H1976–H1984. [DOI] [PubMed] [Google Scholar]
- 48.Slutsky AS, Tremblay LN. Multiple system organ failure. Is mechanical ventilation a contributing factor? Am J Respir Crit Care Med 1998;157:1721–1725. [DOI] [PubMed] [Google Scholar]
- 49.Gattinoni L, Caironi P, Pelosi P, Goodman LR. What has computed tomography taught us about the acute respiratory distress syndrome? Am J Respir Crit Care Med 2001;164:1701–1711. [DOI] [PubMed] [Google Scholar]
- 50.Soutiere SE, Mitzner W. On defining total lung capacity in the mouse. J Appl Physiol 2004;96:1658–1664. [DOI] [PubMed] [Google Scholar]
- 51.Sibilla S, Tredici S, Porro A, Irace M, Guglielmi M, Nicolini G, Tredici G, Valenza F, Gattinoni L. Equal increases in respiratory system elastance reflect similar lung damage in experimental ventilator-induced lung injury. Intensive Care Med 2002;28:196–203. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.