Abstract
Rationale: Chorioamnionitis frequently associates with preterm delivery and increased amniotic fluid IL-1, and causes fetal lung and systemic inflammation. However, chorioamnionitis is also associated with a paradoxical reduction in the incidence of surfactant deficiency–related respiratory distress syndrome in preterm infants.
Objectives: To identify the role of IL-1 signaling in the mediation of pulmonary and systemic inflammation and lung maturation in a fetal sheep model of lipopolysaccharide (LPS) induced chorioamnionitis.
Methods: After confirming the efficacy of recombinant human IL-1 receptor antagonist (rhIL-1ra), fetal sheep were exposed to intraamniotic (IA) injections of Escherichia coli LPS with or without prior IA injections of rhIL-1ra. Preterm lambs were delivered at 82% of term gestation.
Measurements and Main Results: rhIL-1ra decreased IA LPS–induced lung inflammation assessed by decreased lung neutrophil and monocyte influx, inducible nitric oxide synthase expression, lung IL-6 and IL-1β mRNA expression, and airway myeloperoxidase concentrations. rhIL-1ra inhibited IA LPS–induced fetal systemic inflammation assessed by decreased plasma IL-8, protein carbonyls, blood neutrophilia, and the expression of serum amyloid A3 mRNA in the liver. rhIL-1ra also partially blocked the lung maturational effects of IA LPS. Therefore blockade of IL-1 signaling in the amniotic compartment inhibited fetal lung and systemic inflammation and lung maturation in response to LPS-induced chorioamnionitis.
Conclusions: IL-1 plays a central role in the pathogenesis of chorioamnionitis-induced fetal inflammatory responses.
Keywords: respiratory distress syndrome, bronchopulmonary dysplasia, preterm birth, interleukin-1 receptor, innate immunity
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Chorioamnionitis is associated with pulmonary and systemic inflammation, an increase in pulmonary surfactant, and organ injury responses in the fetus. The mediator/s of these responses are not known.
What This Study Adds to the Field
Inhibition of IL-1 signaling in the amniotic compartment decreased pulmonary and systemic inflammation and reversed lung maturation responses in a fetal sheep model of chorioamnionitis induced by intraamniotic injection of lipopolysaccharide.
Approximately 70% of very low birth weight infants born before 28 weeks of gestation are exposed to chorioamnionitis, defined as inflammation of the fetal membranes (1). Although fetal exposures to chorioamnionitis are associated with the benefit of decreased incidence of respiratory distress syndrome (2, 3), chorioamnionitis is associated with increased risks of brain injury, necrotizing enterocolitis, and bronchopulmonary dysplasia (3–6). Although the mechanisms of organ injury associated with chorioamnionitis remain undetermined, pathogen-induced fetal inflammatory responses are thought to play a major role in the pathogenesis (7). Unlike systemic inflammation in the adult, fetal inflammatory responses are more subtle and often clinically asymptomatic. Fetal inflammatory response is clinically recognized by increased plasma IL-6 levels or by umbilical cord inflammation (5, 7). IL-1 is consistently increased in amniotic fluid with infection/chorioamnionitis (8–10). At present there are no treatments for chorioamnionitis-associated fetal inflammation.
In the fetal sheep, intraamniotic injection of LPS causes chorioamnionitis and induces lung maturation (11, 12). However, intraamniotic lipopolysaccharide (LPS) also decreases alveolar septation in the fetal lung (similar to bronchopulmonary dysplasia) (13, 14), induces a systemic inflammatory response, and causes axonal disruption in the subcortical white matter of the fetal brain (similar to periventricular leukomalacia) (15). Intraamniotic LPS robustly induces IL-1β expression in the chorioamnion and fetal lung (16). Intraamniotic or intratracheal injections of IL-1α or IL-1β cause chorioamnionitis, induce lung inflammation, and enhance lung maturation (17, 18). It is noteworthy that intraamniotic injections of other early response cytokines such as tumor necrosis factor (TNF)-α, IL-8, or IL-6 do not cause chorioamnionitis (19, 20). However, it is not known whether IL-1 signaling is required for intraamniotic LPS–induced lung/systemic inflammation or lung maturation.
We tested the hypothesis that IL-1 mediates intraamniotic LPS–induced pulmonary and systemic inflammation and lung maturation. Recombinant human IL-1 receptor antagonist (rhIL-1ra) was used to block the receptor IL-1R1, thereby inhibiting signaling by both IL-1α and IL-1β (21, 22). Preterm fetal lambs were exposed to intraamniotic Escherichia coli LPS and the effects of blockade with rhIL-1ra on fetal inflammation and lung maturation were assessed.
METHODS
Detailed methods are reported in the online supplement.
Animals and Intraamniotic Injections
A total of 77 fetal lambs were studied in Western Australia with approval from the animal care and use committees of the Cincinnati Children's Hospital (Cincinnati, OH), the Department of Agriculture and Food Western Australia (South Perth, WA, Australia), and the University of Western Australia (Perth, WA, Australia). In separate protocols, time-mated Merino ewes with singleton fetuses were randomly assigned to groups of five to nine animals (see Tables S1 and S2 in the online supplement), to receive either 10 mg of LPS (E. coli 055:B5; Sigma, St. Louis, MO), 100 μg of recombinant sheep IL-1α (Protein Express, Cincinnati, OH), or an equivalent 2-ml volume of saline (control) by intraamniotic injection at intervals of 2 or 7 days before caesarean delivery at 124 ± 1 day gestational age (11). IL-1 signaling was inhibited with rhIL-1ra (anakinra [Kineret]; Amgen, Inc., Thousand Oaks, CA). Lambs were given 100 mg of rhIL-1ra or saline (control) via the amniotic fluid only, 3 hours before intraamniotic LPS (10 mg) or saline injection. Animals delivered 2 days after LPS exposure received only 1 dose, whereas animals delivered 6 or 7 days after LPS exposure received two additional intraamniotic 100-mg doses of rhIL-1ra or saline treatment at 2 and 4 days.
rhIL-1ra Levels and Blood Counts
rhIL-1ra levels were measured by a specific ELISA for human rhIL-1ra (R&D Systems, Minneapolis, MN). Automated total white blood cell differential counts were performed with correction for nucleated red blood cells.
Assessment of Inflammation
Bronchoalveolar lavage fluid (BALF) was obtained as reported (11) and cell counts were determined by Diff-Quik staining of cytospins. BALF myeloperoxidase activity was determined by measuring the oxidation of tetramethylbenzidine against standard concentrations of pure myeloperoxidase (Athens Research & Technology, Athens, GA) (23). BALF/plasma IL-8 protein was measured by an ELISA using anti-ovine IL-8 antibodies (Chemicon, Temecula, CA) (23). Determination of IL-1β, IL-6, IL-8, and serum amyloid A3 gene expression in the lung/liver was performed by RNase protection analysis using 10 μg of total RNA (16, 24, 25). Plasma haptoglobin was measured in an ELISA for bovine haptoglobin (ICL, Newberg, OR). Protein carbonyls were measured by derivatizing the samples with dinitrophenylhydrazine followed by an ELISA using an anti-dinitrophenylhydrazine antibody (23). Blinded inflammatory scoring in the lung and liver was performed by counting inducible nitric oxide synthase (NOSII)-positive inflammatory cells in 10 comparable nonoverlapping high-power fields from each animal (four or five animals per group) (26). IL-1β in situ hybridization was performed with a digoxigenin-labeled antisense sheep IL-1β riboprobe (26).
Evaluation of Lung Maturation
Saturated phosphatidylcholine, a major component of surfactant lipid, was extracted from the BALF and quantified by phosphorus assay (12). Surfactant protein mRNAs were measured using 3 μg of total RNA from the lung by S1 nuclease protection assay (12). Lung compliance was evaluated by measuring the deflating limb pressure–volume curve (16).
Data Analysis
Results are given as means ± SEM, except for pharmacokinetic data (reported as means ± SD). Comparisons between three or more groups were performed by analyses of variance with Student-Newman-Keuls tests used for post hoc analyses. Comparison of two groups was done by nonparametric t test (Welch). Statistical significance was accepted at P < 0.05.
RESULTS
Additional results are reported in the online supplement.
rhIL-1ra Inhibits Intraamniotic LPS–induced Fetal Lung Inflammation
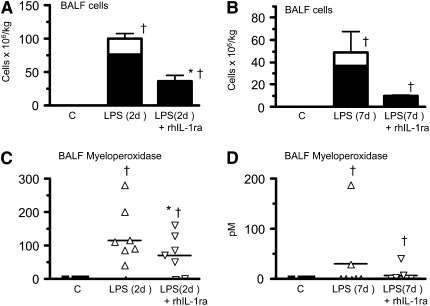
After demonstrating that rhIL-1ra completely blocked IL-1 signaling in vivo (see Figure E1 and Table E3 in the online supplement), we asked whether IL-1 mediated fetal responses to intraamniotic (IA) LPS. Intraamniotic injection of rhIL-1ra before IA LPS decreased neutrophil and monocyte influx in the fetal lung both at 2 days (Figure 1A) and 7 days (Figure 1B) after exposure. Both saline controls and lambs given IA rhIL-1ra alone (data not shown) had no neutrophils or monocytes in BALF. Similarly, IA rhIL-1ra decreased IA LPS–induced increases in BALF myeloperoxidase 2 days after exposure (Figure 1C). Effects on myeloperoxidase activity were variable 7 days after exposure to IA LPS (Figure 1D).
Figure 1.
Recombinant human IL-1 receptor antagonist (rhIL-1ra) decreases intraamniotic LPS-induced lung inflammation. Bronchoalveolar lavage fluid (BALF) neutrophils (solid column) and monocytes (open column) are expressed per kilogram body weight (A) 2 days and (B) 7 days after exposure. BALF myeloperoxidase (C) 2 days and (D) 7 days after exposure. rhIL-1ra decreased intraamniotic LPS-induced BALF inflammatory cell counts and myeloperoxidase expression (†P < 0.05 vs. control, *P < 0.05 vs. intraamniotic LPS, 2 d of exposure).
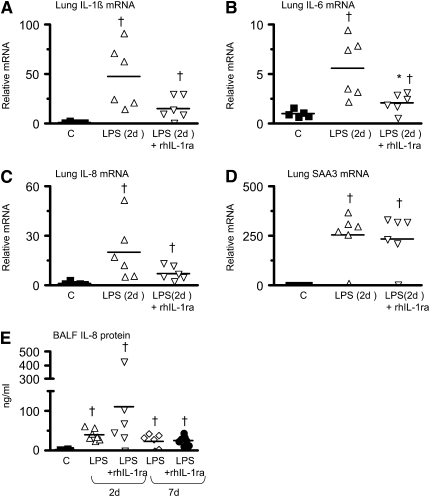
Previous experiments showed increased IL-1β, IL-6, IL-8, and serum amyloid A3 mRNA expression in the fetal lung 2 days after IA LPS with expression returning to baseline values 4–7 days after IA LPS (16, 27). In the present study, pretreatment with rhIL-1ra decreased IA LPS–induced increases in IL-1β and IL-6 mRNA in the fetal lung (Figures 2A and 2B), whereas the decrease in IL-8 mRNA did not reach significance (P = 0.09) (Figure 2C). In contrast, IA LPS–induced expression of serum amyloid A3 mRNA in the fetal lung (Figure 2D) or IL-8 protein in the BALF (Figure 2E) was not inhibited by rhIL-1a. Control fetal lambs had essentially no IL-1β mRNA expression (Figure 3A). Two days after IA LPS exposure, robust expression of IL-1β mRNA was localized to the lung inflammatory cells, with little expression in noninflammatory cells (Figure 3B). Consistent with the mRNA quantitation in Figure 2A, decreased numbers of IL-1β mRNA–expressing inflammatory cells were detected in the rhIL-1ra plus LPS group (Figure 3C).
Figure 2.
Recombinant human IL-1 receptor antagonist (rhIL-1ra) variably decreases intraamniotic LPS-induced lung cytokine expression. Shown is quantification by RNase protection assay of (A) IL-1β, (B) IL-6, (C) IL-8, and (D) serum amyloid A3 (SAA3) mRNAs from fetal lung normalized to L32 (ribosomal protein mRNA), 2 days after exposure. (E) Bronchoalveolar lavage fluid (BALF) IL-8 protein levels 2 and 7 days after exposure. For the RNA measurements, the mean mRNA signal in control animals was given the value of 1 and levels at each time point were expressed relative to controls. rhIL-1ra decreased intraamniotic LPS-induced induction of IL-6 mRNA but not of IL-8 or SAA3 (†P < 0.05 vs. control, *P < 0.05 vs. intraamniotic LPS, 2 d of exposure).
Figure 3.
Cellular expression of IL-1β mRNA. In situ hybridization was performed with digoxigenin-labeled antisense sheep IL-1β riboprobe on 5-μm sections of fetal lung. (A) Saline control showing no IL-1β mRNA expression. (B) Robust expression in lung inflammatory cells (arrows) 2 days after intraamniotic (IA) LPS. (C) Fewer inflammatory cells expressing IL-1β in the 2-day IA LPS plus rhIL-1ra group. Insets show higher magnification of IL-1β expression in the inflammatory cells (n = 4 animals per group). Scale bar: 50 μm.
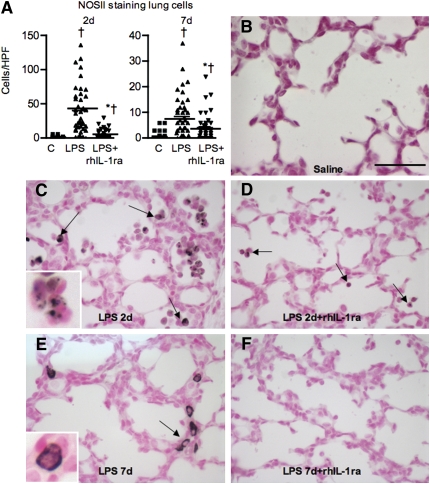
Pretreatment with IA rhIL-1ra decreased intraamniotic LPS–induced activation of neutrophils and monocytes in the fetal lung as demonstrated by reduced NOSII expression (compare Figures 4C, 4D and 4E, 4F). Few neutrophils or monocytes were detected in the lung parenchyma in the control group (Figures 4A and 4B).
Figure 4.
Recombinant human IL-1 receptor antagonist (rhIL-1ra) decreases intraamniotic LPS-mediated induction of inducible nitric oxide synthase (NOSII) expression in lung inflammatory cells. Immunostaining was performed on 5-μm sections of fetal lung. (A) Ten high-power fields (HPFs) in each of four animals per group were scored for cells positive for NOSII immunostaining and are shown as dot plots with means. NOSII expression in the lung sections from (B) saline control, (C) 2 days after intraamniotic LPS, (D) intraamniotic LPS plus rhIL-1ra at 2 days, (E) intraamniotic LPS at 7 days and (F) intraamniotic LPS plus rhIL-1ra at 7 days. NOS II expression was not detected in control lung. Robust NOSII expression was detected in the inflammatory cells (shown by arrow) 2 days after intraamniotic LPS with a decreased expression at 7 days. Note the different patterns of expression at 2 and 7 days [compare (C) and (E); insets show higher magnification]. rhIL-1ra decreased NOSII expression both at 2 and 7 days (†P < 0.05 vs. control, * P < 0.05 vs. the corresponding LPS group). Scale bar: 50 μm.
rhIL-1ra Inhibits Intraamniotic LPS–induced Lung Maturation
Previous experiments demonstrated that surfactant protein mRNAs are induced at 2 days, whereas surfactant lipids and lung compliance improve 6–7 days after IA LPS administration in fetal sheep (16). In the present study, rhIL-1ra decreased IA LPS–induced increases in surfactant protein A, B, and C mRNAs (Figures 5A–5C). Similarly, rhIL-1ra decreased the IA LPS–induced increase in airway saturated phosphatidylcholine, the most abundant surfactant lipid (Figure 5D). The net result was reduced lung compliance in the IA LPS plus rhIL-1ra group compared with the IA LPS group as measured by deflation limb of the pressure–volume curve (Figure 5E).
Figure 5.
Recombinant human IL-1 receptor antagonist (rhIL-1ra) decreases intraamniotic LPS-induced lung maturation. Quantification of surfactant protein mRNA by S1 nuclease protection assay, 2 days after exposure. (A) SP-A, (B) SP-B, and (C) SP-C mRNAs from fetal lung normalized to L32 (ribosomal protein mRNA), 2 days after exposure. The mean mRNA signal in control animals was given the value of 1 and levels at each time point were expressed relative to controls. (D) Bronchoalveolar lavage fluid (BALF) surfactant saturated phosphatidylcholine (Sat PC) and (E) deflating limb pressure–volume curve, 7 days after exposure. rhIL-1ra inhibited intraamniotic LPS-induced lung maturation (†P < 0.05 vs. control, *P < 0.05 vs. intraamniotic LPS, exposure).
rhIL-1ra Inhibits Intraamniotic LPS–induced Systemic Inflammation
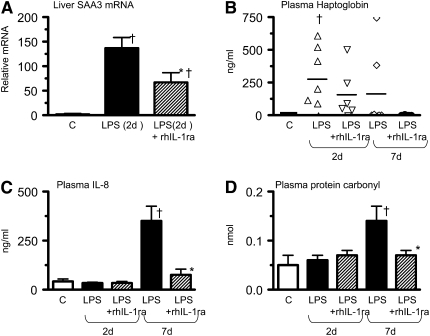
Intraamniotic rhIL-1ra inhibited the IA LPS–induced fetal systemic inflammatory response as measured by serum amyloid A3 mRNA expression in the fetal liver (Figure 6A) as well as plasma IL-8 (Figure 6C) and protein carbonyl levels (a marker of global protein oxidation) (Figure 6D); plasma haptoglobin did not decrease (Figure 6B). IA rhIL-1ra inhibited IA LPS–induced early neutropenia and a later neutrophilia (Table 1), markers that are clinically widely used indicators of systemic inflammation.
Figure 6.
Recombinant human IL-1 receptor antagonist (rhIL-1ra) decreases intraamniotic LPS-induced fetal systemic inflammatory response. Quantification by RNase protection assay of (A) serum amyloid A3 (SAA3) mRNA in the liver 2 days after intraamniotic (IA) LPS. SAA3 mRNA expression was normalized to L32 (ribosomal protein mRNA). The mean mRNA signal in control animals was given the value of 1 and levels at each time point were expressed as the increases relative to controls: (B) plasma haptoglobin, (C) fetal plasma IL-8, and (D) plasma protein carbonyls 2 and 7 days after exposure. rhIL-1ra decreased intraamniotic LPS-induced systemic inflammation. (†P < 0.05 vs. control, *P < 0.05 vs. intraamniotic LPS, exposure).
TABLE 1.
IL-1 RECEPTOR ANTAGONIST PREVENTS INTRAAMNIOTIC LPS-INDUCED CHANGES IN BLOOD LEUKOCYTES
| Group | Blood Neutrophils (× 109/L) | Blood Lymphocytes (× 109/L) | Blood Monocytes (× 109/L) |
|---|---|---|---|
| Control | 0.76 ± 0.08 | 2.1 ± 0.18 | 0.14 ± 0.04 |
| IA LPS (2 d) | 0.16 ± 0.07* | 2.1 ± 0.32 | 0.07 ± 0.02 |
| rhIL-1ra + LPS (2 d) | 0.47 ± 0.2 | 2.2 ± 0.23 | 0.06 ± 0.03 |
| LPS (7 d) | 3.3 ± 0.48* | 2.5 ± 0.23 | 0.35 ± 0.08 |
| rhIL-1ra + LPS (7 d) | 0.46 ± 0.17† | 2.5 ± 0.16 | 0.05 ± 0.03† |
Definition of abbreviations: IA = intraamniotic; rhIL-1ra = recombinant human IL-1 receptor antagonist.
Note: All injections intraamniotic.
P < 0.05 versus controls.
P < 0.05 versus corresponding LPS group.
Pharmacokinetics of rhIL-1ra in Fetal Sheep
After an intravenous injection into fetal sheep, rhIL-1ra had a volume of distribution of 3.7 ± 0.9 L, clearance of 0.7 ± 0.1 L/hour, and a half-life of 3.5 ± 0.6 hours in the fetal plasma. In contrast to the plasma, the half-life of rhIL-1ra was about threefold longer in the amniotic fluid after an intraamniotic injection. Interestingly, the plasma level of rhIL-1ra, 2 days after intraamniotic injection, was only 1% of the amniotic fluid level, reflecting low amniotic to blood diffusion and a higher clearance in the blood.
DISCUSSION
We report the novel finding that IL-1 is a mediator of intraamniotic (IA) LPS–induced lung and systemic inflammation and lung maturation in the preterm fetus. The findings are clinically relevant because the experiments used a model of fetal inflammation induced by administration of LPS in the amniotic cavity, which closely models human chorioamnionitis (28). The chorioamnionitis-induced fetal inflammation causes injury responses in the lung and the brain in both preterm humans as well as preterm sheep (3, 6, 14, 15). A biologically important conclusion from the present study is that IL-1 signaling in the amniotic compartment is critical to the pathogenesis of chorioamnionitis-induced inflammation. Fetal inflammatory responses are different from those in the adult, because the preterm fetus does not respond to TNF-α (19). Therefore, it should not be surprising that whereas IL-1 blockade has variable effects on LPS signaling in the adult, IL-1 blockade significantly decreased LPS-induced inflammation in the fetus in this study.
The biological activity of IL-1 is mediated by two different gene products, IL-1α and IL-1β (21). These two proteins share only 25% amino acid identity, but signal via binding of either ligand to IL-1 receptor-1 (IL-1R1). The IL-1 receptor antagonist (IL-1ra), a member of the IL-1 cytokine family, binds to IL-1R1 and thus antagonizes biological effects of IL-1. Both IL-1α and IL-1β have been implicated in the pathogenesis of systemic inflammatory disorders that respond to IL-1ra treatment (29–31). The fetal tissues that express IL-1β mRNA in response to intraamniotic LPS are the lung and the chorioamnion (16). Although the predominant site of IL-1β expression in the fetal lung is the inflammatory cells, IL-1α mRNA could not be detected in fetuses exposed to intraamniotic LPS (our unpublished data). We also reported that the LPS receptor Toll-like receptor-4 (TLR4) is expressed in all lung cell types in the fetus and other proteins required for LPS signaling, such as lipopolysaccharide-binding protein, can be detected in the fetal airway (26, 32). The fetal airway epithelium can also respond to IL-1 because intratracheal injection of IL-1α induces lung inflammation similar to LPS (18). The present study demonstrated that fetal intraamniotic injection of rhIL-1ra resulted in IL-1 signaling blockade in the amniotic compartment, because little transfer from the amniotic to blood compartment was detected. Taken together, our study suggests that IL-1β secreted by the airway inflammatory cells amplifies LPS-induced lung and systemic inflammation in the fetus. Also, preterm lambs do not have macrophages in the airspaces (33, 34), suggesting that the primary target of IL-1 receptor blockade is likely to be the airway epithelium. However, the precise mechanisms of how blockade of IL-1 signaling in the amniotic compartment decreases fetal inflammation remain to be identified.
Fetal inflammatory response syndrome has been recognized as a distinct, albeit nebulous entity that is associated with subtle increases in umbilical cord plasma cytokine levels in the absence of bacteremia (5, 7). By contrast, systemic inflammatory response syndrome in the adult, generally caused by sepsis or trauma, results in a “cytokine storm” causing multiorgan dysfunction and a high risk of death (35). Despite the subtle increases in plasma cytokines, a systemic inflammatory response induced by chorioamnionitis is postulated to be the proximate cause of fetal organ injury, for example, periventricular leukomalacia (4), necrotizing enterocolitis (3), or bronchopulmonary dysplasia (4–6). In this study, some of the markers of systemic inflammation (plasma haptoglobin and liver serum amyloid A3 expression) increased 2 days after LPS, whereas other markers (plasma IL-8 and protein carbonyls) increased later, suggesting different mechanisms of induction for these indicators of systemic inflammation. Intraamniotic injection of rhIL-1ra effectively decreased intraamniotic LPS-induced induction of liver serum amyloid, plasma IL-8, protein carbonyls, and blood neutrophil counts. However, rhIL-1ra did not significantly reverse intraamniotic LPS-induced increases in plasma haptoglobin and induction of NOSII expression in the fetal liver (see Figure E2 in the online supplement). Taken together, intraamniotic inhibition of IL-1 signaling decreased most but not all indicators of chorioamnionitis/LPS-induced fetal systemic inflammatory response. These results demonstrate that, although IL-1 signaling is an important requirement for LPS effects in chorioamnionitis, other pathways downstream of TLR4 signaling also contributes to fetal inflammation.
Lung inflammation is a major consequence of IA LPS injection (28). IL-1 is an important mediator of IA LPS–induced lung inflammation because rhIL-1ra inhibited IA LPS–induced airway neutrophil and monocyte influx and activation. Transgenic mice expressing IL-1β in the lung epithelium in the perinatal period had pulmonary inflammation and disruption of normal lung architecture (36). Both LPS and IL-1 receptors share common downstream signaling molecules (37), and both can induce the expression of IL-6, IL-8, and monocyte chemotactic protein-1 in the perinatal lung (16, 18, 36). We previously reported that intraamniotic injection of IL-1α and IL-1β (17), but not TNF-α (19) or IL-8 (20), induced robust pulmonary inflammatory cell recruitment, consistent with an important role for IL-1 in chorioamnionitis-induced prenatal inflammation. Because the responses to IL-1α were completely inhibited under our experimental conditions, the present study is informative for inflammatory pathways downstream of TLR4 signaling in the fetus that require IL-1 signaling, in contrast to those that are IL-1 independent. Whereas recruitment and activation of inflammatory cells in the lung were IL-1 dependent, pulmonary expression of the acute-phase reactant serum amyloid A3 was not. Also, intraamniotic rhIL-1ra had dichotomous effects on NOSII and serum amyloid expression in the lung versus the liver, suggesting different mechanisms of induction of these mediators in the lung versus the systemic compartment.
Several studies in adult animals have examined the role of IL-1 signaling in mediating physiological effects of LPS administered both systemically and via the airway. Mice lacking IL-1R1, a major transducer of the IL-1 signal, responded to systemically administered LPS with an acute-phase response indistinguishable from controls (38). Human IL-1 receptor antagonist modestly decreased systemically administered LPS-induced fever but not plasma IL-6 levels in rats and caused modest reversal of LPS-induced appetite suppression in mice (39, 40). Treatment with rhIL-1ra did not prevent intraperitoneal LPS-induced preterm labor in mice (41). Conversely, IL-1 receptor antagonist reduced systemically administered LPS-induced lethality in adult rabbits and mice (42, 43). IL-1 signaling is required for mediating turpentine-induced inflammation, because mice lacking IL-1R1 were resistant to inflammatory responses induced by subcutaneous turpentine (21, 38). Similarly, IL-1 signaling is central to the pulmonary inflammation induced by bleomycin (44). Compared with wild-type mice, mice lacking IL-1R1 had no reduction in airway inflammatory cell influx or airway hyperreactivity induced by LPS given by airway inhalation (45). On the other hand, rhIL-1ra decreased airway neutrophil recruitment induced by intratracheal LPS by 45% (46). Collectively, these studies demonstrate that blockade of IL-1 signaling has variable effects on LPS-induced physiological effects in adult animals. In contrast, our results in the preterm fetus demonstrate that IL-1 signaling in the amniotic compartment is a significant mediator and serves to amplify LPS-induced fetal inflammation.
Preterm fetal sheep have low lung surfactant pools and intraamniotic LPS exposure increases surfactant protein mRNAs followed by increased airway surfactant lipid pools, leading to improved lung compliance (12, 16). In this study, rhIL-1ra decreased intraamniotic LPS-induced pulmonary surfactant protein mRNA, surfactant lipid pool size, and lung compliance. We previously reported that LPS-induced inflammatory cell influx mediated by the integrin CD18 is required to mediate lung maturation (47). The results from this study demonstrate that IL-1 signaling is an important mediator of IA LPS–induced lung inflammatory cell influx and lung maturation.
Because our studies suggest beneficial effects of rhIL-1ra in chorioamnionitis/LPS-induced lung and systemic inflammation, a valid concern would be the apparently deleterious effects on fetal lung mechanics. In several different chorioamnionitis models, fetal lung inflammation consistently causes lung maturation (16–18, 48) and inhibition of inflammatory cell influx in the lung reversed the intraamniotic LPS–induced lung maturation in the fetal sheep (47). Preterm human fetuses exposed to chorioamnionitis also have clinical lung maturation (2). Therefore, reversal of the improved lung mechanics may be a necessary consequence of inhibition of inflammation induced by chorioamnionitis. The two clinical strategies to prevent/treat respiratory distress syndrome in clinical use in preterm infants are antenatal maternal glucocorticoids and postnatal surfactants (49). Effects of the combination of these therapies with rhIL-1ra in chorioamnionitis models remain to be evaluated.
Supplementary Material
Acknowledgments
The authors thank Professor Alexander Vinks (Cincinnati Children's Hospital, Cincinnati, OH) for help with pharmacokinetic modeling, and Amy Whitescarver and Tiffany Crane for expert technical assistance.
Supported by NIH HD-057869 (S.G.K.), AI-069716 and HL-65397 (A.H.J.), Grow Research Institute, University of Maastricht, NWO grant 016.096.141 (B.W.K.), CDA-303261 from the NHMRC, Australia (T.J.M.M.), NHFA and NHMRC Fellowship, Australia (G.R.P.), Viertel SMRF, Australia (J.J.P.), and WIRF. The anakinra was a gift from Amgen.
Presented in part at the Society of Pediatric Research Meeting, Honolulu, Hawaii in May 2008.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200811-1728OC on February 20, 2009
Conflict of Interest Statement: S.G.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. I.N. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. T.J.M.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. G.R.P. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.J.P. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. F.-C.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. B.W.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.P.N. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.I. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.H.J. received the anakinra used in the experiments reported in the manuscript as a gift from Amgen. No grant money other than the drug was received. Amgen was not involved in the planning or execution of the experiments, or in the analysis of the results.
References
- 1.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med 2000;342:1500–1507. [DOI] [PubMed] [Google Scholar]
- 2.Watterberg K, Demers L, Scott S, Murphy S. Chorioamnionitis and early lung inflammation in infants in whom bronchopulmonary dysplasia develops. Pediatrics 1996;97:210–215. [PubMed] [Google Scholar]
- 3.Andrews WW, Goldenberg RL, Faye-Petersen O, Cliver S, Goepfert AR, Hauth JC. The Alabama Preterm Birth study: polymorphonuclear and mononuclear cell placental infiltrations, other markers of inflammation, and outcomes in 23- to 32-week preterm newborn infants. Am J Obstet Gynecol 2006;195:803–808. [DOI] [PubMed] [Google Scholar]
- 4.Alexander J, Gilstrap L, Cox S, McIntire D, Leveno K. Clinical chorioamnionitis and the prognosis for very low birth weight infants. Obstet Gynecol 1998;91:725–729. [DOI] [PubMed] [Google Scholar]
- 5.Gomez R, Romero R, Ghezzi F, Yoon B, Mazor M, Berry S. The fetal inflammatory response syndrome. Am J Obstet Gynecol 1998;179:194–202. [DOI] [PubMed] [Google Scholar]
- 6.Viscardi RM, Muhumuza CK, Rodriguez A, Fairchild KD, Sun CC, Gross GW, Campbell AB, Wilson PD, Hester L, Hasday JD. Inflammatory markers in intrauterine and fetal blood and cerebrospinal fluid compartments are associated with adverse pulmonary and neurologic outcomes in preterm infants. Pediatr Res 2004;55:1009–1017. [DOI] [PubMed] [Google Scholar]
- 7.Romero R, Gomez R, Ghezzi F, Yoon B, Mazor M, Edwin S, Berry S. A fetal systemic inflammatory response is followed by the spontaneous onset of preterm parturition. Am J Obstet Gynecol 1998;179:186–193. [DOI] [PubMed] [Google Scholar]
- 8.Baud O, Emilie D, Pelletier E, Lacaze-Masmonteil T, Zupan V, Fernandez H, Dehan M, Frydman R, Ville Y. Amniotic fluid concentrations of interleukin-1β, interleukin-6 and TNF-α in chorioamnionitis before 32 weeks of gestation: histological associations and neonatal outcome. Br J Obstet Gynaecol 1999;106:72–77. [DOI] [PubMed] [Google Scholar]
- 9.Baergen R, Benirschke K, Ulich T. Cytokine expression in the placenta: The role of interleukin 1 and interleukin 1 receptor antagonist expression in chorioamnionitis and parturition. Arch Pathol Lab Med 1994;118:52–55. [PubMed] [Google Scholar]
- 10.Arntzen K, Kojllesdal A, Halgunset J, Vatten L, Austgulen R. TNF, IL-1, IL-6, IL-8 and soluble TNF receptors in relation to chorioamnionitis and premature labor. J Perinat Med 1998;26:17–26. [DOI] [PubMed] [Google Scholar]
- 11.Jobe AH, Newnham JP, Willet KE, Moss TJ, Ervin MG, Padbury JF, Sly PD, Ikegami M. Endotoxin-induced lung maturation in preterm lambs is not mediated by cortisol. Am J Respir Crit Care Med 2000;162:1656–1661. [DOI] [PubMed] [Google Scholar]
- 12.Bachurski CJ, Ross GF, Ikegami M, Kramer BW, Jobe AH. Intra-amniotic endotoxin increases pulmonary surfactant proteins and induces SP-B processing in fetal sheep. Am J Physiol Lung Cell Mol Physiol 2001;280:L279–L285. [DOI] [PubMed] [Google Scholar]
- 13.Willet KE, Jobe AH, Ikegami M, Brennan S, Newnham J, Sly PD. Antenatal endotoxin and glucocorticoid effects on lung morphometry in preterm lambs. Pediatr Res 2000;48:782–788. [DOI] [PubMed] [Google Scholar]
- 14.Moss TJ, Newnham JP, Willett KE, Kramer BW, Jobe AH, Ikegami M. Early gestational intra-amniotic endotoxin: lung function, surfactant, and morphometry. Am J Respir Crit Care Med 2002;165:805–811. [DOI] [PubMed] [Google Scholar]
- 15.Nitsos I, Rees SM, Duncan J, Kramer BW, Harding R, Newnham JP, Moss TJ. Chronic exposure to intra-amniotic lipopolysaccharide affects the ovine fetal brain. J Soc Gynecol Investig 2006;13:239–247. [DOI] [PubMed] [Google Scholar]
- 16.Kallapur SG, Willet KE, Jobe AH, Ikegami M, Bachurski CJ. Intra-amniotic endotoxin: chorioamnionitis precedes lung maturation in preterm lambs. Am J Physiol Lung Cell Mol Physiol 2001;280:L527–L536. [DOI] [PubMed] [Google Scholar]
- 17.Willet KE, Kramer BW, Kallapur SG, Ikegami M, Newnham JP, Moss TJ, Sly PD, Jobe AH. Intra-amniotic injection of IL-1 induces inflammation and maturation in fetal sheep lung. Am J Physiol Lung Cell Mol Physiol 2002;282:L411–L420. [DOI] [PubMed] [Google Scholar]
- 18.Sosenko IR, Kallapur SG, Nitsos I, Moss TJ, Newnham JP, Ikegami M, Jobe AH. IL-1α causes lung inflammation and maturation by direct effects on preterm fetal lamb lungs. Pediatr Res 2006;60:294–298. [DOI] [PubMed] [Google Scholar]
- 19.Ikegami M, Moss TJ, Kallapur SG, Mulrooney N, Kramer BW, Nitsos I, Bachursky CJ, Newnham JP, Jobe AH. Minimal lung and systemic responses to TNFα in preterm sheep. Am J Physiol Lung Cell Mol Physiol 2003;285:L121–L129. [DOI] [PubMed] [Google Scholar]
- 20.Kallapur SG, Moss TJM, Auten RL, Maeda DY, Ikegami M, Newnham JP, Jobe AH. CXCR2 signaling mediates intraamniotic endotoxin induced lung neutrophil influx in preterm fetal lambs [abstract]. Am J Respir Crit Care Med 2007;175:A92. [Google Scholar]
- 21.Fantuzzi G, Dinarello CA. The inflammatory response in interleukin-1β-deficient mice: comparison with other cytokine-related knock-out mice. J Leukoc Biol 1996;59:489–493. [DOI] [PubMed] [Google Scholar]
- 22.Dinarello C. Interleukin-1. Cytokine Growth Factor Rev 1997;8:253–265. [DOI] [PubMed] [Google Scholar]
- 23.Cheah FC, Jobe AH, Moss TJ, Newnham JP, Kallapur SG. Oxidative stress in fetal lambs exposed to intra-amniotic endotoxin in a chorioamnionitis model. Pediatr Res 2008;63:274–279. [DOI] [PubMed] [Google Scholar]
- 24.Wilson TC, Bachurski CJ, Ikegami M, Jobe AH, Kallapur SG. Pulmonary and systemic induction of SAA3 after ventilation and endotoxin in preterm lambs. Pediatr Res 2005;58:1204–1209. [DOI] [PubMed] [Google Scholar]
- 25.Kallapur SG, Jobe AH, Ikegami M, Bachurski CJ. Increased IP-10 and MIG expression after intra-amniotic endotoxin in preterm lamb lung. Am J Respir Crit Care Med 2003;167:779–786. [DOI] [PubMed] [Google Scholar]
- 26.Kallapur SG, Jobe AH, Ball MK, Nitsos I, Moss TJ, Hillman NH, Newnham JP, Kramer BW. Pulmonary and systemic endotoxin tolerance in preterm fetal sheep exposed to chorioamnionitis. J Immunol 2007;179:8491–8499. [DOI] [PubMed] [Google Scholar]
- 27.Kallapur SG, Kramer BW, Moss TJ, Newnham JP, Jobe AH, Ikegami M, Bachurski CJ. Maternal glucocorticoids increase endotoxin-induced lung inflammation in preterm lambs. Am J Physiol Lung Cell Mol Physiol 2003;284:L633–L642. [DOI] [PubMed] [Google Scholar]
- 28.Kallapur SG, Ikegami M. Physiological consequences of intrauterine insults. Paediatr Respir Rev 2006;7:110–116. [DOI] [PubMed] [Google Scholar]
- 29.Kawaguchi Y, Nishimagi E, Tochimoto A, Kawamoto M, Katsumata Y, Soejima M, Kanno T, Kamatani N, Hara M. Intracellular IL-1α-binding proteins contribute to biological functions of endogenous IL-1α in systemic sclerosis fibroblasts. Proc Natl Acad Sci USA 2006;103:14501–14506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dinarello CA. Blocking IL-1 in systemic inflammation. J Exp Med 2005;201:1355–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakurai T, He G, Matsuzawa A, Yu GY, Maeda S, Hardiman G, Karin M. Hepatocyte necrosis induced by oxidative stress and IL-1α release mediate carcinogen-induced compensatory proliferation and liver tumorigenesis. Cancer Cell 2008;14:156–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hillman NH, Moss TJ, Nitsos I, Kramer BW, Bachurski CJ, Ikegami M, Jobe AH, Kallapur SG. Toll-like receptors and agonist responses in the developing fetal sheep lung. Pediatr Res 2008;63:388–393. [DOI] [PubMed] [Google Scholar]
- 33.Kramer BW, Jobe AH, Ikegami M. Monocyte function in preterm, term, and adult sheep. Pediatr Res 2003;54:52–57. [DOI] [PubMed] [Google Scholar]
- 34.Kramer BW, Joshi SN, Moss TJ, Newnham JP, Sindelar R, Jobe AH, Kallapur SG. Endotoxin-induced maturation of monocytes in preterm fetal sheep lung. Am J Physiol Lung Cell Mol Physiol 2007;293:L345–L353. [DOI] [PubMed] [Google Scholar]
- 35.Matsuda N, Hattori Y. Systemic inflammatory response syndrome (SIRS): molecular pathophysiology and gene therapy. J Pharmacol Sci 2006;101:189–198. [DOI] [PubMed] [Google Scholar]
- 36.Bry K, Whitsett JA, Lappalainen U. IL-1β disrupts postnatal lung morphogenesis in the mouse. Am J Respir Cell Mol Biol 2007;36:32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li X, Qin J. Modulation of Toll-interleukin 1 receptor mediated signaling. J Mol Med 2005;83:258–266. [DOI] [PubMed] [Google Scholar]
- 38.Leon LR, Conn CA, Glaccum M, Kluger MJ. IL-1 type I receptor mediates acute phase response to turpentine, but not lipopolysaccharide, in mice. Am J Physiol 1996;271:R1668–R1675. [DOI] [PubMed] [Google Scholar]
- 39.Smith BK, Kluger MJ. Human IL-1 receptor antagonist partially suppresses LPS fever but not plasma levels of IL-6 in Fischer rats. Am J Physiol 1992;263:R653–R655. [DOI] [PubMed] [Google Scholar]
- 40.Swiergiel AH, Smagin GN, Johnson LJ, Dunn AJ. The role of cytokines in the behavioral responses to endotoxin and influenza virus infection in mice: effects of acute and chronic administration of the interleukin-1-receptor antagonist (IL-1ra). Brain Res 1997;776:96–104. [DOI] [PubMed] [Google Scholar]
- 41.Fidel PL Jr, Romero R, Cutright J, Wolf N, Gomez R, Araneda H, Ramirez M, Yoon BH. Treatment with the interleukin-I receptor antagonist and soluble tumor necrosis factor receptor Fc fusion protein does not prevent endotoxin-induced preterm parturition in mice. J Soc Gynecol Investig 1997;4:22–26. [DOI] [PubMed] [Google Scholar]
- 42.Ohlsson K, Bjork P, Bergenfeldt M, Hageman R, Thompson RC. Interleukin-1 receptor antagonist reduces mortality from endotoxin shock. Nature 1990;348:550–552. [DOI] [PubMed] [Google Scholar]
- 43.Alexander HR, Doherty GM, Buresh CM, Venzon DJ, Norton JA. A recombinant human receptor antagonist to interleukin 1 improves survival after lethal endotoxemia in mice. J Exp Med 1991;173:1029–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gasse P, Mary C, Guenon I, Noulin N, Charron S, Schnyder-Candrian S, Schnyder B, Akira S, Quesniaux VF, Lagente V, et al. IL-1R1/MyD88 signaling and the inflammasome are essential in pulmonary inflammation and fibrosis in mice. J Clin Invest 2007;117:3786–3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moreland JG, Fuhrman RM, Wohlford-Lenane CL, Quinn TJ, Benda E, Pruessner JA, Schwartz DA. TNF-α and IL-1β are not essential to the inflammatory response in LPS-induced airway disease. Am J Physiol Lung Cell Mol Physiol 2001;280:L173–L180. [DOI] [PubMed] [Google Scholar]
- 46.Ulich TR, Yin SM, Guo KZ, del Castillo J, Eisenberg SP, Thompson RC. The intratracheal administration of endotoxin and cytokines. III. The interleukin-1 (IL-1) receptor antagonist inhibits endotoxin- and IL-1–induced acute inflammation. Am J Pathol 1991;138:521–524. [PMC free article] [PubMed] [Google Scholar]
- 47.Kallapur SG, Moss TJM, Ikegami M, Jasman RL, Newnham JP, Jobe AH. Recruited inflammatory cells mediate endotoxin induced lung maturation in preterm fetal lambs. Am J Respir Crit Care Med 2005;172:1315–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moss TJ, Knox CL, Kallapur SG, Nitsos I, Theodoropoulos C, Newnham JP, Ikegami M, Jobe AH. Experimental amniotic fluid infection in sheep: effects of Ureaplasma parvum serovars 3 and 6 on preterm or term fetal sheep. Am J Obstet Gynecol 2008;198:122.e121–122.e128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jobe AH, Ikegami M. Lung development and function in preterm infants in the surfactant treatment era. Annu Rev Physiol 2000;62:825–846. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.