Abstract
XBP-1, a transcription factor that drives the unfolded protein response (UPR), is activated in B cells when they differentiate to plasma cells. Here, we show that in the B cells, whose capacity to secrete IgM has been eliminated, XBP-1 is induced normally on induction of differentiation, suggesting that activation of XBP-1 in B cells is a differentiation-dependent event, but not the result of a UPR caused by the abundant synthesis of secreted IgM. Without XBP-1, B cells fail to signal effectively through the B-cell receptor. The signalling defects lead to aberrant expression of the plasma cell transcription factors IRF4 and Blimp-1, and altered levels of activation-induced cytidine deaminase and sphingosine-1-phosphate receptor. Using XBP-1-deficient/Blimp-1-GFP transgenic mice, we find that XBP-1-deficient B cells form antibody-secreting plasmablasts in response to initial immunization; however, these plasmablasts respond ineffectively to CXCL12. They fail to colonize the bone marrow and do not sustain antibody production. These findings define the role of XBP-1 in normal plasma cell development and have implications for management of B-cell malignancies.
Keywords: BCR signalling, Blimp-1, chemokine receptors, CXCL12, IRF4
Introduction
Plasma cell differentiation begins when a naive B cell recognizes antigen through its B-cell receptor (BCR) in secondary lymphoid organs. A functional BCR consists of a membrane-bound IgM molecule and a disulfide-linked Igα/Igβ heterodimer. On antigen binding, the BCR is recruited into lipid rafts and activated through phosphorylation of the immunoreceptor tyrosine-based activation motifs (ITAM) on Igα/Igβ (Pierce, 2002; Dykstra et al, 2003). Phosphorylated Igα/Igβ then recruits Syk kinase, which, after phosphorylation, transduces signals eventually leading to regulation of transcription factors. Plasma cell differentiation is achieved by downregulation of the transcription factors BCL6 and Pax5, responsible for maintaining the naive B-cell phenotypes, and by upregulation of the canonical plasma cell transcription factors XBP-1, Blimp-1 and IRF4 (Reimold et al, 2001; Shaffer et al, 2002; Klein et al, 2006; Sciammas et al, 2006; Kallies et al, 2007; Martins and Calame, 2008).
XBP-1 is one of the major transcription factors in plasma cell differentiation, and its loss leads to an almost complete absence of plasma cells and circulating immunoglobulins (Reimold et al, 2001; Iwakoshi et al, 2003a, 2003b). XBP-1 is a basic-region leucine zipper type transcription factor that belongs to the CREB/ATF family, and is one of the three major arms of the unfolded protein response (UPR) in mammals. When cells are treated with drugs such as tunicamycin and thapsigargin, or exposed to dithiothreitol, accumulation of misfolded proteins is believed to occur, leading to splicing of XBP-1 by transmembrane kinase and endoribonuclease inositol-requiring enzyme-1 (IRE-1) in an unconventional cleavage and re-ligation reaction (Shen et al, 2001; Yoshida et al, 2001; Calfon et al, 2002). On activation, spliced XBP-1 (XBP-1s) regulates the synthesis of chaperones and other proteins essential for restoration of proper secretory function (Lee et al, 2003, 2005).
To form a fully functional plasma cell, a B cell needs to expand its endoplasmic reticulum (ER) in preparation for increased immunoglobulin secretion; activation of XBP-1 and the UPR allows a B cell to rapidly increase the capacity of the ER. The failure of XBP-1-deficient B cells to generate plasma cells is usually attributed to the accumulation of newly synthesized immunoglobulin, not all of which may fold correctly. The inability of XBP-1-deficient B cells to activate the normal UPR would then lead to ER obstruction caused by an overload of misfolded immunoglobulin and, consequently, cell death (Iwakoshi et al, 2003a, 2003b; van Anken et al, 2003). The current model of XBP-1 in plasma cell differentiation assumes that immunoglobulin synthesis is initiated first, and that misfolded IgM triggers IRE-1-mediated splicing of XBP-1. However, B-cell lines stimulated with lipopolysaccharide (LPS) show XBP-1 activation before an increase in immunoglobulin synthesis (Gass et al, 2002). XBP-1 is also activated at the pro-B-cell stage in the bone marrow, despite the fact that pro-B cells do not secrete large amounts of immunoglobulin or any other known protein that could accumulate in the ER and trigger XBP-1 activation (Brunsing et al, 2008). How XBP-1 deficiency causes the failure of plasma cell differentiation remains to be settled.
To investigate the role of XBP-1 in B-cell differentiation and BCR signalling, we established a hen egg lysozyme (HEL)-specific BCR transgenic (MD4) (Goodnow et al, 1988), conditional XBP-1 knockout mouse model, which allowed us to examine the consequences of antigen-specific BCR activation in an XBP-1-deficient background. Our data define XBP-1 activation as a differentiation-dependent event, rather than a response to large quantities of secreted IgM in B cells. We show that XBP-1 is required for proper signalling through the BCR, whereas IL-4 and TLR signalling remains unchanged in XBP-1-deficient B cells. Lack of XBP-1 causes upregulation of IRF-4 and Blimp-1, establishing an earlier unappreciated feedback loop initiated by XBP-1. In addition, we track plasma cells in vivo using immunized XBP-1KO/MD4/Blimp-1-GFP mice. We find that XBP-1-deficient mice have a robust plasma cell population in the spleen and high titers of serum antibodies after one immunization. This robust antibody response is short lived due to a defect in the plasma cell colonization of long-lived niches in the bone marrow.
Results
XBP-1KO/MD4 B cells do not sustain antibody secretion
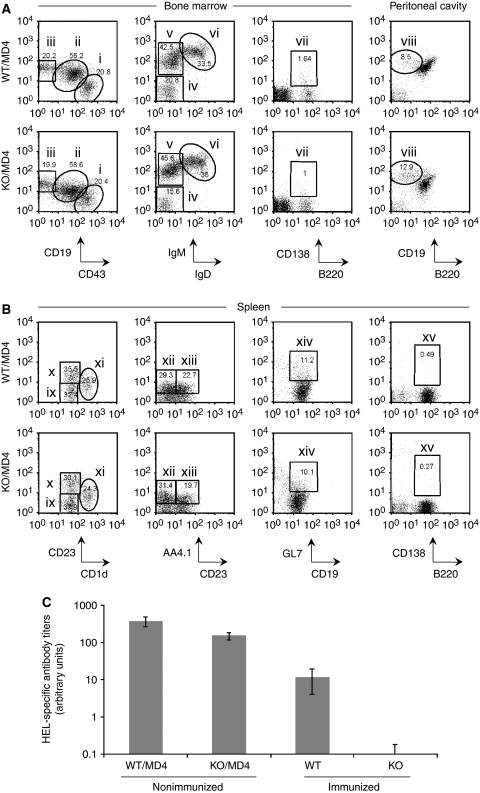
To investigate the role of XBP-1 in B-cell responses to antigen, we generated CD19-Cre × XBP-1flox/flox/MD4 transgenic (XBP-1KO/MD4) mice, in which >95% of B cells express a BCR specific for the HEL. We examined the B-cell compartment (bone marrow, peritoneal cavity and spleen) of XBP-1KO/MD4 mice and found normal numbers of B cells, including pro-B, pre-B and immature B cells in bone marrow, as well as normal B1 and B2 compartments in the peritoneal cavity and spleen. Transitional B-cell populations, marginal zone B cells and germinal centre B cells were also unaffected by XBP-1 deficiency. The number of CD138+ long-lived plasma cells in the bone marrow and spleen was extremely low in these mice, as they expressed the MD4 transgene and had never been exposed to the relevant antigen, HEL (Figure 1A and B). We repeatedly immunized mice with HEL and found that the anti-HEL IgM in the sera of XBP-1KO mice was significantly lower than that of XBP-1WT mice (Figure 1C; see also Figure 7C), a phenotype consistent with the block in plasma cell differentiation seen in XBP-1−/−/RAG2−/− chimeric mice (Reimold et al, 2001). However, to our surprise, unimmunized XBP-1KO/MD4 mice had appreciable levels of circulating antibody. These antibody levels were lower than those seen in XBP-1WT/MD4 mice. Although XBP-1 is required for sustained antibody production, it can proceed in the absence of XBP-1 (Figure 1C). Despite the link between XBP-1 and the UPR, we conclude that at least some of the serum antibodies in XBP-1KO/MD4 mice are properly folded as evidenced by their ability to bind antigen in an enzyme-linked immunosorbent assay (ELISA) (Figure 1C).
Figure 1.
B cells from XBP-1KO/MD4 mice fail to differentiate into plasma cells. (A, B) XBP-1 deficiency leads to reduced production of plasma cells, whereas all other B-cell compartments of the XBP-1KO mice appear normal. B cells were isolated from the bone marrow, peritoneal cavity and spleen, and stained with indicated markers. Cells shown were gated on B220+ or CD19+ populations. The B-cell populations indicated in each panel are as follows: (i) pro-B cells, (ii) pre-B cells, (iii) immature B cells, (iv) pro-B cells, (v) pre-B cells, (vi) immature B cells, (vii) plasma cells, (viii) B-1 cells, (ix) transitional B cells, (x) follicular B cells, (xi) marginal zone B cells, (xii) T-1 transitional B cells, (xiii) T-2 transitional B cells, (xiv) germinal centre B cells and (xv) plasma cells. The numbers indicate the percentages of cells. (C) XBP-1WT/MD4 and XBP-1KO/MD4 mice were not immunized. XBP-1WT and XBP-1KO mice were repeatedly immunized with HEL. The levels of anti-HEL IgM in the sera were determined by ELISA.
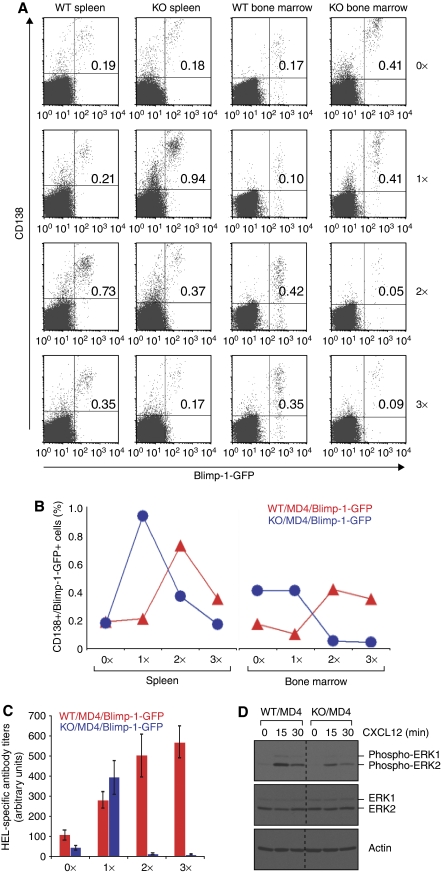
Figure 7.
XBP-1-deficient plasma cells mount only a short lived but robust antibody response due to their failure to colonize into the bone marrow. (A) XBP-1WT/MD4/Blimp-1-GFP and XBP-1KO/MD4/Blimp-1-GFP mice were immunized with HEL at the following time points: days −34, −20 and −7 (3 × immunized); days −20 and −7 (2 × immunized); day −7 (1 × immunized); or unimmunized (0 × immunized). Splenocytes and bone marrow cells from all these mice were isolated on the same day (day 0), stained for CD138 and analysed by flow cytometry. The percentage of Blimp-1-GFP-positive/CD138-positive cells is indicated in the upper right quadrants. The results are representative of two independent experiments. Note that very few cells (0.05 and 0.09%) in the bone marrow of XBP-1KO/MD4/Blimp-1-GFP mice are Blimp-1-GFP-positve and CD138-positive after reimmunization. (B) The percentages of CD138+/Blimp-1-GFP+ B cells from the spleens and bone marrow of immunized mice (shown in A) were plotted. (C) HEL-specific antibody titers in the sera from mice described in (A) were measured by ELISA. (D) Day 4 LPS-stimulated XBP-1WT/MD4 and XBP-1KO/MD4 plasmablasts were stimulated with CXCL12 for indicated time and lysed immediately. Lysates were immunoblotted for phospho-ERK1/2, ERK1/2 and actin.
Protein folding occurs normally in the absence of XBP-1, and unfolded IgM is not required for XBP-1 activation
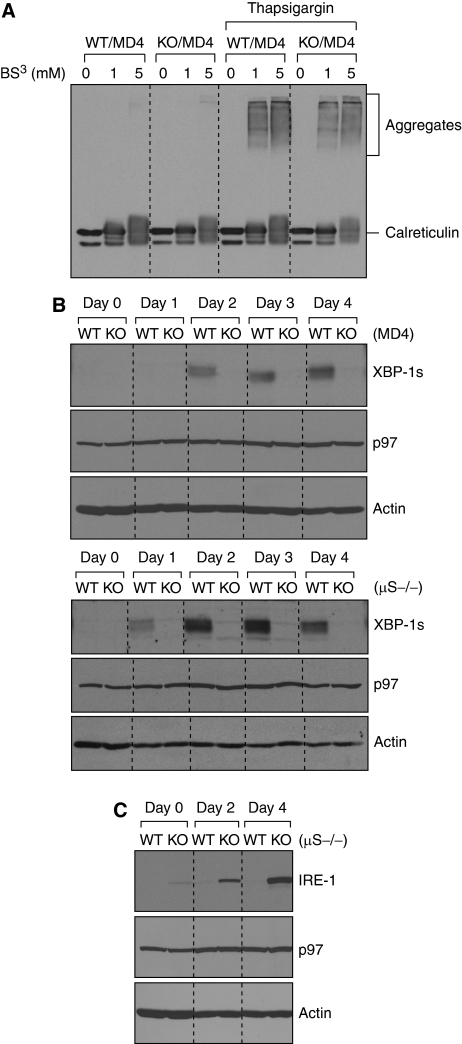
XBP-1 could operate in one of the two ways during plasma cell differentiation. Either a flood of newly synthesized IgM into the ER activates XBP-1 to turn on the UPR, to handle this increased secretory load, or XBP-1 is part of a network of transcription factors that regulate plasma cell function independent of secretory capacity. To test the first hypothesis, we looked for evidence of accumulation of unfolded proteins in XBP-1-deficient B cells. We stabilized potential protein aggregates in lysates by cross-linking using bis[sulfosuccinimidyl]suberate (BS3) before analysis by SDS–PAGE. We did not detect aggregated proteins in either XBP-1WT/MD4 or XBP-1KO/MD4 B cells (Figure 2A). We induced aggregation of ER-resident proteins by exposure of intact cells to thapsigargin, a treatment expected to lead to collapse of calcium gradients across the ER membrane, with a concomitant failure of calcium-dependent chaperones to assist in protein folding. Thapsigargin treatment induced calreticulin-containing high molecular weight aggregates in both XBP-1WT/MD4 and XBP-1KO/MD4 cells to a similar degree. Thus, the presence of XBP-1 did not provide a protective effect against the formation of these protein aggregates (Figure 2A).
Figure 2.
XBP-1 activation is a differentiation-dependent event in B cells, and the lack of XBP-1 leads to IRE-1 upregulation. (A) XBP-1 deficiency does not lead to accumulation of misfolded proteins. Four-day LPS-stimulated XBP-1WT/MD4 and XBP-1KO/MD4 plasmablasts were treated with or without 30 μM thapsigargin for 3 h before lysis. Lysates were treated with the indicated concentrations of the cross-linker bis[sulfosuccinimidyl]suberate (BS3) and immunoblotted for calreticulin. (B) B cells purified from spleens of either XBP-1WT/MD4 and XBP-1KO/MD4 mice (upper three panels) or XBP-1WT/μS−/− and XBP-1KO/μS−/− mice (lower three panels) were cultured in LPS (20 μg/ml) to induce differentiation. Cell lysates were immunoblotted for XBP-1, p97 (AAA-ATPase) and actin. (C) XBP-1WT/μS−/− and XBP-1KO/μS−/− B cells were stimulated by LPS to induce differentiation. Lysates were immunoblotted for IRE-1, p97 and actin.
To test the hypothesis that whether in normal B cells the increase of secreted IgM is responsible for XBP-1 activation, we stimulated naive B cells purified from the spleens of XBP-1WT/μS−/− mice with LPS for 4 days. These B cells neither produce secreted IgM on LPS stimulation (Boes et al, 1998) (see below, Figure 3B), nor is there a massive increase in the synthesis of membrane IgM. We observed XBP-1 activation in both wild-type μS−/− and MD4 B cells (Figure 2B; Supplementary Figure S1), showing clearly that activation of XBP-1 can occur in response to LPS-induced differentiation in the absence of massively increased synthesis of secreted IgM. The levels of XBP-1 decrease in μS−/− B cells after 4 days in culture, whereas its levels in MD4 B cells keep increasing (Figure 2B; Supplementary Figure S1), suggesting that secreted IgM may play a role in sustaining high levels of XBP-1 in the later stages of plasma cell differentiation.
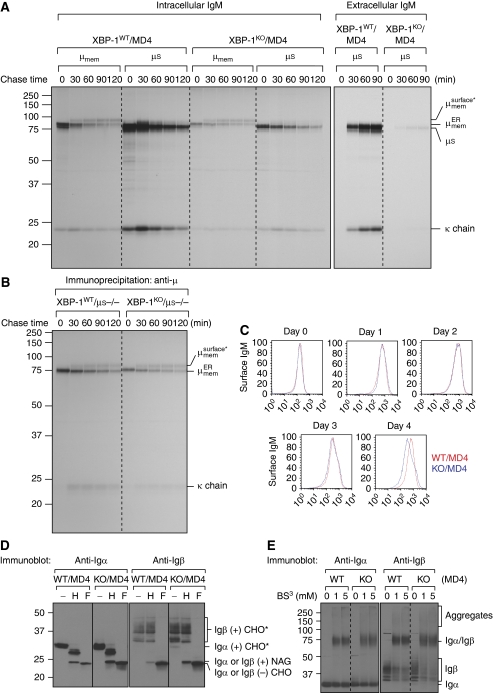
Figure 3.
Transport of membrane-bound IgM and the heterodimeric Igα/Igβ to the cell surface appears to be normal in XBP-1-deficient B cells. (A) Four-day LPS-stimulated XBP-1WT/MD4 and XBP-1KO/MD4 plasmablasts were labelled by 35S-[methionine] and -[cysteine] for 10 min and chased for indicated time. Cells were lysed in Triton X-114 and lysates were subjected to phase separation. The intracellular membrane-bound IgM was immunoprecipitated using the anti-μ antibody from Triton X-114-associated protein fractions, whereas the intracellular secreted IgM was immunoprecipitated from Triton X-114 supernatant fractions. The extracellular secreted IgM was immunoprecipitated from the culture media. The asterisk denotes endo-H-resistant complex glycans. (B) Four-day LPS-stimulated XBP-1WT/μS−/− and XBP-1KO/μS−/− plasmablasts were radiolabelled for 10 min and chased for indicated time. Lysates were immunoprecipitated using the anti-μ antibody. (C) Naive XBP-1WT/MD4 and XBP-1KO/MD4 B cells were stimulated by LPS for 4 days to allow differentiation. Each day cells were surface-stained by an FITC-conjugated anti-μ antibody and analysed by flow cytofluorimetry. (D) Three-day LPS-stimulated XBP-1WT/MD4 and XBP-1KO/MD4 plasmablasts were lysed. Lysates were treated with either endo-H or PNGase F before immunoblotting for Igα or Igβ. CHO, CHO* and NAG represent high mannose-type glycans, complex-type glycans and N-acetylglucosamines, respectively. (E) Four-day LPS-stimulated XBP-1WT/MD4 and XBP-1KO/MD4 plasmablasts were lysed. Lysates were cross-linked with BS3 of indicated concentrations and analysed by immunoblot for Igα or Igβ.
We further examined whether the XBP-1-activating enzyme IRE-1 is expressed properly. We detected only small amounts of IRE-1 protein in wild-type μS−/− B cells even after these cells were induced to differentiate in response to LPS treatment for 4 days (Figure 2C). In XBP-1KO/μS−/− B cells, we observed a massive increase of IRE-1 levels in a differentiation-dependent manner, despite the fact that secreted IgM is absent in these B cells (Figure 2C). Similar results were observed in XBP-1KO and XBP-1KO/MD4 B cells (Supplementary Figure S1 and data not shown). The absence of XBP-1 thus leads to increased synthesis of IRE-1 and shows a negative feedback loop controlled by XBP-1.
XBP-1 deficiency affects synthesis but not surface expression of membrane-bound IgM
To further investigate protein folding in XBP-1-deficient B cells, we focused primarily on proteins necessary for B-cell function that were known to be produced in large quantities, beginning with IgM. Pulse chase experiments were performed in LPS-stimulated XBP-1WT/MD4 and XBP-1KO/MD4 B cells, and membrane IgM was separated from secreted IgM using Triton X-114 phase separation. XBP-1KO/MD4 plasmablasts synthesized substantially less secreted IgM (Tirosh et al, 2005) (Figure 3A) and released only small amounts of IgM into the culture media (Figure 3A). XBP-1KO/MD4 plasmablasts synthesized only slightly less membrane IgM than XBP-1WT/MD4 plasmablasts, but the trafficking of membrane IgM was normal, as assessed by acquisition of complex-type N-linked glycans on membrane μ chains (Figure 3A). The μS−/− system obviates the need for detergent-based IgM separation, and rates of maturation of membrane IgM in XBP-1KO/μS−/− plasmablasts were similar to that observed in XBP-1WT/μS−/− plasmablasts (Figure 3B), confirming that intracellular transport of IgM is normal in the absence of XBP-1. No differences in surface staining of membrane IgM were observed when comparing naive or 1- to 3-day LPS-stimulated XBP-1WT/MD4 and XBP-1KO/MD4 B cells (Figure 3C). Although surface expression of membrane IgM after 4-day LPS stimulation was decreased by an average of 40% (as determined by mean fluorescence intensity) on XBP-1KO/MD4 B cells, such differences were not seen when comparing XBP-1WT/μS−/− and XBP-1KO/μS−/− B cells (data not shown).
XBP-1 deficiency does not alter the synthesis and assembly of Igα and Igβ
As Igα and Igβ are responsible for transduction of signals on antigen binding to the BCR, we investigated whether there is a defect in their synthesis, assembly and exit from the ER when XBP-1 is absent. The synthesis of Igα and Igβ was not affected by the absence of XBP-1, as we detected similar amounts of Igα and Igβ in XBP-1WT/MD4 and XBP-1KO/MD4 plasmablasts (Figure 3D). Igα and Igβ contain two and three N-linked glycosylation sites, respectively; only one glycosylation site on Igα acquired endo-H-resistant complex glycans, whereas all three sites of Igβ acquired complex glycans (Figure 3D). As Igα and Igβ of XBP-1KO/MD4 plasmablasts can acquire complex glycans in the Golgi apparatus, their exit from the ER is normal in the absence of XBP-1. To assess the association of Igα with Igβ, we cross-linked proteins in detergent lysates using BS3 and analysed the appearance of Igα/Igβ heterodimers. We saw no difference in Igα/Igβ dimerization between XBP-1WT/MD4 and XBP-1KO/MD4 plasmablasts (Figure 3E).
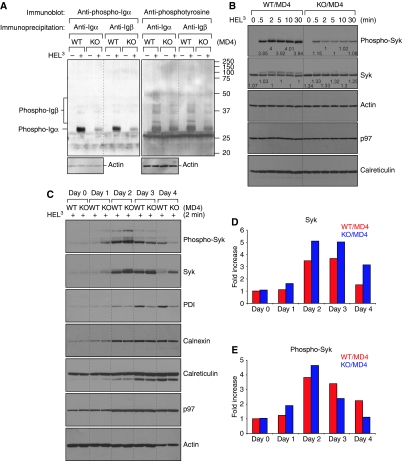
XBP-1-deficient B cells show defective phosphorylation of Igα, Igβ and Syk on antigen-specific activation of the BCR
B cells were harvested from the spleens of XBP-1WT/MD4 and XBP-1KO/MD4 mice, cultured with LPS for 3 or 4 days and activated with trimeric HEL as a physiological means of engaging the BCR through its antigen-binding sites, rather than by cross-linking through conserved portions of the BCR (Kim et al, 2006). XBP-1-deficient B cells showed an approximately six-fold reduction in phosphorylation of Igα and Igβ when compared with their wild-type counterparts (Figure 4A), although total levels of Igα and Igβ were unaffected (Figure 3D and E). As phosphorylation of Syk occurs immediately downstream of phosphorylation of Igα and Igβ, we examined kinetics of Syk phosphorylation in response to trimeric HEL and observed that phosphorylation of Syk is approximately four-fold stronger in XBP-1WT/MD4 than in XBP-1KO/MD4 B cells (Figure 4B).
Figure 4.
XBP-1 deficiency causes defective phosphorylation of Igα, Igβ and Syk in LPS-induced plasmablasts on antigen engagement. (A) LPS-stimulated XBP-1WT/MD4 and XBP-1KO/MD4 plasmablasts were activated by trimeric HEL for 2 min and lysed immediately. Lysates were immunoprecipitated using anti-Igα and anti-Igβ antibodies. The immunoprecipitates were analysed by SDS–PAGE and immunoblotted using an anti-phospho-Igα antibody or an anti-phosphotyrosine antibody. Total lysates were immunoblotted using an anti–actin antibody as a loading control. (B) XBP-1 plays a role in the maintenance of persistent Syk phosphorylation on BCR activation. Day 4 LPS-stimulated XBP-1WT/MD4 and XBP-1KO/MD4 plasmablasts were stimulated with trimeric HEL for indicated time and lysed immediately. Lysates were immunoblotted for phospho-Syk, Syk, actin, p97 and calreticulin. Quantitation of protein bands was performed, and the numbers represent relative band intensity within each gel. (C) Naive XBP-1WT/MD4 and XBP-1KO/MD4 B cells were induced to differentiate into plasmablasts by LPS. At the end of each indicated LPS stimulation time, cells were exposed to trimeric HEL for 2 min and lysed immediately. Day 0 cells were not exposed to LPS. Lysates were immunoblotted for phospho-Syk, Syk, protein disulfide isomerase (PDI), calnexin, calreticulin, p97 and actin. Note the imbalanced Syk phosphorylation between XBP-1WT/MD4 and XBP-1KO/MD4 plasmablasts from day 1 to day 4. (D) Protein bands in the Syk immunoblot in (C) were quantified and data were plotted as fold changes. (E) Protein bands in the phospho-Syk immunoblot in (C) were quantified.
Splenic B cells consist of ∼10% marginal zone and 90% follicular B cells. We sorted marginal zone (CD23medium, CD1dhigh) and follicular (CD23high, CD1dlow) B cells and found reduced Syk phosphorylation in both follicular and marginal zone XBP-1-deficient B cells (Supplementary Figure S2), although the phosphorylation defect is more pronounced in follicular B cells.
To determine when in the course of differentiation this difference in response occurred, we compared total Syk levels and HEL-induced Syk phosphorylation in B cells after exposure with LPS over the course of 4 days (Figure 4C). Initially, there was no difference in total or phosphorylated Syk levels between naive XBP-1-proficient and -deficient B cells, when both were stimulated with trimeric HEL (Figure 4C). After LPS-induced differentiation, XBP-1KO/MD4 B cells consistently expressed higher levels of Syk than XBP-1WT/MD4 B cells (Figure 4D), but their ability to phosphorylate Syk in response to HEL stimulation dropped significantly after 3 and 4 days of LPS-induced differentiation (Figure 4E). Four-day LPS-stimulated B cells showed a complete inversion of the relative amounts of Syk versus phospho-Syk in XBP-1KO/MD4 versus XBP-1WT/MD4 B cells. In the same experiment, we observed that the expression of calnexin was more differentiation dependent than that of calreticulin and the AAA ATPase (p97). The expression level of protein disulfide isomerase (PDI) was not only differentiation dependent, but also XBP-1 dependent (Figure 4C).
Defective BCR signalling in XBP-1-deficient B cells leads to reduced production of IL-6
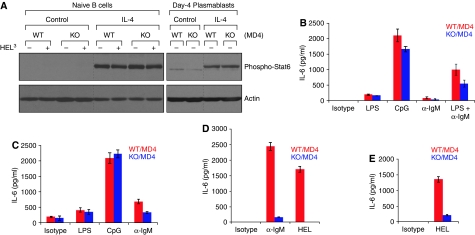
We treated naive and 3-day LPS-induced B cells with IL-4 for 4 and 24 h, respectively, and examined the phosphorylation status of Stat6 (signal transducers and activators of transcription-6). We detected no difference in Stat6 phosphorylation between XBP-1-proficient and -deficient cells in response to IL-4 stimulation, showing that—unlike signalling through the BCR—signalling through the IL-4 receptor is normal in these cells (Figure 5A).
Figure 5.
IgM- or HEL-stimulated XBP-1-deficient B cells produce less IL-6. (A) Naive B cells were cultured in the media with or without IL-4 for 4 h, and some cells were subsequently stimulated by trimeric HEL for 2 min. Besides, naive B cells were cultured in LPS for 3 days and subsequently treated with IL-4 for another 24 h. Lysates were immunoblotted for phospho-Stat6 (upper panels) and actin (lower panels). Naive (B), 2-day LPS stimulated (C) and 4-day LPS stimulated (D) XBP-1WT/MD4 and XBP-1KO/MD4 B cells were cultured with plate-bound LPS, CpG, anti-IgM or HEL. (E) Sorted XBP-1WT/MD4 and XBP-1KO/MD4 follicular B cells (using CD1d and CD23 markers) were stimulated with LPS for 4 days and then cultured with plate-bound HEL. The level of secreted IL-6 in the culture supernatants was measured after 24 h by ELISA. *P<0.005.
Although secreted IgM is the most commonly used readout for B-cell activation, XBP-1-deficient B cells have a known defect in the synthesis of secreted IgM, and thus we measured B-cell activation in response to various stimuli by IL-6 production instead. Naive, 2- and 4-day LPS-induced XBP-1-deficient B cells secreted significantly less IL-6 than their wild-type counterparts when stimulated with antibodies against IgM or with trimeric HEL, but not with LPS or CpG (Figure 5B–D). Sorted XBP-1-deficient follicular B cells also secreted less IL-6 when stimulated with trimeric HEL (Figure 5E). We conclude that signalling through Toll-like receptor (TLR)-4 and TLR-9 is normal. Treatment of XBP-1-deficient B cells with exogenous IL-4 or IL-6 did not restore the production of secreted IgM (Supplementary Figure S3).
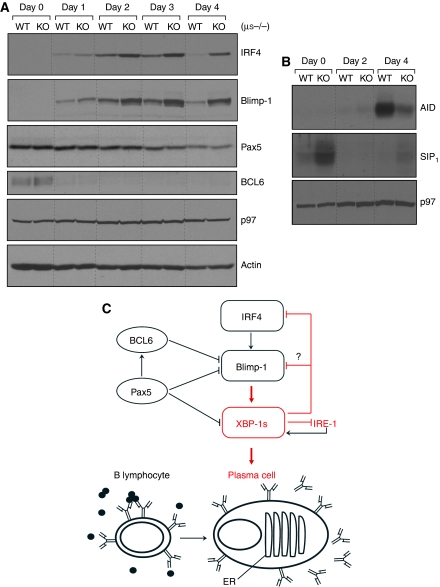
XBP-1 deficiency leads to upregulation of IRF-4 and Blimp-1, and altered levels of activation-induced cytidine deaminase
As signalling through the BCR is compromised by XBP-1 deficiency (Figure 4; Supplementary Figure S2), we investigated the effect of XBP-1 deficiency on transcription factors downstream of BCR activation. Surprisingly, in XBP-1-deficient B cells, IRF4 and Blimp-1, both of which control plasma cell differentiation, were present at elevated levels (Figure 6A). A relative increase in Blimp-1 likewise occurred in XBP-1-deficient B cells in response to engagement of diverse TLR agonists (Supplementary Figure S4). Pax5 and BCL6, transcription factors associated with the naive B-cell state, were unaffected by the lack of XBP-1, and on B-cell differentiation their levels declined as expected, independently of XBP-1 status (Figure 6A). Similar upregulation of IRF4 and Blimp-1 was observed in XBP-1KO/MD4 B cells (Supplementary Figure S5).
Figure 6.
XBP-1 deficiency leads to altered expression of IRF4, Blimp-1, activation-induced cytidine deaminase (AID) and sphingosine-1-phosphate receptor (S1P1). (A) XBP-1WT/μS−/− and XBP-1KO/μS−/− B cells were cultured in LPS to induce differentiation for indicated time. Lysates were immunoblotted for IRF4, Blimp-1, Pax5, BCL6, p97 and actin. (B) XBP-1WT and XBP-1KO B cells were stimulated by LPS to induce differentiation. Lysates were immunoblotted for AID, S1P1 and p97. (C) A model that illustrates the inhibitory effect of XBP-1 on IRF4, Blimp-1 and its activating enzyme IRE-1. The red arrows indicate that the function of IRF4 and Blimp-1 to regulate plasma cell differentiation requires XBP-1.
IRF4 regulates activation-induced cytidine deaminase (AID) expression, class switching and plasma cell differentiation (Klein et al, 2006; Sciammas et al, 2006). Expression of AID depends on LPS-induced differentiation and declines in XBP-1-deficient B cells (Figure 6B), although IRF4 levels are elevated in these cells (Figure 6A; Supplementary Figure S5).
Blimp-1-GFP-positive plasma cells of HEL-immunized XBP-1KO/MD4/Blimp-1-GFP mice do not colonize the bone marrow
Sphingosine-1-phosphate receptor (S1P1) controls the migration of lymphocytes from secondary lymphoid organs into the circulation, followed by their homing to the respective target sites (Rosen and Goetzl, 2005; Schwab and Cyster, 2007). S1P1 levels drop in 2-day LPS-stimulated B cells and rise again in 4-day LPS-stimulated B cells (Figure 6B), a pattern similar to that reported for activated T cells (Matloubian et al, 2004). XBP-1 deficiency leads to upregulation of the S1P1 in naive and 4-day LPS-stimulated B cells (Figure 6B). To examine plasma cell homing in vivo, we immunized XBP-1KO/MD4/Blimp-1-GFP mice with HEL and analysed the formation of Blimp-1-GFP-positive plasma cells. Blimp-1-GFP-positive plasmablasts arose in the spleens of HEL-immunized XBP-1KO/MD4/Blimp-1-GFP mice, and their number decreased in mice immunized twice or three times (Figure 7A and B). As plasmablasts usually migrate to long-lived niches in the bone marrow for sustained antibody production, we looked for the arrival of such cells in the bone marrow. However, significantly fewer XBP-1-deficient Blimp-1-GFP-positive plasma cells migrated to and colonized the bone marrow when compared with XBP-1-proficient mice (Figure 7A and B). Although XBP-1KO/MD4/Blimp-1-GFP mice initially responded to immunization by producing anti-HEL antibodies, the HEL-specific antibody titers rapidly decreased in XBP-1KO/MD4/Blimp-1-GFP mice immunized more than once (Figure 7C). Decreased bone marrow colonization could be due to impaired CXCR4 signalling through interaction with CXCL12, a chemokine secreted by bone marrow stromal cells. Indeed, XBP-1-deficient plasmablasts phosphorylate ERK1/2 less efficiently than their wild-type counterparts in response to stimulation with CXCL12 (Figure 7D).
Discussion
XBP-1 is required for the formation of plasma cells as well as several other secretory cell types, including exocrine gland acinar cells and Paneth cells (Lee et al, 2005; Kaser et al, 2008). Usually triggered by ER stress-induced IRE-1 activation, XBP-1 drives the expression of numerous genes involved in glycoprotein folding and ER homeostasis, with the overall effect of increasing secretory capacity. Thus, the favoured hypothesis for XBP-1 function in plasma cell development is as follows. Activated B cells rapidly increase production of secreted IgM, some fraction of which is misfolded and accumulates in the ER. Misfolded IgM binds to the chaperone BiP, titrating BiP away from IRE-1. IRE-1 dimerizes, self-activates and initiates the splicing of xbp-1 transcripts. The translation product, XBP-1s, then upregulates transcription of ER chaperones, relieving ER stress and allowing the nascent plasma cell to continue producing IgM. In the absence of XBP-1, misfolded IgM presumably accumulates in the ER and leads to apoptosis, thus explaining the lack of plasma cells in XBP-1-deficient mice (Iwakoshi et al, 2003a, 2003b).
The current model of XBP-1 in plasma cells leads to two testable hypotheses. First, it is misfolded IgM that triggers XBP-1 activation, and second, XBP-1-deficient B cells would be expected to have an increase in misfolded proteins. Neither of these hypotheses is supported by the data shown here. B cells from XBP-1WT/μS−/− mice can neither produce secreted IgM, nor do they show an increase in membrane-bound IgM during LPS-induced differentiation. Although class-switching rescues serum immunoglobulin levels in vivo, LPS-stimulated XBP-1WT/μS−/− LPS plasmablasts show virtually no evidence of switching to other isotypes, as showed by the complete lack of any immunoglobulin heavy chains recovered by immunoprecipitation of kappa chains from culture supernatants (McGehee et al, manuscript in preparation). Nonetheless, XBP-1WT/μS−/− plasmablasts activate XBP-1 with similar kinetics as do XBP-1WT/MD4 plasmablasts (Figure 2B; Supplementary Figure S1), demonstrating conclusively that XBP-1 activation is a differentiation-dependent event unlinked to accumulation of misfolded IgM. Formally, we cannot exclude the possibility that proteins other than IgM fail to fold properly when B cells initiate their terminal differentiation programme, and so trigger the UPR. However, for the glycoproteins examined, we observed no obvious folding or trafficking defects.
To directly assess the degree of misfolded proteins in XBP-1-deficient B cells, we cross-linked plasmablast lysates and identified misfolded glycoproteins by their aggregation and association with calreticulin and calnexin (Figure 2A and data not shown). We found no evidence for a global increase in misfolded proteins in XBP-1-deficient plasmablasts. Furthermore, close examination of individual proteins relevant to B-cell function showed no defects in their folding. IgM, both membrane-bound and secreted forms, trafficked through the ER at similar rates (Figure 3A and B) independent of XBP-1 status. The resulting secreted IgM from wild-type and XBP-1-deficient plasmablasts was properly folded, as assessed by its ability to bind antigen, both in an ELISA and by binding to antigen-coated beads (Figure 1C; McGehee et al, manuscript in preparation). Igα and Igβ folding was also normal in the absence of XBP-1 as assessed by rate of synthesis, acquisition of complex glycans, disulfide bond formation and heterodimer stability (Figure 3D and E; McGehee et al, manuscript in preparation). A variety of B-cell glycoproteins, such as MHC I, MHC II, CD1d, CD40, CD80 and CD86, are expressed normally on the surface of XBP-1-deficient plasmablasts (McGehee et al and data not shown). IL-6, itself a glycoprotein, is secreted normally from XBP-1-deficient plasmablasts on ligation of TLRs (Figure 5B and C). Signalling through the IL-4 receptor and through TLRs 4 and 9 is uncompromised in XBP-1-deficient B cells, providing further evidence that these receptors are functional and properly folded (Figure 5A–C).
To better understand the role of XBP-1 in plasma cell differentiation and the defects in XBP-1-deficient cells, we analysed the B cell-specific XBP-1 knockout/MD4 transgenic (XBP-1KO/MD4) mouse, in which B cells express an HEL-specific BCR encoded by a transgene (Goodnow et al, 1988). As anti-IgM reagents do not interact directly with the antigen-binding site, activation of MD4 B cells by trimeric HEL serves as physiological means to cross-link the BCR and explore signalling alterations in the absence of XBP-1 (Kim et al, 2006).
XBP-1-deficient MD4 plasmablasts showed a greatly diminished response to antigen stimulation, producing far less IL-6 than their wild-type counterparts (Figure 5). Closer examination of proximal signalling in XBP-1-deficient plasmablasts showed impaired phosphorylation of Igα, Igβ and Syk on BCR engagement (Figure 4; Supplementary Figure S2). XBP-1-deficient plasmablasts showed a slight increase in Syk phosphorylation when tested after 2 days of LPS culture (Figure 4C and E), but this apparent increase in proximal signalling did not rescue the diminished IL-6 production on BCR engagement (Figure 5C). Both follicular and marginal zone LPS-stimulated B cells showed impaired signalling (Figure 5E; Supplementary Figure S2). Heterogeneity of the total LPS-induced plasmablast populations, therefore, cannot explain the differences observed in BCR signalling between wild-type and XBP-1-deficient plasmablasts. Furthermore, XBP-1-deficient plasmablasts responded normally to IL-4, CpG and LPS stimulation (Figure 5), indicating that signalling downstream of the IL-4 receptor and TLRs 4 and 9 is unaffected by the absence of XBP-1.
How the transcription factor XBP-1 could affect BCR signalling is not immediately obvious. Surface expression of IgM is modestly diminished in XBP-1-deficient plasmablasts, but only after 4 days of culture (Figure 3C), whereas signalling defects are observed earlier (Figure 4C–E). IgM produced by XBP-1-deficient MD4 B cells is fully capable of antigen binding, and IL-6 production by XBP-1-deficient MD4 plasmablasts cannot be rescued by increasing the concentration of trimeric HEL used for stimulation (Figure 1C and data not shown). Several mechanisms could contribute to the BCR-specific signalling defect in XBP-1-deficient cells. First, although we found no evidence for protein misfolding in the absence of XBP-1, we cannot exclude the possibility that some proteins necessary for B-cell function are misfolded or abnormally glycosylated. Full engagement of the BCR requires not only the antigen-binding membrane IgM and its signalling accessories, Igα/Igβ, but also co-receptor proteins, such as CD21 and CD81. Any defect in assembly or modification of these proteins as a result of downregulated PDI (Figure 4C) altered terminal glycosylation or other as yet unidentified alterations could compromise activation of the BCR. Some differences in glycosylation were observed in XBP-1-deficient cells (McGehee et al, manuscript in preparation), but the functional importance of these altered terminal glycans in BCR signalling has yet to be explored. Furthermore, the expression of XBP-1 massively induces phosphatidylcholine synthesis and increases transcription of several other genes involved in lipid synthesis and metabolism (Sriburi et al, 2004, 2007). Changes in lipid composition caused by the lack of XBP-1 may lead to failure of assembling functional lipid raft domains, which are critical for BCR clustering on engagement of antigen. Indeed, we have observed a decrease in sphingomyelin and phosphatidylinositol content in membranes of XBP-1-deficient B cells (McGehee et al, manuscript in preparation). Sphingomyelin is an important component of lipid rafts, and phosphatidylinositol is an essential intermediate in signalling pathways involving phosphatidylinositol-3-phosphate.
Despite the defects in BCR signalling, XBP-1KO/MD4 mice have normal numbers of B cells and B-cell subsets (Figure 1A and B). This is not unexpected, given that mice with far more significant alterations in signalling still produce B cells, albeit with slight shifts in the ratios of marginal zone, follicular and B-1 B cells (Pillai et al, 2004). However, BCR signal strength determines B-cell subset fate in the bone marrow during the immature stage of B-cell development. The large differences in BCR signalling reported here were observed predominately after LPS stimulation (Figure 4) at a time when XBP-1 spliced protein is also present (Figure 2B; Supplementary Figure S1). Thus, B cells in XBP-1 knockout mice are predicted to be similar to the point when they first contact the antigen. We do observe some baseline differences in S1P1 expression by naive XBP-1-deficient splenic B cells (Figure 6B). Although naive B cells do not express XBP-1, expression of XBP-1 in pro-B cells could affect B-cell development (Brunsing et al, 2008).
Blimp-1 is a transcriptional repressor essential for plasma cell development. IRF4 controls transcription of the Blimp-1 gene prdm1 by direct binding to a conserved noncoding sequence between exons 5 and 6 (Sciammas et al, 2006). Although earlier studies have shown that irf4 and prdm1 are neither direct nor indirect targets of XBP-1 (Acosta-Alvear et al, 2007), we observed that both IRF4 and Blimp-1 protein levels are upregulated in XBP-1-deficient plasmablasts (Figure 6A; Supplementary Figure S5). We, therefore, propose that XBP-1 inhibits the expression of IRF4, and consequently Blimp-1, in plasma cells (Figure 6C). Given that IRF4 and Blimp-1 can be expressed in the absence of XBP-1, XBP-1 must be placed downstream of both of these factors, an observation consistent with earlier data showing that IRF4 and Blimp-1 increase xbp-1 transcription (Shaffer et al, 2002; Klein et al, 2006). XBP-1 activation is also regulated post-transcriptionally by IRE-1-mediated splicing to remove 26 nucleotides from xbp-1 mRNA. Of note, XBP-1 deficiency greatly enhances IRE-1 protein levels (Figure 2C; Supplementary Figure S1), demonstrating feedback inhibition of XBP-1 expression on IRE-1, similar to what is seen in hepatocytes (Lee et al, 2008).
We propose that XBP-1 activation in B cells is a differentiation-dependent event, and that the failure of XBP-1-deficient B cells to become plasma cells involves misregulation of key transcription factors, possibly due to altered BCR signalling. Paradoxically, loss of XBP-1 leads to increased IRF4 levels, which cause an increase in Blimp-1, both key transcription factors in plasma cell differentiation. However, despite higher levels of these canonical plasma cell proteins, XBP-1-deficient B cells still do not become plasma cells. This block is apparent not only by the lack of antibody secretion, but also by decreased expression of AID (Figure 6B), a key enzyme in class switch recombination and somatic hypermutation. Thus, at least in tissue culture, XBP-1-deficient B cells appear poised to become plasma cells, yet fail to do so.
To analyse plasma cell formation in vivo, we immunized XBP-1KO/MD4/Blimp-1-GFP mice, which express GFP under control of the Blimp-1 promoter to allow the unambiguous quantitation of plasma cells by flow cytometry. To our surprise, XBP-1-deficient mice developed a robust plasmablast population in the spleen after a single immunization, which correlated with transiently elevated serum levels of anti-HEL antibodies. On successive immunizations, however, the spleen plasmablast population decreased and no plasma cell population could be found accumulating in the bone marrow (Figure 7A and B). Specific antibody titers also declined in the XBP-1-deficient animals with each subsequent immunization (Figure 7C). The half-life of serum IgM in the mouse is 2 days (Vieira and Rajewsky, 1988). Consequently, although XBP-deficient mice can mount a primary antibody response, they do not sustain production of serum antibodies even after reimmunization.
Annexin V staining of spleen plasma cells at each time point showed no differences in apoptosis between wild-type and XBP-1-deficient cells (data not shown). CXCL12, produced by stromal cells, and its receptor CXCR4 are primarily responsible for homing of plasma cell precursors to the bone marrow niches (Muehlinghaus et al, 2005). CXCR4 is expressed normally on the surface of XBP-1-deficient plasma cells, but signalling through CXCR4 is impaired, as shown by decreased ERK1/2 phosphorylation in XBP-1-deficient plasmablasts exposed to CXCL12 (Figure 7D). The blunted response to CXCL12 could in part account for the failure of XBP-1-deficient plasma cells to colonize the bone marrow. In addition, IL-6 contributes to plasma cell maintenance, and immunoglobulin itself has been proposed to sustain long-lived plasma cells, although the mechanism responsible is not clear (Iwakoshi et al, 2003a; Kumazaki et al, 2007). Decreased serum antibody titers and decreased IL-6 produced by B cells could contribute to the lack of long-lived plasma cells in the bone marrow of XBP-1-deficient mice. However, given that XBP-1-deficient MD4 mice in this study have measurable baseline serum antibodies in the same order of magnitude as wild-type MD4 mice (Figures 1C and 7C), and given that IL-6 is produced by many cell types other than B cells, we propose that failure of plasma cells to traffick to the bone marrow is the major cause of lack of sustained antibody production in XBP-1-deficient mice.
The short burst of antibody production observed in immunized XBP-1KO/MD4/Blimp-1-GFP mice is at odds with earlier reports showing that immunized XBP-1-deficient mice have lower serum levels of specific antibodies (Reimold et al, 2001). This discrepancy could be due to the different time points at which the immunized mice were analysed, given that the antibody production we observed occurred only briefly, and only after a single immunization. Alternatively, the MD4 BCR is expressed from a transgene on >95% of B cells, including B-1 cells in the peritoneal cavity and extrafollicular B cells. Thus, immunization in MD4 mice could induce antibody production primarily from these sources, which would not be a major contributing factor in mice with a polyclonal repertoire.
XBP-1 is upregulated in many human malignancies, particularly multiple myeloma, a cancer for which few treatment options are available. Overexpression of XBP-1 in B cells is sufficient to cause a monoclonal gammopathy of undetermined significance in mice, suggesting that abnormal expression of XBP-1 could be a predisposing factor for the development of myeloma (Carrasco et al, 2007). Our finding that XBP-1 activation precedes the UPR in normal plasma cell development offers an attractive possibility. If IRE-1-mediated splicing of XBP-1 is triggered by a specific differentiation-dependent event rather than by the accumulation of misfolded aggregates, then XBP-1 activation itself may be an attractive target for drug therapy.
Materials and methods
Mice
We generated CD19-Cre × XBP-1f/f/MD4 (XBP-1KO/MD4) (Goodnow et al, 1988; Hetz et al, 2008), XBP-1KO/MD4/Blimp-1-GFP (Kallies et al, 2004) and XBP-1KO/μS−/− mice (Boes et al, 1998) by crossing relevant strains. All animals were maintained according to the MIT Committee on Animal Care.
Antibodies and reagents
Antibodies were raised against Igα, Igβ (rabbit polyclonal) and phospho-ITAMs of Igα and Igβ (mouse monoclonal). Antibodies to XBP-1 (Santa Cruz), Blimp-1 (Santa Cruz), IRF4 (Cell Signaling), Pax5 (Santa Cruz), BCL6 (Cell Signaling), Syk (Cell Signaling), phopho-Syk (Cell Signaling), lyn (Cell Signaling), phospho-Stat6 (Cell Signaling), IRE-1α (Cell Signaling), eIF2α (Cell Signaling), AID (Cell Signaling), p44/42 MAPK (Cell Signaling), phospho-p44/42 MAPK (Cell Signaling), CD19 (Santa Cruz), S1P1 (Santa Cruz), actin (Sigma), calreticulin (Stressgen), p97 (Fitzgerald), phosphotyrosine (Upstate) and μ (SouthernBiotech) were obtained commercially. Antibodies for flow cytometry were from BD Pharmingen: IgM (11/41 or R6-60.2), IgD (11-26c.2a), CD1d (1B1), CD19 (MB19-1), CD23 (B3B4), CD43 (1B11), CD138 (281-2), B220 (RA3-6B2), AA4.1 and GL7. Trimeric HEL and anti-PDI were produced in our laboratory. Anti-calnexin was kindly provided by Dr David B Williams (University of Toronto, Canada). LPS and CpG DNA were from Sigma and TIB-MOLBIOL, respectively.
Cell culture
Naive B lymphocytes were purified from mouse spleen by magnetic depletion of CD43-positive cells (Miltenyi Biotech). Naive B cells were cultured in RPMI 1640 media containing 10% FBS with or without LPS (20 μg/ml). Marginal zone and follicular B cells were stained with anti-CD1d and anti-CD23 antibodies and sorted using an FACS Aria (BD Biosciences).
Immunization
Mice were immunized, intraperitoneally, with HEL (50 μg) mixed in complete or incomplete Freund's adjuvant (Sigma).
BCR activation, SDS–PAGE and immunoblot
B cells were activated by trimeric HEL (5 μg/ml) for 2 min unless indicated and lysed as described (Kim et al, 2006). Proteins were resolved by SDS–PAGE, transferred to nitrocellulose and immunoblotted by conventional methods. In some cases, proteins were treated with endoglycosidase H or PNGase F (New England Biolabs). For cross-linking, proteins were treated with BS3 (Pierce). Quantitation of protein bands was performed using the Image Gauge Version 3.0. (Fujifilm).
Pulse chase labelling and immunoprecipitation
Plasmablasts were starved in methionine- and cysteine-free media containing dialysed serum for 1 h, and then pulse labelled for 10 min with 250 μCi/ml of [35S]-methionine and [35S]-cysteine. At the end of each chase point, cells were rinsed twice with PBS and lysed in conventional RIPA buffer containing protease inhibitors. Pre-cleared lysates were incubated with a primary antibody and Protein G-agarose beads, washed, eluted from the beads using reducing Laemmli SDS–PAGE sample buffer and analysed by SDS–PAGE.
Enzyme-linked immunosorbent assay
ELISA analyses of IL-6 production were performed according to the manufacturer's instruction (BD Biosciences). ELISA plates were read using SpectraMax M2 microplate reader (Molecular Devices).
FACS analysis
Live B cells were stained with indicated antibodies and analysed by an FACSCalibur flow cytometer (BD Biosciences).
Triton X-114 phase separation
B cells were lysed in Triton X-114 lysis buffer (10 mM Tris–HCl, pH 7.4; 150 mM NaCl; 1% Triton X-114) containing protease inhibitors. Phase separation was performed as described by Bordier, 1981.
Supplementary Material
Supplementary Figure S1
Supplementary Figure S2
Supplementary Figure S3
Supplementary Figure S4
Supplementary Figure S5
Supplementary Figure Legends
Review Process File
Acknowledgments
We thank Dr Laurie H Glimcher and Dr Ann-Hwee Lee for providing us with XBP-1f/f mice; Dr Stephen L Nutt for providing us with Blimp-1-GFP mice; B Tirosh, YM Kim, J Antos, M Popp, L Buti and B Park for useful discussions. These studies were supported by grants from the NIH (to HLP). AMM was supported by an NSF Graduate Research Fellowship. SKD is supported by a CRI Fellowship.
References
- Acosta-Alvear D, Zhou Y, Blais A, Tsikitis M, Lents NH, Arias C, Lennon CJ, Kluger Y, Dynlacht BD (2007) XBP1 controls diverse cell type- and condition-specific transcriptional regulatory networks. Mol Cell 27: 53–66 [DOI] [PubMed] [Google Scholar]
- Boes M, Esau C, Fischer MB, Schmidt T, Carroll M, Chen J (1998) Enhanced B-1 cell development, but impaired IgG antibody responses in mice deficient in secreted IgM. J Immunol 160: 4776–4787 [PubMed] [Google Scholar]
- Bordier C (1981) Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem 256: 1604–1607 [PubMed] [Google Scholar]
- Brunsing R, Omori-Fitzpatrick S, Weber F, Bicknell AA, Friend L, Rickert R, Niwa M (2008) B and T cell development both involve activity of the unfolded protein response pathway. J Biol Chem 283: 17954–17961 [DOI] [PubMed] [Google Scholar]
- Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D (2002) IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 415: 92–96 [DOI] [PubMed] [Google Scholar]
- Carrasco DR, Sukhdeo K, Protopopova M, Sinha R, Enos M, Carrasco DE, Zheng M, Mani M, Henderson J, Pinkus GS, Munshi N, Horner J, Ivanova EV, Protopopov A, Anderson KC, Tonon G, DePinho RA (2007) The differentiation and stress response factor XBP-1 drives multiple myeloma pathogenesis. Cancer Cell 11: 349–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykstra M, Cherukuri A, Sohn HW, Tzeng SJ, Pierce SK (2003) Location is everything: lipid rafts and immune cell signaling. Annu Rev Immunol 21: 457–481 [DOI] [PubMed] [Google Scholar]
- Gass JN, Gifford NM, Brewer JW (2002) Activation of an unfolded protein response during differentiation of antibody-secreting B cells. J Biol Chem 277: 49047–49054 [DOI] [PubMed] [Google Scholar]
- Goodnow CC, Crosbie J, Adelstein S, Lavoie TB, Smith-Gill SJ, Brink RA, Pritchard-Briscoe H, Wotherspoon JS, Loblay RH, Raphael K, Trent RJ, Basten A (1988) Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature 334: 676–682 [DOI] [PubMed] [Google Scholar]
- Hetz C, Lee AH, Gonzalez-Romero D, Thielen P, Castilla J, Soto C, Glimcher LH (2008) Unfolded protein response transcription factor XBP-1 does not influence prion replication or pathogenesis. Proc Natl Acad Sci USA 105: 757–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwakoshi NN, Lee AH, Glimcher LH (2003a) The X-box binding protein-1 transcription factor is required for plasma cell differentiation and the unfolded protein response. Immunol Rev 194: 29–38 [DOI] [PubMed] [Google Scholar]
- Iwakoshi NN, Lee AH, Vallabhajosyula P, Otipoby KL, Rajewsky K, Glimcher LH (2003b) Plasma cell differentiation and the unfolded protein response intersect at the transcription factor XBP-1. Nat Immunol 4: 321–329 [DOI] [PubMed] [Google Scholar]
- Kallies A, Hasbold J, Fairfax K, Pridans C, Emslie D, McKenzie BS, Lew AM, Corcoran LM, Hodgkin PD, Tarlinton DM, Nutt SL (2007) Initiation of plasma-cell differentiation is independent of the transcription factor Blimp-1. Immunity 26: 555–566 [DOI] [PubMed] [Google Scholar]
- Kallies A, Hasbold J, Tarlinton DM, Dietrich W, Corcoran LM, Hodgkin PD, Nutt SL (2004) Plasma cell ontogeny defined by quantitative changes in blimp-1 expression. J Exp Med 200: 967–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaser A, Lee AH, Franke A, Glickman JN, Zeissig S, Tilg H, Nieuwenhuis EE, Higgins DE, Schreiber S, Glimcher LH, Blumberg RS (2008) XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell 134: 743–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YM, Pan JY, Korbel GA, Peperzak V, Boes M, Ploegh HL (2006) Monovalent ligation of the B cell receptor induces receptor activation but fails to promote antigen presentation. Proc Natl Acad Sci USA 103: 3327–3332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein U, Casola S, Cattoretti G, Shen Q, Lia M, Mo T, Ludwig T, Rajewsky K, Dalla-Favera R (2006) Transcription factor IRF4 controls plasma cell differentiation and class-switch recombination. Nat Immunol 7: 773–782 [DOI] [PubMed] [Google Scholar]
- Kumazaki K, Tirosh B, Maehr R, Boes M, Honjo T, Ploegh HL (2007) AID−/−mus−/− mice are agammaglobulinemic and fail to maintain B220-CD138+ plasma cells. J Immunol 178: 2192–2203 [DOI] [PubMed] [Google Scholar]
- Lee AH, Chu GC, Iwakoshi NN, Glimcher LH (2005) XBP-1 is required for biogenesis of cellular secretory machinery of exocrine glands. EMBO J 24: 4368–4380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AH, Iwakoshi NN, Glimcher LH (2003) XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol 23: 7448–7459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AH, Scapa EF, Cohen DE, Glimcher LH (2008) Regulation of hepatic lipogenesis by the transcription factor XBP1. Science 320: 1492–1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins G, Calame K (2008) Regulation and functions of Blimp-1 in T and B lymphocytes. Annu Rev Immunol 26: 133–169 [DOI] [PubMed] [Google Scholar]
- Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG (2004) Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 427: 355–360 [DOI] [PubMed] [Google Scholar]
- Muehlinghaus G, Cigliano L, Huehn S, Peddinghaus A, Leyendeckers H, Hauser AE, Hiepe F, Radbruch A, Arce S, Manz RA (2005) Regulation of CXCR3 and CXCR4 expression during terminal differentiation of memory B cells into plasma cells. Blood 105: 3965–3971 [DOI] [PubMed] [Google Scholar]
- Pierce SK (2002) Lipid rafts and B-cell activation. Nat Rev Immunol 2: 96–105 [DOI] [PubMed] [Google Scholar]
- Pillai S, Cariappa A, Moran ST (2004) Positive selection and lineage commitment during peripheral B-lymphocyte development. Immunol Rev 197: 206–218 [DOI] [PubMed] [Google Scholar]
- Reimold AM, Iwakoshi NN, Manis J, Vallabhajosyula P, Szomolanyi-Tsuda E, Gravallese EM, Friend D, Grusby MJ, Alt F, Glimcher LH (2001) Plasma cell differentiation requires the transcription factor XBP-1. Nature 412: 300–307 [DOI] [PubMed] [Google Scholar]
- Rosen H, Goetzl EJ (2005) Sphingosine 1-phosphate and its receptors: an autocrine and paracrine network. Nat Rev Immunol 5: 560–570 [DOI] [PubMed] [Google Scholar]
- Schwab SR, Cyster JG (2007) Finding a way out: lymphocyte egress from lymphoid organs. Nat Immunol 8: 1295–1301 [DOI] [PubMed] [Google Scholar]
- Sciammas R, Shaffer AL, Schatz JH, Zhao H, Staudt LM, Singh H (2006) Graded expression of interferon regulatory factor-4 coordinates isotype switching with plasma cell differentiation. Immunity 25: 225–236 [DOI] [PubMed] [Google Scholar]
- Shaffer AL, Lin KI, Kuo TC, Yu X, Hurt EM, Rosenwald A, Giltnane JM, Yang L, Zhao H, Calame K, Staudt LM (2002) Blimp-1 orchestrates plasma cell differentiation by extinguishing the mature B cell gene expression program. Immunity 17: 51–62 [DOI] [PubMed] [Google Scholar]
- Shen X, Ellis RE, Lee K, Liu CY, Yang K, Solomon A, Yoshida H, Morimoto R, Kurnit DM, Mori K, Kaufman RJ (2001) Complementary signaling pathways regulate the unfolded protein response and are required for C. elegans development. Cell 107: 893–903 [DOI] [PubMed] [Google Scholar]
- Sriburi R, Bommiasamy H, Buldak GL, Robbins GR, Frank M, Jackowski S, Brewer JW (2007) Coordinate regulation of phospholipid biosynthesis and secretory pathway gene expression in XBP-1(S)-induced endoplasmic reticulum biogenesis. J Biol Chem 282: 7024–7034 [DOI] [PubMed] [Google Scholar]
- Sriburi R, Jackowski S, Mori K, Brewer JW (2004) XBP1: a link between the unfolded protein response, lipid biosynthesis, and biogenesis of the endoplasmic reticulum. J Cell Biol 167: 35–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirosh B, Iwakoshi NN, Glimcher LH, Ploegh HL (2005) XBP-1 specifically promotes IgM synthesis and secretion, but is dispensable for degradation of glycoproteins in primary B cells. J Exp Med 202: 505–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Anken E, Romijn EP, Maggioni C, Mezghrani A, Sitia R, Braakman I, Heck AJ (2003) Sequential waves of functionally related proteins are expressed when B cells prepare for antibody secretion. Immunity 18: 243–253 [DOI] [PubMed] [Google Scholar]
- Vieira P, Rajewsky K (1988) The half-lives of serum immunoglobulins in adult mice. Eur J Immunol 18: 313–316 [DOI] [PubMed] [Google Scholar]
- Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K (2001) XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107: 881–891 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1
Supplementary Figure S2
Supplementary Figure S3
Supplementary Figure S4
Supplementary Figure S5
Supplementary Figure Legends
Review Process File