Abstract
It has been widely recognized that induction of Phase 2 enzymes is an effective and sufficient strategy for achieving protection against carcinogenesis. Nrf2 is the unifying master regulator of these enzymes and its activation in various tissues, including the urinary bladder, is associated with inhibition of carcinogenesis. 5,6-Dihydrocyclopenta[c][1,2]-dithiole-3(4H)-thione (CPDT) is a highly potent inducer of Phase 2 enzymes and an activator of Nrf2. In vivo, it is particularly effective in the bladder, showing significant effects in this tissue when dosed to rats at levels as low as 0.98 µmol/kg/day (0.17 mg/kg/day). The activities of key phase 2 enzymes, including glutathione S-transferase, NAD(P)H:quinone:oxidoreductase 1 and glutamate cysteine synthetase, and levels of glutathione were elevated by CPDT in rat bladder in vivo and in cultured bladder cells in vitro. In the bladder, enzyme induction and Nrf2 activation appear to occur exclusively in the epithelium. This is highly significant, since almost all bladder cancers develop from the epithelium. Studies in cultured bladder cells using siRNA to knock down Nrf2 or in cells with total Nrf2 knockout showed that the ability of CPDT to induce Phase 2 enzymes depends completely on Nrf2. In conclusion, CPDT potently and preferentially induces Phase 2 enzymes in the bladder epithelium and Nrf2 is its key mediator.
Keywords: dithiolethione, phase 2 enzyme, Nrf2, chemoprevention, bladder cancer
1. Introduction
Phase 2 enzymes are part of the biotransformation machinery of cells and play a major role in defense against carcinogens, oxidants and other toxic chemicals. The cancer-preventive activities of many Phase 2 enzymes have been well documented. Numerous epidemiological studies have shown that individuals who are deficient in Phase 2 enzymes are at increased risk of developing cancer. For example, an inverse association in humans between the incidence of bladder cancer and tissue activities of glutathione S-transferase (GST), NAD(P)H:quinone oxidoreductase (NQO1) and N-acetyltransferase has been reported [1–5]. Animal studies have also shown that susceptibility to cancer increases if a Phase 2 gene is knocked out [6,7]. Moreover, in cultured cells, forced expression of a Phase 2 enzyme through gene transfection protects against carcinogen-induced DNA damage and other cytotoxicities [8,9].
Many Phase 2 enzymes are readily inducible and are often coordinately induced in response to various stimuli. There is compelling evidence that induction of Phase 2 enzymes is an effective and sufficient strategy for achieving protection against carcinogenesis [10]. While the induction of an individual Phase 2 enzyme may involve multiple mechanisms, it is the Keap1-Nrf2-ARE signaling system that unites them and provides the molecular basis for their coordinate induction [11]. Nuclear factor erythroid 2-related factor 2 (Nrf2), is a transcription factor that is normally sequestered by its repressor Kelch-like ECH-associated protein 1 (Keap1). Dissociation of Nrf2 from Keap1 allows the former to heterodimerize with partners such as Maf, to bind to a cis-acting DNA regulatory element - antioxidant response element (ARE), and to promote transcription of the down-stream gene. One or more copies of ARE are known to exist in the 5’-flanking region of many Phase 2 genes. Modification of critical cysteine residues of Keap1 by inducers, which frees Nrf2 from Keap1, has been recognized as a key mechanism of Nrf2 activation [12]. Several animal studies have shown that Nrf2 knockout not only renders the animals more susceptible to chemical carcinogenesis but also abolishes the inhibitory effect of Phase 2 enzyme inducers [13–15].
Dithiolethiones are a well-known class of inducers of Phase 2 enzymes, among which 4-methyl-5-pyrazinyl-1,2-dithiole-3H-3-thione (oltipraz) has been the most extensively studied [16]. Oltipraz showed ability as a wide-spectrum inhibitor of chemical carcinogenesis in preclinical models [17]. Activation of Nrf2 signaling was critical for the chemopreventive activity of oltipraz, as knockout of Nrf2 rendered Phase 2 enzymes nonresponsive to this substance and caused loss of its cancer-preventive activity in animal studies [13,15,18]. However, questions about the usefulness of oltipraz in cancer chemoprevention in humans have been raised, since significant side effects occur in humans at dose levels which show no or questionable chemopreventive efficacy [19,20]. Interestingly, several dithiolethiones have been shown to be markedly more potent than oltipraz in induction of Phase 2 enzymes in preclinical studies. Egner et al [21] showed that 5,6-dihydrocyclopenta[c][1,2]-dithiole-3(4H)-thione (CPDT, see Fig. 1A for its chemical structure) was the most potent inducer of NQO1 in an in vitro cell model among 25 dithiolethiones. Our recent in vivo study showed that while administration of CPDT to rats increased Phase 2 enzyme activities in many tissues, the bladder was the tissue most susceptible to this inductive effect. CPDT showed the highest inductive activity in the bladder among 10 dithiolethiones evaluated in rats, and the degree of induction of GST and NQO1 by this compound was 4.2 and 4.8 fold higher than that by oltipraz under the same treatment conditions [22]. In the present study, the inductive potency and tissue specificity of CPDT have been further delineated. The effect of CPDT on Nrf2 and the importance of Nrf2 in mediating the inductive activity of CPDT have also been elucidated.
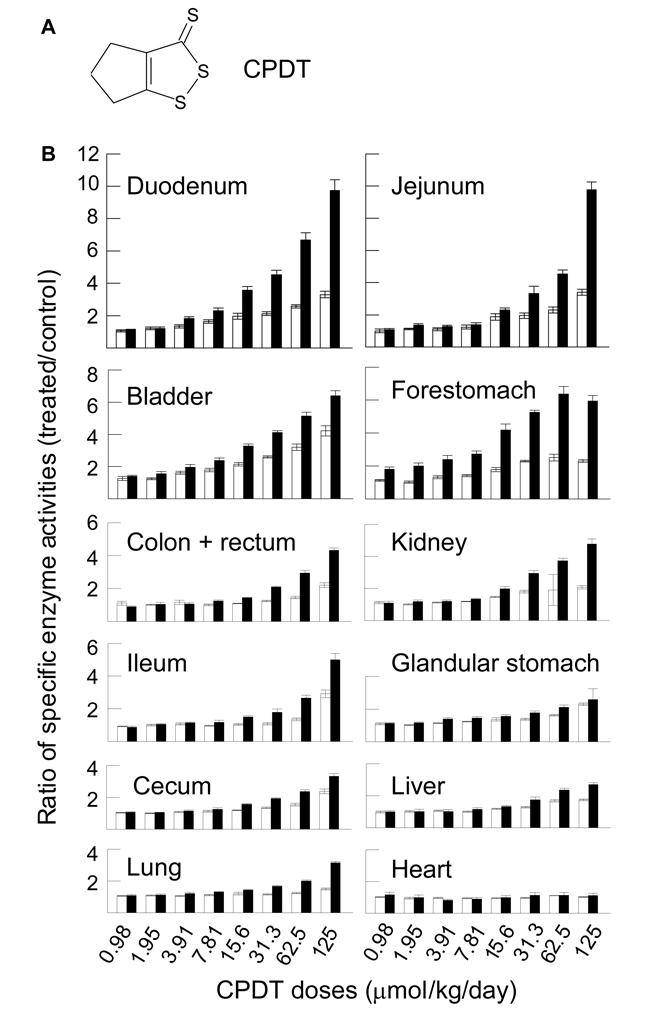
Fig. 1.
Induction of GST and NQO1 by CPDT in vivo: dose-response and organ specificity. A. The chemical structure of CPDT. B. Groups of 6 rats were dosed with CPDT by gavage at the specified doses once daily for 5 days. Another group of 12 rats was dosed with vehicle. The animals were killed on the sixth day and organs were harvested for analysis of GST and NQO1. Each value (mean ± SEM) is the ratio of specific activities (treated/control) of GST (□) and NQO1 (■). The specific enzyme activities (IU/g tissue, mean ± SEM) of GST and NQO1 in the control tissues were, respectively: 3.69 ± 0.14 and 27.9 ± 0.9 (bladder); 4.65 ± 0.16 and 59.9 ± 3.7 (cecum); 2.89 ± 0.09 and 26.6 ± 0.9 (colon and rectum); 27.1 ± 1.6 and 10.6 ± 1.1 (duodenum); 4.17 ± 0.46 and 16.9 ± 1.6 (forestomach); 7.84 ± 0.16 and 64.6 ± 2.5 (glandular stomach); 2.84 ± 0.06 and 1.57 ± 0.2 (heart); 4.04 ± 0.12 and 16.3 ± 1.3 (ileum); 15.0 ± 0.5 and 4.66 ± 0.35 (jejunum); 13.7 ± 0.3 and 6.97 ± 0.37 (kidney); 172 ± 2 and 45.0 ± 1.3 (liver); 6.29 ± 0.21 and 16.1 ± 0.4 (lung). The increase in enzymatic activity was significant at each of the following CPDT doses (P<0.05): All doses in the bladder; ≥15.6 µmol/kg/day (GST) and ≥7.81 (NQO1) in the cecum; ≥62.5 (GST) and ≥7.81 (NQO1) in the colon and rectum; ≥3.91 (GST) and ≥7.81 (NQO1) in the duodenum; ≥3.91 (GST) and all doses (NQO1) in the forestomach; ≥3.91 (GST) and all doses in the glandular stomach; ≥62.5 (GST) and ≥15.6 (NQO1) in the ileum; ≥15.6 (GST) and ≥1.95 (NQO1) in the jejunum; ≥7.81 (GST and NQO1) in the kidney; ≥15.6 (GST) and ≥31.3 (NQO1) in the liver; ≥15.6 (GST) and ≥3.91 (NQO1) in the lung.
2. Materials and methods
2.1. Chemicals
CPDT was synthesized as described previously [23]. Its identity and purity were confirmed by melting point and mass spectrometry. The antibodies for the following proteins, including the catalytic subunit and modulatory subunits of glutamate cysteine synthetase (GCSc and GCSm, catalog #: sc-22755 and sc-55586), NQO1 (catalog #: sc-16464) and Nrf2 (catalog #: sc-16464) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Another anti-NQO1 antibody (catalog #: 3187) was purchased from Cell Signaling Technology (Danvers, MA). The antibodies against GST-α (catalog #: GSTA11-S) and GST-µ (catalog #: GSTM11-S) were purchased from Alpha Diagnostics International (San Antonio, Texas). An antibody recognizing glyceraldehyde-3-phosphate dehydrogenase (GAPDH, Catalog #: MAB374) was purchased from Millipore (Billerica, MA). These antibodies were used in Western blot analyses. Three additional antibodies were purchased for immunohistochemistry, including anti-GST-µ (catalog #: LS-B963) from Lifespan Biosciences (Seattle, WA), anti-Nrf2 (catalog #: sc-722) from Santa Cruz Biotechnology, and anti-NQO1 (catalog #: MA1-16672) from Affinity BioReagents (Golden, CO).
2.2. Cell culture, CPDT treatment, and siRNA treatment
NBT-II rat bladder carcinoma cells were from American Type Culture Collection (ATCC, Manassas, VA) and were grown in Eagle’s Minimum Essential Medium (Mediatech, Manassas, VA), supplemented with 10% (v/v) fetal bovine serum (Life Technologies, Grand Island, NY). Wild type murine embryonic fibroblasts (MEF, Nrf2+/+) and their Nrf-2 knockout counterparts (MEF, Nrf2−/−) were a generous gift of Dr. Masayuki Yamamoto of Tohoku University in Japan [24] and were grown in Iscove’s Modified Dulbecco’s Medium (Mediatech), containing 10% (v/v) heat-inactivated (557°C and 30 min) fetal bovine serum. Normal human bladder epithelial (NHBE) cells were a generous gift of Dr. Jenny Southgate of the University of York in the UK and were grown in Keratinocyte Growth Medium-2 (KGM-2) with SingleQuot Kit (Lonza, Basel, Switzerland). All the cells were maintained in a humidified incubator at 37°C with 5% CO2.
Approximately 1.5 million cells were plated with 10 ml medium in a 10-cm dish overnight and then incubated with CPDT at the desired concentrations for 48 h. NHBE cells were plated in 6-cm dishes (0.7 million cells/dish with 4 ml medium). CPDT was dissolved in DMSO, and the final concentration of DMSO in the media was 0.1% (v/v). Cells were harvested by trypsinization, rinsed once with ice-cold PBS, and processed for measurement of enzyme activities, GSH content and expression levels of various proteins as described below.
To knock down Nrf2, NBT-II cells were treated with Nrf2 stealth RNAi (NFE2L2RSS343557) or negative universal control stealth RNAi 45–2001 (Invitrogen, Carlsbad, California), following the manufacturer’s instructions. Briefly, 1.5 million cells were grown in each 10-cm dish with 10 ml medium overnight and incubated with RNAi in fresh medium for 48 h. RNAi (0.584 nmol) was diluted in Opti-MEM I reduced serum medium (Invitrogen), mixed with lipofectamine 2000, and the mixture added to the medium in each dish. At the end of RNAi treatment, cells were either harvested to check the level of Nrf2 or treated with CPDT for 24 h.
2.3. Animal treatment
Female Sprague-Dawley rats (11–12 weeks of age) were bred at AgResearch, Ruakura Agricultural Research Center, New Zealand. The animals were randomly allocated to treatment groups. They were housed in solid-bottom cages containing bedding of softwood shavings and allowed free access to food (Laboratory Chow, Sharpes Animal Feeds, Carterton, NZ) and tap water. The room temperature was maintained at 21–23°C with a 12 h light-dark cycle. All experimental protocols were approved by the Institutional Animal Ethics Committee.
The rats were used to assess dose-response, time course and tissue specificity of Phase 2 enzyme induction by CPDT. In the first experiment, groups of 6 rats were dosed with freshly prepared solutions of CPDT in soya oil by gavage once daily at 0.98–125 µmol/kg/day (0.17–21.75 mg/kg/day) for 5 days. A control group of 12 rats was dosed with soya oil alone (approximately 0.5 ml/rat/day) for 5 days. In the second experiment, groups of 6 rats were dosed by gavage with CPDT once daily at 3.91 µmol/kg/day (0.68 mg/kg/day) for 2, 5, 8, 11, 14 or 17 days. A control group of 12 rats was dosed with the vehicle for 17 days. The experiment was structured so that all the animals were killed on the same day at the same age. For example, the rats given 17 doses of CPDT began treatment 17 days before killing, and those given 2 doses began treatment 2 days before killing. In both experiments, more rats were used in the control group to ensure that any unexpected loss of animals during the experimental period would not compromise the entire experiment, since data interpretation of each and every treatment group depends on the control group. All rats in both experiments were killed 1 day after the last dose, and various organs were dissected out and stored at −80°C. The contents of the gastrointestinal tract were removed under running water before storage. The specimens were used to measure the enzymatic activities of GST and NQO1. In the third experiment, two groups of 5 rats were dosed by gavage once daily with either CPDT at 125 µmol/kg/day (21.75 mg/kg/day) or vehicle for 5 days and killed on the sixth day to harvest bladder. One half of each bladder was fixed in 10% buffered formalin and the other half was stored frozen at −80°C. The specimens were used for further analysis of Phase 2 enzymes, GSH levels and Nrf2.
2.4. Measurement of GST and NQO1 activities and GSH content
Cell specimens were lysed by sonication (using a Branson’s Model 450 sonifier) in 0.08% digitonin solution containing 2 mM EDTA (pH 7.8). The lysates were cleared by centrifugation before determination of enzyme activities and GSH levels, using 1-chloro-2,4-dinitrobenzene as a GST substrate and menadione as an NQO1 substrate, as previously described [25], and the GSH reductase-coupled 5,5’-dithiobis-2-nitrobenzoic acid assay for measurement of GSH content [26]. For measurement of GST and NQO1 activities in rat organs, tissue specimens were homogenized in ice-cold 0.2% Triton X-100 using a Polytron tissue homogenizer. The homogenates were cleared by centrifugation and measured for GST and NQO1 activities by the methods of Habig et al. [27] and Ernster [28], respectively. For measurement of GSH levels in rat bladder tissues, the specimens were homogenized in 10 mM Tri-HCl, pH 7.4 (21 µl/mg tissue), containing 0.25 M sucrose, 1 mM phenylmethylsulfonyl fluoride (PMSF, Sigma, St Louis, MO) and 1 µg/ml leupeptin (Roche, Indianapolis, IN). The homogenates were assayed for GSH levels as mentioned above. The protein concentrations of all samples were measured by a BCA assay kit (Pierce, Rockford, IL).
2.5. Western blotting
Cells harvested from each dish were suspended in 200 µl cell lysis buffer (Cell Signaling Technology), supplemented with 1 mM PMSF and sonicated using a Branson’s Model 450 sonifier. Nuclear extracts were prepared as previously described [29]. Cells grown in each dish were rinsed once in ice cold PBS and lysed by incubation with 1 ml ice cold buffer A (10 mM HEPES, pH 7.5, 1.5 mM MgCl2, 10 mM KCl, 0.1% Nonidet P-40, 0.5 mM DTT, 0.5 mM PMSF, 1 µg/ml leupeptin and 1 µg/ml pepstatin A [Sigma]) for 10 min. The lysates were transferred to a microfuge tube, vortexed for 10 seconds and allowed to sit on ice for 5 min. The lysates were then centrifuged at 16,060g for 3 min. After removing the supernatant, the pellet was resuspended in buffer C (20 mM HEPES, pH 7.5, 420 mM NaCl, 1.5 mM MgCl2, 25% glycerol, 0.25 mM EDTA, 0.5 mM DTT, 0.5 mM PMSF, 1 µg/ml leupeptin, 1 µg/ml pepstatin) and shaken on ice on a rocking platform for 45 min. The samples were then centrifuged at 16,060g for 10 min at 4°C. The supernatant fractions (nuclear extract) were then mixed 1:6 with buffer D (20 mM HEPES, pH 7.5, 100 mM KCl, 20% glycerol, 0.25 mM EDTA, 0.5 mM DTT, 0.5 mM PMSF, 1 mg/ml leupeptin, 1 mg/ml pepstatin A). Protein levels were determined using Pierce’s BCA assay kit. Each sample (25–50 µg protein) was resolved by SDS-PAGE (6–12%) and transferred to a polyvinylidine difluoride membrane. The membranes were blocked at room temperature for 1 h with either 5% milk or 1x Detector Block (KPL, Gaithersburg, MD), and then probed with an antibody overnight at 4°C. Immunoreactivity was detected by incubation with an HRP-linked secondary antibody (either mouse or rabbit, Amersham, Piscataway, NJ) at room temperature for 1 h, and the band of interest was visualized using either ECL (Pierce) or ECL+ (Amersham) chemiluminescence systems.
2.6. Immunohistochemistry
The formalin-fixed paraffin-embedded bladder tissues were sectioned at 4 µm. Sections were placed on charged slides and dried at 60°C for 1 h. The slides were then deparaffinized with xylene and rehydrated using graded alcohols. Endogenous peroxidase was quenched with aqueous 3% H2O2, and antigen retrieval was carried out as follows: citrate buffer, pH 6.0 in a microwave oven (GST-µ and Nrf2) or in a steamer (NQO1) for 40 min. The slides were then stained using a DAKO autostainer. Briefly, the slides were treated with 0.03% casein in PBS/T for 30 min and then incubated for 1 h with anti-GST-µ (LS-B963, 0.25 mg/ml), anti-Nrf2 (sc-722, 1/600) or anti-NQO1 (MA1-16672, 2 µg/ml). An isotype-matched control was used on a duplicate slide in place of the primary antibody as a negative control. The mouse Envision FLEX+ system (DAKO) was used to amplify the signal of the primary antibody, and the reaction was visualized by DAB+ chromogen (DAKO). The slides were counterstained with hematoxylin. All slides were examined by a single pathologist without reference to treatment group. The immunohistochemical staining pattern was evaluated as follows: for GST-µ and NQO1, the staining was cytoplasmic and was scored as negative, weak, moderate and strong; for Nrf2 the staining was cytoplasmic and nuclear, and effects were evaluated on the basis of the proportion of Nrf2-positive nuclei. Nuclei were evaluated in a total of 250 cells (5 fields of 50 cells each) for each bladder.
2.7. Statistical analysis
Statistical significance of the in vivo data on enzyme activities was tested by ANOVA, followed by the Student-Newman-Keuls multiple comparisons test using Instat software (GraphPad, SanDiego, CA). Because of the limited sample size, statistical significance of other data was tested by ANOVA, followed by Student’s t-test.
3. Results
3.1. Dose-response, organ specificity and time course of Phase 2 enzyme induction by CPDT in vivo
In studies of dose-response, organ specificity and time course of Phase 2 enzyme induction by CPDT, we focused on the effect of CPDT on the enzymatic activities of GST and NQO1, two of the best known cancer preventive Phase 2 enzymes. We have recently shown that rats dosed with CPDT once daily for 5 days at 125 µmol/kg/day exhibited significant increases in both GST and NQO1 activities in multiple organs, with increases in the bladder being greatest [22]. In the present dose-response study, rats were dosed with CPDT by gavage once daily for 5 days at 0.98, 1.95, 3.91, 7.81, 15.6, 31.3, 62.5 and 125 µmol/kg/day (0.17, 0.34, 0.68, 1.36, 2.71, 5.45, 10.88 and 21.75 mg/kg/day). The rats were killed on day 6, and organs were promptly removed for analysis. Dose-dependent inductive effects of CPDT on GST and NQO1 activities were detected in the small intestine, colon/rectum, kidney, stomach, liver and lung, but not in the heart, as shown in Fig. 1B, nor in the spleen (data not shown). Maximal induction of NQO1 (9.72–9.77 fold) occurred in the duodenum and jejunum and maximal induction of GST (4.23 fold) occurred in the bladders of rats treated with the highest CPDT dose. At the lowest CPDT dose level, however, it was most effective in the bladder (elevating GST and NQO1 1.26 and 1.44 fold respectively, P<0.05) and forestomach (elevating NQO1 1.8 fold, P<0.05, without significant effect on GST). Thus, CPDT was a particularly potent inducer of the enzymes in the bladder and forestomach. No significant changes in organ weight were detected in the bladder, heart, ileum, lungs and spleen at any of the CPDT doses. However, significant and dose-dependent increases in organ weight were detected in the liver (up to 27% at ≥15.6 µmol/kg/day or 2.71 mg/kg/day), cecum and forestomach (up to 13% and 177%, respectively, at ≥31.3 µmol/kg/day or 5.45 mg/kg/day), glandular stomach, duodenum and kidney (up to 12%, 18% and 29%, respectively, at ≥62.5 µmol/kg/day or 10.88 mg/kg/day), jejunum and colon/rectum (22% and 13%, respectively, at 125 µmol/kg/day or 21.75 mg/kg/day).
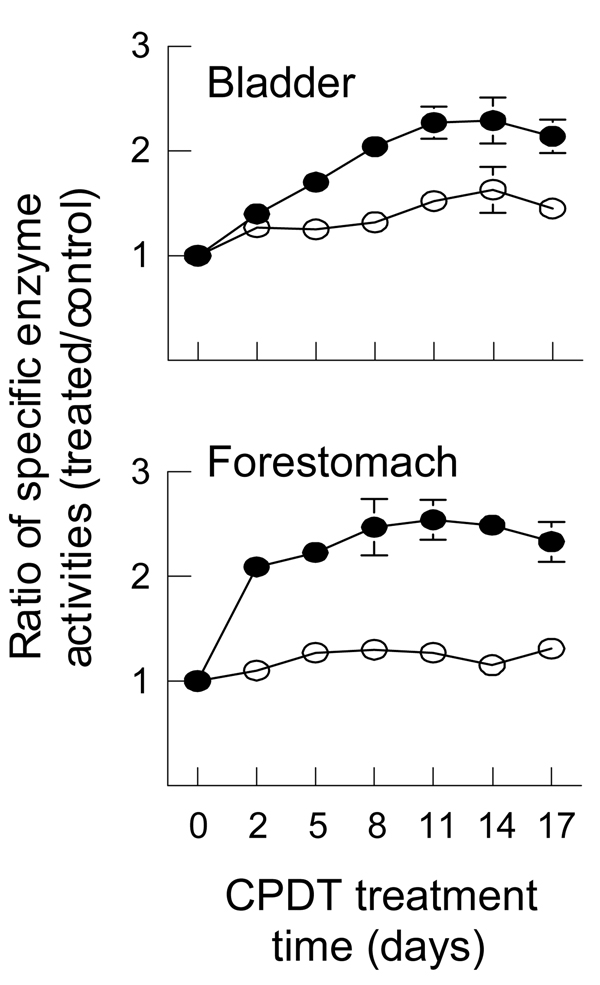
The time course of induction of GST and NQO1 by CPDT was examined in both bladder and forestomach, as these organs were particularly sensitive to CPDT as described above. The rats were dosed with CPDT for 2, 5, 8, 11, 14 and 17 days at 3.91 µmol/kg/day (0.68 mg/kg/day), which was the lowest dose needed to cause significant induction of both GST and NQO1 in both organs after 5 days of treatment (Fig. 1B). As shown in Fig. 2, significant induction of both GST (1.27 fold) and NQO1 (1.4 fold) occurred in the bladder and of NQO1 (2.08 fold) in the forestomach after only two days of CPDT treatment. Maximal enzyme induction appears to be reached after 8–11 days of CPDT treatment, elevating GST and NQO1 by 1.6 and 2.3 fold in the bladder and 1.3 and 2.5 fold in the forestomach, respectively.
Fig. 2.
Time course of induction of GST and NQO1 by CPDT in bladder and forestomach in vivo. Groups of 6 rats were dosed with CPDT at 3.91 µmol/kg/day by gavage once daily for 2, 5, 8, 11, 14 or 17 days. Another group of 12 rats was dosed with vehicle. The animals were killed the day following the the last dose and both bladder and forestomach were harvested for analysis of GST and NQO1. Each value (mean ± SEM) is the ratio of specific activities (treated/control) of GST (□) and NQO1 (■). The specific enzyme activities of GST and NQO1 in the control tissues are provided in Fig. 1. All increases in enzyme activity were significant (P<0.05) except for the GST values at 2 and 14 days in the forestomach.
3.2. Phase 2 enzyme induction and Nrf2 activation by CPDT in the bladder in vivo
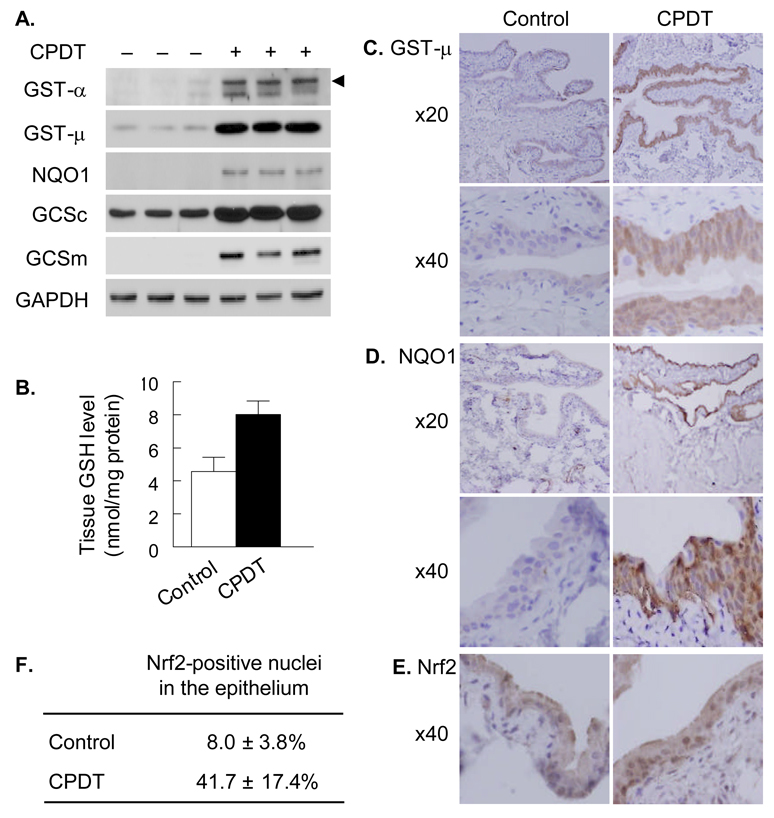
Given that CPDT was most active in the bladder and forestomach but humans do not possess a forestomach, further assessment of the induction of Phase 2 enzymes by CPDT was focused on the bladder. Rats were dosed with CPDT by gavage once daily for 5 days at 125 µmol/kg/day (21.75 mg/kg/day) or with vehicle, and killed 1 day after the last dose. Bladders were removed for examination. A high dose of CPDT was used in this experiment to facilitate the evaluation of the endpoints. CPDT treatment significantly elevated the protein level of NQO1 in the bladder (Fig. 3A), consistent with increased NQO1 enzymatic activity as shown in Fig. 1B. Elevated GST enzymatic activity in the bladder after CPDT treatment (Fig. 1B) resulted apparently from induction of at least GST-α and GST-µ as both GST isozymes were significantly elevated in this tissue (Fig. 3A). However, GST-π was not detectable in the bladder specimens. In addition, CPDT also significantly induced both GCSc and GCSm (Fig. 3A), which is the rate-limiting enzyme in GSH biosynthesis. As expected, CPDT treatment elevated bladder total GSH content by 1.75 fold (Fig. 3B). We next asked where in the bladder the Phase 2 enzymes were induced. Both GST-µ and NQO1 were examined by immunohistochemistry as representatives. Induction of both enzymes occurred predominantly if not exclusively in the epithelium (Fig. 3C and 3D). NQO1 staining in the epithelium of all control bladders were apparently negative but was strong in all CPDT-treated bladders. GST-µ staining in the epithelium of control bladders ranged from weak (3 out of 5 bladders) to moderate (2 out of 5 bladders) but was strong in all CPDT-treated bladders. Although the other Phase 2 enzymes were not examined by immunohistochemistry, it is probably safe to assume that they were also induced by CPDT in the bladder epithelium, in view of the Nrf2 result described below. Nrf2 is known to regulate all the enzymes discussed above and is translocated from cytoplasm to nucleus when activated. Nuclei showing increased Nrf2 staining were mainly detected in the epithelium, but CPDT treatment caused a 5.2 fold increase in the number of such nuclei (Fig. 3E and 3F). This shows that CPDT caused marked activation of Nrf2 in the bladder epithelium and suggests that Nrf2 activation may be responsible for the induction of the Phase 2 enzymes.
Fig. 3.
The inductive effect of CPDT on Nrf2 and Phase 2 genes in the bladder in vivo. Groups of 5 rats were dosed with either vehicle or CPDT at 125 µmol/kg/day for 5 days. The animals were killed one day after the last dose to harvest bladders for analyses. A. Western blot analysis of various Phase 2 proteins in bladder specimens (whole tissue homogenates), showing results from 3 control rats and three CPDT treated rats; GAPDH was used as a loading control. The arrow head points to the GST-α band; the lower band might be caused by non-specific antibody binding. B. Tissue GSH levels; each value is a mean ± SD. The two values are significantly different (P<0.05). C and D. Representative images of immunohistochemical staining of GST-µ and NQO1 in bladder tissues, showing both low (×20) and high (×40) magnifications. E and F. Representative images of immunohistochemical staining of Nrf2 in bladder epithelium and percentage of Nrf2-positive nuclei. Each value is mean ± SD; a total of 250 nuclei in 5 fields were counted in slides from each bladder.
3.3. The inductive activity of CPDT in cell models
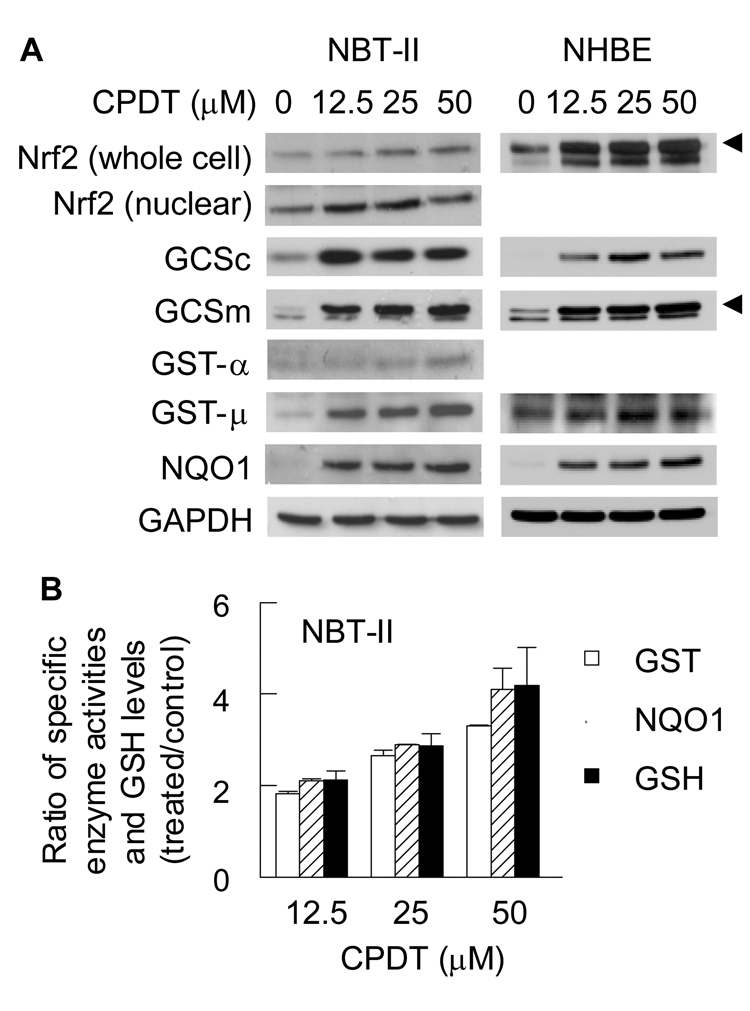
The effects of CPDT on Nrf2 and Phase 2 enzymes were further evaluated in the rat bladder carcinoma NBT-II cell line and in a primary culture of normal human bladder epithelial (NHBE) cells. Use of NBT-II cells permitted us to compare in vitro and in vivo efficacy of CPDT, whereas NHBE cells were employed in order to gauge the human relevance of the animal data. All cells were treated with CPDT at 0, 12.5, 25 and 50 µM for 24 h before being harvested for analysis. CPDT elevated total Nrf2 levels in a dose-dependent manner and promoted its nuclear accumulation in NBT-II cells (Fig. 4A). This indicates that Nrf2 is activated by CPDT, as it is well known that Nrf2 when repressed by Keap1 in the cytoplasm is targeted for degradation, but free Nrf2 is more stable and translocates to the nucleus. We did not measure nuclear levels of Nrf2 in NHBE cells due to the limited availability of these cells, but CPDT strongly elevated total Nrf2 in these cells. A Western blot band that was picked up by the anti-Nrf2 antibody but migrated slightly faster than Nrf2 was similarly elevated by CPDT in NHBE cells but not in NBT-II cells. The identity of this band is unknown at present. In both cell types, CPDT also increased the levels of GCSc, GCSm, GST-µ and NQO1. We also measured the enzymatic activities of GST and NQO1 and GSH levels in CPDT-treated NBT-II cells. As expected, CPDT caused significant and dose-dependent increases in all three endpoints (Fig. 4B). The inductive effects of CPDT on Nrf2 and the Phase 2 enzymes are therefore similar between these cells, and also closely resemble that in the rat bladder in vivo as described above. Interestingly, while GST-α was induced by CPDT in the bladder in vivo and in NBT-II cells in vitro, it was not detected in NHBE cells.
Fig. 4.
Activation of Nrf2 and induction of Phase 2 enzymes by CPDT in cultured bladder cells. Cells were treated with CPDT at the indicated concentrations for 48 h and then harvested for analysis. A. Nrf2 and various Phase 2 enzymes were measured with Western blot analysis. Whole cell extracts were used, except for Nrf2 which were measured in both whole cell extracts and nuclear extracts. GAPDH was used as a loading control. The data are representative of at least two experiments. The specific bands in some blots are indicated with an arrow head; the unidentified band in these blots might result from non-specific antibody binding. B. Measurement of enzyme activities of GST and NQO1 and measurement of GSH levels in whole cell extracts. Each value is a mean ± SD (n=3). All values are significantly different from their controls (<0.05). The values in the control cells were as follows: 34.67 ± 4.49 nmol/mg protein (GSH), 92.64 ± 2.03 nmol/min/mg protein (GST) and 1.35 ± 0.01 µmol/min/mg protein (NQO1).
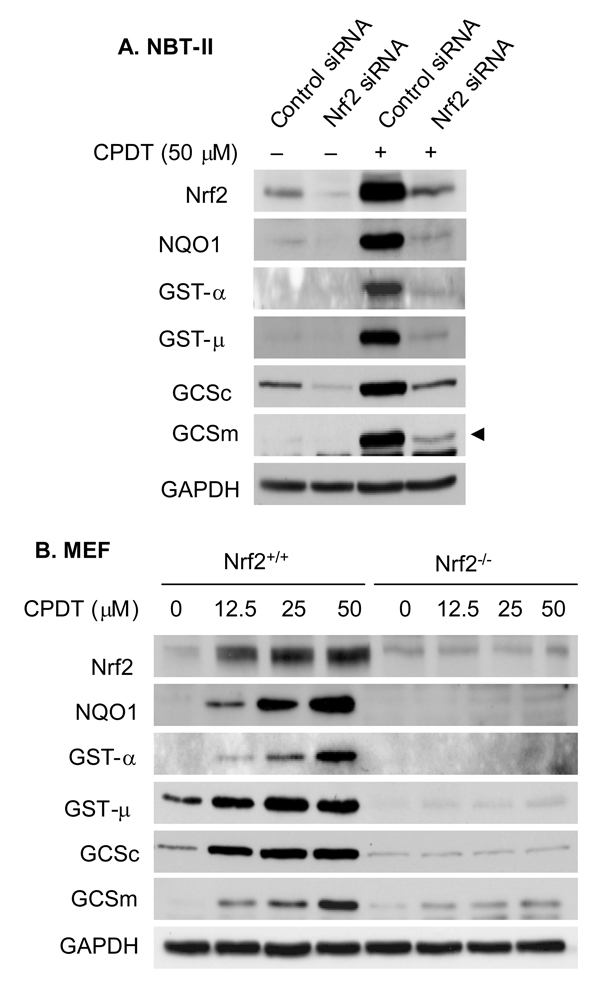
3.4. The role of Nrf2 in Phase 2 enzyme induction by CPDT
We next asked to what extent induction of Phase 2 enzymes by CPDT depended on Nrf2 activation. NBT-II cells were treated with either an Nrf2 siRNA or control siRNA for 48 h and then either harvested or further incubated with CPDT at 50 µM for 24 h. Cellular levels of Nrf2 and Phase 2 enzymes were examined by Western blotting. As shown in Fig. 5A, Nrf2 siRNA markedly attenuated basal levels of Nrf2 and Phase 2 enzymes and almost totally prevented CPDT from elevating Nrf2 and all Phase 2 enzymes. To further clarify the role of Nrf2 in mediating Phase 2 enzyme induction by CPDT, we compared the response of both wild-type MEF and Nrf2-deficient MEF to CPDT. Cells were treated with 0, 12.5, 25 and 50 µM CPDT for 48 h and then harvested for analysis. As expected, CPDT caused dose-dependent increases in Nrf2 levels in wild type MEF but had no effect in Nrf2-deficient MEF (Fig. 5B). A faint band resembling Nrf2 was detected in Nrf2−/− cell lysates, likely resulting from a non-specific reaction of the antibody. More interestingly, CPDT induced all Phase 2 enzymes in a dose-dependent manner in the wild type cells but was totally ineffective in the Nrf2 deficient cells.
Fig. 5.
The role of Nrf2 in Phase 2 enzyme induction by CPDT. NBT-II cells were treated with either scrambled control siRNA or Nrf2 siRNA for 48 h. The cells were either harvested for analysis or further treated with 50 µM CPDT for 24 h before harvest. Both wild type MEF (WT) and Nrf2-deficient MEF (Nrf2-KO) were treated with the indicated concentrations of CPDT for 48 h and then harvested for analysis. Levels of Nrf2 and each Phase 2 enzyme in whole cell lysates were measured using Western blot analysis. GAPDH was used as a loading control. The results are representative of at least two experiments. The GCSm band is indicated with an arrow head; the lower unidentified band might be caused by non-specific antibody binding.
4. Discussion
While oral administration of CPDT to rats induced both GST and NQO1 in a dose-dependent manner in most of the organs examined, it was the bladder and forestomach in which CPDT exhibited the most potent inductive activities. Significant induction of both enzymes in the bladder and NQO1 in the forestomach were detected even at the lowest dose tested (0.98 µmol/kg/day) (Fig. 1). The reason for the exceptional sensitivity of these organs to CPDT is not known with certainty. However, the forestomach serves as a holding chamber for food in rodents, and the exposure of this tissue to orally dosed CPDT may be higher and more prolonged than other tissues. Enzyme induction in the bladder by CPDT occurred exclusively in the epithelium (Fig. 3C–3F), which raises the possibility that CPDT or its active metabolites are delivered to the bladder through urinary excretion. There is a precedent for this phenomenon. Many isothiocyanates, another group of well-known cancer chemopreventive agents, are also particularly active in the bladder and have been shown to be selectively delivered to this tissue through urinary excretion [30,31]. Therefore, further studies on the metabolism and disposition of CPDT may be the key to explaining the high activity of CPDT in the bladder. CPDT appears to be markedly more potent than oltipraz in both bladder and forestomach, as our previous study showed that under the same experimental conditions there was little induction of NQO1 or GST in these organs even when rats were treated with oltipraz at 125 µmol/kg/day [22]. Interestingly, oltipraz was previously shown to inhibit tumorigenesis in both the bladder and forestomach of mice [13,15]. CPDT also appears to be markedly more potent than broccoli sprout extract in which isothiocyanates are the principal if not the sole chemopreventive ingredients. Our recent study showed that administration of a freeze-dried aqueous extract of broccoli sprouts to female Sprague-Dawley rats at an isothiocyanate dose of 40 µmol/kg/day for 14 days increased the activities of GST and NQO1 in the bladder 43% and 141% respectively [32]. However, under identical experimental conditions, comparable increases in the activities of GST and NQO1 in the bladder were observed with CPDT at a dose of only 3.91 µmol/kg/day (Fig. 2). Yet broccoli sprout extract significantly inhibited bladder carcinogenesis in vivo at an isothiocyanate dose of 40 µmol/kg/day [30]. These results raise the possibility that CPDT may be a far more potent and effective inhibitor of carcinogenesis in these organs than oltipraz or isothiocyanates.
Since humans do not have a forestomach, we have focused on the bladder in subsequent studies. As expected, increased activities of GST and NQO1 in the bladders of CPDT-treated rats were associated with increases in their protein levels (Fig. 3A). In the case of GST, CPDT increased the levels of both GST-α and GST-µ Both GST-α and GST-µ consist of several isoforms, but the antibodies available were not capable of further discernment. CPDT also significantly induced both the catalytic and modulatory subunits of GCS (Fig. 3A), the rate-limiting enzyme in GSH biosynthesis. Not surprisingly, induction of GCS was associated with a significant increase in the GSH content of the bladder (Fig. 3B). We further examined the location of the enzyme induction in the bladder, focusing on GST-µ and NQO1. As shown in Fig. 3C and 3D, induction of both enzymes occurred exclusively in the bladder epithelium. This is probably true of the other enzymes as well, since we also found that CPDT not only activated Nrf2 (increased nuclear accumulation) but also such activation was localized in the epithelium (Fig. 3E and 3F). As mentioned previously, Nrf2 is the key transcriptional regulator of many Phase 2 genes, including the genes encoding each of the Phase 2 enzymes mentioned above [11]. Activation of Nrf2 not only suggests that other Nrf2-regulated genes may be stimulated by this compound, but also further highlights the chemopreventive potential of CPDT, as many animal studies have shown that Nrf2 deficiency significantly increases cancer risk [11]. However, the most significant finding is that activation of Nrf2 and induction of Phase 2 enzymes in the bladder by this compound exclusively occurred in the bladder epithelium where nearly all bladder cancers in humans occur [33].
CPDT elicited similar responses of Nrf2 and Phase 2 enzymes as well as elevation of GSH levels in cultured bladder cells, including primary normal human bladder epithelial cells (NHBE) and rat bladder cancer NBT-II cells in vitro (Fig. 4). The finding that the response of NHBE cells to CPDT resembles that of NBT-II cells and bladder in vivo is highly interesting, because it suggests that CPDT may elicit a similar response in the human bladder in vivo. Moreover, the induction of GST-µ and NQO1 by CPDT in NHBE cells is particularly noteworthy, because deficiency of each enzyme has been linked to increased risk of human bladder cancer [2–4]. It is also of note that GST-α was not detected in NHBE cells but was induced in both NBT-II cells and bladder tissues (data not shown). These NHBE cells were apparently obtained from a single human subject, but it is not known if the response of GST-α to CPDT in these cells is representative of that in bladder cells from other human subjects. Since GST-α has also been inversely linked to human bladder cancer risk [1], additional study on the effect of CPDT on this enzyme in bladder cells from other human subjects is warranted. UGT1A was another Phase 2 enzyme whose response to CPDT was not uniform: It did not respond to CPDT in the bladder in vivo and in NHBE cells in vitro but was significantly induced in NBT-II cells (data not shown).
The robust response of NBT-II cells to CPDT and their unlimited availability made them our choice for determination of the role of Nrf2 in induction of Phase 2 enzymes by CPDT. We showed that siRNA-induced Nrf2 knockdown resulted in marked loss and in some cases almost total loss of induction of Phase 2 enzymes by CPDT (Fig. 5A). Since Nrf2 protein in NBT-II cells was not completely eliminated after siRNA treatment, we next compared the response of wild type MEF and Nrf2-deficient MEF to CPDT. While wild type MEF showed a robust response of all Phase enzymes examined as well as Nrf2 itself, there was no induction of NQO1, GST-α, GST-µ and GCSc, and only slight induction of GCSm in Nrf2-deficient MEF. These results show clearly that Nrf2 is essential for CPDT to induce Phase 2 enzymes. In view of the importance of Phase 2 enzyme induction in cancer prevention, Nrf2 may be viewed as a key chemopreventive target of CPDT.
While the present study shows that CPDT potently and preferentially activates Nrf2 and induces Phase 2 enzymes in the bladder epithelium, and the discussion provided above suggests that CPDT is a promising bladder cancer chemopreventive agent, two important questions have not yet been addressed. First, does CPDT actually protect normal bladder epithelium against carcinogenic insult and inhibit bladder cancer development? Second, is CPDT toxic? CPDT at dose levels less than 15.6 µmol/kg/day (2.71 mg/kg/day) was quite effective in inducing Phase 2 enzymes in the bladder in vivo and did not show any signs of toxicity, but at higher dose levels it increased the weight of several organs in rat, including kidney, intestine, liver and stomach (see the result section). Studies to address these questions as well as those to understand the reason for CPDT’s tissue specificity in the activation of Nrf2 and induction of Phase 2 enzymes are warranted.
Acknowledgements
This work was financially supported by the National Cancer Institute (USA) grant R01 CA120533.
Footnotes
Conflict of Interest statement
The authors declare that there are no conflicts of interest.
References
- 1.Komiya Y, Tsukino H, Nakao H, Kuroda Y, Imai H, Katoh T. Human glutathione S-transferase A1 polymorphism and susceptibility to urothelial cancer in the Japanese population. Cancer Lett. 2005;221(1):55–59. doi: 10.1016/j.canlet.2004.10.042. [DOI] [PubMed] [Google Scholar]
- 2.Schulz WA, Krummeck A, Rosinger I, Eickelmann P, Neuhaus C, Ebert T, Schmitz-Drager BJ, Sies H. Increased frequency of a null-allel for NAD(P)H: quinone oxidoreductase in patients with urological malignancies. Pharmacogenetics. 1997;7(3):235–239. doi: 10.1097/00008571-199706000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Salagovic J, Kalina I, Habalova V, Hrivnak M, Valansky L, Biros E. The role of human glutathione S-transferase M1 and T1 in individual susceptibility to a bladder cancer. Physiol. Res. 1999;48(6):465–471. [PubMed] [Google Scholar]
- 4.Park SJ, Zhao H, Spitz MR, Grossman HB, Wu X. An association between NQO1 genetic polymorphism and risk of bladder cancer. Mutat. Res. 2003;536(1–2):131–137. doi: 10.1016/s1383-5718(03)00041-x. [DOI] [PubMed] [Google Scholar]
- 5.Marcus PM, Vineis P, Rothman N. NAT2 slow acetylation and bladder cancer risk: a meta-analysis of 22 case-control studies conducted in the general population. Pharmacogenetics. 2000;10(2):115–122. doi: 10.1097/00008571-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Long DJ, 2nd, Waikel RL, Wang XJ, Perlaky L, Roop DR, Jaiswal AK. NAD(P)H:quinone oxidoreductase 1 deficiency increases susceptibility to benzo(a)pyrene-induced mouse skin carcinogenesis. Cancer Res. 2000;60(21):5913–5915. [PubMed] [Google Scholar]
- 7.Henderson CJ, Smith AG, Ure J, Brown K, Bacon EJ, Wolf CR. Increased skin tumorigenesis in mice lacking pi class glutathione S-transferases. Proc. Natl. Acad. Sci. U. S. A. 1998;95(9):5275–5280. doi: 10.1073/pnas.95.9.5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fields WR, Li Y, Townsend AJ. Protection by transfected glutathione S-transferase isozymes against carcinogen-induced alkylation of cellular macromolecules in human MCF-7 cells. Carcinogenesis. 1994;15(6):1155–1160. doi: 10.1093/carcin/15.6.1155. [DOI] [PubMed] [Google Scholar]
- 9.Joseph P, Jaiswal AK. NAD(P)H:quinone oxidoreductase1 (DT diaphorase) specifically prevents the formation of benzo(a)pyrene quinone-DNA adducts generated by cytochrome P4501A1 and P450 reductase. Proc. Natl. Acad. Sci. U. S. A. 1994;91(18):8413–8417. doi: 10.1073/pnas.91.18.8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Talalay P. Chemoprotection against cancer by induction of phase 2 enzymes. Biofactors. 2000;12(1–4):5–11. doi: 10.1002/biof.5520120102. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Gordon GB. A strategy for cancer prevention: stimulation of the Nrf2-ARE signaling pathway. Mol Cancer Ther. 2004;3(7):885–893. [PubMed] [Google Scholar]
- 12.Dinkova-Kostova AT, Holtzclaw WD, Kensler TW. The role of Keap1 in cellular protective responses. Chem Res Toxicol. 2005;18(12):1779–1791. doi: 10.1021/tx050217c. [DOI] [PubMed] [Google Scholar]
- 13.Ramos-Gomez M, Kwak MK, Dolan PM, Itoh K, Yamamoto M, Talalay P, Kensler TW. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc. Natl. Acad. Sci. U. S. A. 2001;98(6):3410–3415. doi: 10.1073/pnas.051618798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fahey JW, Haristoy X, Dolan PM, Kensler TW, Scholtus I, Stephenson KK, Talalay P, Lozniewski A. Sulforaphane inhibits extracellular, intracellular, and antibiotic-resistant strains of Helicobacter pylori and prevents benzo[a]pyrene-induced stomach tumors. Proc. Natl. Acad. Sci. U. S. A. 2002;99(11):7610–7615. doi: 10.1073/pnas.112203099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iida K, Itoh K, Kumagai Y, Oyasu R, Hattori K, Kawai K, Shimazui T, Akaza H, Yamamoto M. Nrf2 is essential for the chemopreventive efficacy of oltipraz against urinary bladder carcinogenesis. Cancer Res. 2004;64(18):6424–6431. doi: 10.1158/0008-5472.CAN-04-1906. [DOI] [PubMed] [Google Scholar]
- 16.Kensler TW, Groopman JD, Sutter TR, Curphey TJ, Roebuck BD. Development of cancer chemopreventive agents: oltipraz as a paradigm. Chem. Res. Toxicol. 1999;12(2):113–126. doi: 10.1021/tx980185b. [DOI] [PubMed] [Google Scholar]
- 17.Ruggeri BA, Robinson C, Angeles T, Wilkinson J, 4th, Clapper ML. The chemopreventive agent oltipraz possesses potent antiangiogenic activity in vitro, ex vivo, and in vivo and inhibits tumor xenograft growth. Clin. Cancer Res. 2002;8(1):267–274. [PubMed] [Google Scholar]
- 18.Ramos-Gomez M, Dolan PM, Itoh K, Yamamoto M, Kensler TW. Interactive effects of nrf2 genotype and oltipraz on benzo[a]pyrene-DNA adducts and tumor yield in mice. Carcinogenesis. 2003;24(3):461–467. doi: 10.1093/carcin/24.3.461. [DOI] [PubMed] [Google Scholar]
- 19.Jacobson LP, Zhang BC, Zhu YR, Wang JB, Wu Y, Zhang QN, Yu LY, Qian GS, Kuang SY, Li YF, Fang X, Zarba A, Chen B, Enger C, Davidson NE, Gorman MB, Gordon GB, Prochaska HJ, Egner PA, Groopman JD, Muñoz A, Helzlsouer KJ, Kensler TW. Oltipraz chemoprevention trial in Qidong, People's Republic of China: study design and clinical outcomes. Cancer Epidemiol. Biomarkers Prev. 1997;6(4):257–265. [PubMed] [Google Scholar]
- 20.Kelley MJ, Glaser EM, Herndon JE, 2nd, Becker F, Bhagat R, Zhang YJ, Santella RM, Carmella SG, Hecht SS, Gallot L, Schilder L, Crowell JA, Perloff M, Folz RJ, Bergan RC. Safety and efficacy of weekly oral oltipraz in chronic smokers. Cancer Epidemiol. Biomarkers Prev. 2005;14(4):892–899. doi: 10.1158/1055-9965.EPI-04-0585. [DOI] [PubMed] [Google Scholar]
- 21.Egner PA, Kensler TW, Prestera T, Talalay P, Libby AH, Joyner HH, Curphey TJ. Regulation of phase 2 enzyme induction by oltipraz and other dithiolethiones. Carcinogenesis. 1994;15(2):177–181. doi: 10.1093/carcin/15.2.177. [DOI] [PubMed] [Google Scholar]
- 22.Munday R, Zhang Y, Munday CM, Li J. Structure-activity relationships in the induction of Phase II enzymes by derivatives of 3H-1,2-dithiole-3-thione in rats. Chem. Biol. Interact. 2006;160(2):115–122. doi: 10.1016/j.cbi.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 23.Legrand L, Mollier Y, Lozac'h N. Sulfuration des composés organiques (IV). Dithiole-1,2-thiones-3 comportant des substituants hydrocarbonés ou des noyaux condensés. Bull. Soc. Chim. France. 1953:327–331. [Google Scholar]
- 24.Wakabayashi N, Dinkova-Kostova AT, Holtzclaw WD, Kang MI, Kobayashi A, Yamamoto M, Kensler TW, Talalay P. Protection against electrophile and oxidant stress by induction of the phase 2 response: fate of cysteines of the Keap1 sensor modified by inducers. Proc. Natl. Acad. Sci. U. S. A. 2004;101(7):2040–2045. doi: 10.1073/pnas.0307301101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ye L, Zhang Y. Total intracellular accumulation levels of dietary isothiocyanates determine their activity in elevation of cellular glutathione and induction of Phase 2 detoxification enzymes. Carcinogenesis. 2001;22(12):1987–1992. doi: 10.1093/carcin/22.12.1987. [DOI] [PubMed] [Google Scholar]
- 26.Richie JP, Jr., Skowronski L, Abraham P, Leutzinger Y. Blood glutathione concentrations in a large-scale human study. Clin. Chem. 1996;42(1):64–70. [PubMed] [Google Scholar]
- 27.Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferase. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974;249(22):7130–7139. [PubMed] [Google Scholar]
- 28.Ernster L, Danielson L, Ljunggren M. DT diaphorase. I. Purification from the soluble fraction of rat-liver cytoplasm, and properties. Biochim. Biophys. Acta. 1962;58:171–188. doi: 10.1016/0006-3002(62)90997-6. [DOI] [PubMed] [Google Scholar]
- 29.Dignam JD, Lebovitz RM, Roeder RG. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Munday R, Mhawech-Fauceglia P, Munday CM, Paonessa JD, Tang L, Munday JS, Lister C, Wilson P, Fahey JW, Davis W, Zhang Y. Inhibition of urinary bladder carcinogenesis by broccoli sprouts. Cancer Res. 2008;68(5):1593–1600. doi: 10.1158/0008-5472.CAN-07-5009. [DOI] [PubMed] [Google Scholar]
- 31.Tang L, Zhang Y. Isothiocyanates in the chemoprevention of bladder cancer. Curr. Drug Metab. 2004;5(2):193–201. doi: 10.2174/1389200043489027. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y, Munday R, Jobson HE, Munday CM, Lister C, Wilson P, Fahey JW, Mhawech-Fauceglia P. Induction of GST and NQO1 in cultured bladder cells and in the urinary bladders of rats by an extract of broccoli (Brassica oleracea italica) sprouts. J. Agric. Food Chem. 2006;54(25):9370–9376. doi: 10.1021/jf062109h. [DOI] [PubMed] [Google Scholar]
- 33.Kausch I, Bohle A. Bladder cancer. II. Molecular aspects and diagnosis. Eur. Urol. 2001;39(5):498–506. doi: 10.1159/000052495. [DOI] [PubMed] [Google Scholar]