Abstract
This study explored the impact of feeding raw meat to dogs on the fecal prevalence of several enteric bacterial zoonotic pathogens. Campylobacter jejuni was isolated from 1/42 (2.6%) raw meat-fed dogs. Salmonella enterica was isolated from 2/40 (5%) of the raw meat feeds, 6/42 (14%) raw meat-fed dog feces, none of the dogs that did not receive raw meat (P = 0.001), 4/38 (10.5%) of the vacuum cleaner waste samples from households where raw meat was fed, and 2/44 (4.5%) of vacuum cleaner waste samples from households where raw meat was not fed to dogs (P = 0.41). Responses to a questionnaire probing practices and beliefs regarding raw meat feeding that was administered to dog owners demonstrated that dog owners may either not be aware or refuse to acknowledge the risks associated with raw meat-feeding; thus, they may neglect to conduct adequate intervention strategies to prevent zoonoses among themselves and their families.
Résumé
Perceptions, pratiques et conséquences associées aux agents pathogènes d’origine alimentaire et à une alimentation de viande crue pour les chiens. Cette étude a exploré l’impact d’une alimentation de viande crue pour les chiens sur la prévalence fécale de plusieurs bactéries pathogènes entériques zoonotiques. Campylobacter jejuni a été isolé chez 1/42 (2,6 %) des chiens nourris de viande crue. Salmonella enterica a été isolé dans 2/40 (5 %) des aliments de viande crue, dans 6/42 (14 %) des fèces des chiens nourris de viande crue, chez aucun des chiens qui n’avaient pas reçu de viande crue (P = 0,001), dans 4/38 (10,5 %) des échantillons de déchets d’aspirateur provenant de ménages où une alimentation de viande crue était servie et dans 2/44 (4,5 %) des échantillons de déchets d’aspirateur provenant de ménages où la viande crue n’était pas servie aux chiens (P = 0,41). Les réponses à un questionnaire examinant les pratiques et les croyances concernant l’alimentation à base de viande crue qui a été administré aux propriétaires de chiens ont démontré que les propriétaires de chiens n’étaient soit pas au courant ou refusaient de reconnaître les risques associés à une alimentation à base de viande crue; par conséquent, ils peuvent négliger de mettre en place des stratégies d’intervention adéquates afin de prévenir les zoonoses pour eux-mêmes et leur famille.
(Traduit par Isabelle Vallières)
Introduction
An estimated 76 million cases of human bacterial gastroenteritis occur in the United States (US) each year and most of these cases are attributed to the consumption of contaminated foods (1). Likewise, dogs are also susceptible to infection with many of the same bacterial pathogens (2). Although dogs frequently display no clinical signs of illness when colonized with bacterial organisms that cause human disease, excretion of these agents in their feces may pose a zoonotic health threat. In the US, 3% of all salmonellosis cases and as many as 15% of Campylobacter spp. infections may be attributable to contact with companion animals (3). Prevention of disease transmission from dogs to humans, either through increased hygiene or decreased pathogen prevalence in the pet dog population, could have considerable impact on public health, especially among children and immunocompromised individuals, who are at particularly high risk for infection and severe illness (4).
In recent years, there has been increased recognition by the veterinary community of dog owners choosing to feed raw meat to their pets (5–9). Given the frequency with which raw meat products are contaminated with foodborne pathogens, the primary goal of this research was to determine if dogs consuming food containing raw meat were at increased risk for carriage of the 3 most common foodborne bacterial zoonotic pathogens occurring in humans; namely, Salmonella enterica, Campylobacter jejuni, and Escherichia coli O157. A secondary goal was to determine if dogs could contaminate their household environment with Salmonella enterica.
Materials and methods
Recruitment and sample collection
Multiple methods were used to solicit participation among dog owners. Between June 1, 2004, and June 30, 2006, veterinarians and dog breeders were asked, via telephone calls, e-mail, and direct mailing, to recruit an equal number of clients that regularly fed raw meats (including poultry and eggs) and clients that did not feed any raw meats to their dogs. Participation information was also posted on the investigator’s internet homepage (10). Inclusion criteria included the following: 1) that dogs be greater than 1 y of age, 2) that they be considered clinically healthy by the owners, and 3) that they had no history of antibiotic treatment in the past 3 mo. Participants were requested to submit via overnight courier 1) a freshly voided fecal specimen, 2) approximately 50 g of the food being fed to the dog, 3) the contents of a vacuum cleaner bag used in the household in which the dog lived, and 4) a completed questionnaire. Participation was limited to 1 dog per household. All costs associated with testing, including shipping costs, were paid by the investigators. The submitter was assigned to each submission a unique identifier that he/she could use to access the culture results of his/her samples via the Internet.
Microbiological methods
Bacterial pathogens were isolated, using enrichment procedures previously described (11). All fecal and raw food samples were cultured for C. jejuni. Briefly, cotton-tipped swabs were inserted into the raw food or feces and coated evenly with the sample (approximate weight of 0.15 g). Swabs were then inoculated into enrichment broth (CampyThio; Remel, Lenexa, Kansas, USA) and incubated for 48 h at 4°C. The enriched samples were subsequently plated on Columbia Blood Agar containing vancomycin and amphotericin B (Sigma Aldrich, St. Louis, Missouri, USA) and incubated under microaerophilic conditions for an additional 48 h at 42°C. Bacterial colonies with morphology consistent with that of C. jejuni were tested by polymerase chain reaction (PCR) for the C. jejuni-specific mopA gene (12).
For the culture of E. coli O157, 10-g samples of feces and raw food were homogenized with 90 mL of buffered peptone water (BPW) and incubated overnight at 42°C. Escherichia coli O157 was concentrated from 1-mL samples of these homogenates by using anti-O157 specific immunomagnetic beads and an automated immunomagnetic bead separator (BeadRetriever, Dynal BioTech, Oslo, Norway). Beads were plated on sorbitol MacConkey agar plates containing cefixime (50 mg/mL) and tellurite (100 mg/mL) (SMACct) (Invitrogen Corporation, Carlsbad, California, USA). After overnight incubation at 37°C, up to 5 suspect (sorbitol-negative) colonies recovered from each SMACct plate were further screened for the biochemical characteristics of E. coli O157:H7, including lactose fermentation, absence of d-umbelliferyl-β-glucuronide cleavage, and agglutination in a commercially available latex test kit specific for the O157 antigen (Oxoid E. coli O157 Latex test DR0620M; Oxoid, Ogdensburg, New York USA).
For the culture of Salmonella spp. 10 g of feces and raw food and 10 g of vacuum cleaner waste were enriched and homogenated, as described previously for E. coli. One hundred microliters of each homogenate was transferred to 9.9 mL of Rappaport-Vassiliadis (RV) broth and incubated for 18 h at 42°C. One loopful (20 μL) of the broth enrichment was then plated onto a xylose-lysine-tergitol-4 (XLT4) agar plate (Neogen Corp, Lansing, Michigan, USA) and incubated at 37°C overnight. From each XLT4 plate, up to 3 colonies with a morphology typical of that of S. enterica were selected for biochemical screening (triple sugar iron, citrate, and urea agars). One colony from each sample that produced an acid butt and alkaline slant with H2S production on triple sugar iron agar, that was urea-negative, and that was citrate-positive was serogrouped, using group-specific antisera (Becton Dickinson, Sparks, Maryland, USA) and sent for serotyping to the United States National Veterinary Service Laboratory, Ames, Iowa.
Salmonella isolates were further subtyped, using pulsed-field gel electrophoresis (13) and antibiotic resistance phenotyping. Resistance to antibiotics on the United States Department of Agriculture National Antibiotic Resistance Monitoring System (NARMS) panel (amikacin, amoxicillin/clavulanic acid, ampicillin, cefoxitin, ceftiofur, ceftriaxone, chloramphenicol, ciprofloxacin, gentamicin, kanamycin, nalidixic acid, streptomycin, sulfisoxazole, tetracycline, and trimethoprim/sulfamethoxazole) was determined, using the broth dilution method in commercially purchased plates (Sensititer; Trek diagnositics, West Lake, Ohio, USA). Breakpoints for resistance were those used by the NARMS (14).
Questionnaire
Participants were requested to complete a questionnaire assessing demographic data of respondents; sources of information about animal health; and a variety of dog signalment and management practices, such as pet veterinary checkups, exercise options, and hygiene practices, as well as selected beliefs and opinions on pet feeding, such as what, how often, and where dogs were fed. The questionnaires and methods of participant recruitment were reviewed by the Ohio State University Office of Risk Protection and deemed exempt from review by the Institutional Review Board.
Response rates
A total of 92 dog owners agreed to participate in the study. One owner asked to withdraw from the study after the samples had been shipped, but before they had been analyzed. Some participants failed to submit all the requested materials. As such, a total of 91 fecal samples were screened, 42 from raw meat-fed (RMF) dogs and 49 from not raw meat-fed (NRMF) dogs. Forty raw meat-containing feeds were analyzed. The contents of 82 vacuum cleaner bags were tested, 38 and 44 bags from households feeding raw meat and not feeding raw meat, respectively. Two questionaires from households not feeding raw meat were not completed. All other submitted fecal samples were accompanied with completed questionnaires.
Statistical analyses
Two separate research questions were evaluated. First, whether specific dog management practices were associated with the isolation of Salmonella from the feces and if the isolation rate of Salmonella from vacuum cleaner dust was related to the type (raw versus not raw) of feed used in the home. These hypotheses were tested by using the chi-squared tests for homogeneity. Second, if there were differences in human behavior, beliefs, and dog management practices between owners who did and those who did not feed raw meat. These hypotheses were tested by comparing the distribution of survey responses between groups, using the Kolmogrov-Smirnov two-sample test. Difference in amount of money spent on feed was compared, using a two-sample t-test. The analyses were all conducted by using an appropriate software product (SAS for Windows version 9.0; SAS Institute, Cary, North Carolina, USA) with type-I error set at 0.05.
Results
Campylobacter jejuni was isolated from the feces of 1/42 [2.4%; 95% confidence interval (CI): 0.06–12.8] of the RMF dogs and 0/49 (0%; 95% CI: 0–7.2) of the NRMF dogs. None of the raw food samples tested positive for C. jejuni. Escherichia coli O157 was not recovered from any fecal or raw food sample. Salmonella enterica was recovered from the feces of 6/42 (14.3%; 95% CI: 5.4–28.5) of the RMF dogs and 0/49 (0%; 95% CI: 0–5.8) of the NRMF dogs (Table 1); this difference in S. enterica prevalence between feeding groups was significant (P ≤ 0.05). Salmonella enterica was also isolated from 2/40 (5%; 95% CI: 0.6–16.9) of the raw food specimens and 6/82 (7.3%; 95% CI: 2.7–15.2) of the vacuum cleaner waste samples. Salmonella enterica was recovered more frequently in vacuum cleaner waste from households with RMF dogs than from those with NRMF dogs (4/38, 10.5% versus 2/44, 4.5%), but this difference was not statistically significant (P = 0.41).
Table 1.
Sources, serovars, and antibiotic resistance of Salmonella enterica isolated
|
Salmonella serovar isolation
|
|||||
|---|---|---|---|---|---|
| Feed type | Sample | In food | In feces | In vacuum | Resistance |
| Raw | 1 | Anatum var. 15+ | aPansusceptible | ||
| 1 | Hadar | Str,Tet | |||
| 2 | Heidelberg | Tet | |||
| 2 | Heidelberg | Tet | |||
| 3 | 4,5,12:i:- | Amp, Amo, Tri | |||
| 4 | Worthington | Tet | |||
| 5 | Hadar | Str, Tet | |||
| 6 | Kentucky | Pansusceptible | |||
| 6 | St. Paul | Pansusceptible | |||
| 7 | 6,7:e,h:- | Pansusceptible | |||
| 8 | Berta | Amp, Tet | |||
| 9 | London | Pansusceptible | |||
| Not raw | 10 | Anatum | Pansusceptible | ||
| 11 | Newport | Pansusceptible | |||
Pansusceptible to all antimicrobials tested; Str — streptomycin; Tet — tetracycline; Amp — ampicillin; Amoamoxicilllin/calavulanic acid; Tri — Trimethoprim/sulfamethoxazole.
Several different serovars of S. enterica were recovered from the feed, feces, and vacuum contents (Table 1). Notably, S. Heidelberg was isolated from both the fecal sample and the vacuum cleaner contents from 1 household; these isolates had similar antimicrobial resistance phenotypes, but unique pulsed-field gel electrophoresis profiles (data not shown). Seven of the 14 Salmonella isolates were susceptible to all the antimicrobials tested (Table 1). Three isolates were resistant only to tetracycline, 2 isolates were resistant only to tetracycline and streptomycin, 1 isolate was resistant only to tetracycline and ampicillin, and 1 isolate was resistant only to ampicillin, amoxicillin/clavulanic, and trimethoprim/sulfamethoxazole.
Recovery of S. enterica from the feces was associated with the feeding of raw meat and exposure to livestock, but with none of the other potential risk factors examined in this study (Table 2). Exposure to livestock (cattle, sheep, goats, pigs, and horses) was higher among RMF dogs than among NMRF dogs (12/40, 30% versus 4/48, 10%; P = 0.01), but the fecal carriage of Salmonella among RMF dogs was not statistically different between dogs with or without livestock exposure (3/28, 7.5% versus 3/12, 25%; P = 0.25).
Table 2.
Factors associated with fecal carriage of Salmonella. All statistically different factors are more common among raw meat-fed dogs and owners of raw meat-fed dogs
| Variable | OR | 95% CI | P |
|---|---|---|---|
| Inclusion of any raw ingredients in diet | Undefineda | < 0.01 | |
| Inclusion of the following specific feed ingredients | |||
| Raw beef | Undefined | < 0.01 | |
| Raw chicken | Undefined | < 0.01 | |
| Raw pork | 16.9 | 2–142 | 0.21 |
| Raw eggs | 7.5 | 1.3–44.6 | 0.01 |
| Bones | 5.5 | 0.6–49.4 | 0.09 |
| Pig ears | 9.8 | 1.1–88 | 0.01 |
| Rawhide | Undefined | 0.22 | |
| Cooked table scraps | 1.7 | 0.3–10.2 | 0.52 |
| Deli meats | 2.0 | 0.38–10.8 | 0.39 |
| Feed supplements (neutraceuticals, probiotics) | 1.3 | 0.2–8.7 | 0.72 |
| Dog has contact with livestock | 4.9 | 0.89–26.6 | 0.05 |
| Dog confined to fenced yard | Undefined | 0.21 | |
| Dog can run in park or other place dogs have visited | 0.34 | 0.4–3.1 | 0.32 |
| Dog is always on a leash | 0.74 | 0.1–4.3 | 0.74 |
| Dog has contact with cats | 0.4 | 0.04–3.6 | 0.41 |
| Frequency feed offered | NAb | 0.41 | |
| Frequency of cleaning of waterbowl | NA | 0.61 | |
| Frequency of cleaning of food bowl | NA | 0.45 | |
The complete absence of Salmonella-positive dogs in 1 of the 2 groups make the odds ratio (OR) undefinable.
Not Applicable, multiple ordinal responses.
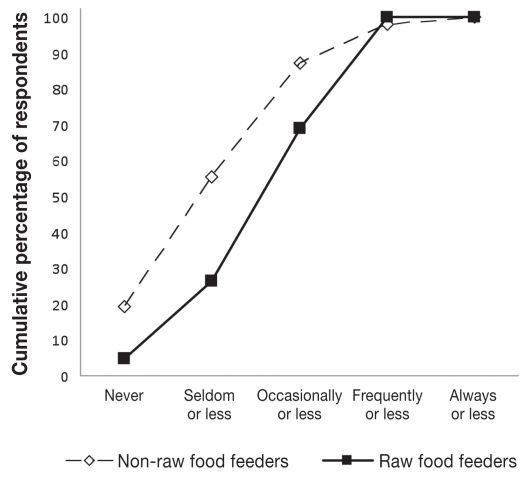
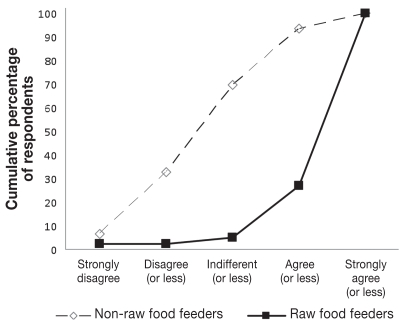
Dog owners feeding raw meat and not feeding raw meat were similar in terms of gender, level of education, level of reported income, age, and number of children in the household (Table 3). However, certain beliefs, practices, dog management factors, and influences on feed selection were divergent among the 2 groups (Table 3, Figure 1). While only 2 of 41 (5%) individuals who fed raw food strongly agreed, disagreed, or were indifferent with the statement “Diets containing raw meat are healthy for dogs,” 32 of 46 (70%) individuals who did not feed raw meat disagreed to some extend or were indifferent with this statement (Figure 1B). Owners feeding raw products spent $63, standard error (Sx̄) = 8, per month on raw foods, whereas those who did not feed raw feed meats spent $39, Sx̄ = 6, per month (P = 0.01). Raw poultry products (83%), beef (80%), eggs (38%), and pork (14%) were typically included in the raw rations. Feeding of particular meat products from various species was often correlated (data not show).
Table 3.
Owner beliefs, practices, and perceptions associated with the feed type provided to dogs
| P-value | |
|---|---|
| Demographics of Respondents | |
| Owner gender | 0.26 |
| Owner level of education | 0.13 |
| Owner income | 0.68 |
| Owner age | 0.95 |
| Number of children in household | 0.62 |
| Owner Beliefs | |
| Raw food is healthy for dogs | < 0.01a |
| Raw food is hazardous for dogs | < 0.01b |
| Commercial food is hazardous to dogs | < 0.01 |
| Choice of dog food has environmental impact | 0.05 |
| Choice of dog food reflects level of care for pet | 0.32 |
| Pet foods should contain only organic ingredients | 0.08 |
| Owner Practices | |
| Consumes raw fish | 0.03 |
| Consumes pink ground beef | 0.81 |
| Consumes undercooked (runny) eggs | 0.30 |
| Consumes raw shellfish | 0.57 |
| Consumes raw cookie dough | 0.28 |
| Consumes raw sprouts | 0.23 |
| Increasing frequency of choosing organic foods for self and family | 0.01c |
| Follows a vegetarian lifestyle | 0.48 |
| Increasing frequency of recycling of packaging materials | 0.61 |
| Dog Management | |
| Prepares dog food at home | < 0.01 |
| Feeds dog cooked table scraps | 0.02 |
| Feeds dog dietary supplement | 0.04 |
| Dog not vaccinated in last 3 years | 0.03 |
| Increasing interval since dogs’ last veterinary examination | 0.28 |
| Factors influencing feed selection | |
| Absence of preservatives | < 0.01 |
| Perceived nutritional value | 0.01 |
| Presence or absence of specific feed ingredients | 0.23 |
| Perceived pet preference | 0.49 |
| Perceived ability of feed to cause diseases | 0.12 |
| Perceived ability of feed to prevent diseases | < 0.01 |
| Product freshness | 0.14 |
| Veterinary consultation | 0.16 |
| Feed costs | 0.27 |
See Figure 1 for examples of response distribution.
All other variables, except this factor, were more common among raw meat fed dogs and owners of raw meat fed dogs than those animals that did not receive raw meat.
Figure 1A.
Distribution of owners’ choice of food selection for self.
Figure 1B.
Owners’ views on the statement “Raw feed is healthy for dogs’ health.”
Discussion
In this study, although dogs that were fed raw meat were more likely to shed Salmonella in their feces than dogs that were not, Salmonella may contaminate the household environment and serve as a source of Salmonella exposure to humans in households with dogs, regardless of the diet fed. Human exposure to Salmonella-positive dogs and contaminated household environments has public health implications, particularly for the very young, the elderly, pregnant women, and otherwise immunocompromised individuals.
Previous studies have reported the frequency of microbial contamination of raw foods intended for dogs (5,6,8,15,16). Campylobacter spp. were not found in a combined total of 313 samples of raw meat dog foods in either of 2 previous studies (5,6). In another study, 1 of the 2 raw feeds analyzed was positive for E. coli O157 (15). Prevalence values for Salmonella contamination of raw meat dog food range from 5% to 50% (5,6,8,15,16).
The frequency of food contamination can be influenced by the feed ingredients, the prevalence of the pathogens in the live animals at the time of slaughter, and the degree of hygiene during processing, transportation, and storage. Certain meat products are more likely than others to be contaminated with specific pathogens. For example, at slaughter, E. coli O157 is most likely to contaminate foods of bovine origin and not other meats (17). Therefore, exclusion of beef products from raw meat diets for dogs will reduce the likelihood of this organism in the feed.
Furthermore, there are seasonal variations in carriage rates and contamination rates of several foodborne pathogens by food-producing animals (18,19,20) Prevalence is highest in cattle in the summer for E. coli O157, Salmonella, and Campylobacter (18,19). Likewise, the prevalence of Campylobacter spp. in poultry also increases in the warmer months (21). The frequency of pathogen contamination of meats intended for human consumption has decreased in recent years. Nevertheless, approximately 0.17% of ground beef samples test positive for E. coli O157 (22) and as many as 25% and 80% of poultry samples test positive for Salmonella spp. and Campylobacter spp. respectively, depending upon the source (20). In summary, pathogen contamination in raw meats, even those intended for human consumption, occurs. Sixty percent of raw meat commercially available for dog foods are contaminated with generic E. coli, indicating that microbial contamination of these products at the time of either slaughter or processing is common (6). The frequency of Salmonella contamination of raw-meat diets found in this study was similar to that in other reports (5,6,8).
It is assumed that dogs become colonized with Salmonella spp., Campylobacter spp., and E. coli O157 from foodborne sources (23,24). The frequency of foodborne transmission of bacterial zoonotic pathogens to dogs is related to the frequency and dose of exposure, as well as the pathogens’ propensity to colonize the gastrointestinal tract of dogs, which may be modulated by both pathogen characteristics and host defenses. Carriage of Campylobacter spp. and E. coli O157 in healthy dogs is reported to be as high as 50% and 3%, respectively (25,26). The low prevalence of Campylobacter spp. and E. coli O157 identified in the fecal samples cultured in this study precluded drawing conclusions on the impact of raw meat feeding on the prevalence of these pathogens in dogs.
Most of the work on the subject of raw meat feeding has been related to the carriage of Salmonella spp., with prevalence values ranging from 1% to 69% (2). Based on the use of a single fecal culture, Joffe and Schlesinger (8) reported that 3/10 RMF dogs shed Salmonella compared with 0/10 NRMF dogs. In a more recent study by Finley et al (27), 44% of dogs fed Salmonella-infected raw meat diets excreted the organism intermittently in their feces starting from 1 to 7 d post exposure and continuing for up to 7 d. Given that not every lot of food was expected to be contaminated and fecal shedding is intermittent, the power to identify matching dog food/fecal pairs was low. Multiple samples from the dogs in both feeding groups may have resulted in higher prevalence estimates in this study, yielded more food/fecal pairs positive, and identified similar serotypes among specimens.
The onset of and duration of shedding are related to dose of exposure, the serovar, and host factors (23,27). Furthermore, the Salmonella serovar present in the feces could represent a serovar that was consumed anytime in the previous week, not only that present in the feed the day immediately prior to stool collection. For example, it is possible that the dog from whose feces S. Heidelberg was isolated had been exposed previously to multiple strains of S. Heidelberg, or that the organism underwent clonal turnover during passage through the intestinal tract of the dog (28). Many of the Salmonella serovars isolated during the course of this study have previously been reported in food-producing animals, retail meats, dogs, and as causes of human disease, particularly in neonates or other immunocompromised individuals. Therefore, all these serovars isolated should be considered potential pathogens (29–34).
Another public health concern related to Salmonella infections is the emergence and increase in antibiotic resistance among Salmonella serovars spp. Many isolates in this study were susceptible to the panel of antibiotics tested. Resistance to 1 (tetracycline) or 2 (tetracycline and ampicillin or streptomycin) antibiotics was common. Typically, approximately 40% to 50% of Salmonella spp. recovered from retail meats and from live animals are resistant to tetracycline (35,36). Likewise, resistance to streptomycin ranges from 30% to 37% among Salmonella spp. from these sources (35,36). In this study, only 1 isolate was resistant to more than 2 (n = 3) of the antimicrobial agents tested. Multidrug (> 3) resistance, including resistance to important classes of drugs used extensively in human medicine, such as ciprofloxacin or 3rd generation cephalosporins, was not observed. The infrequent antimicrobial resistance observed in this study may be a reflection of the serovars and clonal groups recovered from these sources, the absence of selective pressure in the presumptively healthy dogs, or animals from which the meat was derived (37).
Although Salmonella spp., Campylobacter spp., and E. coli O157 in humans are primarily foodborne pathogens, there are several other important sources of exposure to these microorganisms, such as water, direct contact with animals, and the environment. Living in a household with a dog or cat is considered to be a significant risk factor for humans to acquire campylobacteriosis (38). In 1 study, subtypes of C. jejuni recovered from poultry products, dogs, and humans in the population were indistinguishable, indicating an association between these sources (25). Several cases of E. coli O157 infection among children have been linked to contact with infected dogs (37).
The exact route and frequency of transmission of pathogens from pet dogs to humans has not been reported in the literature. However, petting animals and handling contaminated objects can readily transfer pathogens from the pet’s fur or the object to the owners’ hands (38). Possibly more importantly, as evidenced by findings in previous reports (39–41) and this study, the home environment can be an indirect source of contamination. Thus, when environmental contamination in the home is widespread, both personal hygiene (hand washing) and household sanitation are critical in preventing transmission, especially if the environment is contaminated with a large number of organisms. Most infant salmonellosis cases are acquired not from food, but from household environmental sources (41,42).
In this study, although RMF dogs were more frequent carriers of Salmonella, it was not possible to unequivocally implicate the raw foods as the source of the Salmonella in either the dogs or the environment. The contamination of the raw meat samples could have been present at the time of purchase or have occurred in the home. Likewise, dogs may have been exposed and the household environment contaminated with Salmonella spp. from other than canine sources in the environment, including, but not limited to, owners, wildlife, or other domestic animals (40,43,44). Multivariable logistic modeling is a useful tool in determining the contributions of individual risk factors in this study while adjusting for the confounding effects of other covariates; however, in this study, the effective sample size of positive results precluded this particular statistical method from being employed, so only univariable analyses were performed.
Participation in this study was completely voluntary, submitted specimens were based on convenience sampling and the design of the study was not randomized, so participation among individuals could have contributed to a bias in the results. Laboratory workers were not intentionally blinded to the source of the samples as they arrived; however, standard operating procedures, including the number of suspect colonies to be selected from each plate, were followed.
Michel (45) proposed that owners choose diets for their pets based on affective (feeling-based) decision-making processes, such as how the pet fits within the family social structure and how the owners perceive the pet’s reaction to the food; on ideological principles like agricultural sustainability or vegetarianism; and on principles of empowerment wherein “the pet owner becomes invested in the well-being of her or his companion.” The responses to this survey confirm that all of these driving forces play a role in owners’ decisions on feed selection. Although both groups of owners similarly (highly) ranked the affective measures of pets’ preference and the choice of food as a measure of concern for their pets, owners that fed raw food more frequently held stronger opinions concerning the impact of pet food choice on the environment and more often selected organic products for themselves (ideological rationale for pet food selection) (Figure 1A). Furthermore, the perceived disease prevention and treatment effects (empowerment) that raw food provided was a stronger rationale for dietary selection among those who fed raw meat than among those who did not include raw meats in their pet’s diet. This latter point underscores the idea that those feeding raw meat, in addition to following their affective and ideological principles, make rational decisions about feeding based on the information they consider to be true. Unfortunately, the large number of reputed health and therapeutic benefits of raw diets lack scientific validation. In addition, the ideological principles and affective decisions may be anthropomorphically biased, assuming that the same factors that make for sound decisions for owners apply equally to animals. To reiterate what was stated by Michel (45), all of these factors must be considered when developing communication strategies for pet owners.
In addition to veterinary consultation, development and dissemination of credible literature about the health implications of various pet diets, especially in media perused by those choosing alternative diets for their dogs (such as the Internet, newspapers, magazines, and books), would be helpful in order to provide science-based information to pet owners, so that they are able to make better informed decisions. However, because of the affective and ideological components involved in the choice to feed raw meat, even the most comprehensive scientific review will not persuade all owners to change their feeding practices.
Since raw meats are frequently contaminated with pathogens, eliminating these uncooked meats from dogs’ diets may be the single most effective method to reduce prevalence of canine infection with these pathogens. Many of the food safety concerns related to feeding raw meat to pet dogs could be avoided by cooking the raw meat components. But, first, it will be necessary to further understand the perceived barriers to this type of intervention before this important food safety and public health intervention strategy will be adopted.
Acknowledgment
The authors thank the pet owners who participated and made this study possible. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office ( hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
Authors’ contributions
Drs. Lenz and Kauffman were involved with the recruitment of the participants, the microbiological analyses, and reading the manuscript. Drs. Joffe and LeJeune designed the study and were involved with the recruitment of the participants, the data analysis and interpretation, and the preparation of the manuscript. Dr. Zhang was involved with determining the antibiotic resistance, the pulsed-field gel electrophoresis, the analysis and interpretation of data, and reading the manuscript.
Funding for this research was provided, in part, by Nestlé Purina’s Veterinary Student Summer Research Grant Program awarded to Dr. Jennifer Lenz.
References
- 1.Mead PS, Slutsker L, Dietz V, et al. Food-related illness and death in the United States. Emerg Infect Dis. 1999;5:607–625. doi: 10.3201/eid0505.990502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.LeJeune JT, Hancock DD. Public health concerns associated with feeding raw meat diets to dogs. J Am Vet Med Assoc. 2001;219:1222–1225. doi: 10.2460/javma.2001.219.1222. [DOI] [PubMed] [Google Scholar]
- 3.Stehr-Green JK, Schantz PM. The impact of zoonotic diseases transmitted by pets on human health and the economy. Vet Clin North Am Small Anim Pract. 1987;17:1–15. doi: 10.1016/s0195-5616(87)50601-5. [DOI] [PubMed] [Google Scholar]
- 4.Koehler KM, Lasky T, Fein SB, et al. Population-based incidence of infection with selected bacterial enteric pathogens in children younger than five years of age, 1996–1998. Pediatr Infect Dis J. 2006;25:129–134. doi: 10.1097/01.inf.0000199289.62733.d5. [DOI] [PubMed] [Google Scholar]
- 5.Weese JS, Rousseau J, Arroyo L. Bacteriological evaluation of commercial canine and feline raw diets. Can Vet J. 2005;46:513–516. [PMC free article] [PubMed] [Google Scholar]
- 6.Strohmeyer RA, Morley PS, Hyatt DR, Dargatz DA, Scorza AV, Lappin MR. Evaluation of bacterial and protozoal contamination of commercially available raw meat diets for dogs. J Am Vet Med Assoc. 2006;228:537–542. doi: 10.2460/javma.228.4.537. [DOI] [PubMed] [Google Scholar]
- 7.Morley PS, Strohmeyer RA, Tankson JD, Hyatt DR, Dargatz DA, Fedorka-Cray PJ. Evaluation of the association between feeding raw meat and Salmonella enterica infections at a Greyhound breeding facility. J Am Vet Med Assoc. 2006;228:1524–1532. doi: 10.2460/javma.228.10.1524. [DOI] [PubMed] [Google Scholar]
- 8.Joffe DJ, Schlesinger DP. Preliminary assessment of the risk of Salmonella infection in dogs fed raw chicken diets. Can Vet J. 2002;43:441–442. [PMC free article] [PubMed] [Google Scholar]
- 9.Finley R, Reid-Smith R, Weese JS. Human health implications of Salmonella-contaminated natural pet treats and raw pet food. Clin Infect Dis. 2006;42:686–691. doi: 10.1086/500211. [DOI] [PubMed] [Google Scholar]
- 10.OARDC, Center for Diagnostic Assays. LeJeune Jeffrey T. [Last accessed February 5, 2009];Web site, research raw dog food. Available from www.oardc.ohio-state.edu/fahrp/lab_pages/lejeune/lejeune.htm.
- 11.Dodson K, LeJeune J. Escherichia coli O157:H7, Campylobacter jejuni, and Salmonella prevalence in cull dairy cows marketed in northeastern Ohio. J Food Prot. 2005;68:927–931. doi: 10.4315/0362-028x-68.5.927. [DOI] [PubMed] [Google Scholar]
- 12.Stucki U, Frey J, Nicolet J, Burnens AP. Identification of Campylobacter jejuni on the basis of a species-specific gene that encodes a membrane protein. J Clin Microbiol. 1995;33:855–859. doi: 10.1128/jcm.33.4.855-859.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis MA, Hancock DD, Besser TE, et al. Correlation between geographic distance and genetic similarity in an international collection of bovine faecal Escherichia coli O157:H7 isolates. Epidemiol Infect. 2003;131:923–930. doi: 10.1017/s0950268803008884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.United States Department of Agriculture, Agricultural Research Service [homepage on the Internet], Search. Enter Antimicrobial-Salmonella Year 2004. Athens, Georgia: c2004. [Last accessed February 6, 2009]. Available from http://www.ars.usda.gov/SP2UserFiles/Place/66120508/NARMS/narms_2004/04Table1.pdf. [Google Scholar]
- 15.Freeman LM, Michel KE. Evaluation of raw food diets for dogs. J Am Vet Med Assoc. 2001;218:705–709. doi: 10.2460/javma.2001.218.705. [DOI] [PubMed] [Google Scholar]
- 16.Chengappa MM, Staats J, Oberst RD, Gabbert NH, McVey S. Prevalence of Salmonella in raw meat used in diets of racing greyhounds. J Vet Diagn Invest. 1993;5:372–377. doi: 10.1177/104063879300500312. [DOI] [PubMed] [Google Scholar]
- 17.Torrance M, Isaacson R, editors. Microbial Food Safety in Animal Agriculture: Current Topics. Hoboken, New Jersey: Blackwell Publ; 2003. [Google Scholar]
- 18.Stanley K, Jones K. Cattle and sheep farms as reservoirs of Campylobacter. J Appl Microbiol. 2003;94 (Suppl):104S–113S. doi: 10.1046/j.1365-2672.94.s1.12.x. [DOI] [PubMed] [Google Scholar]
- 19.Barkocy-Gallagher GA, Arthur TM, Rivera-Betancourt M, et al. Seasonal prevalence of Shiga toxin-producing Escherichia coli, including O157:H7 and non-O157 serotypes, and Salmonella in commercial beef processing plants. J Food Prot. 2003;66:1978–1986. doi: 10.4315/0362-028x-66.11.1978. [DOI] [PubMed] [Google Scholar]
- 20.Jorgensen F, Bailey R, Williams S, et al. Prevalence and numbers of Salmonella and Campylobacter spp. on raw, whole chickens in relation to sampling methods. Int J Food Microbiol. 2002;76:151–164. doi: 10.1016/s0168-1605(02)00027-2. [DOI] [PubMed] [Google Scholar]
- 21.Willis WL, Murray C. Campylobacter jejuni seasonal recovery observations of retail market broilers. Poult Sci. 1997;76:314–317. doi: 10.1093/ps/76.2.314. [DOI] [PubMed] [Google Scholar]
- 22.News Releases. Enter About FSIS, News and Events. United States Department of Agriculture [homepage on the Internet]; Washington DC: Feb, 2005. c2004. [Last accessed February 6, 2009]. FSIS Ground Beef Sampling Shows Substantial E. coli O157:H7 Decline in 2004. Available from: http://www.fsis.usda.gov/News_&_Events/NR_022805_01/index.asp. [Google Scholar]
- 23.Green C. Salmonellosis. In: Green C, editor. Infectious Diseases of the Dog and Cat. Philidelphia: WB Saunders; 1998. pp. 235–240. [Google Scholar]
- 24.Fox J. Philadelphia: WB Saunders; 1998. Campylobacter infections. In: Greene C, ed. Infectious Diseases of the Dog and Cat; pp. 226–229. [Google Scholar]
- 25.Workman SN, Mathison GE, Lavoie MC. Pet dogs and chicken meat as reservoirs of Campylobacter spp. in Barbados. J Clin Microbiol. 2005;43:2642–2650. doi: 10.1128/JCM.43.6.2642-2650.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hancock DD, Besser TE, Rice DH, Ebel ED, Herriott DE, Carpenter LV. Multiple sources of Escherichia coli O157 in feedlots and dairy farms in the northwestern USA. Prev Vet Med. 1998;35:11–19. doi: 10.1016/s0167-5877(98)00050-6. [DOI] [PubMed] [Google Scholar]
- 27.Finley R, Ribble C, Aramini J, et al. The risk of Salmonellae shedding by dogs fed Salmonella-contaminated commercial raw food diets. Can Vet J. 2007;48:69–75. [PMC free article] [PubMed] [Google Scholar]
- 28.Akiba M, Sameshima T, Nakazawa M. Clonal turnover of enterohemorrhagic Escherichia coli O157:H7 in experimentally infected cattle. FEMS Microbiol Lett. 2000;184:79–83. doi: 10.1111/j.1574-6968.2000.tb08994.x. [DOI] [PubMed] [Google Scholar]
- 29.Rigby CE, Pettit JR, Baker MF, Bentley AH, Salomons MO, Lior H. Sources of Salmonellae in an uninfected commercially-processed broiler flock. Can J Comp Med. 1980;44:267–274. [PMC free article] [PubMed] [Google Scholar]
- 30.Park JK, Seok WS, Choi BJ, et al. Salmonella enterica serovar London infections associated with consumption of infant formula. Yonsei Med J. 2004;45:43–48. doi: 10.3349/ymj.2004.45.1.43. [DOI] [PubMed] [Google Scholar]
- 31.Olsen JE, Sorensen M, Brown DJ, Gaarslev K, Bisgaard M. Plasmid profiles as an epidemiological marker in Salmonella enterica serovar Berta infections. Comparison of isolates obtained from humans and poultry. APMIS. 1992;100:221–228. [PubMed] [Google Scholar]
- 32.Gotoff SP, Boring JR, Lepper MH. An epidemic of Salmonella saint-paul infections in a convalescent home. Am J Med Sci. 1966;251:16–21. doi: 10.1097/00000441-196601000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Chittick P, Sulka A, Tauxe RV, Fry AM. A summary of national reports of foodborne outbreaks of Salmonella Heidelberg infections in the United States: Clues for disease prevention. J Food Prot. 2006;69:1150–1153. doi: 10.4315/0362-028x-69.5.1150. [DOI] [PubMed] [Google Scholar]
- 34.Casey PG, Butler D, Gardiner GE, et al. Salmonella carriage in an Irish pig herd: Correlation between serological and bacteriological detection methods. J Food Prot. 2004;67:2797–2800. doi: 10.4315/0362-028x-67.12.2797. [DOI] [PubMed] [Google Scholar]
- 35.Department of Agriculture Agricultural Research Service [homepage on the Internet] Enter SP2 User files Table 3. Athens, Georgia: c2004. [Last accessed February 6, 2009]. United States Total percent Salmonella isolates sensitive, intermediate, or resistant regardless of clinical status. Available from: http://ars.usda.gov/SP2UserFiles/Place/66120508/NARMS/narms_2004/04%20Table%203.pdf. [Google Scholar]
- 36.United States Food and Drug Administration [homepage on the Internet] Animal Veterinary, Antimicrobial resistance. Table 9. College Park, MD: c2004. [Last accessed February 6, 2009]. Search Antimicrobial Resistance among Salmonella Isolates (N = 324), 2004. Available from http://www.fda.gov/cvm/Documents/Table9b2004.pdf. [Google Scholar]
- 37.Trevena WB, Hooper RS, Wray C, Willshaw GA, Cheasty T, Domingue G. Vero cytotoxin-producing Escherichia coli O157 associated with companion animals [letter] Vet Rec. 1996;138:400. [PubMed] [Google Scholar]
- 38.Hald B, Madsen M. Healthy puppies and kittens as carriers of Campylobacter spp., with special reference to Campylobacter upsaliensis. J Clin Microbiol. 1997;35:3351–3352. doi: 10.1128/jcm.35.12.3351-3352.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buma R, Maeda T, Kamei M, Kourai H. Pathogenic bacteria carried by companion animals and their susceptibility to antibacterial agents. Biocontrol Sci. 2006;11:1–9. doi: 10.4265/bio.11.1. [DOI] [PubMed] [Google Scholar]
- 40.Rice DH, Hancock DD, Roozen PM, et al. Household contamination with Salmonella enterica. Emerg Infect Dis. 2003;9:120–122. doi: 10.3201/eid0901.020214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haysom IW, Sharp K. The survival and recovery of bacteria in vacuum cleaner dust. J R Soc Health. 2003;123:39–45. doi: 10.1177/146642400312300114. [DOI] [PubMed] [Google Scholar]
- 42.Haddock RL, Nocon FA. Infant salmonellosis and vacuum cleaners. J Trop Pediatr. 1994;40:53–54. doi: 10.1093/tropej/40.1.53. [DOI] [PubMed] [Google Scholar]
- 43.Rice DH, Hancock DD, Vetter RL, Besser TE. Escherichia coli O157 infection in a human linked to exposure to infected livestock. Vet Rec. 1996;138:311. [PubMed] [Google Scholar]
- 44.Rice DH, Hancock DD, Besser TE. Faecal culture of wild animals for Escherichia coli O157:H7. [letter] Vet Rec. 2003;152:82–83. doi: 10.1136/vr.152.3.82. [DOI] [PubMed] [Google Scholar]
- 45.Michel KE. Unconventional diets for dogs and cats. Vet Clin North Am Small Anim Pract. 2006;36:1269–1281. vi–vii. doi: 10.1016/j.cvsm.2006.08.003. [DOI] [PubMed] [Google Scholar]