Abstract
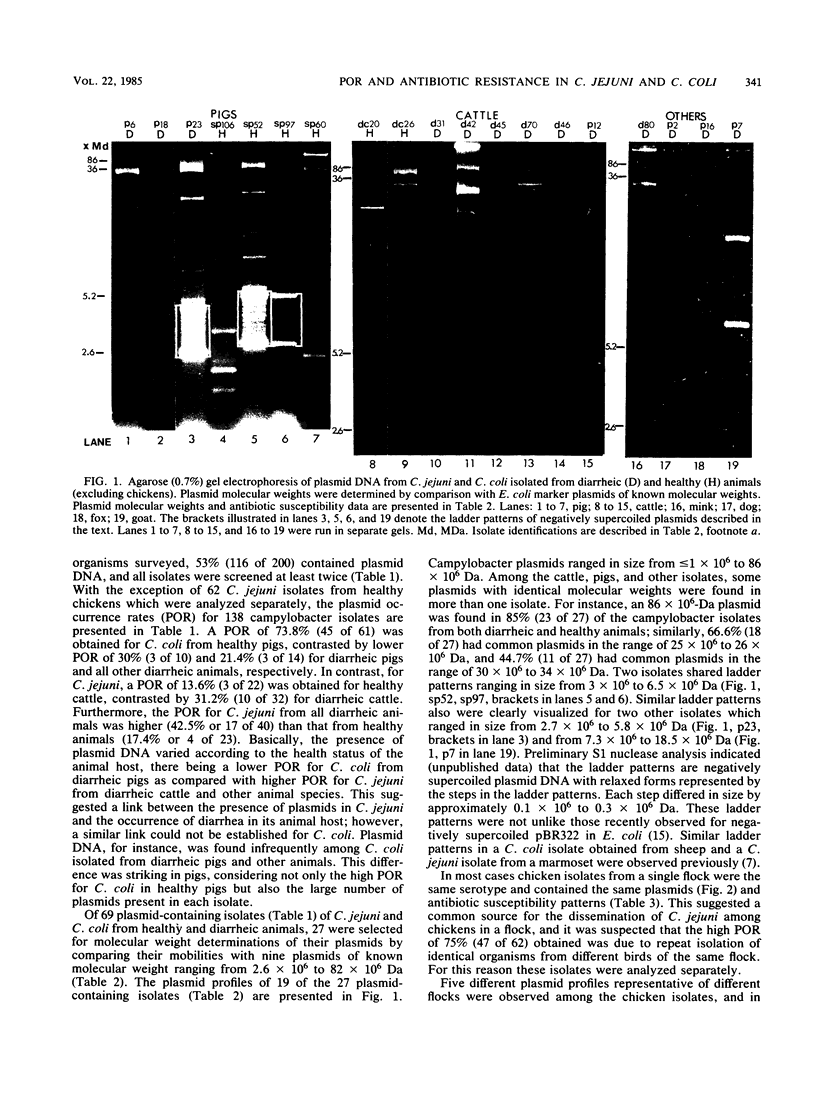
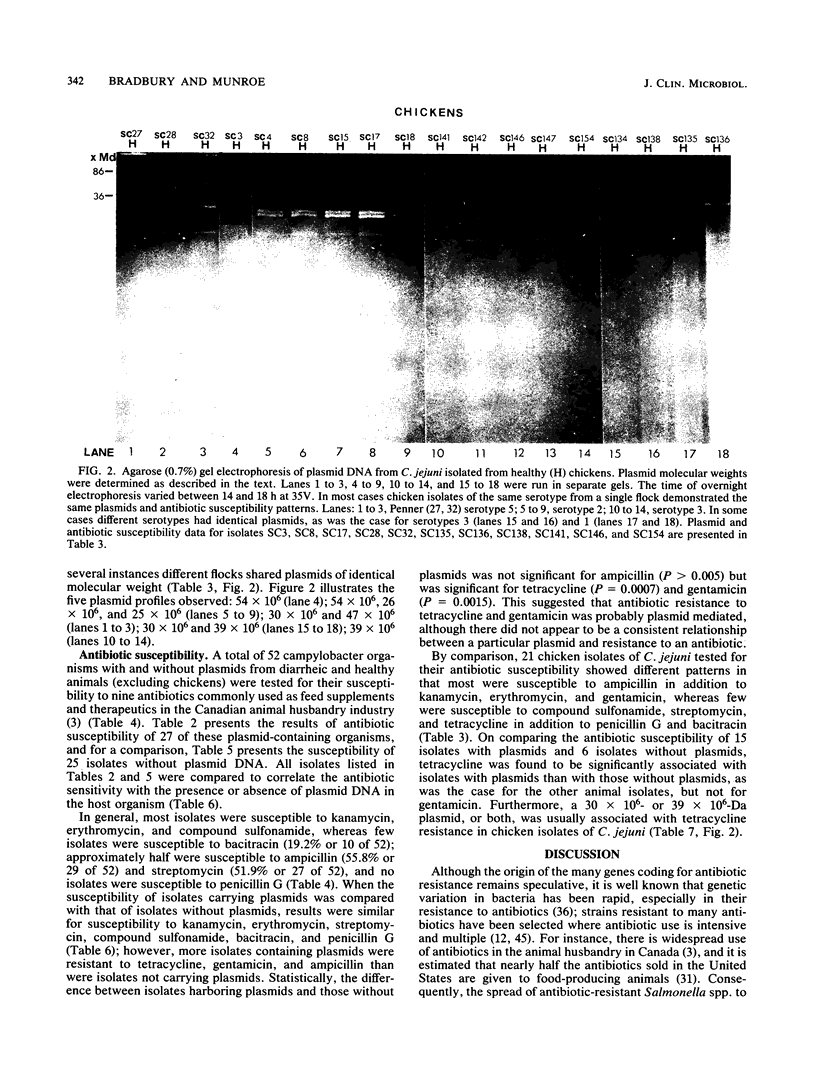
Serologically defined strains of Campylobacter jejuni and Campylobacter coli from healthy and diarrheic animals were examined for the occurrence of plasmid DNA in association with the antibiotic susceptibility of the bacterial host and the health status of the animal host. Of all campylobacter organisms surveyed, 53% (116 of 200) contained plasmid DNA. A plasmid occurrence rate of 73.8% was obtained for C. coli from healthy pigs, contrasted by lower plasmid occurrence rates for C. coli from diarrheic pigs (30%) and from all diarrheic animals (21.4%). For C. jejuni, in contrast, only 13.6% of healthy cattle contained plasmid DNA, contrasted by a higher plasmid occurrence rate of 31.2% from diarrheic cattle. A high plasmid occurrence rate of 75.8% was observed for C. jejuni from healthy chickens. Campylobacter plasmids ranged in size from less than or equal to 1 to 86 megadaltons. Antibiotic susceptibility for 52 animal isolates (excluding chickens) indicated that most isolates were susceptible to kanamycin, erythromycin, gentamicin, tetracycline, and compound sulfonamide, whereas few were susceptible to bacitracin (19.2%); approximately half were susceptible to ampicillin (55.8%) and streptomycin (51.9%), and no isolates were susceptible to penicillin G. More isolates containing plasmids were resistant to ampicillin, tetracycline, and gentamicin than were isolates not carrying plasmids, there being a statistically significant difference for tetracycline and gentamicin, which suggested that these two antibiotics were probably plasmid mediated. The antibiotic susceptibility patterns of 21 chicken isolates of C. jejuni, by contrast, were different in that most were susceptible to ampicillin in addition to kanamycin, erythromycin, and gentamicin, whereas few wer susceptible to compound sulfonamide, streptomycin, and tetracycline in addition to penicillin G and bacitracin. A 30- or 39-megadalton plasmid, or both, common to many of the chicken isolates was usually associated with tetracycline resistance.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahart J. G., Burton G. C., Blenden D. C. The influence of antimicrobial agents on the percentage of tetracycline-resistant bacteria in faeces of humans and animals. J Appl Bacteriol. 1978 Apr;44(2):183–190. doi: 10.1111/j.1365-2672.1978.tb00789.x. [DOI] [PubMed] [Google Scholar]
- Blaser M. J., Reller L. B. Campylobacter enteritis. N Engl J Med. 1981 Dec 10;305(24):1444–1452. doi: 10.1056/NEJM198112103052404. [DOI] [PubMed] [Google Scholar]
- Blaser M. J., Wells J. G., Feldman R. A., Pollard R. A., Allen J. R. Campylobacter enteritis in the United States. A multicenter study. Ann Intern Med. 1983 Mar;98(3):360–365. doi: 10.7326/0003-4819-98-3-360. [DOI] [PubMed] [Google Scholar]
- Bopp C. A., Birkness K. A., Wachsmuth I. K., Barrett T. J. In vitro antimicrobial susceptibility, plasmid analysis, and serotyping of epidemic-associated Campylobacter jejuni. J Clin Microbiol. 1985 Jan;21(1):4–7. doi: 10.1128/jcm.21.1.4-7.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury W. C., Marko M. A., Hennessy J. N., Penner J. L. Occurrence of plasmid DNA in serologically defined strains of Campylobacter jejuni and Campylobacter coli. Infect Immun. 1983 May;40(2):460–463. doi: 10.1128/iai.40.2.460-463.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury W. C., Pearson A. D., Marko M. A., Congi R. V., Penner J. L. Investigation of a Campylobacter jejuni outbreak by serotyping and chromosomal restriction endonuclease analysis. J Clin Microbiol. 1984 Mar;19(3):342–346. doi: 10.1128/jcm.19.3.342-346.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butzler J. P., Skirrow M. B. Campylobacter enteritis. Clin Gastroenterol. 1979 Sep;8(3):737–765. [PubMed] [Google Scholar]
- Campbell A. Evolutionary significance of accessory DNA elements in bacteria. Annu Rev Microbiol. 1981;35:55–83. doi: 10.1146/annurev.mi.35.100181.000415. [DOI] [PubMed] [Google Scholar]
- Elwell L. P., Shipley P. L. Plasmid-mediated factors associated with virulence of bacteria to animals. Annu Rev Microbiol. 1980;34:465–496. doi: 10.1146/annurev.mi.34.100180.002341. [DOI] [PubMed] [Google Scholar]
- Holmberg S. D., Osterholm M. T., Senger K. A., Cohen M. L. Drug-resistant Salmonella from animals fed antimicrobials. N Engl J Med. 1984 Sep 6;311(10):617–622. doi: 10.1056/NEJM198409063111001. [DOI] [PubMed] [Google Scholar]
- Horiuchi H., Takagi M., Yano K. Relaxation of supercoiled plasmid DNA by oxidative stresses in Escherichia coli. J Bacteriol. 1984 Dec;160(3):1017–1021. doi: 10.1128/jb.160.3.1017-1021.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. M., Abbott J. D., Painter M. J., Sutcliffe E. M. A comparison of biotypes and serotypes of Campylobacter sp. isolated from patients with enteritis and from animal and environmental sources. J Infect. 1984 Jul;9(1):51–58. doi: 10.1016/s0163-4453(84)94498-0. [DOI] [PubMed] [Google Scholar]
- Karmali M. A., Fleming P. C. Campylobacter enteritis in children. J Pediatr. 1979 Apr;94(4):527–533. doi: 10.1016/s0022-3476(79)80004-9. [DOI] [PubMed] [Google Scholar]
- Karmali M. A., Fleming P. C. Campylobacter enteritis. Can Med Assoc J. 1979 Jun 23;120(12):1525–1532. [PMC free article] [PubMed] [Google Scholar]
- Karmali M. A., Penner J. L., Fleming P. C., Williams A., Hennessy J. N. The serotype and biotype distribution of clinical isolates of Campylobacter jejuni and Campylobacter coli over a three-year period. J Infect Dis. 1983 Feb;147(2):243–246. doi: 10.1093/infdis/147.2.243. [DOI] [PubMed] [Google Scholar]
- Klipstein F. A., Engert R. F. Properties of crude Campylobacter jejuni heat-labile enterotoxin. Infect Immun. 1984 Aug;45(2):314–319. doi: 10.1128/iai.45.2.314-319.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey R. W. Evolution of microorganisms and antibiotic resistance. Lancet. 1984 Nov 3;2(8410):1022–1025. doi: 10.1016/s0140-6736(84)91117-6. [DOI] [PubMed] [Google Scholar]
- Marri L., Musmanno R. A., Coratza G., Figura N., Molina A. M. Does the occurrence of plasmids in Campylobacter jejuni and Campylobacter coli isolates correlate with antibiotic resistance? Eur J Clin Microbiol. 1984 Oct;3(5):446–447. doi: 10.1007/BF02017372. [DOI] [PubMed] [Google Scholar]
- McMyne P. M., Penner J. L., Mathias R. G., Black W. A., Hennessy J. N. Serotyping of Campylobacter jejuni isolated from sporadic cases and outbreaks in British Columbia. J Clin Microbiol. 1982 Aug;16(2):281–285. doi: 10.1128/jcm.16.2.281-285.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills S. D., Bradbury W. C. Human antibody response to outer membrane proteins of Campylobacter jejuni during infection. Infect Immun. 1984 Feb;43(2):739–743. doi: 10.1128/iai.43.2.739-743.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munroe D. L., Prescott J. F., Penner J. L. Campylobacter jejuni and Campylobacter coli serotypes isolated from chickens, cattle, and pigs. J Clin Microbiol. 1983 Oct;18(4):877–881. doi: 10.1128/jcm.18.4.877-881.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent M. E., Bone D. H., Datta N. A transposon, Tn732, encoding gentamicin/tobramycin resistance. Nature. 1979 Nov 22;282(5737):422–423. doi: 10.1038/282422a0. [DOI] [PubMed] [Google Scholar]
- O'Brien T. F., Hopkins J. D., Gilleece E. S., Medeiros A. A., Kent R. L., Blackburn B. O., Holmes M. B., Reardon J. P., Vergeront J. M., Schell W. L. Molecular epidemiology of antibiotic resistance in salmonella from animals and human beings in the United States. N Engl J Med. 1982 Jul 1;307(1):1–6. doi: 10.1056/NEJM198207013070101. [DOI] [PubMed] [Google Scholar]
- Penner J. L., Hennessy J. N., Mills S. D., Bradbury W. C. Application of serotyping and chromosomal restriction endonuclease digest analysis in investigating a laboratory-acquired case of Campylobacter jejuni enteritis. J Clin Microbiol. 1983 Dec;18(6):1427–1428. doi: 10.1128/jcm.18.6.1427-1428.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner J. L., Hennessy J. N. Passive hemagglutination technique for serotyping Campylobacter fetus subsp. jejuni on the basis of soluble heat-stable antigens. J Clin Microbiol. 1980 Dec;12(6):732–737. doi: 10.1128/jcm.12.6.732-737.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott J. F., Munroe D. L. Campylobacter jejuni enteritis in man and domestic animals. J Am Vet Med Assoc. 1982 Dec 15;181(12):1524–1530. [PubMed] [Google Scholar]
- Ruiz-Palacios G. M., Torres J., Torres N. I., Escamilla E., Ruiz-Palacios B. R., Tamayo J. Cholera-like enterotoxin produced by Campylobacter jejuni. Characterisation and clinical significance. Lancet. 1983 Jul 30;2(8344):250–253. doi: 10.1016/s0140-6736(83)90234-9. [DOI] [PubMed] [Google Scholar]
- Saunders J. R. Genetics and evolution of antibiotic resistance. Br Med Bull. 1984 Jan;40(1):54–60. doi: 10.1093/oxfordjournals.bmb.a071948. [DOI] [PubMed] [Google Scholar]
- Skirrow M. B., Benjamin J. '1001' Campylobacters: cultural characteristics of intestinal campylobacters from man and animals. J Hyg (Lond) 1980 Dec;85(3):427–442. doi: 10.1017/s0022172400063506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. W. Anti-microbial drugs in animal feeds. Nature. 1968 May 25;218(5143):728–731. doi: 10.1038/218728a0. [DOI] [PubMed] [Google Scholar]
- Taylor D. E., Bryner J. H. Plasmid content and pathogenicity of Campylobacter jejuni and Campylobacter coli strains in the pregnant guinea pig model. Am J Vet Res. 1984 Oct;45(10):2201–2202. [PubMed] [Google Scholar]
- Taylor D. E., De Grandis S. A., Karmali M. A., Fleming P. C. Transmissible plasmids from Campylobacter jejuni. Antimicrob Agents Chemother. 1981 May;19(5):831–835. doi: 10.1128/aac.19.5.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. E., Garner R. S., Allan B. J. Characterization of tetracycline resistance plasmids from Campylobacter jejuni and Campylobacter coli. Antimicrob Agents Chemother. 1983 Dec;24(6):930–935. doi: 10.1128/aac.24.6.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenover F. C., Bronsdon M. A., Gordon K. P., Plorde J. J. Isolation of plasmids encoding tetracycline resistance from Campylobacter jejuni strains isolated from simians. Antimicrob Agents Chemother. 1983 Feb;23(2):320–322. doi: 10.1128/aac.23.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlfall E. J., Ward L. R., Rowe B. Spread of multiresistant strains of Salmonella typhimurium phage types 204 and 193 in Britain. Br Med J. 1978 Oct 7;2(6143):997–997. doi: 10.1136/bmj.2.6143.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATANABE T. Infective heredity of multiple drug resistance in bacteria. Bacteriol Rev. 1963 Mar;27:87–115. doi: 10.1128/br.27.1.87-115.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]




