Abstract
Apligraf® (Organogenesis, Canton, MA) is a bi-layered bioengineered skin substitute and was the first engineered skin US Food and Drug Administration (FDA)-approved to promote the healing of ulcers that have failed standard wound care. Constructed by culturing human foreskin-derived neonatal fibroblasts in a bovine type I collagen matrix over which human foreskin-derived neonatal epidermal keratinocytes are then cultured and allowed to stratify, Apligraf provides both cells and matrix for the nonhealing wound. Its exact mechanism of action is not known, but it is known to produce cytokines and growth factors similar to healthy human skin. Initially approved by the FDA in 1998 for the treatment of venous ulcers greater than one-month duration that have not adequately responded to conventional therapy, Apligraf later received approval in 2000 for treatment of diabetic foot ulcers of greater than three weeks duration. Herein, we review the use of Apligraf in the treatment of chronic venous leg ulcers and diabetic foot ulcers. Our goal is to provide a working understanding of appropriate patient selection and proper use of the product for any physician treating this segment of the aging population.
Keywords: wound healing, Apligraf ®, venous leg ulcer, diabetic foot ulcer
Venous leg ulcers (VLU) and diabetic foot ulcers (DFU) are among the most prevalent of chronic wounds and along with pressure ulcers comprise a majority of nonhealing wounds in the aging population. It is estimated that venous ulcers affect approximately 1% of the world’s population, a number that increases with increasing age (Trent et al 2005). In the US, VLU affect up to 2.5 million patients per year (Brem et al 2004). Similar in overall prevalence, DFU affect up to 68 per 1000 people with diabetes per year, and over half of these patients develop an infection, often osteomyelitis, with 20% requiring some form of amputation during the course of their ulcer treatment (Wu and Armstrong 2005). Besides having a negative impact on a patient’s quality of life (Mathias et al 2000; Goodridge et al 2006), both types of chronic ulcers pose a major economic burden on the medical community.
While the underlying pathophysiology of ulcer development differs between the chronic wound types, a commonality exists once ulcers develop. VLU are thought to develop as a result of venous hypertension or sustained ambulatory venous pressures, a consequence of an abnormal venous system (typically diseased veins or valves) leading to venous reflux (Hess and Kirsner 2003). DFU are most often a consequence of longstanding diabetes leading to either neuropathy (the most common cause of foot ulcers) or vascular disease (or a combination of both). Once an ulcer develops and does not proceed to healing, the chronic wound environment takes on its own unique characteristics which include excessive proteases, increased cellular senescence, and increased bacterial bioburden. Growth factors are often deficient and unavailable for healing in this environment.
History of Apligraf ®
Apligraf ® (Organogenesis, Canton, MA), a tissue-engineered biological dressing, was the first composite tissue analog of any kind to become commercially available. It was initially approved by the US Food and Drug Administration (FDA) in 1998 for the treatment of venous ulcers greater than one-month duration that had not adequately responded to conventional therapy, and it received approval in 2000 for treatment of diabetic foot ulcers of greater than three weeks duration. It has shown promise as an effective clinical treatment of chronic nonhealing wounds, such as VLU and DFU, and has been utilized in a variety of acute and chronic wounds to facilitate healing.
The initial published experience with Apligraf (previously known as Graftskin® and Living Skin Equivalent) in patients was its use as a skin substitute for acute surgical wounds (Eaglstein et al 1995) where it was found to produce better than expected healing in post-surgical sites (mainly used after cancer excisions). In a study comparing the skin substitute with secondary intention healing after Mohs micrographic or excisional surgery (Gohari et al 2002), the human skin substitute produced a more pliable, less vascular scar with a better cosmetic appearance than those healing by secondary intention. Living skin equivalent was also compared with autograft or polyurethane film in acute partial thickness donor site wounds and was found to have no toxic effect or clinically apparent rejection. It was found to decrease pain at the operative site compared with patients whose wounds were covered with polyurethane film (Muhart et al 1999). Apligraf has also been shown to expedite healing in excised (Waymack et al 2000) and full-thickness (Hayes et al 2001) burn wounds. Other reported successful uses include the following: the treatment of epidermolysis bullosa, showing that Apligraf treated areas healed faster than the areas treated with conventional therapy (Falabella et al 1999; Streit et al 2001); the treatment of chronic leg ulcers secondary to hydroxyurea that failed standard wound care (Flores et al 2000); chronic nonhealing pressure ulcers failing standard therapy (Brem et al 2000); ulcerative sarcoidosis that was unresponsive to steroid treatment (Falabella et al 2000); traumatic avulsion wounds in patients with age or steroid related dermal atrophy (Maier et al 2002); severe eroded and ulcerated actinic purpura (Banta and Kirsner 2002); bullous morphea (where a decrease in surrounding fibrosis was also appreciated) (Martin and Kirsner 2003); and in the repair of cicatricial ectropion (Culican and Custer 2002), among others.
To obtain FDA approval, two successful pivotal trials were performed using Apligraf for the treatment of VLU and DFU. The first was titled, “A randomized controlled trial of the allogeneic human skin equivalent was evaluated for the safety, efficacy, and immunological impact in the treatment of venous ulcers” (Falanga et al 1998). This multi-center study was completed on 293 patients in an outpatient setting and examined VLU receiving either compression therapy alone compared with compression therapy and serial (up to 5) applications of the human skin equivalent, Apligraf. This study found that treatment with bioengineered skin was more effective than compression therapy at its primary endpoint of 6 months, with 63% versus 49% of patients having completely healed, respectively, and the median time to complete wound closure was 61 versus 181 days, respectively, in the two groups. Interestingly, treatment with the skin substitute was most effective in the more difficult wounds, those larger than 10 cm2, and ulcers present for greater than 6 months duration. Adverse events were similar in both groups, with no signs or symptoms of rejection detected in vitro to bovine collagen or to alloantigens expressed on keratinocytes or fibroblasts in the bioengineered skin. This was the first study demonstrating Apligraf as an effective and safe mode of treatment for chronic, nonhealing VLU.
The pivotal trial for DFU using Apligraf was titled, “A randomized controlled prospective trial investigating the effectiveness of Apligraf ® in the treatment of non-infected, non-ischemic, chronic plantar diabetic foot ulcers” (Veves et al 2001). Conducted in 24 centers in the US, 208 patients were randomly assigned to ulcer treatment either with the bioengineered skin or saline-moistened gauze. Other standard wound care measures were employed, including extensive surgical debridement and adequate foot off-loading in both groups. The Apligraf was applied at the beginning of the study and weekly thereafter for a maximum of 4 weeks (maximum of five applications) or until complete healing occurred. Outcomes were assessed at the 12 week follow up visit. At 12 weeks 56% of the Apligraf-treated patients achieved complete wound healing in comparison with 38% of the control group, with the median time to complete closure being 65 days for the Apligraf-treated group and 90 days in the control group. The odds ratio for complete healing for the Apligraf-treated group was 2.14 (95% confidence interval [CI] 1.23–3.74). The rate of adverse reactions was similar in both groups. However, importantly, osteomyelitis and lower limb amputations were both less frequent in the Apligraf-treated group. This study demonstrated the benefit of Apligraf in comparison with standard wound therapy for the treatment-resistant DFU.
Economic impact of Apligraf ®
Controlled clinical studies have shown that Apligraf is more effective and economical at healing chronic VLU (Schonfeld et al 2000; Curran and Plosker 2002; Fivenson and Scherschun 2003) and DFU (Veves et al 2001; Redekop et al 2003) than standard wound care therapy alone. As noted, its use has been found to decrease the risk of development of osteomyelitis (Veves et al 2001) and the risk of amputation in diabetic patients (Veves et al 2001; Redekop et al 2003), and improve the quality of life of patients with chronic VLU, mainly by reducing wound pain (Mathias et al 2000).
Ulcer-related medical costs of patients with either VLU or DFU following treatment with Apligraf are lower in comparison with conventional wound care therapy (Harding et al 2000; Schonfeld et al 2000; Curran and Plosker 2002; Fivenson and Scherschun 2003; Redekop et al 2003). Among the most difficult to heal patients with VLU are those whose ulcers have been present greater than 1-year duration and in that population Apligraf compared with standard compression therapy was 3 times more effective than control group at achieving complete wound closure at 8 weeks (Falanga 2000). Another study comparing patients with chronic non-healing VLU (mean duration of ulceration upon entering study was 23.9 months and median ulcers size of 13.5 cm2) treated with standard compression therapy or Apligraf reported a 0.72 cm2 increase in mean ulcer size per week in the control group, while the Apligraf-treated group had an average decrease of 2.37 cm2 per week. Medical costs per unit change in ulcer size were lower following Apligraf treatment relative to cost of the control group (Fivenson and Scherschun 2003). Similarly, in the DFU pivotal trial described above, ulcers treated with Apligraf had an increase in the amount of ulcer-free time by 1.53 months (Veves et al 2001).
Composition and mechanism of action of Apligraf ®
Apligraf is composed of neonatal fibroblasts which are initially placed in a bovine type I collagen matrix. Over time a neodermis produced by the neonatal fibroblasts develops. The dermal component is overlaid by neonatal epidermal keratinocytes which grow initially to a monolayer and then allowed to stratify. Apligraf does not contain any antigen-presenting cells such as Langerhans cells, dermal dendritic cells, endothelial cells, melanocytes, or inflammatory cells such as leucocytes. The cells (fibroblasts and keratinocytes) that constitute Apligraf do not persist indefinitely and appear to be relatively short lived in most patients (less than 4 weeks) (Phillips et al 2002; Hu et al 2006), depending on the wound treated. Apligraf is recognized as being immunologically inert, showing no clinical or laboratory evidence of rejection (Briscoe et al 1999; Eaglstein et al 1999).
The mechanism of action of Apligraf in the promotion of wound healing is not fully understood; however, it is known to produce a great number of cytokines and growth factors (Streit and Braathen 2000; Falanga et al 2002) which are thought to be responsible for stimulating differentiation and proliferation in an otherwise senescent, nonhealing wound bed. As previously mentioned, it has been noted by several authors that wounds treated with the living skin substitute demonstrate faster and better healing with less fibrosis. While it is not completely clear why this occurs, it is perhaps due to the nature of the graft itself, as it is constructed of neonatal fibroblasts and neonatal keratinocytes and may, therefore, stimulate a more primitive or fetal-like development of the underlying healing skin.
Appropriate patient selection for Apligraf ®
In determining the appropriate patient on whom to use Apligraf, the wound type has to be accurately identified. For VLU this implies the appearance of a medially-based ulcer, the presence of hemosiderin deposition, increased vascular markings, and evidence of periulcer fibrosis or lipodermatosclerosis. Confirmatory tests, such as a duplex ultrasound, may be performed and can be helpful. The diagnosis of a DFU coincides with evidence of neuropathy, usually in patients with a long-standing history of poorly controlled diabetes, and may be confirmed by simple bedside testing with a monofilament, or, if needed, by more extensive testing such as nerve conduction studies (Dickinson et al 2002; Meerwaldt et al 2005). For both patients with VLU and DFU, arterial blood flow should be evaluated and intervention considered if compromise exists (usually by referral to a vascular surgeon).
Next, the patient should have received or be receiving standard of care as this is critical to the concept of the non-healing wound. A nonhealing wound is defined as a wound that does not adequately respond to conventional, standard therapy within a three to four week period of time (Cavorsi et al 2006). For VLU, standard therapy entails moist wound care, debridement, control of infection, and the use of a multilayered compression, and for DFU, debridement, control of infection, moist wound care, and off-loading (with shoes, walkers, or contact casts).
As previously stated, it is the patient with the nonhealing wound that is suitable for treatment with Apligraf. For both VLU and DFU, two methods exist to define a nonhealing wound. The first is failure to improve (to reduce in size) over a 3–4 week course of standard of care (Davis et al 2006), and the second is the presence of negative predictive markers (or risk factors). Negative predictive markers that have been identified for VLU include: prolonged duration of ulcer (>6 months), large wound size (>5 cm), a history of failure of prior therapy, extensive lipodermatosclerosis, location below the malleolus, and coexisting site infection (Cavorsi et al 2006). For DFU, negative predictive markers include wound duration and size, site infection, depth of ulceration, location on mid-plantar area or heel, extreme foot deformity, inadequate weight off-loading, patient noncompliance, and poor glycemic control (Cavorsi et al 2006).
Proper use of Apligraf
With either VLU or DFU, any underlying disease associated conditions that may interfere with healing should be addressed, both for the purpose of treatment as well as for prevention of recurrence. For VLU, the management of venous insufficiency and associated edema with high-compression therapy and infection control are essential. While for DFU, therapeutic off-loading and proper shoe selection along with glycemic control and infection control are the mainstays of avoiding wound exacerbation.
Prior to application of Apligraf, the wound bed should be optimized, a term that has been called “wound bed preparation.” For VLU, wound bed preparation consists of debridement of any slough, necrotic, and possible fibrotic material. For DFU, wound bed preparation begins with sharp debridement to remove, not solely the debris, slough, and necrotic tissue within the wound, but also all of the calloused tissue surrounding the ulcer. Apligraf should not be applied to infected wounds.
To ensure product viability, the Apligraf (that comes in a sealed poly bag) should be examined with the accompanying pH color chart to ensure proper pH. The expiration date on the bag, which is typically 10 days after its arrival, should also be noted. If the product is satisfactory, the bag is opened and the Apligraf and its container are removed. The Apligraf is removed from the container using sterile forceps to gently push the tissue from the edge of the plate, being careful not to remove the polycarbonate membrane supporting the tissue on the media. If folding of the Apligraf occurs, it can be placed dermal side down on the wound bed, sprinkled with additional saline and adjusted. Apligraf can also be fenestrated or meshed to allow for wound drainage.
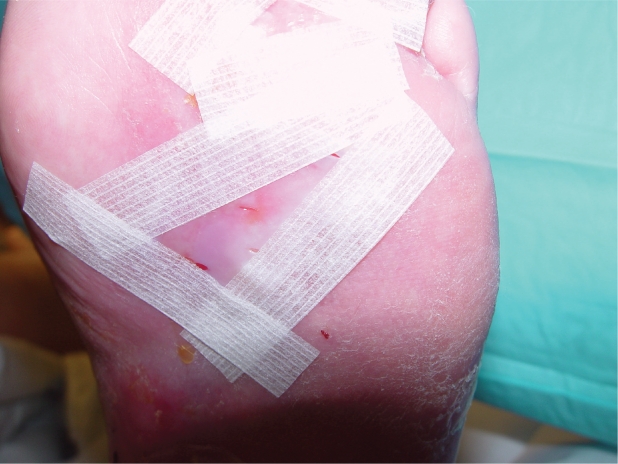
Apligraf should be applied immediately after opening (Figure 1). It is important to keep track of proper product orientation, as it must be placed dermal side down (glossier side) onto the wound bed. The product can be cut to the size of the wound but should overlap the wound margin by 2–3 mm. Every effort should be made for the Apligraf to contact all the wound margins. If a portion of the wound must be left uncovered, it should be in the center of the wound bed, as epithelization most often occurs from the edge of chronic wounds. Air pockets should be removed to ensure contact of Apligraf with the patient’s wound bed. To adhere the Apligraf is important and can be accomplished in a number of ways, such as using steri-strips (Figure 2). Cover the wound with a primary dressing, and in cases of DFU, a secondary foam dressing and maintenance off-loading is essential; while in VLU a compression dressing is added and applied from the toes to the knees.
Figure 1.
Apligraf ® as it is received from the manufacturer. Note that the epidermal side is dull-appearing and facing upwards.
Figure 2.
Apligraf ® that has been fenestrated and applied directly to the wound bed. It is held in place with steri-strips.
The primary dressing is kept in place for 5–7 days, while secondary dressings may be changed more frequently (depending on the dressing type). Reapplication of the product is at the discretion of the physician, but generally is not repeated for 4–6 weeks. This allows time for the wound to respond to stimulating properties of the product. In practice, 1–2 applications of the product usually results in healing. Patients requiring more than three applications to get a healing response will not benefit from additional applications.
Discussion
Apligraf has proven to be a valuable and cost effective treatment of chronic nonhealing VLU and DFU, both conditions that affect a large part of the aging population. Its effectiveness at wound healing has shown to offset the added cost of the product. However, for Apligraf to be used optimally, it requires rapid identification of the nonhealing wound in the setting of good wound care practice. It also requires proper patient selection, and proper use of the product. Apligraf, a living cell therapy, has been an exciting addition to the field of wound healing, and has established itself as an asset to the physician interested in treating a variety of wounds, especially for those wounds that are difficult to heal.
References
- Banta MN, Kirsner RS. Modulating diseased skin with tissue engineering: actinic purpura treated with Apligraf. Dermatol Surg. 2002;28:1103–6. doi: 10.1046/j.1524-4725.2002.02116.x. [DOI] [PubMed] [Google Scholar]
- Brem H, Balledux J, Bloom T, et al. Healing of diabetic foot ulcers and pressure ulcers with human skin equivalent: a new paradigm in wound healing. Arch Surg. 2000;135:627–34. doi: 10.1001/archsurg.135.6.627. [DOI] [PubMed] [Google Scholar]
- Brem H, Kirsner RS, Falanga V. Protocol for the successful treatment of venous ulcers. Am J Surg. 2004;188:1–8. doi: 10.1016/S0002-9610(03)00284-8. [DOI] [PubMed] [Google Scholar]
- Briscoe DM, Dharnidharka VR, Isaacs C, et al. The allogeneic response to cultured human skin equivalent in the hu-PBL-SCID mouse model of skin rejection. Transplantaton. 1999;67:1590–9. doi: 10.1097/00007890-199906270-00014. [DOI] [PubMed] [Google Scholar]
- Cavorsi J, Vicari F, Wirthlin DJ, et al. Best-practice algorithms for the use of a bilayered living cell therapy (Apligraf) in the treatment of lower-extremity ulcers. Wound Repair Regen. 2006;14:102–9. doi: 10.1111/j.1743-6109.2006.00098.x. [DOI] [PubMed] [Google Scholar]
- Culican SM, Custer PL. Repair of cicatricial ectropion in an infant with harlequin ichthyosis using engineered human skin. Am J Ophthalmol. 2002;134:442–3. doi: 10.1016/s0002-9394(02)01567-2. [DOI] [PubMed] [Google Scholar]
- Curran MP, Plosker GL. Bilayered bioengineered skin substitute (Apligraf): a review of its use in the treatment of venous leg ulcers and diabetic foot ulcers. BioDrugs. 2002;16:439–55. doi: 10.2165/00063030-200216060-00005. [DOI] [PubMed] [Google Scholar]
- Davis SC, Martinez L, Kirsner R. The diabetic foot: the importance of biofilms and wound bed preparation. Curr Diab Rep. 2006;6:439–45. doi: 10.1007/s11892-006-0076-x. [DOI] [PubMed] [Google Scholar]
- Dickinson PJ, Carrington AL, Frost GS, et al. Neurovascular disease, antioxidants and glycation in diabetics. Diabetes Metab Res Rev. 2002;18:260–72. doi: 10.1002/dmrr.305. [DOI] [PubMed] [Google Scholar]
- Eaglstein WH, Alvarez OM, Auletta M, et al. Acute excisional wounds treated with a tissue-engineered skin (Apligraf) Dermatol Surg. 1999;25:195–201. doi: 10.1046/j.1524-4725.1999.08186.x. [DOI] [PubMed] [Google Scholar]
- Eaglstein WH, Iriondo M, Laszlo K. A composite skin substitute (Graftskin) for surgical wounds. A clinical experience. Dermatol Surg. 1995;21:839–43. doi: 10.1111/j.1524-4725.1995.tb00709.x. [DOI] [PubMed] [Google Scholar]
- Falabella AF, Schachner LA, Valencia IC, et al. The use of tissue-engineered skin (Apligraf) to treat a newborn with epidermolysis bullosa. Arch Dermatol. 1999;35:1219–22. doi: 10.1001/archderm.135.10.1219. [DOI] [PubMed] [Google Scholar]
- Falabella AF, Valencia IC, Eaglstein WH, et al. Tissue-engineered skin (Apligraf) in the healing of patients with epidermolysis bullosa wounds. Arch Dermatol. 2000;136:1225–30. doi: 10.1001/archderm.136.10.1225. [DOI] [PubMed] [Google Scholar]
- Falanga VJ. Tissue engineering in wound repair. Adv Skin Wound Care, 2000;13:15–19. [PubMed] [Google Scholar]
- Falanga V, Isaacs C, Paquette D, et al. Wounding of bioengineered skin: cellular and molecular aspects after injury. J Invest Dermatol. 2002;119:653–60. doi: 10.1046/j.1523-1747.2002.01865.x. [DOI] [PubMed] [Google Scholar]
- Falanga V, Margolis D, Alvarez O, et al. Rapid healing of veous ulcers and lack of clinical rejection with an allogeneic cultured human skin equivalent. Human Skin Equivalent Investigators Group. Arch Dermatol. 1998;134:293–300. doi: 10.1001/archderm.134.3.293. [DOI] [PubMed] [Google Scholar]
- Fivenson D, Scherschun L. Clinical and economic impact of Apligraf for the treatment of nonhealing venous leg ulcers. Int J Dermatol. 2003;42:960–5. doi: 10.1111/j.1365-4632.2003.02039.x. [DOI] [PubMed] [Google Scholar]
- Flores F, Eaglstein WA, Kirsner RS. Hydroxyurea-induced leg ulcers treated with Apligraf. Ann Intern Med. 2000;132:417–18. doi: 10.7326/0003-4819-132-5-200003070-00017. [DOI] [PubMed] [Google Scholar]
- Gohari S, Gambla C, Healey M, et al. Evaluation of tissue-engineered skin (human skin substitute) and secondary intention healing in the treatment of full thickness wounds after Mohs micrographic or excisional surgery. Dermatol Surg. 2002;28:1107–14. doi: 10.1046/j.1524-4725.2002.02130.x. [DOI] [PubMed] [Google Scholar]
- Goodridge D, Trepman E, Sloan J, et al. Quality of life of adults with unhealed and healed diabetic foot ulcers. Foot Ankle Int. 2006;27:274–80. doi: 10.1177/107110070602700408. [DOI] [PubMed] [Google Scholar]
- Harding K, Cutting K, Price P.2000The cost-effectiveness of wound management protocols of care Br J Nurs 9S6, S8, S10passim [DOI] [PubMed] [Google Scholar]
- Hayes DW, Jr, Webb GE, Mandracchia VJ, et al. Full-thickness burn of the foot: successful treatment with Apligraf. A case report. Clin Podiatr Med Surg. 2001;18:179–88. [PubMed] [Google Scholar]
- Hess CT, Kirsner RS. Orchestrating wound healing: assessing and preparing the wound bed. Adv Skin Wound Care. 2003;16:246–57. doi: 10.1097/00129334-200309000-00015. [DOI] [PubMed] [Google Scholar]
- Hu S, Kirsner RS, Phillips T, et al. Evaluation of Apligraf ® persistence and basement membrane restoration in donor site wounds: a pilot study. Wound Repair Regen. 2006;14:427–33. doi: 10.1111/j.1743-6109.2006.00148.x. [DOI] [PubMed] [Google Scholar]
- Maier JP, Lippitt C, Mooney EK. Use of tissue-engineered skin in the dermal atrophy patient with traumatic avulsion injuries. Ann Plast Surg. 2002;49:67–72. doi: 10.1097/00000637-200207000-00011. [DOI] [PubMed] [Google Scholar]
- Martin LK, Kirsner RS. Ulcers caused by bullous morphea treated with tissue-engineered skin. Int J Dermatol. 2003;42:402–4. doi: 10.1046/j.1365-4362.2003.01763.x. [DOI] [PubMed] [Google Scholar]
- Mathias SD, Prebil LA, Boyko WL, et al. Health-related quality of life in venous leg ulcer patients successfully treated with Apligraf: a pilot study. Ads Skin Wound Care. 2000;13:76–8. [PubMed] [Google Scholar]
- Meerwaldt R, Links TP, Graaff R, et al. Increased accumulation of skin advanced glycation end-products precedes and correlates with clinical manifestation of diabetic neuropathy. Diabetologia. 2005;48:1637–44. doi: 10.1007/s00125-005-1828-x. [DOI] [PubMed] [Google Scholar]
- Muhart M, McFalls S, Kirsner RS, et al. Behavior of tissue-engineered skin: a comparison of a living skin equivalent, autograft, and occlusive dressing in human donor sites. Arch Dermatol. 1999;135:913–18. doi: 10.1001/archderm.135.8.913. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Manzoor J, Rojas A, et al. The longevity of a bilayered skin substitute after application to venous ulcers. Arch Dermatol. 2002;138:1079–81. doi: 10.1001/archderm.138.8.1079. [DOI] [PubMed] [Google Scholar]
- Redekop WK, McDonnell J, Verboom P, et al. The cost effectiveness of Apligraf treatment of Diabetic foot ulcers. Pharmacoeconomics. 2003;21:1171–83. doi: 10.2165/00019053-200321160-00003. [DOI] [PubMed] [Google Scholar]
- Schonfeld WH, Villa KF, Fastenau JM, et al. An economic assessment of Apligraf (Graftskin) for the treatment of hard-to-heal venous leg ulcers. Wound Repair Regen. 2000;8:251–7. doi: 10.1046/j.1524-475x.2000.00251.x. [DOI] [PubMed] [Google Scholar]
- Streit M, Bohlen LM, Braathen LR. Ulcerative sarcoidosis successfully treated with Apligraf. Dermatology. 2001;202:367–70. doi: 10.1159/000051685. [DOI] [PubMed] [Google Scholar]
- Streit M, Braathen LR. Apligraf—a living human skin equivalent for the treatment of chronic wounds. Int J Artif Organs. 2000;23:831–3. [PubMed] [Google Scholar]
- Trent JT, Falabella A, Eaglstein WH, et al. Venous ulcers: pathophysiology and treatment options. Ostomy Wound Manage. 2005;51:35–54. [PubMed] [Google Scholar]
- Veves A, Falanga V, Armstrong DG, et al. Graftskin, a human skin equivalent, is effective in the management of noninfected neuropathic diabetic foot ulcers: a prospective randomized multicenter clinical trial. Diabetes Care. 2001;24:290–5. doi: 10.2337/diacare.24.2.290. [DOI] [PubMed] [Google Scholar]
- Waymack P, Duff RG, Sabolinski M. The effect of a tissue engineered billayered living skin analog over meshed split-thickness autografts on the healing of excised burn wounds. The Apligraf Burn Study Group. Burns. 2000;26:609–19. doi: 10.1016/s0305-4179(00)00017-6. [DOI] [PubMed] [Google Scholar]
- Wu S, Armstrong DG. Risk assessment of the diabetic foot and wound. Int Wound J. 2005;2:17–24. doi: 10.1111/j.1742-4801.2005.00085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]