Abstract
Objective:
To review the pharmacological properties and the available clinical data of full length parathyroid hormone (PTH) in post-menopausal osteoporosis.
Sources:
A MEDLINE search was completed, together with a review of information obtained from the manufacturer and from the medicine regulatory agencies.
Study and data selection:
Studies were selected according to relevance and availability. Relevant information (design, objectives, patients’ characteristics, outcomes, adverse events, dosing, etc) was analyzed.
Results:
Different studies have shown that, when administered intermittently as a subcutaneous injection in the abdomen, PTH increases bone mineral density (BMD) and prevents vertebral fractures. On completion of PTH therapy (up to 24 months), there is evidence that sequential treatment with alendronate is associated with a therapeutic benefit in terms of increase in BMD. Further trials are necessary to determine long-term safety and the role of PTH in combination with other treatments for osteoporosis and the effect of repeated cycles of PTH followed by an anti-catabolic agent. There are currently no completed comparative trials with other osteoporosis treatments.
Conclusions:
Full length PTH, given intermittently as an abdominal subcutaneous injection, appears to be a safe and efficacious treatment option for high risk osteoporosis. More data are needed to determine its specific role in osteoporosis treatment.
Keywords: postmenopausal osteoporosis, anabolic therapy, PTH (1–84)
Introduction
Musculoskeletal diseases, mainly rheumatoid arthritis, osteoarthritis, and osteoporosis are associated among older people with a loss of independence and a need for additional support or admission to residential care. In the European Union (EU), more than 15%–20% of medical consultations in primary care are related to musculoskeletal disease (ECHCPD 2006). Osteoporosis has been defined as “a skeletal disease characterized by compromised bone strength predisposing a person to an increased risk of fracture” (NIH 2001). The incidence and prevalence of osteoporosis is greater in females and increases with age. Future projections in the EU (15 members) show an increase in the annual number of hip fractures from 414 thousand in 2000 to 972 thousand by 2050. Over the same period, the prevalence of vertebral fractures would rise from 23.7 million to 37.3 million (ECHCPD 2006). More than 1.5 million Americans suffer osteoporotic fractures every year (half of them are vertebral fractures) with an annual cost of nearly $14 billion to the US healthcare system (Riggs and Melton 1995; Ray et al 1997). By 2020, that figure is expected to increase to 14 million cases of osteoporosis and over 47 million cases of low bone mass (NOF 2002). This increase in cases could cause the number of hip fractures to double or triple by 2040 (Schneider and Guralnik 1990).
Bone loss produces fewer and thinner bony spicules, also loss of horizontal bracing rods, which leads to unsupported vertebral trabeculae and further weakening of bone integrity (Rosen 2006). Many osteoporotic patients have lost more than 30% of their peak bone mass and continue to have fractures after being treated with antiresorptive drugs. These anticatabolic drugs (bisphosphonates, calcitonin, and selective estrogen receptor modulators) are capable in reducing bone markers and modestly increasing bone density to 8%–10% at three years (Riggs and Parfitt 2005).
Therefore, the availability of effective anabolic drugs that possess the potential to increase bone mass and reduce fracture risk dramatically has meant the onset of a new era in osteoporosis therapy.
The anabolic activity of the parathyroid hormone (PTH) (1–84) and the N-terminal fragment and/or analogues of PTH have been investigated for many years. Parathyroid hormone increases trabecular bone density by stimulating bone formation (Dempster et al 1993; Morley et al 1997). Markers of bone formation and resorption increase during PTH treatment (Hodsman et al 1993), and vertebral bone density increases markedly. (Hodsman et al 1997).
Historical perspective
Parathyroid glands were discovered in the late 19th century. The discussion about their function lasted until the first quarter of the 20th century as reviewed by Boothby in 1921. The death from tetany caused in animals by the removal of all of the parathyroid glands and their separate function from that of the thyroid were already known in 1891 (Gley 1891). Fifteen years later, Erdheim (1906) showed the lack of parathyroid tissue in patients dying from postoperative tetany after thyroid surgery. Not without controversy, MacCallum proposed that the function of the parathyroid glands was the control of blood calcium (MacCallum and Voegtlin 1908), which was finally shown in 1925 by Collip (1925). Another 35 years were needed to purify and characterize the active principle of the glands (Aurbach 1959). As early as 1929, Albright and colleagues (Bauer et al 1929) and later Selye (1932) observed that PTH, in fact yet a “peptide extract”, could also be anabolic to the skeleton, producing increases in bone tissue in animals. Hence, not surprisingly, the first clinical trial of PTH (1–34) for osteoporosis in humans was conducted in 1976 (Reeve et al 1976). However, animal studies showing that the net effect on bone mineral density (BMD) depends on the balance between anabolic and catabolic actions on bone and that this balance is determined by the regimen of administration, appeared six years later (Tam et al 1982; Podbesek et al 1983).
Parathyroid hormone physiology
Parathyroid hormone, together with 1, 25-dihydroxyvitamin D, are the two major hormones modulating calcium and phosphate homeostasis. PTH is most responsible for maintaining serum-ionized calcium concentrations within a narrow range through its actions in the kidney—to stimulate renal tubular calcium reabsorption and the conversion of 25-hydroxyvitamin D to 1,25-dihydroxyvitamin D and thereby the calcium absorption and in the bone—to increase bone turnover (Brown et al 1995; Potts and Juppner 1996). PTH acts in bone to increase the number and activity of osteoblasts and osteoclasts, and increases bone turnover. With sustained elevations in PTH, osteoclastic activity could exceed that of osteoblasts, which results in a net release of calcium from bone and a decrease in BMD. On the contrary, single daily injections of PTH could increase osteoblastic activity preferentially, thereby increasing BMD and bone strength. (Tam et al 1982; Podbesek et al 1983).
The biological actions of PTH are mediated through binding to, at least, two distinct high affinity cell-surface receptors specific for the N-terminal and C-terminal regions of the molecule, both of which are required for normal bone metabolism. The N-terminal portion of the molecule is primarily responsible for the anabolic effects of intermittently administered PTH. These effects are mediated in the osteoblastic cells by multiple cellular pathways and involve the induction of several target genes as insulin-like growth factor-I (IGF-I), Runx2, amphiregulin 1, alpha hydroxylase, transforming growth factor (TGF)-beta, RANKL, or mononuclear phagocyte colony-stimulating factor (M-CSF) (Rosen 2003). The consequence is the enhancement of the recruitment and activation of the osteoblasts increasing bone mass and bone strength. The C-terminal portion of the molecule has antiresorptive activity and is necessary for normal regulation of N-terminal fragment activity: Several C-terminal fragments of PTH, derived both by direct secretion from the parathyroid glands and by postsecretory proteolysis of PTH, normally circulate in blood at concentrations several times higher than that of intact PTH. There is convincing evidence that parathyroid gland secretion of PTH is stimulated by hypocalcemia, whereas secretion of C-terminal fragments is promoted by hypercalcemia. Certain C-terminal PTH fragments inhibit osteoclast formation and bone resorption via direct effects on cells of the hematopoietic lineage through C-terminal fragment receptors, suggesting the presence of a negative feedback loop aimed to restrain release of calcium from bone into blood when it is not needed. Thus, these C-terminal PTH receptors may protect the skeleton by limiting bone resorption and disbalance the equilibrium in favour of net bone formation (Murray et al 2005). Moreover, the C-terminal PTH receptors are extraordinarily common in osteocytes—the most abundant of bone cells—where they regulate gap-junctional communication and cell survival and communication (Divieti et al 2001). These facts could suggest a major role in the crucial mechanosensory function of the osteocyte network. On the other hand, the C-terminal PTH receptors do not recognize any sequences within PTH-related peptide (PTHrp). This could provide target cells with a means to discriminate between PTH and PTHrp (Murray et al 2005). Nevertheless, the C terminal receptor for pTH is still not universally accepted therefore only the PTH1R receptor is considered specific.
Description of the drug
Recombinant human PTH (1–84) is obtained by recombinant DNA technology. PTH is produced as a fusion protein. Post-translational processing involves the cleavage of the leader sequence, leaving the mature protein as a single-chain 84 amino-acids polypeptide (9.4 kDa) whose sequence is identical to that of the full-length native endogenous human PTH. It has no disulfide bonds and no glycosylation sites. The approved dose in the EU is 100 μg administered as a daily subcutaneous injection in the abdomen for a duration of up to 2 years.
Preclinical studies
Pharmacology
The effects of PTH on BMD, strength, and architecture were assessed in two pivotal studies: one in rat and one in monkey (Mosekilde et al 1991; Ejersted et al 1993; Jerome et al 2001; Fox et al 2007). In rat, treatment with PTH for 12 months produced a dose-related gain in cortical and trabecular bone mass, associated with increased bone strength. In monkey, PTH treatment for 39 weeks increased bone formation markers to a greater extent than bone resorption markers; and, at the high doses employed, resulted in significant increases in trabecular bone mass and increases in vertebral and femoral neck bone strength. There was a negative inotropic effect of PTH in vitro that is considered not clinically relevant. Concerns over potential cardiovascular effects of PTH appear to be relevant only when PTH concentrations are sufficiently high to produce a catabolic effect on bone.
Pharmacokinetics
Pharmacokinetics (PK) parameters were determined in rats, dogs, and monkeys in several studies. The lymphatic system does not play an important role in the uptake of PTH from a subcutaneous injection site. In dogs, absorption was much slower than in rats and the tmax was considered to be on average 1.5 h for both sexes. Studies performed with monkeys revealed that the absorption was slower than in rats, and tmax values ranged from 35 minutes to 1.5 h (average 50 minutes) for both sexes (Shrader and Ragucci 2005; White and Ahmad 2005).
Toxicology
Repeat-dose toxicity studies were conducted in the rat, dog, and cynomolgus monkey. The sensitivity of the dog to PTH-induced hypercalcemia and subsequent renal damage precluded the use of this animal as a non-rodent species. Carcinogenicity studies in rats receiving near lifetime daily injections showed dose dependent exaggerated bone formation and an increased incidence of bone tumors, including osteosarcoma. Due to the differences in bone physiology in rats and humans, the clinical relevance of these findings is minor. A 2-year study demonstrated no increase in osteosarcoma risk in rats treated with low-dose PTH (1–84) when compared with controls. Nevertheless, although osteosar-comas have not been observed in clinical trials, it is recommended in that the treatment time does not exceed 24 months. PTH is not recommended during pregnancy.
Clinical studies
Pharmacology
The subcutaneous administration of 100 μg of PTH into the abdomen produces a rapid increase in plasma PTH levels that peaks at 1 to 2 hours after dosing with an average half life of about 1.5 hours. The absolute bioavailability of 100 μg of PTH after subcutaneous administration in the abdomen is 55%. PTH absorption was slightly slower from the thigh than from the abdomen resulting in a plasma PTH profile that has a lower peak level and longer duration with a greater increase in predosing levels and a higher incidence of patients with values above the upper limit of normal. The clearance of PTH is primarily hepatic and, in a lesser extent, renal (Martin et al 1976). A great within-subject variability was observed for maximal concentration and for the area under the 24-hours curve; nevertheless, the variability is reduced at the recommended dose and when administered in the abdomen. No adjustment is necessary in patients with mild to moderate renal impairment (creatinine clearance >30 ml/min) or mild to moderate hepatic impairment (Child-Pugh scale 7–9 points). No differences in PTH pharmacokinetics were detected with regard to age or race. No interaction with other medicinal products seems to be probable (Shrader and Ragucci 2005; White and Ahmad 2005).
Clinical efficacy
The clinical development of PTH in the treatment of post-menopausal osteoporosis included one phase II dose ranging study, one pivotal phase III placebo-controlled study (TOP), an open-label extension study (OLES), and two phase III active control studies (PaTH, and POWER) (Table 1).
Table 1.
Clinical development of full length PTH
| Study | Groups / n |
|---|---|
| A phase II double-blind, placebo-controlled, parallel group study to assess the safety and efficacy of 3 doses of PTH (50, 75, and 100 μg) in the treatment of postmenopausal osteoporosis | Placebo (n = 55)
PTH 50 μg (n = 52) PTH 75 μg (n = 55) PTH 100 μg (n = 55) |
| TOP. An 18-month double-blind, placebo-controlled, phase III trial with a 12-month analysis of the effect of PTH on fracture incidence in women with postmenopausal osteoporosis / patients were eligible to continue in OLES | Placebo (n = 1246)
PTH 100 μg (n = 1286) |
| PaTH. PTH, and ALN in combination for the treatment of osteoporosis | Year 1:
100 μg PTH +placebo (n = 119) Placebo + 10 mg ALN (n = 59) 100 μg PTH + 10 mg ALN (n = 59) |
| POWER. A phase III trial of PTH in women with low bone mass on stable estrogen replacement therapy | HRT + Placebo (n = 90)
HRT + PTH 100 μg (n = 90) |
| PaTH. PTH and ALN in combination for the treatment of osteoporosis | Year 2:
100 μg PTH + Placebo/10 mg ALN (n = 50) Placebo + 10 mg ALN /10 mg ALN (n = 55) 100 μg PTH + 10 mg ALN/ 10 mg ALN (n = 56) 100 μg PTH/10 mg ALN (n = 53) |
| Rittmaster et al 2000. Enhancement of bone mass in osteoporotic women with PTH followed by ALN | 10 mg ALN (n = 66) |
| OLES. An 18-month open-label extension study (OLES) of the safety and efficacy of PTH, in women with postmenopausal osteoporosis who participated in TOP | placebo/PTH 100 μg (n = 900)
PTH 100 μg/ PTH |
| Study. 24 month safety includes patients from TOP and OLE and not just OLE. | 100 μg (n = 781) |
Abbreviations: ALN, alendronate; HRT, hormone replacement therapy; OLES, open-label extension study; PTH, parathyroid hormone;TOP, treatment of osteoporosis with parathyroid hormone.
Dose ranging study
Hodsman and colleagues (2003) have published the results of a randomized, placebo-controlled, double blind, multi-center, parallel-group dose response study. Postmenopausal women (aged 50 to 75 years) with osteoporosis (lumbar spine BMD ≤2.5 SD below the mean of healthy young women) were randomized to receive PTH 50 μg (n = 52), PTH 75 μg (n = 55), PTH 100 μg (n = 55)n or placebo (n = 55) during 12 months. All the subjects received calcium (1000 to 1500 mg/day) and vitamin D3 (400 IU/day). The primary objective was the change from baseline in lumbar spine BMD (L1-L4) with an intention-to-treat (ITT) approach. Lumbar spine BMD increased significantly from the baseline in a time and dose-dependent fashion in all PTH groups but not in the placebo group (0.4% placebo; 1.6% 50 μg; 3.1% 75 μg; 4% 100 μg). The 12-months results confirmed the greater effect in the lumbar spine of the 100 μg dose (+7.8%) compared with the placebo (0.9%), 50 μg (3.1%), and 75 μg (4.9%) dose groups. BMD underestimated the anabolic effect of PTH in lumbar spine because bone area increased significantly. A non-significant decrease (−0.9%) in total hip BMD occurred during the first 6 months with the 100 μg dose, but this trend reversed (+1.6%) during the second 6 months. Overall, no significant change was detected in the femoral neck. The bone turnover markers increased during the first 6 months and maintained subsequently. The compliance was excellent (>95%), and treatment was generally safe and well tolerated. Dose-related incidences of transient hypercalcemia occurred, but only one patient (100 μg group) was withdrawn because of repeated hypercalcemia. No serious adverse events were reported.
Pivotal study
The TOP (Treatment of osteoporosis with parathyroid hormone) study is an international, multi center, randomized (1:1), double-blind, placebo-controlled, parallel group, phase III study to compare the effects of 18 months of treatment with PTH or placebo on the incidence of new and/or worsened thoracic and lumbar vertebral fractures in postmenopausal women with osteoporosis receiving calcium and vitamin D3 supplements. Recently, as well as partial results (Recker et al 2004; Dempster et al 2005; Bogado et al 2005, 2006; Greenspan et al 2005; Miller and Greenspan 2005; Silverman et al 2006), the peer-reviewed article has been published (Greenspan et al 2007).
To be eligible, participants had to be postmenopausal women, 45 years of age or older. If younger than 54 years, BMD had to be ≥3.0 standard deviations (SD) below mean peak bone mass or ≥2.5 SD with at least 1 prevalent vertebral fracture; if ≥55 years of age, BMD had to be ≥2.5 SD below mean pike bone mass or ≥2.0 SD with at least 1 prevalent vertebral fracture. Patients were randomized to receive either 100 μg PTH or placebo administered as a daily subcutaneous injection together with daily oral calcium (700 mg) and vitamin D3 (400 IU) supplements. To reduce the expected potential increases in serum and urine calcium concentrations after PTH administration, protocol-specified management algorithms were predesigned. As a consequence of the findings of osteosarcoma in a rat carcinogenicity study with teriparatide (fragment 1–34 of PTH) the study duration was reduced from 36 months to 18 months.
The primary efficacy endpoint was the incidence of new and/or worsened vertebral fractures (T4 to L4; by means of a semi-quantitative 4-point grading scale -0 to 3- corresponding to no reduction ≥20%, 20%–25%, 25%–40% or >40% reduction in anterior, middle, and/or posterior vertebral height in a blind fashion). Secondary efficacy variables were incidences of vertebral fractures at month 12; hip and wrist; and other clinical fracture; changes in height; changes in BMD assessed by dual X-ray absorptiometry (DXA); bone mineral content (BMC) and bone mineral area (BMA) of the lumbar spine, proximal femur, whole body, and forearm; changes in cortical and trabecular bone compartments assessed by quantitative computed tomography (QCT) and peripheral QCT (pQCT) at lumbar spine, hip, forearm, distal femur, and central tibia (QCT substudy); and by bone histomorphometry at the iliac crest.
The study was powered to detect at least 90% of the times a difference between the treatment groups based on the assumption of a 60% reduction in fracture rate (30% in those with reduced dosage of PTH) and a fracture rate in the control group of 4.6%–5% (drop-out up to 20%). The needed number of patients was 2600. The study was conducted on an intention-to-treat- basis (including all patients randomised and who received ≥1 dose of double-blind study medication) by last observation carried forward methodology.
From 10,749 subjects screened, 2679 subjects were randomized to receive either 100 μg PTH (1286 patients) or placebo (1246 patients) administered as a daily subcutaneous injection in the thigh or in the abdomen. The drop-out rate was greater than 30% (35.8% in the PTH group and 29.6% in the placebo group). Hypercalcemia (4 patients, 1 in placebo group [0.1] and 3 in PTH group [0.2]).and hypercalciuria (39 patients, 15 [1.2%] in the placebo group and 24 [1.9%] in the PTH group) were more common in PTH-treated patients. Serious adverse events appeared in 39 subjects (1.5%); 22 (1.8%) in the control group, and 17 (1.3%) in the PTH group. Twenty-three subjects (1.8%) in the placebo group and 20 (1.6%) in the PTH group were noncompliant.
The study population was comprised mostly of Caucasian (≈85%) and more than two thirds were younger than 74 years without prevalent vertebral fractures (placebo, 81.1%; PTH, 81.6%). The majority of patients with prevalent vertebral fractures had, in fact, only one fracture (75%). The groups were comparable in terms of medical history, concomitant medications, weight, height, body mass index (BMI), prevalent vertebral fractures, and BMD (T-score; Lumbar spine: placebo −2.96, PTH −3.02) although the duration of bisphosphonate treatment was longer in the placebo group.
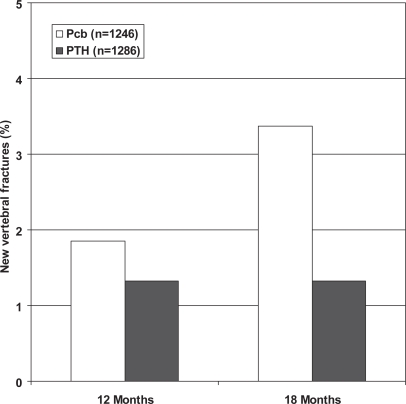
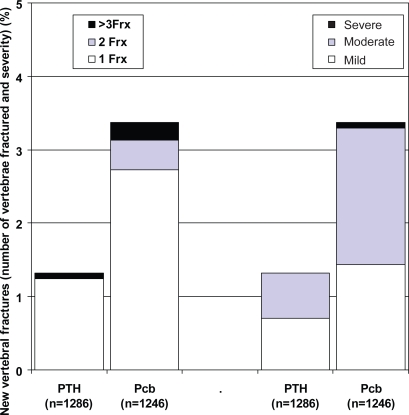
At 18 months, there was approximately a 60% reduction in the risk of a new vertebral fracture in the PTH treated subjects (relative risk ratio: 0.42 [95% confidence interval: 0.24, 0.72], p = 0.001 in the ITT analysis) (Figure 1 and 2). Only one patient had more than one vertebral fracture and another had a worsening in the severity of a prevalent vertebral fracture. The difference was not significant at month 12 (p = 0.290). The incidence of nonvertebral fractures did not show significant differences (Placebo 73/1246 (5.86%), PTH 71/1286 (5.52%); p = 0.714) as occurred when non-vertebral fractures were analysed according to localization (hip, wrist, other).
Figure 1.
New vertebral fractures in PTH and Pcb groups. TOP study.
Abbreviations: Pcb, placebo; PTH, parathyroid hormone; TOP, treatment of osteoporosis with parathyroid hormone.
Figure 2.
Number (left) and severity (right) of new fractures in PTH and Pcb groups. TOP study.
Abbreviations: Pcb, placebo; PTH, parathyroid hormone; TOP, treatment of osteoporosis with parathyroid hormone.
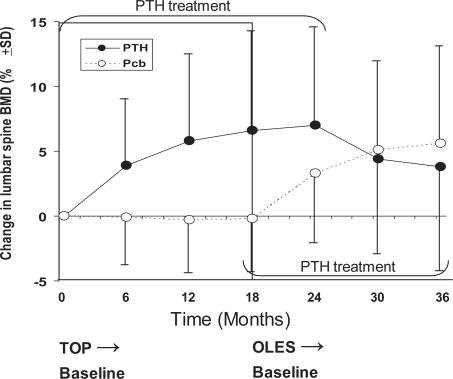
PTH treatment induced a sustained increase in lumbar spine BMD (+6.5% at 18 months) whereas the BMD for the placebo group remained at or below baseline throughout the study (−0.3% at 18 months, p < 0.001) (Figure 3). The changes in BMD for femoral sites of measurement were similar except for an initial decrease in total hip BMD at 6 months followed by an increase at 12 and 18 months. One hundred and twenty-two patients were randomized in an ancillary study to assess changes in areal and volumetric cortical and trabecular bone volume evaluated by QCT and pQCT (62 in the placebo group and 60 in PTH group). Eighty-two patients (46 in the placebo group and 36 in the PTH group) completed the study. The volumetric trabecular BMD (vTbBMD) evaluated in the lumbar vertebra L3 increased at month 3 and 12 (+54.2%), after which declined to +38.3% at month 18. The changes in placebo group were +19% at month 3 with a subsequent decrease reaching baseline levels by month 18. The change in vTbBMD was significant between PTH and placebo group at months 12 and 18.
Figure 3.
Changes in lumbar spine BMD (%) during the TOP study and its extension (OLES).
Abbreviations: BMD, bone mineral density; OLES, open-label extension study; TOP, treatment of osteoporosis with parathyroid hormone.
The results of the bone biopsies from 16 women have been published as abstracts (Recker et al 2004; Dempster et al 2005) and as rapid original article (Fox et al 2005). Histomorphometricaly and in microcomputed tomography, trabecular bone showed increased bone volume (+48%), trabecular number (+21%) and bone formation rate, with reduced trabecular separation what improves connectivity and bone strength compared with placebo.
Open-label extension study
The women who had completed or had prematurely discontinued from TOP study were invited to enter in an OLES up to a maximum of 24 months. The primary objective was to evaluate the safety of continued administration of PTH during this period. Other objectives were to evaluate the changes in BMD (on ITT basis), the incidence of vertebral and nonvertebral clinical fractures, the changes in height, biochemical markers of bone turnover, and bone histomorphometry; quality of life, treatment satisfaction, assessments of dietary calcium intake, and immunological responses to PTH or Escherichia coli proteins were also measured as were the duration of the effect on BMD after discontinuation of PTH treatment.
All patients received PTH given as a daily 100 μg subcutaneous injection (the patients whose study drug schedule had been reduced to one 100 μg injection every second day as a consequence of hypercalcemia or hypercalciuria received the same dose as they were following at the end of TOP study). All patients also received oral calcium (700 mg) and vitamin D3 (400 IU) supplements unless they had discontinued calcium supplements in the TOP study.
A total of 1681 patients received open-label PTH. Treatment with PTH was discontinued when a patient reached a maximum total exposure of 24 months in TOP and OLES combined (Figure 3). Approximately 62% of the PTH/PTH treatment group completed 24 months of treatment, and 29% of the placebo/PTH group completed 18 months of treatment. As expected, the baseline lumbar spine BMD for the OLES study was lower in the patients receiving placebo in the previous TOP study. Nevertheless, there were no differences between the treatment groups in baseline total hip and femoral neck T-score. The changes in lumbar spine BMD during TOP and OLES studies are shown in Figure 3. The discontinuation of PTH treatment after 24 months of total exposure was associated to a decrease in BMD although the mean final BMD was higher than the baseline value in the TOP study. In the subset of patients with available measurements for the forearm BMD, a total loss of 6.1% was observed. During the OLES study there were six new fractures (and one worsened fracture) in the placebo/PTH group and 2 new fractures (and 4 worsened fractures) in those treated with PTH for 24 months. Nonvertebral clinical fractures were more common in the placebo/PTH group (29 patients) than in the PTH/PTH group (19 patients).
Combination study and sequential study
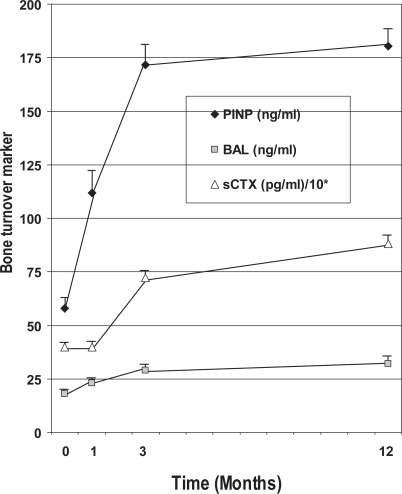
The efficacy of PTH and alendronate (ALN) as mono-therapy and in combination for the treatment of postmenopausal osteoporosis was evaluated in a randomized, placebo-controlled, 2 year, multicenter, double-blind, two periods factorial trial (Black et al 2003, 2005; Bauer et al 2006) The inclusion criteria were postmenopausal women (aged 55 to 85 years), with BMD (T-score) < −2.5 at the lumbar spine or proximal femur or > −2.0 plus, at least, one additional risk factor for fracture. 238 women were randomized to receive during 12 months PTH 100 μg sc/daily, ALN 10 mg/day, or a combination of both (period I). in the second year of the study (period II), women in the original PTH group were randomly assigned to receive either ALN or matching placebo (Pcb) in a double-blind fashion, and women in the other two groups (combination group and ALN group) received open ALN. All participants received daily calcium (500 mg/day) and vitamin D (400 IU/day) supplements. During the first year, the BMD at the spine increased in all the treatment groups (PTH, 6.3%; PTH + ALN, 6.1%; ALN, 4.6%), without significant differences in the increase between the PTH group and the combination-therapy group. Increases in BMD at the total hip were 1.9%, 0.3%, and 2.8% for the 3 groups, respectively. The volumetric density of trabecular bone at the spine increased more with PTH alone (about twice) than with combination therapy or alendronate alone. In the PTH group, the cortical volume at the hip increased, the volumetric density decreased, and the bone mineral content was unchanged, which is consistent with the previously demonstrated actions of PTH (induction of new bone not fully mineralized and increased cortical porosity) (Dempster et al 2001; Misof et al 2003). Bone formation markers increased markedly in the PTH group, but not in the combination therapy group. Bone resorption decreased in the combination therapy group and the alendronate group. No relationship between baseline turnover and 1-year change in DXA and QCT BMD were detected in women treated with PTH alone. As expected in an anabolic therapy, bone turnover markers increased significantly (Figure 4). The patients with greater increases in the short term (1 or 3 months) bone turnover, particularly in the formation marker PINP (an extension peptide derived from procollagen molecule), showed greater increases in areal BMD. The relationship was even greater with volumetric hip and spine BMD assessed by QCT, Each standard deviation increase in the 3-month change of PINP was associated with a 21% greater increase in QCT spine trabecular BMD (Bauer et al 2006).
Figure 4.
Changes in bone turnover markers (formation: N-propeptide of type I collagen [PINP], bone specific alkaline phosphatase [BALP]; resorption: serum C-terminal telopeptide of type I collagen [sCTX]) during one year of therapy with PTH. TOP study.
Abbreviations: PTH, parathyroid hormone; TOP, treatment of osteoporosis with parathyroid hormone.
PTH for one year followed by one year of ALN treatment significantly increased lumbar spine areal BMD by 12% (p < 0.001), compared with PTH + ALN, followed by ALN (8% and 11%), and ALN for two years (8% and 6%). The difference was particularly evident for BMD in trabecular bone at the spine on QCT (+31% in the PTH followed by ALN group compared with +14% in the PTH followed by placebo group, p < 0.001). During year two, compared with the PTH followed by Pcb group, patients in the PTH followed by ALN group showed a significant further increase in lumbar spine and hip BMD (4.9% and 3.6%, respectively). Trabecular BMD at the spine and hip was also significantly increased in this group. The PTH followed by Pcb group experienced a significant decrease in lumbar spine BMD (−1.7%) without significant changes in hip or radius BMD. Trabecular BMD was also significantly decreased in the spine and hip. There was no significant change in cortical volumetric BMD in either the PTH followed by ALN or PTH followed by Pcb groups. However, there were increases in both cortical BMD (4.6%) and cortical volume (4.4%) in the PTH followed by ALN group. (Black et al 2005).
Other studies
Rittmaster and colleagues (2000) enrolled a subset of the patients originally included in the dose ranking study in a 1-year open-label extension of treatment with daily ALN (10 mg). 66/75 patients completed the study. There were statistically significant differences for lumbar spinal BMD mean percentage change from baseline in favour of PTH 75 μg and 100 μg followed by ALN compared with placebo followed by ALN. For the approved dose the increases in lumbar spine, hip, and total body BMD were 14.6%, 4.5%, and 6.1%, respectively.
The POWER Study is a phase III, randomized, double-blind, placebo-controlled, parallel-group, multicenter study, investigating the effect of PTH in women with low bone mass on stable estrogen replacement therapy. The results are yet unpublished except in abstract form (Fogelman et al 2005). Almost all patients discontinued the study treatment due to the published data on the risk of long-term hormone replacement therapy (HRT), and the study was terminated after all patients had received at least 18 months of study drug treatment. 180 patients were randomized (90 HRT; 90 HRT + PTH). The increase for lumbar spine BMD was significantly greater in the PTH group compared with the placebo group (7.1% vs 1.1%, p < 0.001). For the femoral neck, there was also a statistically significant increase in BMD (2.3% vs 0.5%, p < 0.05). This study confirmed the known effect of PTH on BMD and bone turnover parameters.
Safety
More than 3000 patients have received PTH in the main studies. Overall, PTH was well tolerated during the clinical trials. The most common treatment-related adverse events in the clinical trials and with greater incidence in PTH treated subjects were hypercalciuria (44% vs 21%), headache (29% vs 23%), hypercalcemia (27% vs 4%), nausea (22% vs 7%), and dizziness (12% vs 8%). Arthralgia and back pain had a similar incidence in PTH and placebo groups (22% and 20%, respectively). The proportion of patients with injection site-related adverse events was also similar in both groups (Table 2). The most frequent serious adverse events in patients treated with PTH were hypercalcemia and events related to anemia.
Table 2.
Adverse event experienced in the clinical development of full length PTH
| Very common (>1/10) n (%) | Common (>1/100 and <1/10) n (%) | Uncommon (>1/1000 and <1/100) n (%) | |
|---|---|---|---|
| Renal and urinary disorders | |||
| Hypercalcuria | 39.3 | ||
| Urine calcium/creatinine ratio increased | 2.9 | ||
| Urine calcium increased | 2.2 | ||
| Metabolism and nutrition disorders | |||
| Hypercalcemia | 25.3 | ||
| Blood calcium increased | 3.1 | ||
| Blood alkaline phosphatase increased | 0.8 | ||
| Anorexia | 0.6 | ||
| Blood uric acid increased | 0.6 | ||
| Gastrointestinal disorders | |||
| Nausea | 13.5 | ||
| Vomiting | 2.5 | ||
| Constipation | 1.8 | ||
| Dyspepsia | 1.3 | ||
| Diarrhea | 1.0 | ||
| Abdominal pain | 0.8 | ||
| Nervous system disorders | |||
| Headache | 9.3 | ||
| Dizziness | 3.9 | ||
| Dysgeusia | 0.8 | ||
| Parosmia | 0.7 | ||
| General disorders and administration site conditions | |||
| Injection site erythema | 2.6 | ||
| Fatigue | 1.8 | ||
| Asthenia | 1.2 | ||
| Injection site irritation | 0.9 | ||
| Musculoskeletal, connective tissue, and bone disorders | |||
| Muscle cramp | 1.1 | ||
| Pain in extremity | 1.1 | ||
| Back pain | 1.0 | ||
| Cardiac disorders | |||
| Palpitations | 1.0 | ||
| Infections and infestations | |||
| Influenza | 0.5 | ||
Abbreviations: PTH, parathyroid hormone.
In most cases, hypercalcemia was transient and was reported most frequently in the first 3 months of treatment. It was managed during the clinical programme by monitoring laboratory values and the use of a prespecified management algorithm involving withdrawal of calcium and vitamin D supplementation and, if necessary, PTH administration every other day. Considering the mechanism of action of PTH, its combined use with cardiac glucosides may predispose patients to digitalis toxicity if hypercalcemia develops. Renal calculi were more frequent in the PTH group than in the placebo group. Caution is therefore recommended in patients treated with cardiac glucosides and those with active or previous urolithiasis. A significant elevation in uric acid was also detected in patients treated with PTH but the incidence of gout, arthralgia, and nephrolithiasis were similar in PTH and placebo groups.
Discussion
The antifracture efficacy of PTH has been documented in postmenopausal women with low BMD, with or without prevalent vertebral fractures. Patients treated with PTH had a 61% relative risk reduction of a new vertebral fracture at month 18 compared with patients in the placebo group although a reduction in the incidence of hip fracture—a secondary efficacy endpoint in the pivotal study—has not been shown.
BMD increased in PTH-treated patients. This increase could be considered as lower than expected in relation to teriparatide (Neer et al 2001), the other bone building treatment, which shares N-terminal portion of the molecule primarily responsible for the anabolic effects. Nevertheless, the efficacy in reducing vertebral fractures was similar to other therapies with a similar mechanism of action.
As with other treatments for osteoporosis, although the relative risk reduction with PTH was similar in patients with and without prevalent fractures, the absolute risk reduction was much higher in patients with prevalent vertebral fractures. PTH has its greater antifracture efficacy when administered in the abdomen and in high risk patients (BMD (T-score) at the lumbar spine ≤ −3, prevalent vertebral fracture, advancing age, additional risk factors for osteoporosis), as stated by the European Medicines Agency in the European Public Assessment Report (EMEA 2006). On completion of PTH therapy (up to 24 months), there is evidence that sequential treatment with ALN (by extrapolation, with bisphosphonates) was associated with a therapeutic benefit in terms of increase in BMD.
From the safety point of view, PTH was well tolerated during clinical trials. Nevertheless, patients treated with 100 μg PTH more frequently reported hypercalciuria, hypercalcemia, and nausea: adverse events that were considered related to the treatment. No dose adjustment is necessary in patients with mild to moderate renal impairment (creatinine clearance 30 to 80 ml/min). There is no data available in patients with severe renal impairment and should therefore not be used in these patients. No dose adjustment is needed for patients with mild or moderate hepatic impairment (total score of 7 to 9 on the Child-Pugh scale). PTH should therefore not be used in patients with severe hepatic impairment. Dose adjustment based upon age is not required. PTH is also contraindicated in patients with hypersensitivity to PTH or to any of the excipients, those who have previously received radiation therapy to the skeleton, with pre-existing hypercalcemia and other disturbances in the phosphocalcic metabolism, with metabolic bone diseases other than primary osteoporosis (including hyperparathyroidism and Paget’s disease of the bone) and with unexplained elevations of bone-specific alkaline phosphatase (EMEA 2006).
At present time, several points need to be addressed. The potential effects of the C-terminal portion of the molecule and the effects on wrist and nonaxial fractures should be studied. The same is valid for the long-term safety (more than 2 years) and the possibility of repeated cycles of PTH followed by a bisphosphonate. More bone histomorphometry data are also needed. The investigation of the clinical profile of patients suffering hypercalciuria and hypercalcemia, also the optimal pattern of use in this subset of patients have to be investigated. In the meantime, as stated in the product information by the European Medicines Agency, patients initiated on PTH therapy should be monitored at months 1, 3, and 6 for elevated levels of serum (20 to 24 hours after the administration of PTH) and/or urinary calcium. Monitoring beyond 6 months is not recommended for patients with normal total serum calcium at 6 months. In patients with persistent elevated serum calcium (after excluding underlying disease. ie, hyperparathyroidism), calcium and vitamin D supplementation should be withdrawn and, if necessary, PTH should be administered as 100 μg every other day. If elevated calcium levels continue, PTH therapy should be stopped and the patient monitored.
In conclusion, full length PTH is the more recent incorporation into the therapeutic arsenal available for the therapy of osteoporosis. Full length PTH provides, together with significant increases in BMD and bone turnover markers, an important reduction in the risk of new vertebral fractures in postmenopausal women with high risk osteoporosis.
References
- Aurbach GD. Isolation of parathyroid hormone after extraction with phenol. J Biol Chem. 1959;234:3179–81. [PubMed] [Google Scholar]
- Bauer DC, Garnero P, Bilezikian JP, et al. Short-term changes in bone turnover markers and bone mineral density response to parathyroid hormone in postmenopausal women with osteoporosis. J Clin Endocrinol Metab. 2006;91:1370–5. doi: 10.1210/jc.2005-1712. [DOI] [PubMed] [Google Scholar]
- Bauer W, Aub JC, Albright F. Studies of calcium phosphorus metabolism: study of bone trabeculae as ready available reserve supply of calcium. J Exp Med. 1929;49:145–62. doi: 10.1084/jem.49.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black DM, Bilezikian JP, Ensrud KE, et al. One year of alendronate after one year of parathyroid hormone (1–84) for osteoporosis. N Engl J Med. 2005;353:555–65. doi: 10.1056/NEJMoa050336. [DOI] [PubMed] [Google Scholar]
- Black DM, Greenspan SL, Ensrud KE, et al. The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med. 2003;349:1207–15. doi: 10.1056/NEJMoa031975. [DOI] [PubMed] [Google Scholar]
- Bogado CE, Lang TF, Zanchetta JR, et al. Longitudinal changes in trabecular and cortical bmd and indices of bone strength assessed by quantitative computed tomography at the hip of postmenopausal osteoporotic women treated with parathyroid hormone (1–84) for 18 months. J Bone Miner Res. 2006;21(Suppl 1):S296. [Google Scholar]
- Bogado CE, Zanchetta JR, Mango A, et al. Effects of parathyroid hormone 1–84 on cortical and trabecular bone at the hip as assessed by QCT: results at 18 months from the TOP study. J Bone Miner Res. 2005;36(Suppl 2):S22. [Google Scholar]
- Boothby WM. The parathyroid glands: a review of the literature. Endocrinology. 1921;5:403–40. [Google Scholar]
- Brown EM, Pollak M, Seidman CE, et al. Calcium-ion-sensing cell-surface receptors. N Engl J Med. 1995;333:234–40. doi: 10.1056/NEJM199507273330407. [DOI] [PubMed] [Google Scholar]
- Collip JB. The extraction of a parathyroid hormone which will prevent or control parathyroid tetany and which regulates the level of blood calcium. J Biol Chem. 1925;63:395–438. [Google Scholar]
- Dempster DW, Cosman F, Kurland ES, et al. Effects of daily treatment with parathyroid hormone on bone microarchitecture and turnover in patients with osteoporosis: a paired biopsy study. J Bone Miner Res. 2001;16:1846–53. doi: 10.1359/jbmr.2001.16.10.1846. [DOI] [PubMed] [Google Scholar]
- Dempster DW, Cosman F, Parisien M, et al. Anabolic actions of parathyroid hormone on bone. Endocr Rev. 1993;14:690–709. doi: 10.1210/edrv-14-6-690. [DOI] [PubMed] [Google Scholar]
- Dempster DW, Moreau IA, Varela A, et al. Treatment of postmenopausal osteoporotic women with parathyroid hormone 1–84 for 18 months improves trabecular bone architecture: a study of iliac crest biopsies using microcomputed tomography. J Bone Miner Res. 2005;36(suppl 2):S98. [Google Scholar]
- Divieti P, Inomata N, Chapin K, et al. Receptors for the carboxyl-terminal region of PTH(1–84) are highly expressed in osteocytic cells. Endocrinology. 2001;142:916–25. doi: 10.1210/endo.142.2.7955. [DOI] [PubMed] [Google Scholar]
- [ECHCPD] European Commission Health & Consumer Protection Directorate 2006. Dissemination of health information and data [online]. Accessed 14 November 2006. URL: http://ec.europa.eu/health/ph_information/dissemination/diseases/musculo_en.htm#rate
- Ejersted C, Andreassen TT, Oxlund H, et al. Human parathyroid hormone (1–34) and (1–84) increase the mechanical strength and thickness of cortical bone in rats. J Bone Miner Res. 1993;8:1097–101. doi: 10.1002/jbmr.5650080910. [DOI] [PubMed] [Google Scholar]
- Erdheim J. Ueber tetania parathyreopriva. Weiner Klinishe Wachen-schrift. 1906;19:716–17. [Google Scholar]
- Fogelman I, Christiansen C, Fordham J, et al. Safety and efficacy of PTH (1–84) at 18 and 24 months in women with postmenopausal osteoporosis receiving hormone therapy: results from the POWER study. J Bone Miner Res. 2005;36(suppl 2):S21. [Google Scholar]
- Fox J, Miller MA, Newman MK, et al. Treatment of skeletally-mature ovariectomized rhesus monkeys with parathyroid hormone 1–84 for 16 months increases bone formation and density and improves trabecular architecture and biomechanical properties at the lumbar spine. J Bone Mineral Res. 2007;22:260–73. doi: 10.1359/jbmr.061101. [DOI] [PubMed] [Google Scholar]
- Fox J, Miller MA, Recker RR, et al. Treatment of postmenopausal osteoporotic women with parathyroid hormone 1–84 for 18 months increases cancellous bone formation and improves cancellous architecture: A study of iliac crest biopsies using histomorphometry and micro computed tomography. J Musculoskelet Neuronal Interact. 2005;5:356–7. [PubMed] [Google Scholar]
- Gley E. Sur la toxicité des urines des chiens thyroidectomisés: contribution à l’étude des fonctions du corps thyroide. Comptes Rendus de la Société de Biologie. 1891;3:366–8. [Google Scholar]
- Greenspan SL, Bone HG, Marriott TB, et al. Preventing the first vertebral fracture in postmenopausal women with low bone mass using PTH (1–84): results from the TOP study. J Bone Miner Res. 2005;36(suppl 2):S56. [Google Scholar]
- Greenspan SL, Bone HG, Ettinger MP, et al. Effect of recombinant human parathyroid hormona (1–84) on vertebral fracture and bone mineral density in postmenopausal women with osteoporosis. Ann Intern Med. 2007;146:326–39. doi: 10.7326/0003-4819-146-5-200703060-00005. [DOI] [PubMed] [Google Scholar]
- Hodsman AB, Fraher LJ, Ostbye T, et al. An evaluation of several biochemical markers for bone formation and resorption in a protocol utilizing cyclical parathyroid hormone and calcitonin therapy for osteoporosis. J Clin Invest. 1993;91:1138–48. doi: 10.1172/JCI116273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodsman AB, Fraher LJ, Watson PH, et al. A randomized controlled trial to compare the efficacy of cyclical parathyroid hormone versus cyclical parathyroid hormone and sequential calcitonin to improve bone mass in postmenopausal women with osteoporosis. J Clin Endocrinol Metab. 1997;82:620–8. doi: 10.1210/jcem.82.2.3762. [DOI] [PubMed] [Google Scholar]
- Hodsman AB, Hanley DA, Ettinger MP, et al. Efficacy and safety of human parathyroid hormone (1–84) in increasing bone mineral density in postmenopausal osteoporosis. J Clin Endocrinol Metab. 2003;88:5212–20. doi: 10.1210/jc.2003-030768. [DOI] [PubMed] [Google Scholar]
- Jerome CP, Burr DB, Van Bibber T, et al. Treatment with human parathyroid hormone (1–34) for 18 months increases cancellous bone volume and improves trabecular architecture in ovariectomized cynomolgus monkeys (Macaca fascicularis) Bone. 2001;28:150–9. doi: 10.1016/s8756-3282(00)00430-0. [DOI] [PubMed] [Google Scholar]
- MacCallum WG, Voegtlin C. On the relation of the parathyroid to calcium metabolism and the nature of tetany. Johns Hopkins Hospital Bulletin. 1908;19:91–2. doi: 10.1111/j.1753-4887.1976.tb05769.x. [DOI] [PubMed] [Google Scholar]
- Martin K, Hruska K, Greenwalt A, et al. Selective uptake of intact parathyroid hormone by the liver: differences between hepatic and renal uptake. J Clin Invest. 1976;58:781–8. doi: 10.1172/JCI108529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller PD, Greenspan S. PTH (1–84) prevents vertebral fracture in postmenopausal women with higher fracture risk: results from the TOP study. Osteoporosis Int. 2005;16(S4):S32. [Google Scholar]
- Misof BM, Roschger P, Cosman F, et al. Effects of intermittent parathyroid hormone administration on bone mineralization density in iliac crest biopsies from patients with osteoporosis: a paired study before and after treatment. J Clin Endocrinol Metab. 2003;88:1150–6. doi: 10.1210/jc.2002-021988. [DOI] [PubMed] [Google Scholar]
- Morley P, Whitfield JF, Willick GE. Anabolic effects of parathyroid hormone on bone. Trends Endocrinol Metab. 1997;8:225–31. doi: 10.1016/s1043-2760(97)00060-x. [DOI] [PubMed] [Google Scholar]
- Mosekilde L, Sogaard CH, Danielsen CC, et al. The anabolic effects of human parathyroid hormone (hPTH) on rat vertebral body mass are also reflected in the quality of bone, assessed by biomechanical testing: a comparison study between hPTH-(1–34) and hPTH-(1–84) Endocrinology. 1991;129:421–8. doi: 10.1210/endo-129-1-421. [DOI] [PubMed] [Google Scholar]
- Murray TM, Rao LG, Divieti P, et al. Parathyroid hormone secretion and action: evidence for discrete receptors for the carboxyl-terminal region and related biological actions of carboxyl- terminal ligand. Endocr Rev. 2005;26:78–113. doi: 10.1210/er.2003-0024. [DOI] [PubMed] [Google Scholar]
- Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–41. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- [NIH] NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285:785–95. doi: 10.1001/jama.285.6.785. [DOI] [PubMed] [Google Scholar]
- [NOF] National Osteoporosis Foundation 2002. America’s bone health: the state of osteoporosis and low bone mass in our nation. 2002 [online]. Accessed on 20 November 2006. URL: http://www.nof.org/advocacy/prevalence/
- Podbesek R, Edouard C, Meunier PJ, et al. Effects of two treatment regimes with synthetic human parathyroid hormone fragment on bone formation and the tissue balance of trabecular bone in greyhounds. Endocrinology. 1983;112:1000–6. doi: 10.1210/endo-112-3-1000. [DOI] [PubMed] [Google Scholar]
- Potts JT, Juppner H. Parathyroid hormone: molecular biology and regulation. In: Bilezikian JP, Raisz LG, Rodan GA, editors. Principles of bone biology. New York: Academic Pr; 1996. pp. 325–41. [Google Scholar]
- [EMEA] European Medicines Agency 2006. Authorised medicines for human use—Preotact H-659-00-00 product information [online]. Accessed 18 November 2006. URL: http://www.emea.eu.int/human-docs/Humans/EPAR/preotact/preotact.htm
- Ray NF, Chan JK, Thamer M, et al. Medical expenditures for the treatment of osteoporotic fractures in the United States in 1995: report from the National Osteoporosis Foundation. J Bone Miner Res. 1997;12:24–35. doi: 10.1359/jbmr.1997.12.1.24. [DOI] [PubMed] [Google Scholar]
- Recker RR, Bare SP, Miller MA, et al. Treatment of osteoporotic women with parathyroid hormone 1–84 for 18 months improves cancellous bone formation and structure: a bone biopsy study. J Bone Miner Res. 2004;19(suppl 1):S97. [Google Scholar]
- Reeve J, Hesp R, Williams D, et al. Anabolic effect of low doses of a fragment of human parathyroid hormone on the skeleton in postmenopausal osteoporosis. Lancet. 1976;1(7968):1035–8. doi: 10.1016/s0140-6736(76)92216-9. [DOI] [PubMed] [Google Scholar]
- Riggs BL, Melton LJ., 3rd The worldwide problem of osteoporosis: insights afforded by epidemiology. Bone. 1995;17:505S–11S. doi: 10.1016/8756-3282(95)00258-4. [DOI] [PubMed] [Google Scholar]
- Riggs BL, Parfitt AM. Drugs used to treat osteoporosis: the critical need for a uniform nomenclature based on their action on bone remodeling. J Bone Miner Res. 2005;20:177–84. doi: 10.1359/JBMR.041114. [DOI] [PubMed] [Google Scholar]
- Rittmaster RS, Bolognese M, Ettinger MP, et al. Enhancement of bone mass in osteoporotic women with parathyroid hormone followed by alendronate. J Clin Endocrinol Metab. 2000;85:2129–34. doi: 10.1210/jcem.85.6.6614. [DOI] [PubMed] [Google Scholar]
- Rosen CJ. The cellular and clinical parameters of anabolic therapy for osteoporosis. Crit Rev Eukaryot Gene Expr. 2003;13:25–38. doi: 10.1615/critreveukaryotgeneexpr.v13.i1.30. [DOI] [PubMed] [Google Scholar]
- Rosen HN.2006. Epidemiology and causes of osteoporosis [online]. In: UpToDate, Rose BD (ed). Accessed 20 November 2006. URL: http://patients.uptodate.com/topic.asp?file=minmetab/15864
- Schneider EL, Guralnik JM. The aging of America. Impact on health care costs. JAMA. 1990;263:2335–40. [PubMed] [Google Scholar]
- Selye H. On the stimulation of new bone-formation with parathyroid extract, irradiated ergosterol. Endocrinology. 1932;16:547–58. [Google Scholar]
- Shrader SP, Ragucci KR. Parathyroid hormone (1–84) and treatment of osteoporosis. Ann Pharmacother. 2005;39:1511–16. doi: 10.1345/aph.1G146. [DOI] [PubMed] [Google Scholar]
- Silverman SL, Miller PD, Mathisen A, et al. Effect of parathyroid hormone (1–84) in reducing new vertebral fractures regardless of baseline vertebral fracture status in patients with a wide range of clinical risk factors. J Bone Miner Res. 2006;21(Suppl 1):S173. [Google Scholar]
- Tam CS, Heersche JN, Murray TM, et al. Parathyroid hormone stimulates the bone apposition rate independently of its resorptive action: differential effects of intermittent and continuous administration. Endocrinology. 1982;110:506–12. doi: 10.1210/endo-110-2-506. [DOI] [PubMed] [Google Scholar]
- White H, Ahmad A. PREOS NPS (Allelix/Nycomed) Curr Opin Investig Drugs. 2005;6:1057–66. [PubMed] [Google Scholar]