Abstract
Alzheimer’s disease is the most common form of dementia in industrialized countries. In the European Union, about 54% of dementia cases are believed to be due to Alzheimer’s disease. The condition is an age-related neurodegenerative disorder characterized by multiple cognitive deficiencies, including loss of memory, judgment, and comprehension. These manifestations are accompanied by behavioral and mood disturbances. Although no cure has yet been discovered for Alzheimer’s disease, symptomatic therapies are now widely available and offer significant relief to patients and benefits to caregivers in terms of reduced care burden. At the start of the 21st century, health technology assessments recommended three agents for the symptomatic treatment of mild to moderate Alzheimer disease: rivastigmine, donepezil, and galantamine. Rivastigmine (Exelon®, Novartis Basel—Switzerland) is a slowly reversible inhibitor of acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE), while donepezil (Aricept®, Pfizer, New York, USA) and galantamine (Reminyl®, Janssen, New Jersey,USA) show no functional inhibition of BuChE, and are considered AChE-selective, rapidly-reversible inhibitors. The efficacy of all three agents has been evaluated in large, double-blind, placebo-controlled clinical trials of up to 6 months’ duration. Rivastigmine treatment in mild to moderate Alzheimer’s disease improves cognition, activities of daily living, and global function.
Keywords: acetylcholinesterase inhibitors, Alzheimer’s disease, donepezil, galantamine, rivastigmine
Alzheimer’s disease: clinical features
The symptoms of Alzheimer’s disease (AD) are directly related to the degeneration of cholinergic neurons of the cortex and hippocampus, which results in lower levels of acetylcholine and a reduction of cholinergic transmission (Davies and Maloney 1976). This cholinergic hypothesis led to the development of cholinesterase inhibitors, which act by inhibiting the two enzymes responsible for the degradation of acetylcholine: acetylcholinesterase (AChE) or butyrylcholinesterase (BuChE).
Cholinesterase (ChE) inhibitors are currently a symptomatic intervention for AD. Their clinical benefit is thought to derive primarily from an increase in synaptic acetylcholine (ACh) levels, leading to enhanced cholinergic neurotransmission which improves activities of daily living (ADL), behavior, and cognitive performance.
At the start of the 21st century, health technology assessments recommended three agents for the symptomatic treatment of mild to moderate AD: rivastigmine, donepezil, and galantamine (Clegg et al 2001; Doody et al 2001). Rivastigmine (Exelon®, Novartis, Basel, Switzerland) is a slowly reversible inhibitor of AChE and BuChE, while donepezil (Aricept®, Pfizer, New York, USA) and galantamine (Reminyl®, Janssen, New Jersey, USA) show no functional inhibition of BuChE, and are considered AChE-selective, rapidly-reversible inhibitors (Weinstock 1999). The efficacy of all three agents have been evaluated in large, double-blind, placebo-controlled clinical trials of up to 6 months duration.
Rivastigmine tartrate is a pseudo-irreversible, carbamate inhibitor that inhibits both AChE and BuChE selective for the brain compared with that in peripheral tissue. As a carbamate, rivastigmine binds to AChE which cleaves the rivastigmine molecule, releasing a phenolic cleavage product that is almost pharmacologically inert and is rapidly excreted via the kidneys.
The carbamate moiety remains bound to the esteratic site of the enzyme for much longer than is the case for the acetate moiety during the hydrolysis of ACh so that the enzyme is inactivated for some time after the parent molecule has disappeared from the circulation. The other consequence of this mechanism of action is that rivastigmine does not rely upon the hepatic cytochrome P450 system for either inactivation or elimination.
As rivastigmine has relatively low protein-binding characteristics, the potential for significant interactions with other drugs is minimal, which is an important feature for a medication intended for use in elderly individuals who typically take many different medications for concurrent illnesses (Table 1). Rivastigmine also exhibits selectivity for the G1 form of AChE and BuChE. The enzyme exists in several forms, the most abundant and important of which in normal individuals is the G4 form. With aging, and especially in AD, however, the amount of the G4 form of AChE falls progressively and it has been postulated that the G1 form plays a progressively more important role in hydrolyzing ACh at cholinergic synapses as AD advances. Rivastigmine inhibits the G1 form, which may mean that its efficacy will be presented.
Table 1.
Pharmacological features of rivastigmine
| Variable | |
|---|---|
| Class | Carbamate |
| No. daily doses | 2 |
| ChE inhibition | |
| Reversibility | Pseudoreversibile |
| BuChE/AchE ratio in vitroa | 1.9 |
| Elimination/metabolism | Renal |
| ADAS-cog changeb | −4.94; −2.58 |
| Completion rate (% of patients)c | 65, 67 |
| Behavioural effectsd | + |
| Adverse events: | |
| Hepatoxixity | X |
| Gastrointestinal | V |
| Other (≥10%) | Asthenia, headache, dizziness |
Notes: aRatio of IC50s (concentrations of drug required to inhibit BuChE and AchE activity by 50%). A smaller ratio indicates greater relative inhibition of BuChE;
Mean difference in ADAS-Cog scores at trial end-point (12 to 30 weeks) between active treatment and placebo in pivotal trials (2 pivotal trials for each drug except galantamine; range given for 4 trials for this drug);
Percentage of patients completing the pivotal trials (2 trials for each drug except galantamine;
As assessment by the total NPI score.
Abbreviations: AChE, acetylcholinesterase; ADAS-cog, Alzheimer’s Disease Assessment Scale—cognitive suscale; BuChE, butyrylcholinesterase; CPY, cytochrome P450 enzyme; NPI, Neuropsychiatric Inventory score; +, indicates improvement.
It shows preferential selectivity for the hippocampus and cortex (Darvesh et al 1998), those regions of the brain in which cholinergic deficits are most pronounced in AD. This results in higher synaptic levels of the neurotransmitter and improved function of cholinergic receptors. It has been proposed that since both AChE and BuChE degrade acetylcholine in the human brain (Mesulam et al 2002), the inhibition of both enzymes may lead to more potent biological effects, and greater, more sustained clinical benefits (Greig et al 2001; Ballard 2002; Poirier 2002).
Acetylcholinesterase and BuChE are two enzymes that have different roles in healthy individuals and subjects with AD. In healthy individuals, 80% of the enzymatic activity is carried out by AChE located predominantly in neurons. AChE is highly selective for acetylcholine hydrolysis, whereas BuChE also acts on substrates. It has been shown (Xie et al 2001) that in the case of AChE deficiency, BuChE is capable of compensating for AChE function. The two enzymes can be distinguished in terms of kinetics, in that AChE is more efficient at low substrate concentrations and inhibited at high concentrations. At high concentrations BuChE becomes very efficient, and it probably supports the hydrolysis of excess AChE. Both BuChE and AChE exist in different forms: a G4 comprised of 4 globular proteins and a G1 form with a single subunit. The G1 and G4 forms are present in different brain regions. The G4 form is more abundant in healthy subjects, whereas the G1 form plays a minor role. In AD, AChE activity is reduced to 55%–65%, whereas BuChE activity is increased and the ratio of BuChE to AChE changes from 0.5 to 11 and eventually activity becomes exclusively BuChE. It has been shown that BuChE may also have a role in the aggregation of β-amyloid (Aβ) that occurs in the early stages of AD and above all in the stages of senile plaque formation (Perry et al 1978; Guillozet et al 1997). Both AChE and BuChE accumulate within the plaques and in the neurofibrillary tangles (Mesulam and Geula 1994). In AD, the G1 form of BuChE increases by 30%–60% and accumulates in the beta-amyloid plaques, which are correlated to plaque density and pathogenicity. The K variant of BuChE may be associated with a greater susceptibility for developing AD, in particular in subjects who carry the apolipoprotein E4 (ApoE4) allele. Rivastigmine, which inhibits both AChE and BuChE, has been shown to protect against the formation of Aβ.
Pharmacokinetics
The absorption time of rivastigmine after oral administration (tmax) ranges from 0.8 to 167 hours. Absorption is >90%. Concomitant food intake reduces absorption of the drug and decreases concentration by 30%. Protein binding is quite low at approximately 40%; 40%–50% of the drug is associated with red blood cells. Rivastigmine is converted immediately to ZNS 144–666 at the site of action in the central nervous system (CNS) by cholinesterases and then enters the hepatic blood stream where it undergoes N-demethylation. The agent’s elimination half-life is less than two hours. Elimination is complete (90%) 24 hours after administration. The cerebrospinal fluid (CSF) concentration of rivastigmine falls of 1.4–3.8 hours and the drug is eliminated rapidly with a tmax rapidly from the CSF with a half-life of 0.31–0.95 hours. A dose-dependent relationship was identified at the CSF level, with inhibition of both AChE and BChE. For dosages of 2, 6, 10, and 12 mg/day mean AChE inhibition was 20%, 46%, 55.6%, and 61.7%, whereas BChE inhibition was 23.9%, 76.6%, 54.9%, and 61%, although wide variability among patients was observed for the BChE values.
Drug interactions
Rivastigmine is not significantly metabolized by the hepatic microsomal cytochrome P450 system because of its low protein binding, so no clinically significant drug interactions are expected. Studies on healthy volunteers support this hypothesis (Anand et al 1996; Spencer and Noble 1998). In fact no interactions have been reported between rivastigmine and dygoxin, warfarin, diazepan, or fluoxetine (Polinsky 1998; Grossberg et al 2000). In addition, Polinsky’s retrospective analysis of clinical trials demonstrated no increase in adverse events relative to placebo in patients who were taking antianginal agents, antiacids, antihypertensive agents, calcium channel blockers, estrogens, antihistamines, and benzodiazepines.
Efficacy of rivastigmine in subjects with mild and moderate Alzheimer’s disease
Rivastigmine, like other cholinesterase inhibitors, produces modest improvements in cognitive function and slows cognitive decline versus placebo. Rivastigmine has been evaluated in the treatment of subjects with mild to moderate AD. The primary indicators of response to treatment in AD are: the Alzheimer’s Disease Assessment Scale–Cognitive section (ADAS-Cog), which assesses cognitive function, and the Clinician Interview-Based Impression of Change-plus (CIBIC-plus), which evaluates global function.
The ADAS-cog has a score ranging from 0 to 70, with higher scores indicating greater alterations of cognitive function (memory, language, orientation, and executive function). Only the patient is assessed. Data from clinical trials are presented as changes or delta, indicating changes in score relative to baseline.
The 7-point CIBIC assesses global change relative to baseline and measures cognitive, behavioral, and functional symptoms based on interviews with the patient and the caregiver (1, marked improvement; 4, no change; 7, marked decline).
Secondary measures of clinical efficacy of the drug are the Mini Mental State Examination (MMSE) and the caregiver-rated Progressive Deterioration Scale (PDS). The MMSE has a maximum score of 30, and high scores above 26 indicate mild cognitive deterioration. The PDS is a second-choice test that measures the ADL and includes an assessment of the caregiver’s quality of life. The PDS is made up of 29 items with a total score between 0 and 100. Clinical trials typically report changes relative to baseline. Instruments used to evaluate the efficacy of rivastigmine in treating AD are shown in Table 2.
Table 2.
Instruments used to evalutate the efficacy of rivastigmine in treating Alzheimer’s disease
| Instrument | Symptoms or domains assessed | Source of information | Range of scale and interpretation |
|---|---|---|---|
| Valutation of cognitive functions | |||
| Alzheimer’s disease assessment scale | Cognition (memory, language, orientation, praxis) | Patient | 0–70 points 0 = no errors 70 = severe impairment |
| Mini Mental State Examination | Cognition (memory, language, orientation, attention, praxis), | Patients | 0–30 points;30 = no errors, 0 = severe impairment |
| Valutation of global functions | |||
| Clinician interview based impression of change scale | Global assessment of behaviour, general psychopathology, cognition, and activities of daily living | Patient and caregiver | 1–7 points 1,2,3 = minimal improvement 4 = no change 5,6,7 = marked deterioration |
| Progressive deterioration scale | Activities of daily living | Caregiver | 29 items Scores range from 0 to 100 |
Two multicenter trials of rivastigmine have been conducted on patients aged 45 to 90 years randomized to placebo or rivastigmine at a dose of 1–4 mg/day or 6–12 mg/day in a forced-dosage titration scheme. The assessment tools used were: ADAS-cog, CIBIC, PDS, and MMSE conducted at baseline and after 12, 18, and 26 weeks of treatment (Table 3). In the two combined studies, the completion rates for placebo, rivastigmine 1–4 mg/day, and rivastigmine 6–12 mg/day were 85%, 85%, and 66%, respectively. The main cause of drop-outs were adverse events. In one trial (Corey-Bloom et al 1998), efficacy measured with ADAS-cog, CIBIC, and MMSE was dose-dependent in patients receiving 6–12 mg. This group of subjects demonstrated a greater improvement compared with the placebo group from baseline to 26 weeks. The study by Rosler and colleagues (1999) found that cognitive function worsened progressively in the placebo group, with a mean deterioration of 1.41 points over the 29 weeks of treatment. ADAS-cog improved by 1.17 in subjects treated with high-dose rivastigmine. The difference between the two groups (placebo vs rivastigmine 6–12 mg) was statistically significant at week 12, 18, and 26 of treatment. As regards the CIBIC at week 26, subjects treated with placebo demonstrated a mean decline of 4.34 points. Instead, the patients treated with high doses of rivastigmine demonstrated a mean improvement of 3.93 points. PDS scores at week 26 showed a statistically significant difference between patients on placebo and those receiving high-dose rivastigmine. At 26 weeks the subjects who received high-dose rivastigmine showed a significant improvement on MMSE and PDS compared with the placebo group.
Table 3.
Summary of rivastigmine clinical trials
| Study | No. of Pts | Study duration (wks) | Dosage (mg/day) | Results (%) at week 26 vs Baseline | ||
|---|---|---|---|---|---|---|
| ADAS-cog | CIBIC-plus | MMSE | ||||
| Agid et al 1998 | 402 | 13 | Placebo | Not done | 29.9a | 0.0 |
| 4 | Not done | 31.5 | 0.0 | |||
| 6 | Not done | 42.7 | 0.3 | |||
| Corey-Bloom et al 1998 | 699 | 26 | Placebo | 4.15 | 4.39 | −0.79 |
| 1–4 | 2.27 | 4.23b | −0.33 | |||
| 6–12 | −0.79c | 4.20d | 0.30b | |||
| Rosler et al 1999 | 725 | 26 | Placebo | 1.34 | 4.38 | −0.47 |
| 1–4 | 1.37 | 4.24 | −0.62 | |||
| 6–12 | −0.26b | 3.91d | 0.21b | |||
Notes: asubjects scored 1 or 2;
p < 0.05;
p < 0.001;
p < 0.01.
Abbreviations: ADAS-cog, Alzheimer’s Disease Assessment Scale–Cognitive section; CIBIC-plus, Clinician Interview-Based Impression of Change-plus; MMSE, Mini Mental State Examination; Pts, patients.
The PDS was used to assess ADL (eg, ability to dress and eat independently, social interaction, participation in housework and hobbies, awareness of time, and handling of financial matters) in trials with rivastigmine. After 26 weeks of treatment, significantly more patients receiving rivastigmine 6–12 mg/day showed a ≥10% improvement in the PDS score compared with placebo in both trials (25 and 29% vs 15% and 19%; p < 0.01) (Corey-Bloom et al 1998; Rosler et al 1999).
Two pivotal trials have shown that rivastigmine 6–12 mg/day significantly improved cognitive function (assessed by the ADAS-cog) after 26 weeks of treatment compared with placebo in patients with mild to moderate probable AD (Corey-Bloom et al 1998; Rosler et al 1999). The effects of rivastigmine on cognitive function were dose related, with an estimated 0.28-point improvement in mean ADAS-cog score for every 1 mg/day increase in dosage (Anand et al 2000). Rivastigmine was also superior to placebo (p < 0.01) on the CIBIC-plus scale, which measures global functioning (cognition, functioning, and behavior) (Farlow et al 2000). Preliminary results from a long term extension study (Farlow et al 2000) of one of these trials (Corey-Bloom et al 1998) suggested that the benefits of rivastigmine, as measured by ADAS-cog scores, persisted over a 104-week study period.
Rivastigmine had a positive effect on the rate of cognitive decline in subjects with severe AD. After 26 weeks, there was a small improvement (0.2) relative to baseline in the mean ADAS-cog score of subjects treated with rivastigmine, whereas there was a mean decline of 6.3 points in subjects receiving placebo (observed case [OC] population; p < 0.001). In the intention to treat-last observation carried forward (ITT-LOCF) population there was no change from baseline in rivastigmine-treated subjects, compared with a 6.1-point decline in the placebo group (treatment difference, p < 0.001).
The efficacy of rivastigmine was also assessed in terms of the proportion of subjects in whom cognitive function was sustained or improved from baseline after 6 months of treatment (mean change from baseline in ADAS-cog>0). In total, 46% of the subjects treated with rivastigmine either improved or showed no deterioration, compared with 9% of subjects treated with placebo (OC population; p < 0.001). The respective figures in the ITT-LOCF population were 44% for rivastigmine and 7% for placebo (p = 0.001).
After 26 weeks, subjects treated with rivastigmine showed a mean change from baseline of −0.8 points on the MMSE, compared with −2.5 points in the placebo group (OC population; p = 0.02). The respective figures in the ITT-LOCF population were −0.8 and −2.5 points (p = 0.02).
Subjects on rivastigmine showed less decline of ADL than those on placebo. In the placebo group, functioning declined by a mean of 6.5 points in the PDS six-item score after 6 months. In contrast, the mean decline among subjects treated with rivastigmine was 1.6 points. In the ITT-LOCF population the decline was 2.0 points for rivastigmine and 6.3 points for placebo.
The recommended dosage range for rivastigmine in patients with mild to moderate AD is 6–12 mg/day administered orally in two separate doses. Rivastigmine should be started at a dosage of 1.5 mg twice daily, and increased in 1.5-mg increments as tolerated. At least two weeks should separate each increase in dose
To reduce the possibility of severe vomiting in patients who have interrupted rivastigmine therapy for more than several days, treatment should be restarted with the lowest daily dose. The dosage should then be titrated to the patient’s previous maintenance dosage
Efficacy of rivastigmine in subjects with moderate to severe Alzheimer’s disease
The efficacy of rivastigmine in the treatment of behavioral and psychological symptoms of dementia (BPSD) has also been studied in patients with moderate to severe AD living in long-term care facilities.
A number of open-label prospective studies in nursing home patients have also investigated the effects of rivastig-mine on neuropsychiatric and behavioral symptoms associated with AD (Cummings et al 2000a; Bullock et al 2001; Etemad et al 2001). One US study assessed the effects of 26-weeks treatment with rivastigmine (3–12 mg/day) in 173 patients with AD (mean MMSE = 9.2) (Cummings et al 2000a). Rivastigmine was associated with a significant 3.25-point overall decrease in the mean Neuropsychiatric Inventory–Nursing Home (NPI-NH) total score after 26 weeks, indicating that behavioral symptoms had improved.
Similar data have been described in a interim report from a second US open-label 26-week study of rivastigmine (12 mg/day). Preliminary data obtained from 181 patients (MMSE = 10.6) reported that mean the NPI-NH total score decreased by approximately 4 points (Etemad et al 2001).
In a 6-month study (Bullock et al 2001) in 113 patients with severe AD (mean MMSE = 10.9), >53% of patients showed improvements on all NPI-NH items and had a mean improvement of 0.7 points on the MMSE. More than 40% of patients had ≥30% improvement from baseline in NPI-NH scores.
Cummings (2003) conducted a prospective, 26-week, open-label study at 12 primary centers in a a total of 29 nursing homes in the US. The subjects were all nursing home residents with moderate to severe probable AD receiving rivastigmine 3–12 mg/day for 26 weeks. Following 6-months of rivastigmine treatment, a statistically significant improvement (p < 0.05) from baseline was observed in the following eight disturbances in subjects with that specific symptom present at baseline: delusion, hallucination, agitation, apathy/indifference, irritability, aberrant motor behavior, night-time behavior, and appetite/eating change. There were statistically significant improvements in depression/dysphoria, anxiety, euphoria/elation, and disinhibition.
A prospective, multicenter 26-week open-label extension to a 26-week open-label study of rivastigmine treatment was carried out in patients with MMSE scores of 6–15 inclusive, residing in nursing home (Aupperle et al 2004). Rivastigmine (3–12 mg/day) significantly improved neuropsychiatric and behavioral symptoms compared with baseline in observed cases and last observation carried forward analyses. Global function was stabilized or improved in more than half of the patients.
A recent study (Doraiswamy et al 2001) demonstrated that cognitive function, ADL performance, and global function were significantly better in patients treated with rivastigmine than those treated with placebo. The efficacy of rivastigmine versus placebo in patients with moderate to moderately severe AD is shown in Figures 1, 2, and 3.
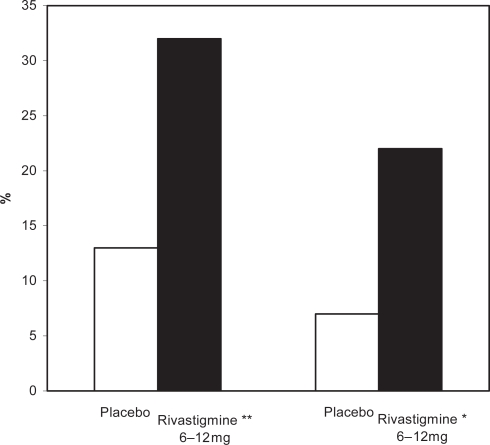
Figure 1.
Percentage of patients with moderate to moderately severe AD showing clinically relevant improvements on the ADAS-cog after 52 weeks.
Notes: *p < 0.05 versus original placebo group; **p = 0.116 versus original placebo group.
Abbreviations: AD, Alzheimer’s disease; ADAS-cog, Alzheimer’s Disease Assessment Scale–Cognitive section.
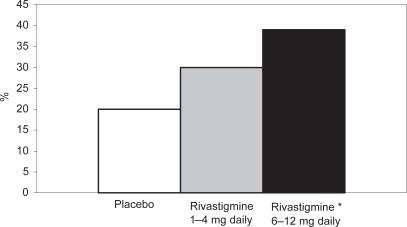
Figure 2.
Percentages of patients in the high- and low-dose rivastigmine group and the placebo group on the PDS after 26 weeks.
Note: * p = 0.02 vs placebo
Abbreviations: PDS, Progressive Deterioration Scale.
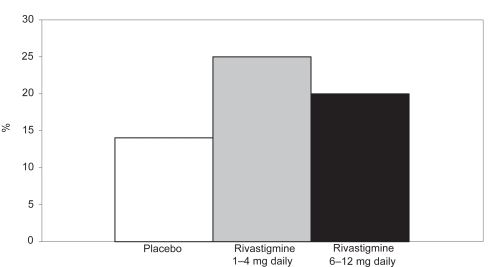
Figure 3.
Percentages of patients in the high- and low-dose rivastigmine group and the placebo group on CIBIC-plus after 26 weeks.
Abbreviations: CIBIC-p, Clinician Interview-Based Impression of Change-plus.
Effects on behavioral symptoms
Behavioral disturbances are a frequent occurrence in patients with AD and related dementia. Many of these behaviors are disruptive and unmanageable in the home care setting, resulting in the institutionalization of many patients. The peak intensity of different behavioral symptoms varies with disease severity and many of the symptoms are present long before the clinical diagnosis of the disease is established. Although antipsychotics have been somewhat effective in controlling the neuropsychiatric symptoms of AD, their use has been limited by side effects including extrapyramidal symptoms, sedation, dystonia, and hypotension. In addition, the use of psychotropic medication in this population to control behavioral abnormalities is tempered by the potential for overuse, resulting in chemical restraint. Therefore, a reduction in behavioral disturbances with a medication that lacks the traditional side effects of psychotropic drugs while improving other areas, such as cognitive and ADL, is an important treatment goal.
There is growing evidence that, in addition to their effects on cognition, cholinesterase inhibitors also exert beneficial psychotropic effects in patients with AD (Cummings et al 2000a). Although this may be a class effect, it is possible that the exact effects and potency of each agent differ (Cummings et al 2000a).
Recent clinical data demonstrate that rivastigmine provides long-term improvement of behavioral symptoms in patients with AD, as assessed by either the patient or care-giver. In patients with mild to moderate disease, rivastigmine improved or stabilized behavioral symptoms over a 2-year period. Although, as expected, the incidence of behavioral symptoms in this patient group was low, mood disorders (anxieties, phobias, and affective disturbances) were significantly improved versus baseline (p = 0.001) and symptoms of aggression, activity disturbances, hallucinations, and paranoid and delusional symptoms were stabilized versus baseline after 2 years (Rosler et al 1998).
In nursing-home patients with moderate to severe disease, rivastigmine improved behavioral symptoms as assessed by the NPI-NH scale for up to 1 year (Cummings et al 2000a, 2000b). Forty-nine percent of patients showed a ≥30% reduction from their baseline score after 52 weeks’ treatment (Cummings et al 2000a). This change is viewed as clinically significant and similar to that achieved with conventional psychotropic agents. Almost half of the patient group who required antipsychotics at baseline no longer needed them after 52 weeks’ treatment with rivastigmine (Cummings 2000).
Further supportive evidence for the effects of rivastigmine on behavioral symptoms comes from a recent randomized, double-blind, placebo-controlled trial in patients with dementias with Lewy bodies (DLB). In this patient group, fluctuating cognitive symptoms are also accompanied by neuropsychiatric symptoms including delusions, visual hallucinations, and sleep disturbances. Almost twice as many patients receiving rivastigmine (mean dosage 9.4 mg/day; n = 59) showed clinically relevant improvements on the NPI-NH as those treated with placebo after 20 weeks (n = 61) (McKeith et al 2000).
Meta-analyses evaluating the effects of rivastigmine on behaviors associated with mild to moderate AD have been performed and presented in poster form. Data were derived from a pooled population of 1840 patients for whom scores were available on the behavioral component of the Clinician’s Interview-Based Impression of Change Plus Caregiver Input Scale (CIBIC-plus adapted from BEHAVE-AD) from three 6-month, double-blind, placebo controlled regulatory trials of rivastigmine in patients with mild to moderate AD (mean MMSE = 19.9) (Agid et al 1998; Corey-Bloom et al 1998; Rosler et al 1999). The results after 6 months of treatment with rivastigmine 6 to 12 mg/day suggested interesting differential effects on individual symptoms. Despite a strong placebo effect, patients with symptoms at baseline had significant improvements in paranoid and delusional symptoms compared with placebo (p = 0.002 and p = 0.046 respectively). With regard to the prevention of symptom emergence, rivastigmine treatment appeared to prevent the emergence of activity disturbances compared with placebo (p = 0.016). The authors of these poster presentations suggested that rivastigmine may improve existing psychotic symptoms in patients with mild to moderate AD.
An open-label extension of a 6-month, double-blind, placebo-controlled regulatory study of rivastigmine in patients with mild to moderate AD (n = 725; mean baseline MMSE score, 10–26) reported significant sustained effects on BPSD for up to 2 years (Rosler et al 1998). At all times after baseline, BPSD were significantly better in the patients who had received rivastigmine for the entire study compared with those who had received placebo for the first 6 months (p < 0.05). With regard to specific items on BEHAVE-AD, symptoms of hallucinations, aggressiveness, activity disturbances, and paranoid and delusional ideation were improved for ≥2 years in patients receiving rivastigmine.
Rivastigmine has also demonstrated behavioral benefits in patients with DLB, including patients with both DLB and AD. In a double-blind, placebo-controlled study (n = 120; mean MMSE = 17.9) (McKeith et al 2000) in patients with DLB, receipt of rivastigmine 2 to 12 mg/day was associated with improvements in psychiatric symptoms as assessed using the NPI. Hallucinations and psychotic features resolved almost completely in more than half of patients receiving rivastigmine. At the end of the study period, NPI scores remained at baseline levels in patients who received rivastigmine for 96 weeks.
An open-label exploratory study of rivastigmine (Reading et al 2001), in which patients were titrated over 6–8 weeks to the maximum tolerated dose and then maintained for a further 6 weeks at this maximum dose, investigated the safety and tolerability in treating parkinsonian hallucinosis and cognitive impairment in diagnosed Parkinson’s disease patients with dementia and hallucinations. Significant improvements from baseline in MMSE (20.4 to 25.4; p < 0.005) and total NPI (39.6 to 15.2; p < 0.004) were seen in patients treated with rivastigmine. Hallucinations and sleep disturbances seemed particularly sensitive to the effects of rivastigmine with significant improvements in NPI (p < 0.03 and p < 0.02, respectively).
Tolerability
As with other cholinesterase inhibitors, the adverse events most commonly associated with rivastigmine are cholinergic in nature. These include nausea, vomiting, diarrhea, and anorexia and were reported in 14% to 50% of patients receiving rivastigmine 6 to 12 mg/day compared with 2% to 11% of placebo recipients in clinical trials (Corey-Bloom et al 1998; Rosler et al 1999). Other events occurring significantly more frequently with rivastigmine 6 to 12 mg/day than with placebo are dizziness, headache, fatigue, malaise, sweating, asthenia, somnolence, dyspepsia, and sinusitis (p < 0.05).
Most events are mild to moderate in intensity, dose-related, and of limited duration. In the pivotal clinical trials, adverse events were reported most frequently during the titration phase and may have been partly a function of the fixed titration schedule demanded by the trial protocol. About one quarter of patients receiving rivastigmine 6 to 12 mg/day discontinued treatment because of adverse events (Corey-Bloom et al 1998; Rosler et al 1999).
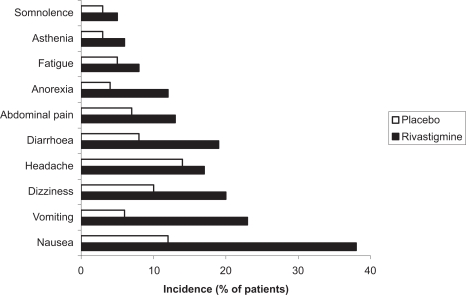
An episode of severe vomiting with esophageal rupture has been reported after re-initiation of rivastigmine therapy at an inappropriate single dose (4.5 mg) after treatment was interrupted for weeks (Babic et al 2000). No clinically relevant changes in laboratory or vital signs (including hepatic enzymes) were observed with rivastigmine 6 to 12 mg/day, except for a ≥7% decrease in body weight reported in about one-fifth of patients (Corey-Bloom et al 1998; Rosler et al 1999). Common adverse events observed with rivastigmine therapy are shown in Figure 4 (Spencer and Noble 1998).
Figure 4.
Common adverse events observed with rivastigmine therapy.
Comparative studies
All acetylcholinesterase inhibitors have proved to be more effective than placebo in randomized double-blind clinical trials. Lopez-Pousa and colleagues (2005) conducted a study to determine the differential efficacy of the acetylcholinesterase inhibitors with respect to a historical sample of AD patients that were not treated. After a study period of 6 months they found no statistically significant differences between donepezil, rivastigmine, and galantamine in terms of efficacy, and all of the drugs significantly slowed the decline in cognitive function associated with AD. Another study conducted by our group (Aguglia et al 2004) produced similar results. A systematic review (Takeda et al 2006) on the use of inhibitors has highlighted that the three drugs have similar efficacy. Adverse effects are prevalently gastrointestinal in type and more common in treated patients (Clegg et al 2002; Wolfson et al 2002). The 2006 Cochrane Review states that there is no evidence of any difference with respect to clinical efficacy despite the differences in the modes of action of the three inhibitors. From the point of view of adverse events, donepezil is the best tolerated, although gradual dose increments may prevent adverse events with rivastigmine and galantamine.
A comparative study of the efficacy and tolerability of donepezil and rivastigmine was conducted by Wilkinson and colleagues (2002) on 111 patients with mild to moderate AD. The two drugs (donepezil at 10 mg/day and rivastigmnine at 12 mg/day) showed equal improvement in cognitive function on ADAS-cog at 4 and at 12 weeks from baseline, while donepezil was better tolerated than rivastigmine: 87.5% of donepezil-treated patients and 47.3% of rivastigmine-treated patients remained on the maximum approved dose of each drug until the end of the study.
Efficacy of rivastigmine in dementia with Lewy bodies
The study by McKeith and colleagues (2000) is a multi-center, placebo-controlled, double-blind study involving 120 patients with DLB from Spain, the UK, and Italy. The subjects received 12 mg of rivastigmine daily or placebo for 20 weeks, followed by three weeks rest. Subjects were administered the NPI at baseline and at weeks 12, 20, and 23; in addition, a computerized cognitive assessment system was used and the patients underwent physical examination and laboratory tests. The authors found that patients receiving rivastigmine were less apathetic and anxious and had fewer delusions and hallucinations than the control subjects. Compared with the placebo group, patients receiving rivastigmine (37.63%) showed a 30% improvement from baseline in the computerized cognitive assessment and in the neuropsychiatric tests, as well as performing faster and better above all in the attentional tests. After discontinuation of treatment, the differences between rivastigmine and placebo tended to disappear. Adverse events (nausea, vomiting, and anorexia) were more frequent with rivastigmine than with placebo, but the safety and tolerability of the drug in these patients were judged acceptable.
Rivastigmine treatment in AD improves cognition, ADL, and global function; rivastigmine is used in mild to moderate AD (Polinsky 1998; Wilkinson et al 2004). Rivastigmine binds to the AChE molecule in a pseudo-irreversible fashion; the acetyl moiety of AChE is dissociated rapidly, but the carbamyl moiety remains for some time longer. Rivastig-mine is metabolized by the synapse rather than by hepatic cytochrome enzymes (Polinsky 1998). It has a half-life at the synapse of 9 hours and should be administered twice daily. The starting dose is 1.5 mg twice daily (3 mg/day), to be gradually increased to 6, 9, and 12 mg/day. A period of 1–2 weeks or more should separate each attempt to increase the dose, depending on patient tolerance.
Rivastigmine preferentially inhibits cerebrospinal fluid (CSF) AChE over peripheral AChE or BuChE (Kennedy et al 1999). After treatment with rivastigmine for 12 months, activity of the CSF and plasma AChE decreases by 46% and that of BuChE by 65% relative to baseline (Darreh-Shori et al 2002). Rivastigmine causes a mild selective upregulation of AChE-R. Changes in the ratio of AChE-R-AChE-S splice variants correlated with sustained cognition at 12 months (Almkvist et al 2004; Darreh-Shori et al 2004). There is significant correlation between plasma AChE inhibition and cognition, particularly as regards attention (Darreh-Shori et al 2004). Rivastigmine seems to have some role in the modulation of amyloid precursor protein processing (Racchi et al 2004).
Rivastigmine at a dose of 6–12 mg/day appears to improve or prevent disruptive behaviors and neuropsychiatric symptoms in patients with advanced AD (Finkel 2004). A recent study (Emre et al 2004) and a Cochrane review (Wild et al 2003) concluded that patients with DLB suffering from behavioral disturbances or psychiatric disorders benefit from rivastigmine treatment, if they can tolerate it.
Rivastigmine for dementia associated with Parkinson’s disease
Emre and colleagues (2004) reported in selected patients who had received a clinical diagnosis of mild to moderate Parkinson’s disease over the past two years were randomized to placebo or rivastigmine 3–12 mg/day for 24 weeks. Efficacy variables were the scores on the ADAS-Cog, ADCS-CGIC (Alzheimer’s Disease Cooperative Study—Clinician’s Global Impression of Change), the MMSE, the 10-item NPI, Cognitive Drug Research power of attention tests, the Verbal Fluency test, and the CDT.
A total of 541 patients were enrolled and 410 completed the study. The outcomes were better among patients treated with rivastigmine than among those receiving placebo; the differences between the two groups were, however, moderate and similar to those reported in trials of rivastigmine for AD. Patients treated with rivastigmine had a mean improvement of 2.1 points in the 70-point ADAS-Cog score, from a baseline score of 23.8, as compared with a 0.7-point worsening in the placebo group, from a baseline score of 24.3 (p < 0.001). A clinically significant improvement in the ADCS-CGIC scores was seen in 19.8% of patients in the rivastigmine group and in 14.5% of those in the placebo group, and a clinically significant worsening was seen in 13% and in 23.1%, respectively (mean score at 24 weeks, 3.8 and 4.3, respectively; p = 0.007). The most frequent adverse events were nausea (29.0% of patients in the rivastigmine-treated group and 11.2% of those in the placebo group), vomiting (16.6% and 1.7%, p < 0.001) and tremor (10.2% and 3.9%, p = 0.01).
In this placebo-controlled study, rivastigmine was associated with a moderate improvement in dementia associated with Parkinson’s disease but also with higher rates of nausea, vomiting, and tremors.
Rivastigmine for vascular dementia
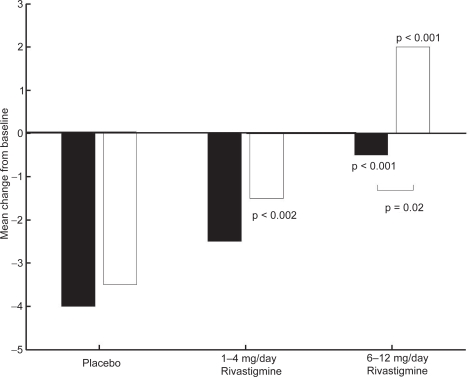
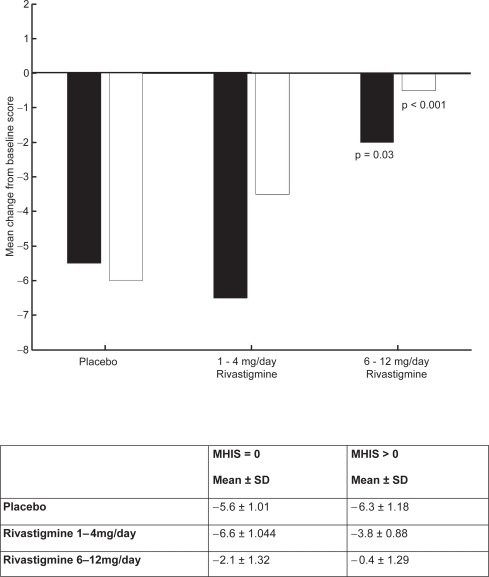
Kumar and colleagues (2000) reported that rivastigmine was associated with improvements in a wide range of efficacy measures (cognitive performance, ADL, and disease severity) in patients with and without vascular risk factors. The effects of treatment were greater in subjects with vascular risk factors, Modified Hachinski Ischemic Score (MHIS) > 0, (ADAS-Cog, PDS, MMSE, and GDS mean change from baseline scores). The treatment difference at 26 weeks for MHIS > 0 patients receiving rivastigmine 6–12 mg/day versus placebo was 6.15 points on the ADAS-Cog. While the mean 6–12 mg/day and placebo difference on ADAS-Cog was larger in patients with vascular risk factors compared with those without, this difference in effect size is attributed to the greater improvement from baseline in the MHIS > 0 category 6–12 mg group.
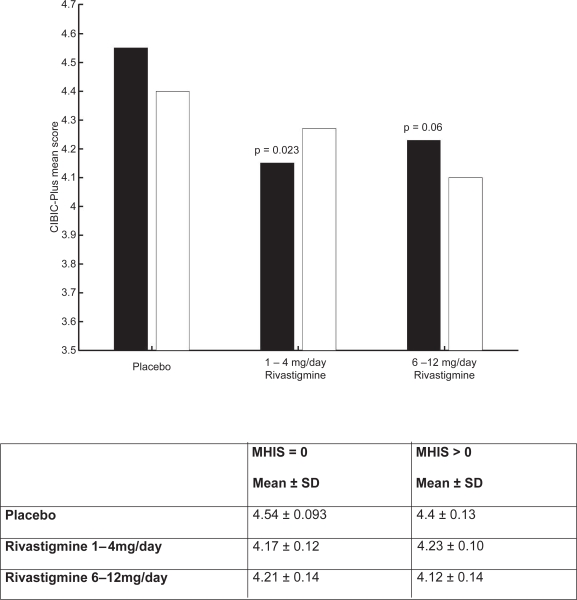
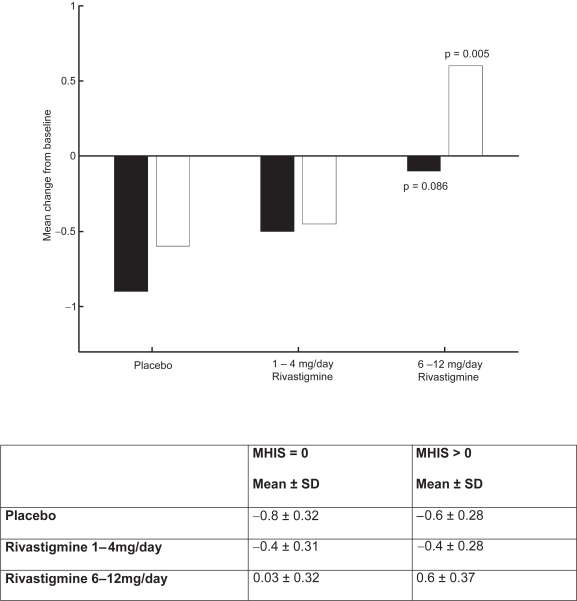
The clinical utility of the cognitive effects is supported by the benefits seen in the ADL. Whereas a significant improvement was seen in AD patients with or without vascular risk factors, the treatment differences were greater for the group with vascular risk factors. These effects were also reinforced by the GDS and MMSE scores observed in these patients. The CIBIC-Plus results show greater benefits in the MHIS > 0 category for 6–12 mg/day and 1–4mg/day. In addition, no statistically significant difference was observed in the MHIS > 0 group, 6–12 mg/day, compared with the placebo group MHIS > 0 (Figures 5, 6, 7, 8).
Figure 5.
ADAS-cog mean change from baseline scores at week 26.
Note: ▪ MHIS = 0; □ MHIS = 1
Abbreviations: ADAS-cog, Alzheimer’s Disease Assessment Scale–Cognitive section; MHIS, Modified Hachinski Ischemic Score.
Figure 6.
PDS mean change from baseline score at week 26.
Notes: ▪ MHIS = 0; □ MHIS>0.
Abbreviations: MHIS, Modified Hachinski Ischemic Score; PDS, Progressive Deterioration Scale; SD, standard deviation.
Figure 7.
CIBIC-plus mean score for population.
Notes: ▪ MHIS = 0; □ MHIS > 0.
Abbreviations: CIBIC-plus, Clinician Interview-Based Impression of Change-plus; MHIS, Modified Hachinski Ischemic Score; SD, standard deviation.
Figure 8.
MMSE mean change from baseline score at week 26.
Note: ▪ MHIS = 0; □ MHIS > 0.
Abbreviations: MHIS, Modified Hachinski Ischemic Score; MMSE, Mini Mental State Examination; SD, standard deviation.
Rivastigmine for subcortical vascular dementia
Moretti and colleagues (2004) have recently described the different studies on rivastigmine in the treatment of vascular dementia (VaD). Given the range of clinical syndromes in VaD, all open-label clinical trials have been conducted on patients with subcortical VaD (Roman et al 2000). This form of VaD is characterized by executive dysfunctions (Roman and Royall 1999), abnormal gait, urinary urgency, and incontinence, resulting from the interruption of prefrontal-subcortical circuits due to lacunar stroke and white-matter lesions (Roman et al 2004).
Rivastigmine was used in small open-label studies of patients with subcortical VaD followed for 12 months (Moretti et al 2001) and 22 months (Moretti et al 2002); rivastigmine proved useful in stabilizing cognitive function and ADL, with an improvement in cognitive function, planning, and behavior, and in reducing caregiver stress. Moretti and colleagues (2002) concluded that long-term rivastigmine treatment in patients with subcortical VaD is safe and effective. The improvements were in the domains that characterize subcortical VaD. The effects of rivastigmine have yet to be validated in large-scale, Phase III, randomized double-blind placebo-controlled clinical trials.
As regards the use of rivastigmine in patients with AD and CVD, Kumar and colleagues (2000) compared outcomes in AD patients with and without risk factors; cognitive effects were seen in both groups but the patients with AD and vascular risk factors experienced greater benefits. These conclusions were confirmed by Erkinjuntti and colleagues (2002) in a 104-week open-label study. Compared with nonhypertensive AD patients, significant differences were seen on the PDS in the hypertensive group. Erkinjuntti and colleagues (2003) analyzed 725 AD patients treated with rivastigmine according to the presence of arterial hypertension at baseline. Rivastigmine 6–12 mg/day improves PDS outcomes more than placebo in hypertensive subjects (p = 0.031) and nonhypertensive subjects (p = 0.035). All patients receiving rivastigmine 6–12 mg/day had higher CIBIC-plus scores compared with the placebo group. The benefits on disease progression experienced by the patients with AD and hypertension could be due to the effects of the drug on cerebrovascular factors or to a greater cholinergic deficit of patients with AD and hypertension.
Neuroprotection
Acetylcholinesterase inhibitors (AChEls) are the mainstay of pharmacological treatment of AD. Andin’s study (2005) provides the first evidence that the glutamatergic system is modulated following AChE inhibition by rivastigmine; a finding which is likely to be of importance for the clinical effects. An in situ hybridization technique (using digoxigenin-labeled cRNA probes) was used to elucidate changes in mRNA expression of the neuronal glutamate transporter, rat excitatory amino carrier 1 (rEAAC1), after treatment with the AChEl rivastigmine. Compared with saline-treated rats, the rats subchronically (3 days) and chronically (21 days), but not acutely, treated with rivastigmine showed a significant increase in rEAAC1 mRNA expression in the hippocampal areas cornu anterior 1 (CA1), CA2, CA3 and dentate gyrus (p<0.01), but not in cortical areas.
Numerous studies have investigated the neurotoxic effects of the abnormal production of ß-amyloid. Mesulam and Geula (1994) hypothesized a two-stage evolution of ß-amyloid plaques with a secondary development of local neurotoxicity due to the increase of butyrylcholinesterase at the plaque level. A study conducted by Venneri and colleagues (2005) to monitor white matter density in a group of 26 patients treated with the three inhibitors showed less worsening of parietotemporal atrophy in subjects taking rivastigmine. This finding provided empirical evidence that dual inhibition may have potential neuroprotective effects.
Rivastigmine, with its dual action on AChE and BuChE especially in the hippocampus and neurocortex, may lead to an increase in acetylcholine concentration and a reduction in noradrenaline and tau in the rat hippocampus. Trabace and colleagues (2000) found that rivastigmine also affects the glutaminergic system leading to increased glutamate concentration in the rat hippocampus, although the mechanisms are not completely clear. A study by Andin and colleagues (2005) has shown that rivastigmine modulates glutaminergic activity by interfering with the regulation of activity of some genes. This finding could have implications for the efficacy of rivastigmine in the treatment of AD.
Conclusion
The regional brain atropy which accompanies the cognitive and functional decline in AD has, in principle, been related to progressive neuropathological changes in that disorder. Studies investigating the possible neurotoxic processes related to the distinctive neuropathology of AD have emphasized the possible role of abnormal β-amyloid production. They have argued for a two-stage evolution of senile amyloid plaques with a secondary development of local neurotoxicity, for which increased levels of BChE in the plaque structure may be a marker and putative toxic agent (Mesulam and Geula 1994). As a test of concept, this proposed neurodegenerative process might be investigated clinically by taking advantage of the differing actions of the cholinesterase inhibitors that are widely used in the hope of achieving symptomatic benefits for AD patients. Donepezil and galantamine are selective for AChE inhibition, whereas rivastigmine provides an additional inhibitory action on BChE and might have an effect on local plaque toxicity.
References
- Agid Y, Dubois B, Anand R, et al. Efficacy and tolerability of rivastigmine in patients with dementia of the Alzheimer type. Curr Ther Res. 1998;59:837–45. [Google Scholar]
- Aguglia E, Onor ML, Saina M, et al. An open-label, comparative study of rivastigmine, donepezil and galantamine in a real-world setting. Curr Med Res Opin. 2004;20:1747–52. doi: 10.1185/030079904X6273. [DOI] [PubMed] [Google Scholar]
- Almkvist O, Dareh-Shori T, Stefanova E, et al. Preserved cognitive function after 12 months of treatment with rivastigmine in mild Alzheimer’s disease in comparison with untreated AD and MCI patients. Eur J Neurol. 2004;11:253–61. doi: 10.1046/j.1468-1331.2003.00757.x. [DOI] [PubMed] [Google Scholar]
- Anand R, Gharabawi G, Enz A. Efficacy and safety results of early phase studies with Exelon (ENA 713) in Alzheimer’s Disease: an overview. J Drug Dev Clin Pract. 1996;8:1–8. [Google Scholar]
- Anand R, Messina J, Hartman R. Dose-response effect of rivastigmine in the treatment of Alzheimer’s disease. Int J Geriatr Psychopharmacol. 2000;2:68–72. [Google Scholar]
- Andin J, Enz A, Gentsch C, et al. Rivastigmine as a modulator of the neuronal glutamate transporter rEAAC1 mRNA expression. Dement Geriatr Cogn Disord. 2005;19:18–23. doi: 10.1159/000080966. [DOI] [PubMed] [Google Scholar]
- Aupperle PM, Koumaras B, Chen M, et al. Long-term effects of rivastigmine treatment on neuropsychiatric and behavioral disturbances in nursing home residents with moderate to severe Alzheimer’s disease: results of a 52-week open label study. Curr Med Res Opin. 2004;20:1605–12. doi: 10.1185/030079904125004204. [DOI] [PubMed] [Google Scholar]
- Babic T, Banfic L, Papa J, et al. Spontaneous rupture of oesophagus related to rivastigmine. Age Aging. 2000;29:370–1. doi: 10.1093/ageing/29.4.370. [DOI] [PubMed] [Google Scholar]
- Ballard CG. Advances in the treatment of Alzheimer’s disease: benefits of dual cholinesterase inhibition. Eur Neurol. 2002;47:64–70. doi: 10.1159/000047952. [DOI] [PubMed] [Google Scholar]
- Bullock R, Moulais R, Steinwachs KC, et al. Effects of rivastigmine on behavioral symptoms in nursing home patients with Alzheimer’s disease. Int Psychogeriatric. 2001;13(suppl 2):242S. [Google Scholar]
- Clegg A, Bryant J, Nicholson T, et al. Clinical and cost-effectiveness of donepezil, rivastigmine and galantamine for Alzheimer’s disease: a rapid and systematic review. Health Technol Assess. 2001;5:1–137. doi: 10.3310/hta5010. [DOI] [PubMed] [Google Scholar]
- Corey-Bloom J, Anand R, Veach J, et al. A randomized trial evaluating the efficacy and safety of ENA 713 (rivastigmine tartrate), a new acetylcholinesterase inhibitor, in patients with mild to moderately severe Alzheimer’s disease. Int J Geriatr Psychopharmacol. 1998;2:68–72. [Google Scholar]
- Cummings JL, Anand R, Koumaras B, et al. Rivastigmine provides behavioral benefits to Alzheimer’s disease patients residing in a nursing home: findings from a 26 week trial. Neurology. 2000a;54(suppl 3):A468–9. [Google Scholar]
- Cummings JL, Anand R, Koumaras B, et al. Behavioral benefits in Alzheimer patients residing in a nursing home following 52 weeks of rivastigmine treatment. American Psychiatric Association, Annual Meeting; May 13–18; Chicago. 2000b. pp. 212–13. [Google Scholar]
- Cummings JL. Cholinesterase inhibitors: a new class of psychotropic compounds. Am J Psychiatry. 2000;157:4–15. doi: 10.1176/ajp.157.1.4. [DOI] [PubMed] [Google Scholar]
- Cummings JL. Use of cholinesterase inhibitors in clinical practice: evidence-based recommendations. Am J Geriatr Psychiatry. 2003;11:131–45. [PubMed] [Google Scholar]
- Darreh-Shori T, Almkvist O, Guan ZZ, et al. Sustained cholinesterase inhibition in AD patients receiving rivastigmine for 12 months. Neurology. 2002;59:563–72. doi: 10.1212/wnl.59.4.563. [DOI] [PubMed] [Google Scholar]
- Darreh-Shori T, Hellstrom-Lindahl E, Flores-Flores C, et al. Long-lasting acetylcholinesterase splice variations in anticholinesterase-treated Alzheimer’s disease patients. J Neurochem. 2004;88:1102–13. doi: 10.1046/j.1471-4159.2003.02230.x. [DOI] [PubMed] [Google Scholar]
- Darvesh S, Grantham DL, Hopkins DA. Distribution of butyrylcholinesterase in human amygdala and hippocampal formation. J Comp Neurol. 1998;393:374–90. [PubMed] [Google Scholar]
- Davies KL, Maloney AJ. Selective loss of central cholinergic neurons in Alzheimer’s disease. Lancet. 1976;2:1403. doi: 10.1016/s0140-6736(76)91936-x. [DOI] [PubMed] [Google Scholar]
- Doody RS, Stevens JC, Beck C, et al. Practice parameter: management of dementia (an evidence-based review). Report of the quality standards subcommittee of the American Academy of Neurology. Neurology. 2001;56:1154–66. doi: 10.1212/wnl.56.9.1154. [DOI] [PubMed] [Google Scholar]
- Doraiswamy PM, Krishnan KRR, Anand R, et al. Long-term effects of rivastigmine in moderately severe Alzheimer’s disease. Does early initiation of therapy offer sustained benefits? Prog Neuropsychopharmacol Biol Psychiatry. 2001;26:705–12. doi: 10.1016/s0278-5846(01)00326-8. [DOI] [PubMed] [Google Scholar]
- Emre M, Aarsland D, Albanese A, et al. Rivastigmine for dementia associated with Parkinson’s Disease. N Engl J Med. 2004;351:2509–18. doi: 10.1056/NEJMoa041470. [DOI] [PubMed] [Google Scholar]
- Erkinjuntti T, Skoog I, Lane R, et al. Rivastigmine in patients with Alzheimer’s Disease and concurrent hypertension. Int J Clin Practice. 2002;56:791–6. [PubMed] [Google Scholar]
- Erkinjuntti T, Skoog I, Lane R, et al. Potential long-term effects of rivastigmine on disease progression may be linked to drug effects on vascular changes in Alzheimer brains. Int J Clin Pract. 2003;57:756–60. [PubMed] [Google Scholar]
- Etemad B, Anand R, Hartman R. Behavioural and cognitive benefits of rivastigmine in nursing home patients with Alzheimer’s disease and related dementias: a 26-week follow-up. Poster presented at the 10th International Congress of the International Psychogeriatric Association; 9-–14 Sseptember; Nice, France. 2001. [Google Scholar]
- Farlow M, Messina J, Anard R. Long-term cognitive benefits associated with the use of rivastigmine in the treatment of Alzheimer’s disease: results following two years of treatment. J Am Geriatr Soc. 2000;48:108. [Google Scholar]
- Finkel SI. Effects of rivastigmine on behavioral and psychological symptoms of dementia in Alzheimer’s disease. Clin Ther. 2004;26:980–90. doi: 10.1016/s0149-2918(04)90172-5. [DOI] [PubMed] [Google Scholar]
- Greig NH, Utsuki T, Yu Q, et al. A new therapeutic target in Alzheimer’s disease treatment: attention to butyrylcholinesterase. Curr Med Res Opin. 2001;17:159–65. doi: 10.1185/0300799039117057. [DOI] [PubMed] [Google Scholar]
- Grossberg GT, Stahelin HB, Messina JC, et al. Lack of adverse pharmacodynamic drug interactions with rivastigmine and twenty-two classes of medications. Int J Geriat Psychiatry. 2000;15:242–7. doi: 10.1002/(sici)1099-1166(200003)15:3<242::aid-gps110>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Guillozet A, Smiley JF, Mash DC, et al. Butyrycholinesterase in the life cycle of amyloid plaques. Ann Neurol. 1997;42:909–18. doi: 10.1002/ana.410420613. [DOI] [PubMed] [Google Scholar]
- Kennedy JS, Polinsky RJ, Johnson B, et al. Preferential cerebrospinal fluid acetylcholinesterase inhibition by rivastigmine in humans. Int Clin Psychopharmacol. 1999;19:513–21. doi: 10.1097/00004714-199912000-00005. [DOI] [PubMed] [Google Scholar]
- Kumar V, Anand R, Messina J, et al. An efficacy and safety analysis of Exelon in Alzheimer’s disease patients with current vascular risk factor. Eur J Neurol. 2000;7:159–69. doi: 10.1046/j.1468-1331.2000.00046.x. [DOI] [PubMed] [Google Scholar]
- Lopez-Pousa S, Turon-Estrada A, Garre-Olmo J, et al. Differential efficacy of treatment with acetylcholinesterase inhibitors in patients with mild and moderate Alzheimer’s disease over a 6-month period. Dement Geriatr Cogn Disord. 2005;19:189–95. doi: 10.1159/000083498. [DOI] [PubMed] [Google Scholar]
- McKeith I, Del Ser T, Spano PF, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomised, double-blind, placebo-controlled international study. Lancet. 2000;356:2031–6. doi: 10.1016/S0140-6736(00)03399-7. [DOI] [PubMed] [Google Scholar]
- Mesulam M, Geula C. Butyrylcholinesterse reactivity differentiates the amyloid plaques of aging from those of dementia. Ann Neurol. 1994;36:722–7. doi: 10.1002/ana.410360506. [DOI] [PubMed] [Google Scholar]
- Mesulam M, Guillozet A, Shaw P, et al. Widely spread butyrylcholinesterase can hydrolyse acetylcholine in the normal and Alzheimer brain. Neurobiol Dis. 2002;9:88–93. doi: 10.1006/nbdi.2001.0462. [DOI] [PubMed] [Google Scholar]
- Moretti R, Torre P, Antonello RM, et al. Rivastigmine in subcortical vascular dementia: a comparison trial on efficacy and tolerability for 12 months follow-up. Eur J Neurol. 2001;8:361–2. doi: 10.1046/j.1468-1331.2001.00224.x. [DOI] [PubMed] [Google Scholar]
- Moretti R, Torre P, Antonello RM, et al. Rivastigmine in subcortical vascular dementia: a open 22-month study. J Neurol Sci. 2002;203:141–6. doi: 10.1016/s0022-510x(02)00280-0. [DOI] [PubMed] [Google Scholar]
- Moretti R, Torre P, Antonello RM, et al. Rivastigmine in vascular dementia. Expert Opin Pharmacother. 2004;5:1399–410. doi: 10.1517/14656566.5.6.1399. [DOI] [PubMed] [Google Scholar]
- Perry EK, Perry RH, Blessed G, et al. Changes in brain cholinesterases in senile dementia of Alzheimer type. Neuropathol Appl Neurobiol. 1978;4:273–7. doi: 10.1111/j.1365-2990.1978.tb00545.x. [DOI] [PubMed] [Google Scholar]
- Poirier J. Evidence that the clinical effects of cholinesterase inhibitors are related to potency and targeting of action. Int J Clin Pract Suppl. 2002;127:6–19. [PubMed] [Google Scholar]
- Polinsky RJ. Clinical pharmacology of rivastigmine: a new-generation acetylcholinesterase inhibitor for the treatment of Alzheimer’s disease. Clin Ther. 1998;20:643–7. doi: 10.1016/s0149-2918(98)80127-6. [DOI] [PubMed] [Google Scholar]
- Racchi M, Mazzucchelli M, Porrello E, et al. Acetylcholinesterase inhibitors: a novel activities of old molecules. Pharmachol Res. 2004;50:441–51. doi: 10.1016/j.phrs.2003.12.027. [DOI] [PubMed] [Google Scholar]
- Reading PJ, Luce AK, McKeith IG. Rivastigmine in the treatment of parkinsonian psychosis and cognitive impairment: preliminary findings from an open trial. Mov Disord. 2001;16:1171–4. doi: 10.1002/mds.1204. [DOI] [PubMed] [Google Scholar]
- Roman GC, Royall DR. Executive control function: a rational basis for diagnosis of vascular dementia. Alzheimer Dis Assoc Disord. 1999;13(suppl 3):S69–S80. doi: 10.1097/00002093-199912003-00012. [DOI] [PubMed] [Google Scholar]
- Roman GC, Erkinjutti T, Wallin A, et al. Subcortical ischaemic vascular dementia. Lancet Neurol. 2000;1:426–36. doi: 10.1016/s1474-4422(02)00190-4. [PF 26] [DOI] [PubMed] [Google Scholar]
- Roman GC, Sachdev P, Royall DR, et al. Vascular cognitive disorders: a new diagnostic category updating vascular cognitive impairment and vascular dementia. J Neurol Sci. 2004;226(1–2):81–7. doi: 10.1016/j.jns.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Rosler M, Anand R, Cicin-Sain A, et al. Efficacy and safety of rivastigmine in patients with Alzheimer’s disease: international randomised controlled trial. BMJ. 1999;318:633–8. doi: 10.1136/bmj.318.7184.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosler M, Retz W, Retz-Junginger P, et al. Effects of two-year treatment with the cholinesterase inhibitor rivastigmine on behavioural symptoms in Alzheimer’s disease. Behav Neurol. 1998;11:211–16. doi: 10.1155/1999/168023. [DOI] [PubMed] [Google Scholar]
- Spencer CM, Noble S. Rivastigmine. A review of its use in Alzheimer’s Disease. Drugs Aging. 1998;13:391–411. doi: 10.2165/00002512-199813050-00005. [DOI] [PubMed] [Google Scholar]
- Takeda A, Loveman E, Clegg A, et al. A systematic review of the clinical effectiveness of donepezil, rivastigmine and galantamine on cognition, quality of live and adverse events in Alzheimer’s disease. Int J Geriatr Psychiatry. 2006;21:17–28. doi: 10.1002/gps.1402. [DOI] [PubMed] [Google Scholar]
- Trabace L, Coluccia A, Gaetani S, et al. In vivo neurochemical effects of the acetylcholinesterase inhibitor ENA713 in rat hippocampus. Brain Res. 2000;865:268–71. doi: 10.1016/s0006-8993(00)02266-6. [DOI] [PubMed] [Google Scholar]
- Venneri A, McGeown WJ, Shanks MF. Empirical evidence of neuroprotection by dual cholinesterase inhibition in Alzheimer’s disease. Neuroreport. 2005;16:107–10. doi: 10.1097/00001756-200502080-00006. [DOI] [PubMed] [Google Scholar]
- Weinstock M. Selectivity of cholinesterase inhibition. CNS Drugs. 1999;12:307–23. [Google Scholar]
- Wild R, Pettit T, Burn A. Cholinesterase inhibitors for dementia with Lewy bodies. Cochrane Database Syst Rev. 2003;3:CD003672. doi: 10.1002/14651858.CD003672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson DG, Francis PT, Schwam E, et al. Cholinesterase inhibitors used in the treatment of Alzheimer’s disease: the relationship between pharmacological effects and clinical efficacy. Drugs Aging. 2004;21:453–78. doi: 10.2165/00002512-200421070-00004. [DOI] [PubMed] [Google Scholar]
- Wilkinson DG, Passmore AP, Bullock R, et al. A multinational, randomised, 12-week, comparative study of donepezil and rivastigmine in patients with mild to moderate Alzheimer’s disease. Int J Clin Pract. 2002;56:441–6. [PubMed] [Google Scholar]
- Wolfson C, Oremus M, Shukla V, et al. Donepezil and rivastigmine in the treatment of Alzheimer’s disease: a best-evidence synthesis of the published data on their efficacy and cost-effectiveness. Clin Ther. 2002;24:862–86. doi: 10.1016/s0149-2918(02)80004-2. [DOI] [PubMed] [Google Scholar]
- Xie W, Stribley JA, Chatonnett A, et al. Postnatal development delay and supersensitivity to organophosphate in gene targeted mice lacking acetylcholinesterase. J Pharm Exp Therapeutics. 2001;293:896–902. [PubMed] [Google Scholar]