Abstract
Benign prostatic hyperplasia (BPH) is a frequent cause of lower urinary symptoms, with a prevalence of 50% by the sixth decade of life. Hyperplasia of stromal and epithelial prostatic elements that surround the urethra cause lower urinary tract symptoms (LUTS), urinary tract infection, and acute urinary retention. Medical treatments of symptomatic BPH include; 1) the 5α-reductase inhibitors, 2) the α1-adrenergic antagonists, and 3) the combination of a 5α-reductase inhibitor and a α1-adrenergic antagonist. Selective α1-adrenergic antagonists relax the smooth muscle of the prostate and bladder neck without affecting the detrussor muscle of the bladder wall, thus decreasing the resistance to urine flow without compromising bladder contractility. Clinical trials have shown that α1-adrenergic antagonists decrease LUTS and increase urinary flow rates in men with symptomatic BPH, but do not reduce the long-term risk of urinary retention or need for surgical intervention. Inhibitors of 5α-reductase decrease production of dihydrotestosterone within the prostate resulting in decreased prostate volumes, increased peak urinary flow rates, improvement of symptoms, and decreased risk of acute urinary retention and need for surgical intervention. The combination of a 5α-reductase inhibitor and a α1-adrenergic antagonist reduces the clinical progression of BPH over either class of drug alone.
Keywords: prostatic hyperplasia, 5α-reductase, dutasteride
Introduction
Benign prostatic hyperplasia (BPH) refers to stromal and glandular epithelial hyperplasia that occurs in the zone of the prostate that surrounds the urethra. Histopathologic BPH is often associated with lower urinary tract symptoms (LUTS), characterized by urinary frequency and urgency, a sensation of incomplete bladder emptying, a weak and interrupted urinary stream, straining to initiate urination, and nocturia. The prevalence of BPH increases with increasing age, and moderate to severe symptoms occur in up to 40% of men after age 60. Symptoms are evaluated with validated instruments such as the American Urologic Association (AUA) Symptom Index. Each of seven symptoms (frequency, urgency, weak stream, intermittency, incomplete emptying, straining to urinate, and nocturia) are scored by the patient on a 0–5 scale based on their frequency. A score of less than 7 indicates mild symptoms; a score of 8–19 indicates moderate symptoms, and a score of greater than 19 indicates severe symptoms. In addition to symptoms that may have a negative impact on the quality of life, BPH can result in acute urinary retention, recurrent urinary tract infections (UTI), bladder stones, urinary incontinence, gross hematuria, and renal failure.
The natural history of BPH is unpredictable in individual men. In a study of men who were followed expectantly for 5 years without treatment, 31% reported symptomatic improvement whereas 16% reported symptomatic worsening (Ball et al 1981). Men with symptomatic BPH have a 23% lifetime risk of developing acute urinary retention if left untreated (Jacobsen et al 1996). A man over age 60 years with obstructive symptoms has a 20-year probability of undergoing surgery related to the prostate of 39% (Arrighi et al 1991).
The AUA and the European Association of Urology have published recommendations for the evaluation of men with LUTS, and the treatment of men with symptomatic BPH. Medical therapies recommended by these two organizations include the α1-adrenergic antagonists terazosin, doxazocin, tamsulosin, and alfuzosin and the 5α-reductase inhibitors finastereide and dutasteride (Roehrborn et al 2003).
Selective α1-adrenergic antagonists relax the smooth muscle of the prostate and bladder neck without affecting the detrussor muscle of the bladder wall, thus decreasing the resistance to urine flow without compromising bladder contractility. Randomized, placebo-controlled clinical trials have shown that α1-adrenergic antagonists decrease LUTS and increase urinary flow rates in men with symptomatic BPH. However, a positive placebo effect was also demonstrated for both symptoms score and peak urinary flow rates in these trials. Common side effects include dizziness, headache, asthenia, and postural hypotension, which occur in 5%–9% of patients (Roehrborn and Schwinn 2004). Tamsulosin is the most uroselective α1-adrenergic antagonist approved for use in the treatment of symptomatic BPH. Clinical trials have shown postural hypotension was observed less frequently with tamsulosin than with either terazosin or doxazocin (Lepor 1998).
Dihydrotestosterone (DHT) is the product of the conversion of testosterone by the enzyme 5α-reductase, and is produced in the tissues of the liver, skin and organs that originate from the mesonephric duct, such as the prostate. Within the prostate, locally produced DHT acts in a paracrine fashion to stimulate growth. Inhibitors of 5α-reductase decrease production of DHT within the prostate resulting in decreased prostate volume, increased peak urinary flow rates, and improvement in symptoms scores. Studies have also shown that 5α-reductase inhibitors reduce serum levels of prostate specific antigen and reduce the overall risk of prostate cancer (Thompson et al 2003). Side effects of 5α-reductase inhibitors include erectile dysfunction, decreased libido, ejaculatory dysfunction, and gynecomastia, which occur in less than 5% of patients (Abramowicz 2002). Dutasteride inhibits both type 1 and type 2 5α-reductase isoenzymes, whereas finasteride inhibits only type 2.
Combination therapy with the α1-adrenergic antagonist doxazocin and the 5α-reductase inhibitor finasteride has been shown to significantly reduce the overall risk of clinical progression of BPH compared with the use of either drug alone. The Medical Therapy of Prostate Symptoms (MTOPS) Trial studied 3047 men age 50 and older with moderate to severe symptomatic BPH for a period of 4.5 years. Patients were randomized to receive 1) finasteride and placebo, 2) doxazocin and placebo, 3) finasteride and doxazocin, and 4) both placebos. The primary outcome was clinical progression of BPH. Secondary outcome measures included changes in AUA symptom score and peak urinary flow rate, and the risk of receiving surgical therapy for symptomatic BPH.
Compared with the placebo group, the risk of clinical progression of BPH, reported as rate per 100 person-years, was reduced by 39% in the doxazocin group (p < 0.001), by 34% in the finasteride group (p = 0.002), and by 66% in the combination therapy group (p < 0.001). The risk of clinical progression in the combination therapy group was significantly reduced compared with either the doxazocin or the finasteride group (p < 0.001). Reduction in the AUA Symptom Index score occurred in all groups and was significantly greater than placebo in all of the drug treatment groups. Improvement in the symptoms score after 4 years was significantly greater in the combination therapy group than either the doxazocin group (p = 0.006) or in the finasteride group (p < 0.001). Peak urinary flow rates increased in all drug groups over the placebo group (p < 0.001 for each pair wise comparison). Compared with the placebo group, the risk of receiving surgical therapy for symptomatic BPH was reduced by 64% in the finasteride group (p < 0.001), and by 67% in the combination therapy group (p < 0.001) (McConnell et al 2003).
Pharmacology, mode of action, and pharmacokinetics of dutasteride
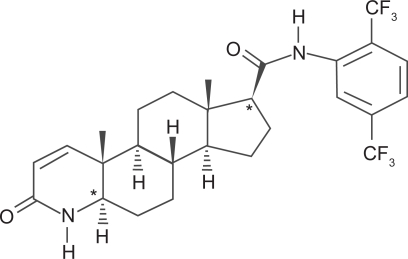
Dutasteride is in a drug class known as 17β-substituted 4-aza-steroids with the chemical name (5α, 17β)-N {2, 5, bis(trifluoromethyl) phenyl}-3-oxo-4-azaandrost-1-ene-17-carboxamide. The structural formula is shown in Figure 1. It is a competitive inhibitor of type 1 and type 2 5α-reductase isoenzymes. It forms a stable complex with a slow rate of dissociation and does not bind the androgen receptor. The bioavailability of dutasteride is approximately 60% and peak serum concentration is achieved after 2–3 hours. The time to steady state is dose dependent, and at a dose of 0.5 mg/day is approximately 3 months. More than 99.5% of circulating dutasteride is bound to plasma proteins, and has a volume of distribution of 300 to 500 liters. Clearance is linear at 0.58 liters/hour, resulting in a half-life of up to 5 weeks. Pharmacokinetic data is summarized in Table 1. Dutasteride is detectable (>0.1 ng/mL) in the serum 4–6 months after discontinuation of treatment. The drug is extensively metabolized by cytochrome-P3A4 in the liver and is excreted mainly in the feces. Only trace amounts are excreted in the urine. Following dosing to steady state, 5 major metabolites are detectable in the serum. The major metabolite, 6β-hydroxydutasteride, has pharmacological activity comparable with dutasteride (Bramson et al 1997).
Figure 1.
Dutasteride structural formula.
Table 1.
Pharmacokinetics of dutasteride 0.5 mg/day
| Bioavailability | 60% |
| Steady state | 3 months |
| Peak serum concentration | 2–3 hours |
| Volume of distribution | 511 liters |
| Elimination half-life | 5 weeks |
Caution should be used when administering the drug in patients with hepatic impairment, and in patients who take drugs that inhibit cytochrome-P3A4 (verapamil, diltiazem, and ritonavir) because the pharmacokinetics and risk of side effects may be altered. No other significant drug interactions have been identified.
In three pooled two-year studies, the most frequent side effects were impotence, decreased libido, gynecomastia, and ejaculatory dysfunction. The incidence of side effects compared with placebo was increased from 4% to 7.3% for impotence, 2.1% to 4.2% for decreased libido, 0.7% to 2.3% for gynecomastia, and 0.8% to 2.2% for ejaculatory dysfunction (Roehrborn et al 2002).
No dose adjustment is necessary in the elderly. Of 2617 men treated with dutasteride in three pivotal studies, 60% were age 65 and over and 15% were age 75 and over. In these studies no overall difference in safety or efficacy was observed between older and younger patients. No change in bone mineral density as measured on dual energy X-ray absorptiometry was observed, when compared with placebo. Plasma lipid profiles were unaffected (PDR 2004).
Dutasteride is not indicated for use in women and the pharmacokinetics have not been investigated in patients younger than 18 years. Due to potential adverse effects on normal development of the male reproductive tract, use of dutasteride should be avoided in men less than 18 years of age, and is absolutely contraindicated in women of reproductive age (PDR 2004).
Efficacy and safety of dutasteride
The efficacy and safety of dutasteride was studied in three parallel, randomized, placebo-controlled trials over a period of two years. A total of 4325 men age 50 years or older with moderate to severe symptomatic BPH (AUA symptom score of 12 or more and peak urinary flow rate of 15 ml/sec or less) and prostate volume greater than 30 cm3 were randomized to receive either dutasteride 0.5 mg/day or placebo. Primary endpoints measured were changes in prostate volume, peak urinary flow rate, risk of surgical intervention, safety and tolerability, and measurements of serum prostate-specific antigen (PSA), testosterone, and DHT (Roehrborn et al 2002).
The mean improvement in symptom score in the placebo groups was 2.3 (± 6.8) to 14.7 (± 7.2) from a baseline of 17.1 (± 6.1, p < 0.001). In the dutasteride groups, a 4.5-point (± 6.6) improvement to a mean of 12.2 (± 6.6) was significant compared with baseline of 17.0 (± 6.0, p < 0.001) and compared with placebo groups (p < 0.001). Peak urinary flow rates in the placebo groups increased by 0.6 ml/sec (± 4.7) to 11.2 ml/sec (± 4.8), which was significant compared with baseline of 10.4 ml/sec (± 3.6, p < 0.001). Peak urinary flow rates in the dutasteride groups increased by a mean of 2.2 ml/sec (± 5.2) to 12.5 (± 5.6), which was significant compared with baseline of 10.1 ml/sec (± 3.5, p < 0.001), and to the placebo groups (p < 0.001). After 24 months of therapy, the mean total prostate volume increased by 12.4% in the placebo groups and decreased by 20.4% in the dutasteride groups (p < 0.001). The relative risk of acute urinary retention in the dutasteride groups compared with the placebo groups was 0.43, or a risk reduction of 57% (p < 0.001). The relative risk of surgical intervention in the dutasteride groups compared with the placebo groups was 0.52, or a risk reduction of 48% (p < 0.001). Significant decreases in serum DHT, PSA, and an increase in serum testosterone were noted in the dutasteride groups compared with the placebo groups (p < 0.001 for each comparison) (Roehrborn et al 2002).
Adverse events attributed to the study drug were reported in 14% of men in the placebo groups and in 19% of men in the dutasteride groups. A higher proportion of men in the dutasteride groups than in the placebo groups reported impotence, reduced libido, ejaculatory dysfunction, and gynecomastia (Roehrborn et al 2002). Results are summarized in Table 2.
Table 2.
Adverse events from dutasteride efficacy and safety study
| Dutasteride | Placebo | |
|---|---|---|
| Impotence | 7.3% | 4.0% |
| Decreased libido | 4.2% | 2.1% |
| Ejaculation disorder | 2.2% | 0.8% |
| Gynecomastia | 2.3% | 0.7% |
Data from several open-label extensions of the above three parallel, randomized placebo-controlled trials have recently been reported. These studies show continued tolerability and efficacy after 48 months of treatment. In these trials less than 1% of the men withdrew from the open-label extension because of sexual function adverse events. Approximately 1% of men withdrew because of gynecomastia. Patients who received dutasteride during both study phases showed greater improvement in symptom scores and urinary peak flow rates than those initially receiving placebo (Roehrborn et al 2004, 2005; Schulman et al 2006).
Effect on quality of life
Data regarding the impact on quality of life of the 4325 men in the three randomized, double-blind, placebo controlled, 2-year studies detailed above have also been reported. At 2 years, dutasteride, but not placebo, resulted in clinically and statistically significant improvements from baseline in the BPH Impact Index (BII) score after 6 months of treatment. This net improvement from baseline increased from 6 months to 2 years (O’Leary et al 2003).
Additional data were recently reported from a separate prospective, multi-center, open-label study evaluating the improvement in symptoms and quality of life in patients with symptomatic BPH treated with dutasteride. Patients received dutasteride 0.5 mg/day for 24 weeks. The primary endpoint was the proportion of patients who achieved at least a 3-point decrease from baseline in International Prostate Symptom Score (IPSS). The secondary endpoints were changes in quality of life (IPSS item 8) and patient discomfort and satisfaction, assessed using the visual analogue scale (VAS).
A total of 366 patients from 72 centers were included in the study. After 24-weeks of treatment with dutasteride, 72.5% of patients achieved at least a 3-point reduction in IPSS. The mean IPSS score decreased from 15.3 (± 6.4) at baseline to 9.1 (± 5.6, p < 0.001) at 24 weeks. Patients with more severe symptoms had a higher probability of improvement in the IPSS. There were statistically significant decreases in all individual IPSS items, with greater improvement seen in items related to irritative and obstructive voiding symptoms than in those items related to storage symptoms.
Patient quality of life improved as assessed by IPSS item 8 with a mean improvement of 38.7% from baseline (p < 0.001). Patient discomfort and satisfaction also significantly improved. The mean VAS for discomfort decreased from 48.9 at baseline to 28.6 (p < 0.001) at 24 weeks. Patient VAS scores for satisfaction increased from a baseline at both 12 and 24 weeks (p < 0.001).
Overall, 77 (19%) patients had at least one adverse event related to dutasteride during the study period, including erectile dysfunction (7%), decreased libido (4%), and gynecomastia (2%) (Desgrandchamps et al 2006).
Dutasteride in prostate cancer prevention and treament
An in-depth discussion of the role of 5α-reductase inhibitors in the prevention and treatment of prostate cancer is beyond the scope of this review, however the clinical implications of long-term use of finasteride or dutasteride in the treatment of BPH must be considered in light of new information from recent trials.
In the Prostate Cancer Prevention Trial (PCPT) 18 882 men aged 55 years or older with a PSA of 3.0 ng/ml or less were randomized to receive finasteride 5 mg or placebo daily for 7 years. Prostate biopsies were performed at the end of the 7 year study period, or if the annual PSA level (adjusted for the effect of finasteride) was greater than 4 ng/ml, or if the digital rectal exam (DRE) was suspicious for prostate cancer. The primary endpoint measure was the diagnosis of prostate cancer. The study was terminated 15 months early on the recommendations of the data and safety monitoring committee because the study objective had been met. Prostate cancer was detected in 18.4% of men in the finasteride group and in 24.4% of men in the placebo group, a relative risk reduction of 24.8% (p < 0.001). However, of the men who developed prostate cancer, high-grade prostate cancer was reported in a higher proportion of men in the finasteride group than in the placebo group (37% versus 22%, p < 0.001) (Thompson et al 2003). Androgen deprivation therapy, including inhibition of DHT by finasteride, results in specific histopathologic changes in prostatic adenocarinoma, which can result in a “grading bias.” In the PCPT, most of the high-grade cancers were reported in the earlier phase of the study, a finding that is at odds with the development of more aggressive cancers with prolonged finasteride treatment (Bostwick et al 2004).
An ongoing study, the Reduction by Dutasteride of Prostate Cancer Events (REDUCE) Trial will use dutasteride in a group of men identified at increased risk of developing prostate cancer to determine if this dual 5α-reductase inhibitor will be an effective chemoprevention agent. Eligible men must be between ages 50 and 75 and have a PSA between 2.5 ng/ml and 10 ng/ml (age 50–60 years) or between 3.0 ng/ml and 10 ng/ml (age > 60 years), and a free PSA of 25% or lower at baseline. Prostate biopsies will be performed at 2 years and at 4 years to evaluate for prostate cancer. Additionally, free and total PSA will be measured every 6 months, with adjustment of the PSA of patients randomized to the dutasteride arm. The primary endpoint is biopsy-proven prostate cancer after 2 and 4 years of treatment. The study also aims to address the issue of high-grade tumors prospectively.
As discussed above, dutasteride inhibits both type 1 and type 2 5α-reductase isoenzymes, whereas finasteride inhibits only type 2. Type 2 5α-reductase is the predominant isoenzyme in normal and hyperplastic prostate tissue. Recent studies have shown that immunohistochemical staining of type 1 is enhanced in prostate cancer tissues compared with BPH epithelium. This suggests that a dual inhibitor of 5α-reductase isoenzymes, such as dutasteride, may be a reasonable agent to evaluate for prevention of prostate cancer (Gomella 2005).
As information from ongoing trials is compiled, physicians who recommend 5α-reductase inhibitors for the treatment of symptomatic BPH should discuss the effect of therapy on the histopathology of prostate biopsies and the potential for risk of high-grade prostate cancer with their patients.
In addition to its potential role in the prevention of prostate cancer, dutasteride has been evaluated as a component of neoadjuvant androgen deprivation therapy before brachytherapy for prostate cancer. In a recently reported study, 31 patients opted for cytoreductive therapy with bicalutamide 50 mg and dutasteride 0.5 mg daily. All patients underwent transrectal ultrasound volumetric study of the prostate gland, with ellipsoid volume determination of the prostate gland and transition zone, before initiation of the neoadjuvant therapy and at 3 months after initiation. After the 3-month course of combination therapy, the average prostate volume decreased by 33.6% using the volumetric determination, and decreased by 34.6% using the ellipsoid volume determination. The average transition zone volume decreased from 20.8 cm3 to 12.4 cm3, a reduction of 39.8% (Merrick et al 2006). Although these data are not the product of a randomized, placebo-controlled trial, the results are consistent with previous studies of androgen deprivation therapy, and dutasteride may be a reasonable alternative to the use of luteinizing hormone-releasing hormone (LHRH) agonists, which have been associated with major side effects
Conclusions
Dutasteride is the only dual inhibitor of 5α-reductase approved for use in the treatment of men with symptomatic BPH. Few drug interactions have been identified. The use of this drug is contraindicated in men under age 18 and in women due to the potential adverse effects on normal development of the male reproductive tract. Side effects occur in less than 5% of patients and include impotence, loss of libido, ejaculatory dysfunction, and gynecomastia. There is a potential risk of developing high-grade prostate cancer, although the overall risk of prostate cancer is reduced. Ongoing trials may support the use of dutasteride in the prevention of cancer and its role in the treatment of prostate cancer is also being evaluated. Treatment in men with moderate to severe lower urinary tract symptoms results in improved symptoms, increased urinary flow rate, a reduced risk of urinary retention and need for surgical intervention, and an improvement in quality of life. Inhibition of 5α-reductase reduces the clinical progression of BPH, an effect that is further enhanced by the addition of an α1-adrenergic receptor antagonist.
References
- Abramocicz M, editor. Dutasteride (Avodart) for benign prostatic hyperplasia. Med Lett Drug Ther. 2002;44:109–10. [PubMed] [Google Scholar]
- Arrighi HM, Metter EJ, Guess HA, et al. Natural history of benign prostatic hyperplasia and the risk of prostatectomy. The Baltimore Longitudinal Study of Aging. Urology. 1991;38(Suppl. 1):4–8. doi: 10.1016/0090-4295(91)80191-9. [DOI] [PubMed] [Google Scholar]
- Ball AJ, Fenely RCL, Abrams PH. The natural history of untreated “prostatism”. BJU Int. 1981;53:613–16. doi: 10.1111/j.1464-410x.1981.tb03273.x. [DOI] [PubMed] [Google Scholar]
- Bostwick DG, Qian J, Civantos F, et al. Does finasteride alter the pathology of the prostate and cancer grading? Clin Prostate Cancer. 2004;2:228–35. doi: 10.3816/cgc.2004.n.004. [DOI] [PubMed] [Google Scholar]
- Bramson HN, Herman D, Batchelor KW, et al. Unique preclinical characteristics of GG745, a potent dual inhibitor of 5AR. J Pharmacol Exp Ther. 1997;282:1496–502. [PubMed] [Google Scholar]
- Desgrandchamps F, Droupy S, Irani J, et al. Effect of dutasteride on the symptoms of benign prostatic hyperplasia, and patient quality of life and discomfort in clinical practice. 2006. BJU Int. 2006;98:83–8. doi: 10.1111/j.1464-410X.2006.06241.x. [DOI] [PubMed] [Google Scholar]
- Gomella LG. Chemoprevention using dutasteride: The Reduce Trial. Curr Opin Urol. 2005;15:29–32. doi: 10.1097/00042307-200501000-00007. [DOI] [PubMed] [Google Scholar]
- Jacobsen SJ, Girman CJ, Guess HA, et al. Natural history of prostatism: Longitudinal changes in voiding symptoms in community dwelling men. J Urol. 1996;155:595–600. doi: 10.1016/s0022-5347(01)66461-9. [DOI] [PubMed] [Google Scholar]
- Lepor H. Phase III multicenter placebo-controlled study of tamsulaosin in benign prostatic hyperplasia. Tamsulosin Investigator Group. Urol. 1998;51:892–900. doi: 10.1016/s0090-4295(98)00126-5. [DOI] [PubMed] [Google Scholar]
- McConnell JD, Roehrborn CG, Bautista OM, et al. The long-term effect of doxazocin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003;349:2449–51. doi: 10.1056/NEJMoa030656. [DOI] [PubMed] [Google Scholar]
- Merrick GS, Butler WM, Wallner KE, et al. Efficacy of neoadjuvant bicalutamide and dutasteride as a cytoreductive regimen before prostate brachytherapy. Urol. 2006;68:116–20. doi: 10.1016/j.urology.2006.01.061. [DOI] [PubMed] [Google Scholar]
- O’Leary MP, Roehrborn C, Andriole G, et al. Improvements in benign prostatic hyperplasia-specific quality of life with dutasteride, the novel dual 5α-reductase inhibitor. BJU Int. 2003;92:262–6. doi: 10.1046/j.1464-410x.2003.04310.x. [DOI] [PubMed] [Google Scholar]
- [PDR] Thompson Micromedics . Physicians desk reference. Montvale, NJ: Thompson MicroMedex; 2004. pp. 1456–9. [Google Scholar]
- Roehrborn CG, Boyle P, Nickel JC, et al. Efficacy and safety of a dual inhibitor of 5α-reductase types 1 and 2 (dutasteride) in men with benign prostatic hyperplasia. Urol. 2002;60:434–41. doi: 10.1016/s0090-4295(02)01905-2. [DOI] [PubMed] [Google Scholar]
- Roehrborn C, McConnell J, Barry M, et al. AUA guideline on management of benign prostatic hyperplasia (2003). Chapter 1: Diagnosis and treatment recommendations. J Urol. 2003;170:530–47. doi: 10.1097/01.ju.0000078083.38675.79. [DOI] [PubMed] [Google Scholar]
- Roehrborn CG, Schwinn DA. α1-Adrenergic receptors and their inhibitors in lower urinary tract symptoms and benign prostatic hyperplasia. J Urol. 2004;171:1029–35. doi: 10.1097/01.ju.0000097026.43866.cc. [DOI] [PubMed] [Google Scholar]
- Roehrborn CG, Lukkarinen O, Mark S, et al. Long-term sustained improvement in symptoms of benign prostatic hyperplasia with the dual 5α-reductase inhibitor dutasteride: results of 4-year studies. BJU Int. 2005;96:572–7. doi: 10.1111/j.1464-410X.2005.05686.x. [DOI] [PubMed] [Google Scholar]
- Schulman C, Pommerville P, Hofner K, et al. Long-term therapy with dual 5α-reductase inhibitor dutasteride is well tolerated in men with symptomatic benign prostatic hyperplasia. BJU Int. 2006;97:73–9. doi: 10.1111/j.1464-410X.2005.05909.x. [DOI] [PubMed] [Google Scholar]
- Thompson IM, Goodman PJ, Tangen CM, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349:215–24. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]