Abstract
The primary role of the immune system is to protect the organism against pathogens, but age-associated alterations to immunity increase the susceptibility of the elderly to infectious disease. The exact nature of these changes is still controversial, but the use of screening procedures, such as the SENIEUR protocol to exclude underlying illness, helped to better characterize the changes actually related to physiological aging rather than pathology. It is generally agreed that the most marked changes occur in the cellular immune response reflecting profound alterations in T cells. Much of this is due to thymic involution as well as changes in the proportions of T cell subpopulations resulting from antigen exposure, and altered T cell activation pathways. However, a body of data indicates that innate immune responses, including the critical bridge between innate and adaptive immunity, and antigen presenting capacity are not completely resistant to senescence processes. The consequences of all these alterations are an increased incidence of infections, as well as possibly cancers, autoimmune disorders, and chronic inflammatory diseases. The leading question is what, if anything, can we do to prevent these deleterious changes without dangerously dysregulating the precarious balance of productive immunity versus immunopathology? There are many potential new therapeutic means now available to modulate immunosenescence and many others are expected to be available shortly. One main problem in applying these experimental therapies is ethical: there is a common feeling that as ageing is not a disease; the elderly are not sick and therefore do not require adventurous therapies with unpredictable side-effects in mostly frail individuals. Animal models are not helpful in this context. In this chapter we will first briefly review what we think we know about human immunosenescence and its consequences for the health status of elderly individuals. We will then discuss possible interventions that might one day become applicable in an appropriate ethical environment.
Keywords: immunosenescence, T cells, phagocytic cells, nutrition, vaccination, exercise, CMV, inflammaging, IRP, immunorestorative therapies
Immunosenescence
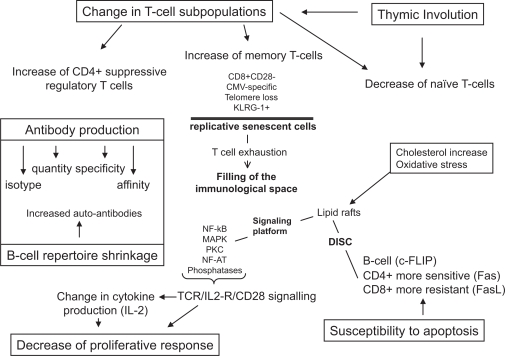
As human beings reach ever older average and maximal ages, the emergence of many diseases associated with aging becomes more apparent. The incidence of infections, cancers, and chronic inflammatory diseases such as atherosclerosis and neurodegenerative diseases increases with age (Wick et al 2000). Although we still do not know what is the exact cause of aging; we recognize that changes of the immune system play an important role both in the aging process and in the increase of age-related diseases (Meyer 2005). The immune system is a complex interactive system composed of many different players that are not all altered in the same manner and do not contribute equally to aging. We should perhaps conceptualize immunosenescence as dysregulation of a homeostatically constantly adapting system, the inputs and outputs of which are still only crudely defined, let alone the pathways linking these (Pawelec 2003). At the whole organism level, many studies have documented changes in endocrine and neural function, cardiovascular, muscle, and skeletal health, as well as regulation of glucose metabolism (Kyriazis 2005). It must be borne in mind that these diverse physiological changes also affect the immune system, although very few investigations address these issues, especially in humans. Although numerous studies on age-associated immune alterations exist (collectively known as “immunosenescence”) (Miller 1996; Pawelec and Solana 1997), the exact nature of these is still controversial because of differences between species, the lack of definition of physiological aging rendering difficult the exclusion of some latent disease states, nutritional, genetic, and environmental differences, amongst other factors (Ligthart 2001). However, the clinical consequences of the decreased immune response with aging seem quite clear. These are mainly the increased incidence and severity of infections, and possibly also cancers and autoimmune disorders (Meyer 2005). Many elderly subjects actually die from infections even if the cause of death given by the attending physician is very different. The hallmark of immunosenescence is the overwhelming decrease in T cell function with aging (Vasto et al 2006). There are also changes in the other parts of the immune system, but they are much less marked, and may often be secondary to changes in the T cells (not only the T cell-dependent B cells, but also innate components sensitive to T cell feedback, especially antigen-presenting cells [APC]) (Figure 1). Such age-related changes of the immune response are multifactorial, but it is reasonable to think that the extra-cellular milieu is very important.
Figure 1.
The immune system in the elderly. The function and parameters which change during aging are summarized in this figure.
Abbreviations: CMV, cytomegalovirus; DISC, death-inducing signaling complex; IL-2, interleukin-2; KLRG-1, killer cell lectin-like receptor subfamily G member 1; NF-AT, nuclear factor of activated T cells; NF-κB, nuclear factor κB; MAPK, mitogen-activated protein kinase; PKC, protein kinase C, TCR, T cell receptor.
Effects of the aging environment on the immune system
Cells of the adaptive immune system may be affected by nonspecific sequels of the aging process such as oxidative stress or glycation (Pahlavani and Harris 1998; Poggioli et al 2004). The former, resulting from an imbalance between the production of free radicals and antioxidant defences, is a characteristic of the aging process and may affect immune cells. The most susceptible molecules are DNA, proteins, and lipids. Indeed, DNA damage increases and repair decreases in lymphocytes with aging (Pawelec 2000). Alterations in lipids may lead to an increase of oxidised lipid products in the cell membrane such as 7-keto cholesterol which could interfere with effective lipid raft functioning in the membrane. The protein alterations induced by free radicals interfere with their functions and may compromise enzyme activity. The clearance of these modified proteins by molecules such as methionine sulfoxide reductases A (MrsA) is altered with aging (Petropoulos and Friguet 2005). The formation of advanced glycation end products (AGE) can alter cell functions and cause a constant inappropriate cellular stimulation with resulting telomere shortening. Together these nonspecific changes could contribute to a cellular stress response resulting in the low grade inflammation commonly observed in aged individuals. Finally, the role of protein-energy malnutrition (PEM) may be relevant, because as many as 5% of free living elderly suffer from PEM. This percentage is much higher (up to 50%) in hospitalized or frail elderly subjects. PEM is a general immunosuppressant, causing a decrease in delayed-type hypersensitivity (DTH), interleukin (IL)-2 production, and T cell proliferation as well as antibody responses (Lesourd 1997, 1999). Of course, this decrease in immune responses due to PEM may be relatively easily corrected by nutritional supplementation (High 2001). Among the necessary micronutrients required in addition to protein supplementation, the most studied has been zinc (Mocchegiani et al 2000a). Zinc deficiency has been linked to profound effects on cell-mediated and humoral responses. Malnutrition, via T cell function alteration, is a major risk factor for infections. Moreover, infections themselves induce malnutrition and generate a vicious circle between malnutrition and infection. One of the mechanisms induced by reactive oxygen species is DNA damage, particularly at the mitochondria level. Indeed such damage leads to decreased adenosine triphosphate (ATP) formation with ageing. In consequence, nutritional requirements are increased to compensate for this lack of ATP (Voorips et al 1993). This cannot compensate properly for mitochondrial inefficacy (Roberts et al 1994).
Adaptive immune system
Thymic involution
The thymus is the central organ for T cell maturation (Rubin and Kretz-Rommel 2001). Thus, from the outset, much attention was focused on the thymus as responsible for the defective production of naïve T cells, ie, those able to respond to newly encountered antigen, via alterations in the induction of their maturation and differentiation, as well as in the well-established reduction in their numerical output (Aspinall 2000). Experimental data in animals and humans clearly showed that age-associated involution of the thymus precedes the failure of T cell functions (Hirokawa and Utsuyama 1989; Fagnoni et al 2000). This leads to the explanation that thymic involution could be of major influence in immunosenescence. It is of note that the exact cause of this age-associated thymic involution is still not known although many parameters such as the hormonal status are involved (Hadden 1998). Unlike age-associated involution, thymic involution caused by stress, or pregnancy seems completely reversible (Rijhsinghani et al 1996). However, recent studies have shown that even the involuted thymus of probably most elderly people is still able to produce naïve T cells albeit at much reduced levels (Berzins et al 1998). Manipulations for prevention or reversing thymic involution including insertion of a transgene for a complete T cell receptor (TCR) (Lacorrazza et al 1999) or Fas, as well as extrathymic factors such as zinc, melatonin, IL-7, and thyroid hormones have been explored and sporadically reported to be effective in certain animal models (Virts et al 2006). These still controversial interventions will be discussed in greater detail later in this chapter.
Phenotype changes: role of continuous antigenic stimulation
Paralleling thymic involution, a change in peripheral T cell subpopulations is found in aging (Effros 2004). The number of naïve T cells decreases, while memory T cells increase. Many expansions of these memory T cells are within the CD8+ T cell subset, which are mostly negative for the important monomorphic T cell costimulator CD28 (Vallejo et al 1999). Moreover, these CD8+ memory T cells have shortened telomeres, suggesting an extensive replicative history. The loss of the CD28 co-receptor provides an explanation for the telomere shortening and proliferation arrest because signaling via this molecule is essential for telomerase induction and prevention of telomere shortening (Pawelec 2000). These cells may be in a replicatively senescent state in accordance with Hayflick’s limit (Effros and Pawelec 1997). They may fill the “immunological space” without being able to proliferate, to secrete IL-2 or to die by apoptosis. This leads to an alteration of T cell-mediated and T cell-dependent functions. Many of these CD8+ T cells in humans are found to carry receptors for cytomegalovirus (CMV) antigens (Khan et al 2002; Ouyang et al 2003). Because almost all of the elderly individuals during their life encounter many antigens, usually of infectious origin, but conceivably also cancer antigens, continuous antigenic stress exhausts the immune system and creates cells possessing a phenotype of replicative senescence. During normal ageing, accumulations of such “exhausted” T cells predominantly specific for persistent Herpes viruses occur (not only CMV, but also Epstein-Barr virus [EBV] and possibly herpes simplex virus [HSV]). These cells, mostly CD8+ T cells, but also some CD4+ T cells, commonly express inhibitory receptors such as the recently-described killer cell lectin-like receptor G1 (KLRG-1) (Ouyang et al 2003). Thus, the reason for much of this shift in T cell subpopulations towards CD8+ T cells terminally-differentiated memory cells may be infectious (Pawelec et al 2004).
Profound age-related changes occur also in the CD4+ T cell compartment, whether at rest or during activation. CD4+ T cells also require CD28 for adequate activation; with aging, the proportion of CD4+ cells expressing little or no CD28 increases significantly, along with telomere shortening in both CD4+ CD28− and CD4+ CD28+ populations (Valenzuela and Effros 2002). Other marked changes occur in CD4+ sub-populations as well, both in terms of the percentage of cells expressing a certain molecule, and the density of expression of that molecule. For example, interestingly, CD4+ CD25+ cells with low-level CD4 expression also accumulate in the blood of otherwise healthy elderly people (Dejaco et al 2006). This may parallel the reported age-associated increase in suppressive T regulatory cells (Gregg et al 2004). It has been shown that decreased levels of CD28 expression is directly related to a significant elongation of “reaction time”, required by the CD4+ cells stimulated in vitro before they start first mitosis (Witkowski and Bryl 2004). Interestingly, studies on centenarians did not reveal such a striking shift, suggesting that these selected “successfully aged” individuals were genetically and epigenetically resistant to these chronic viral infections or the exhausting effect of these infections on their immune system (Cossarizza et al 1996). Together, these and many other reported changes in T cell subpopulations seem to be universal and thus far inevitable in that they are present in all individuals, but their quantitative differences could contribute to differences in the impact of immunosenescence in a major way.
Alterations to T cell activation pathways
T cells need to be activated by an antigen presented by an APC (Friedl and Gunzer 2001). The best known of these are dendritic cells (DC), although monocytes/macrophages, B cells, and neutrophils are also able to present antigens. Once the antigens are presented in the context of MHC restriction, naive T cells become activated, produce IL-2 and other cytokines, and enter differentiation pathways resulting in the development of different T cell subsets, according to the priming conditions (Nel and Slaughter 2002). A universal requirement for any T cell response is marked proliferation to accomplish the clonal expansion critical for generating sufficient T cells to combat the source of antigen. Thereafter, controlled cell death (apoptosis) restores the numerical balance in the immune system, but results in the retention of memory T cells competent to respond more effectively to rechallenge by the same pathogen. Memory T cells possess different stimulatory requirements than naïve T cells, but both naïve and memory cells respond less effectively with age.
The primary receptor for T cell activation is the antigen-specific TCR which delivers “signal 1”. However, this is not sufficient for full activation, and to avoid anergy induction, the T cell requires a second signal, delivered by co-receptors (Nel and Slaughter 2002). Functionally the most important of these is designated CD28, which delivers the second signal for sustaining T cell activation (Riley and June 2005). In addition, activation via CD28 is very important for re-expression of the TCR, for the recruitment and stabilization of T cell lipid rafts to the “immunological synapse” and for the stimulation of the phosphoinositide kinase-3 (PI3K) signaling pathway (Tavano et al 2004). Furthermore, CD28 co-stimulation synergizes with TCR activation and induces IL-2, IL-4, IL-5, tumor necrosis factor (TNF), and granulocyte macrophage colony-stimulating factor production via nuclear factor-kappa B (NF-κB) activation (Wang et al 2004). More co-stimulation is required for the activation of naïve cells than for memory cells. Moreover, the activation of CD28 protects T cells from activation-induced cell death (AICD) (Borthwick et al 2000). It is now well-established that CD28 expression is decreased with aging in both CD8+ and CD4+ lymphocytes; however this affects more the CD8+ T cells (Larbi, Dupuis, et al 2006). This diminution of CD28 signaling can contribute to the decrease of telomerase up-regulation as well as to decreased IL-2 production, leading to decreased or delayed proliferation (Kaltoft 1998). Thus, changes either in co-receptor number or signal transduction could have far-reaching effects on T cell functions with aging including decreased proliferative responses. It is very important that in addition to providing the second signal, CD28 is also responsible for the activation of the cellular metabolism of proteins, lipids, and carbohydrates (Kane and Weiss 2003). Counterbalancing activatory costimulators such as CD28, there are also inhibitory co-receptors, such as the CD28-related cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4), amongst others. Changing relative levels of activatory and inhibitory receptors with age are likely to influence T cell activation.
Activation is the result of the formation of an immune synapse between the APC and the T cell via the recruitment of lipid rafts. Lipid rafts are membrane microdomains which are by their structures specialized in signal transduction (Simons and Ikonen 1997). They are mainly composed of cholesterol, sphyngolipids, and lipidated signaling molecules. Their coalescence (“lipid rafting”) is essential for signal transduction. Following their coalescence the molecule Lck is autophosphorylated, activating ZAP-70 and leading to the activation of linker of activated T cells (LAT). LAT is a transducing signaling molecule platform. From this point, several other signaling pathways are activated such the protein kinase C (PKC), mitogen activated protein kinases (MAPKs), and PI3K (He et al 2005). The decline in these signaling pathways leads to a decrease in transcription factors in T cells such as NF-κB and nuclear factor of activated T cells (NF-AT). NF-κB regulates the expression of many cytokines including IL-1, IL-2, IL-6, IL-8, TNF-α, and adhesion molecules (Wang et al 2004). These signaling pathways converge to assure an appropriate activation of T cells (He et al 2005).
There are several age-associated alterations in these T cell activation pathways, as has been shown in experimental animals (Sadighi and Miller 2005) and humans (Fulop et al 2005). The most important changes occur in CD4+ T cells resulting in the decreased production of IL-2 and clonal expansion (Schindowski et al 2002). There are no changes in TCR number at the cell surface, but the number of CD28 molecules decreases with aging (Vallejo et al 1999). Thus, although the antigen receptor numbers do not decrease, their functions are altered. Almost all molecules of the signaling pathways elicited by TCR or IL-2R were found to be altered in T cells with aging (Pawelec et al 2001; Larbi, Douziech, et al 2004). There is a decrease in the early tyrosine phosphorylation and generation of second messengers, calcium mobilization, and the translocation to the membrane of PKC. The intermediate steps of the signaling pathways including Raf-Ras-MAPKs activation are also decreased. This decline of transmembrane signaling leads to the decreased activation of transcription factors, especially NF-κB and NF-AT (Pahlavani et al 1995; Ponnappan, Trebilcock, et al 1999; Ponnappan, Zhong, et al 1999). As these factors play a crucial role in the expression of genes involved in the immune response, their decrease leads to the altered production of cytokines with aging in T cells. These are mainly IL-2, IL-6, and TNF-α (Rink et al 1998). Investigations in the late 80’s already suggested that biochemical and biophysical alterations of the cell membrane could be responsible for the altered immune response with aging (Maczek et al 1998). Alterations in the lipid composition and fluidity of the cell membrane were found. The situation was further clarified by the discovery of lipid rafts (Simons and Ikonen 1997; Laude and Prior 2004). As mentioned above, both the composition and function of lipid rafts are critical for T cell activation (Taner et al 2004). Cholesterol, the major stabilizing component of lipid rafts, is increased two-fold with aging and this effectively renders the membrane less fluid or more rigid (Larbi, Dupuis, et al 2004; Larbi, Douziech, et al 2004). This decreases the possibility of recruiting signaling molecules such as Lck and LAT, resulting in impaired coalescence and altered immune synapse formation. Recently, a group led by Miller showed that CD4+ T cells from old mice, which are the most affected by the aging process, have defects in their activation process and a deficiency in the formation of immunosynapses during stimulation by antigen-presenting cells. They showed that CD4+ T cells from old mice express unusually high levels of glycosylated forms of the T cell glycoprotein CD43, particularly on a subset of functionally anergic cells expressing P-glycoprotein. T cells from old mice also show a decline in the association of CD43 with cytoskeletal matrix and in the proportion of T cells that can exclude CD43 from the synapse (Garcia and Miller 2003; Garcia et al 2005). These data support the idea that changes in T cell surface glycosylation could play an important role in immunosenescence. Currently we do not know why this alteration of the membrane occurs with aging. An intrinsic alteration of cholesterol metabolism in the elderly could contribute to these lipid changes (Simons and Ikonen 2000). Thus, an alteration in the membrane composition of T cells with aging seems to play a fundamental role in their decreased functions and as such in immunosenescence.
Recently, the role of counter-activating tyrosine and serine/threonine phosphatases was investigated in T cell activation (Hermiston et al 2002). There is more and more experimental evidence that the balance between tyrosine kinases and phosphatases is essential for the maintenance of the resting state and for activation (Hamerman and Lanier 2006). Only a few data exist concerning phosphatase activity in T cells with aging. Our own studies using cholesterol repletion of T cells from young subjects, being a partial aging model of T cells (to mimic the increased T cell membrane cholesterol content seen in the elderly) suggested alterations in the phosphatase activities (our unpublished data). Furthermore, as alterations have been found concerning Src homology domain-containing protein tyrosine phosphatase-1 (SHP-1) activity in neutrophils (Fortin et al 2006), it can be supposed that phosphatase activities might be largely altered in T cells with aging as well.
Taken together, it can be concluded that with aging there is an alteration in T cell activation due to a dysregulation of the intracellular signaling pathways via an alteration of the T cell membrane composition leading to altered functions such as proliferation and IL-2 production.
Activation-induced T cell death (AICD)
Once clonally-expanded T cells have cleared the source of antigen, the majority should die to terminate the immune response (Gupta 2000; Larbi and Fulop 2004). As alluded to above, this occurs through apoptosis, which is a tightly regulated cellular process, involving several death receptors such as Fas (CD95). There is a fine balance between the anti- and pro-apoptotic molecules in T cells, with an important contribution from molecules of the Bcl-2 family and caspases (Werner et al 2002). The latter are either initiators (eg, caspase 8) or executioners (eg, caspases 3, 7, and 9). Mitochondria are important players in this death process. AICD is a major mechanism to avoid the accumulation of superfluous T cell clonal expansions (Fas et al 2006). Dysregulation of this essential control mechanism in the elderly would be expected to cause many problems. It was recently shown that AICD is increased in specific T cell subsets including the CD4+ apoptosis-sensitive T-helper 1 cells (Th1) from old mice, as a direct consequence of their decreased levels of CD28-mediated co-stimulation, which otherwise may protect stimulated cells from apoptosis (Hsu et al 2005). One of the mechanisms by which CD28 signaling protects against apoptosis is by preventing CD95 ligand (FasL) up-regulation and by increasing the expression of the anti-apoptotic proteins c-FLIP short and Bcl-xL (Kirchoff et al 2000). Increased susceptibility of CD4 cells to apoptosis may thus contribute to immunosenescence (Pawelec et al 1996) whereas the reciprocal may be true in CD8 cells. Thus, it has been shown that defective Fas-mediated apoptosis is one of the major mechanisms associated with the accumulation of senescent CD8+ T cells in very old mice. Decreased AICD of CD8+ T cells was found in old mice compared with CD4+ T cells which may contribute to the expansion of CD8+ T cells at the expense of CD4+ T cells with aging (Effros 2004). This decreased apoptosis in CD8+ T cells is primarily associated with defects in the up-regulation of FasL after activation and a decrease in Fas apoptosis signaling (Hsu et al 2001). It is of note that the susceptibility to AICD of T cell subsets is differential and dependent not only on the expression of CD28 but also on other intracellular and extracellular factors, including also aberrant T cell receptor downstream-signaling pathways. These findings were further corroborated by our own studies showing that AICD is also delayed in T-lymphocytes from elderly donors by impaired Fas expression following stimulation (Larbi, Muti, et al 2006). Lipid raft disrupting agents such as methyl-b-cyclodextrin have also been used to demonstrate that lipid rafts play a role in these age-related events of apoptosis commitment and resistance. Because Fas did not localize to lipid rafts with aging during AICD, in contrast with young subjects, we can hypothesize that the formation of the death-inducing signaling complex (DISC) is also impaired in the elderly (Larbi, Muti, et al 2006). In contrast, it was found that lymphocytes from elderly humans over-expressed CD95 at the protein as well as the mRNA level, which induces apoptosis in the presence of FasL during AICD (Gupta et al 2004). Both the density of CD95 expressed on lymphocytes as well as the number of CD95+ T cells is progressively increased during the course of aging and reaches the highest levels in donors of the sixth through the seventh decades. Identical findings were reported on mitogenic stimulation of T cells from elderly subjects. CD95 levels then decrease again at greater ages and in the very oldest donors, values are the same as those observed in young adults. The decrease is mainly due to the loss of CD4+ T cells because of their increased sensitivity to apoptosis (Salvioli et al 2003). Not only is the expression of CD95 increased with age, but this is linked to increased CD95-induced apoptosis as well as to constitutive activity of caspase-3 and 8 (Gupta et al 2003). It can therefore be concluded that in aging there is a progressive imbalance between the apoptosis of CD4+ and CD8+ T cells. As discussed here, T cell activation can also initiate their apoptosis. It seems paradoxical that although CD4+ T cells maintain levels of CD28 to a greater extent than CD8+ T cells, it is the latter that are more resistant to apoptosis. This could, however, be due to defective CD28 signaling in CD4+ T cells. In fact, although CD8+ T cells lose their CD28 co-receptor more readily, they are intrinsically less dependent on CD28 for activation than CD4+ T cells (Larbi, Muti, et al 2006). However, in the elderly, many are in a replicatively senescent state, and are correspondingly resistant to apoptosis and activation (Gupta et al 2005). Thus, the activation of T cell subsets sets the stage for their susceptibility to apoptosis. Alterations in apoptosis of T cells may be an important mechanism in immunosenescence and related diseases such as autoimmunity.
B lymphocytes
There is much less information on the functional changes of B lymphocytes with aging. We know that the quantity of specific antibodies is decreased (Lazuardi et al 2005). In addition, changes in antibody specificity, isotype, affinity, and affinity maturation have been described in old age (Dailey et al 2001; Howard et al 2006). This manifests itself in the clinical observation that vaccination in elderly subjects with a new antigen such as influenza or tetanus is much less effective in terms of resulting specific antibody response compared with young subjects, although the perceived problem in this context is most likely primarily T cell deficiencies. The dysregulation of the humoral immune response leads to the production of low-affinity immunoglobin M (IgM) antibodies (LeMaoult et al 1997). It has also been shown that the B cell repertoire is changed during the aging process. The expression of the RAG-1 gene could be a major influence (Ben Yehuda et al 1994) in this age-related change. One other possibility to explain such age-related decreases in antibody responses is the E2A-encoded transcription factor E47, which regulates many B cell functions including class switch recombination and somatic hypermutation, and which is altered with ageing (Frasca et al 2005). Thus, the age-associated loss of ability to generate an efficient humoral immune response results in part from reduced B cell generation (Banerjee et al 2002). This may in turn fill the immunological space with antigen-experienced B cells. Since their Ig repertoire is shrunken (to previous antigenic stimulation), these cells make a lower response (in quality) to new antigenic stimulation (Johnson and Cambier 2004). At the same time, the quantity of auto-antibodies (both organ specific and nonspecific) increases. During life many self proteins become transformed to nonself as result of changes to their structure due to free radical-mediated oxidative damage or glycation. Thus, these proteins begin to be seen as foreign by the immune system and induce the production of a new type of auto-antibodies.
The notion that CD27 is a marker of memory B cells is now becoming accepted (Agematsu et al 2000). Signaling via CD27 promotes the differentiation of memory B cells to immunoglobulin-secreting cells. The proportion of CD27+ CD19+ memory B cells is increased whereas the proportion of CD27− CD19+ naïve B cells is significantly decreased (Gupta et al 2005). This may cause the reduced antibody response to novel antigens. Moreover, it has been shown that following B cell receptor stimulation in aged mice the cells did not undergo apoptosis to the same extent as in the young. This was explained by a higher level of cellular FLICE-inhibitory protein (cFLIP) expression that leads to procaspase inactivation. Together, this could result in the persistence of self and nonself specific B cells (Montes et al 2006). This is correlated with the fact that B cell clonal expansion persists in old mice along with the appearance of B cell lymphomas (Szabo et al 2004).
While reduced proliferative responses in aging is a well-known phenomenon (Whisler et al 1991), the signaling defects resulting in this in B cells are mainly unknown. For instance, PKC activity as well as protein tyrosine kinases (PTK) phosphorylation is significantly reduced in B cells from aged donors following Ig stimulation (Whisler and Grants 1993). Further studies are needed to understand the role of B cell signaling in age-related changes. It is of note that dendritic cells were also shown to exhibit age-related changes that ultimately lead to decreased B cell response (Aydar et al 2003).
Innate immune system
The innate immune response is composed of phagocytic cells, natural killer (NK) cells, and the complement system. Its role is as the first barrier to any invaders and to alert the adaptive system to intruding pathogens. It provides a very efficient system to initiate the inflammatory process and to finish it by the restitutio ad integrum. For many years, the consensus was that the innate immune response does not change appreciably with aging. However, more sophisticated recent analyses indicate that there are age-associated alterations, decreasing either defence capacity or its modulatory activity towards adaptive immune responses (Sudeghi et al 1999; Fulop et al 2004).
Phagocytic cells
The first cells to arrive at the site of invasion or tissue lesions are neutrophils, followed by monocytes. Their main functions are chemotaxis, phagocytosis, and the killing of the invaders (Mortensen and Zhong 2000). Macrophages kill bacteria, viruses, parasites, and tumor cells either directly or through the release of various mediators such as IL-1, TNF-α, and interferon (IFN)-γ. These mediators are able to influence and activate other immune cells (Scapini et al 2000). All these activities depend on specific receptors initiating various signaling pathways. The chemotaxis is mediated by cell adhesion molecules towards a gradient of chemokines. Recognition is via toll-like receptors (TLRs) which recognize more or less specifically the pathogen associated molecular pattern (PAMP) of the aggressors (Medzhitov 2001). Finally, effector functions are mediated by the Fc-receptor family and complement receptors. It is now accepted that the innate immune response not only has direct effector functions, but can also influence the adaptive immune response (Medzhitov and Janeway 2000).
Experimental results showed that some phagocytic cell functions were altered even in healthy SENIEUR elderly (Fulop et al 2001). The most affected functions were chemotaxis and intra- and extracellular killing, whereas phagocytosis seemed to be conserved with aging. However, these data are still controversial even now, with most investigators continuing to believe that the innate immune response does not change with age, despite accumulating evidence showing that the number of activated CD14dim/CD16bright monocytes is increasing with aging (Sudeghi et al 1999). Concomitantly the production of proinflammatory cytokines IL-1 and IL-6 is also increasing. Additionally, the tumor cell cytotoxicity of monocytes is decreased with aging because of decreased free radical production under stimulation (Fulop et al 1984; Provinciali and Smorlesi 2005). Similarly, neutrophils were shown to mediate decreased cytotoxicity towards tumor, bacteria, and yeast cells. Moreover, recently it became evident that neutrophils may contribute to the modulation of the adaptive immune response via their various cytokine and chemokine products, and that this is also impaired with aging (Scapini et al 2000). Although the number of receptors such as TLRs does not seem to change on human neutrophils and monocytes, there is clearly an alteration in their activation. This contrasts with the decrease of TLR numbers found on macrophages of old mice (Boehmer et al 2005). However, in both cases the functions of TLRs were found to be altered with aging. Ligands for TLR2 are Gram-positive bacteria, while a Gram-negative bacterial product, lipopolysaccharide (LPS), is a ligand for TLR4; both of them are found on neutrophils. The signal transduction pathway initiated by these interactions is mediated initially by an adaptor molecule, MyD88, recruiting various serine-threonine kinases, IL-1 receptor-associated kinases (IRAKs), and finally leading to NF-κB translocation (O’Neill 2006). Among IRAK family proteins, IRAK-4 and IRAK-1 play major roles in signal transduction under LPS stimulation. However, for some TLRs such as TLR3, the signal transduction pathway is independent from the activation of MyD88 pathway (Yamamoto et al 2002). Recently, TLRs were also found to move into lipid rafts on cell activation. The displacement of TLRs to lipid rafts in neutrophils is deficient with aging, leading to an alteration of IRAK activation (Fulop et al 2004). This in turn leads to the alteration of NF-κB activation and ultimately to altered chemokine and cytokine production. Thus, the recognition-induced activation of phagocytic cells is altered with aging. The FcR-dependent killing by neutrophils and monocytes, including free radical and protease production, are severely impaired with aging. This leads to decreased efficiency of elimination of the pathogen. Thus, besides reductions in their phagocytic activity, all the other activities of these cells are also altered with aging (Burns 2004).
Antigen presentation
It is often said that there are few or no age-associated changes in the very important initiating and regulatory function of APC for the adaptive immune response. However, aging may affect the antigen presenting capacity of these cells by influencing their antigen processing capacity, the presence of costimulatory receptors such as CD80/CD86 on their surface, or the levels of cytokines produced in their microenvironment (Miller et al 1994; Donnini et al 2002). Recently, it was shown that the proteasome activity which is essential to antigen processing is altered with aging (Carrard et al 2002). Proteasomes are multi-catalytic enzymes involved in the turnover of most cellular proteins and are also responsible for generating antigenic peptides. We do not know exactly which components of the proteasome are altered with aging, either expression, changes in the proteasome subunits or the formation of inhibitory molecules. Nevertheless, with aging, it seems that the structure and the activity of the proteasome is affected (Ponnappan, Zhong, et al 1999). Thus, it can be supposed that antigen processing via the degradation of the ingested protein is altered with aging. Moreover, very recently it has been shown that localized antigen selection influenced by TLR4 signaling could impact on the maturation of MHC class II complexes (Blander and Medzhitov 2006). Moreover, the aging environment is also favorable to the premature activation and maturation of DC, for example, due to increased levels of TNF-α, oxidative stress, prostaglandins, and IL-6. Moreover, IL-6 can favor the development of monocytes into macrophages instead of DC (Chomarat et al 2000). These cells become mainly inflammatory cells and not APC, further increasing the inflammatory state in aging.
An important alteration affecting APCs is costimulatory activity. For effective and sustained T cell activation, the co-receptors of T cells must be activated and triggered by structures on the APC (Friedl and Gunzer 2001). Alterations either in the number or signal transduction capacity of co-receptors leads to a decreased T cell response. One of the most important and well-characterized co-receptors on T cells is the CD28 molecule, as already described above. CD28 interacts with CD80 and CD86 on APC. Probably the numbers of major histocompatibility complex (MHC)-II, CD80, and CD86 co-receptors on APC do not change appreciably with aging, according to most available data. However, little data exists concerning changes of other co-receptors such as CD40L (CD154) and ICOS, which is a third member, together with CD152, of the CD28 co-receptor family (Carreno and Collins 2002). ICOS may modulate immune responses by increasing the secretion of IL-10. Preliminary data indicate that with aging there is an increase in ICOS expression on T cell clones (Pawelec 2000). Recently, it has been shown that LFA-4, an integrin receptor, may also act as a co-stimulatory molecule in synergy with CD28. The CD154/CD40 ligand pair also seems to be decreased with ageing. This area also requires deeper exploration in the near future for a better understanding of immune response changes and the appearance of immune-related diseases with ageing.
Co-stimulatory activity is also dependent on the pattern recognition receptors including the TLRs. As mentioned, these TLRs recognize the PAMPs shared by large groups of microbial components which result either in the signaling of the presence of pathogens to APCs or the processing of the pathogens for antigen presentation. In aged mice, the expression of TLRs has been shown to decrease (Boehmer et al 2005). In contrast, in humans their number was not decreased, but their function decreased. These changes, either in TLR number in mice or in TLR function in humans have important consequences for antigen presentation resulting in altered activation of the innate and adaptive immune response with aging. TLR stimulation ultimately results in the secretion of proinflammatory cytokines which recruit cells of the adaptive immune system. That is why the function of TLRs is very important not only for an adequate innate response, but also for the adaptive immune response.
Apoptosis
The termination of the inflammatory process must be tightly regulated. This is mediated as in the case of T cells by the death of the participating cells via apoptosis. The most studied example is the apoptosis of neutrophils (Fulop et al 2004). They die spontaneously without proinflammatory stimulation; however, in the presence of proinflammatory stimuli they can survive for at least 72 hours. Neutrophils cannot be rescued from apoptosis by proinflammatory cytokines in the aged as opposed to the young. This is probably the result of altered survival signaling pathways with aging. These specific pathways include the janus kinases and signal transducers and activators of transcription (Jak/STAT), PI3K, and MAPK pathways (Larbi et al 2005; Fortin et al 2006). The signaling alterations result in an imbalance of the Bcl-2 pro- and anti-apoptotic family members. While the expression of the anti-apoptotic molecule Mcl-1 is decreased, that of the pro-apoptotic molecule Bax is increased. This favours a pro-apoptotic status even under proinflammatory stimulation (Fulop et al 2002). There is also an increase in caspase 3 activity in neutrophils with aging (Fortin et al 2006). The underlying mechanisms responsible for all these signaling alterations are not fully elucidated.
NK cells
Natural killer cells are also an important part of the innate immune system because of their capacity to recognize cells altered either as a result of viral infections, oxidative modifications, or carcinogenic transformation. Cytotoxic effector functions are mediated by granules containing granzyme B and perforin. There are clear alterations of NK cells and their functions in aging (Borrego et al 1999). These changes have mostly been studied and described in old mice and much less is known in humans, although age-associated increases in numbers of NK cells have often been described, together with a decrease of cytotoxicity at the single cell level. There are contradictory data concerning NK cell cytotoxicity in response to IL-2 or IFNs. Most studies showed that these cytokines used alone or in combination elicited better NK cytotoxic responses in the elderly than in young subjects (Sansoni et al 1993). However, recent data seem to support an alteration in the proliferative capacity of NK cells stimulated by IL-2, as well as a decrease in CD69 expression. Signal transduction as assessed by Ca2+ mobilization was also found to be altered in NK cells of healthy elderly subjects (Borrego et al 1999). Similar contradictory data exist for lymphokine-activated killer cells (LAK). NK cells are also able to modulate positively or negatively the adaptive immune response via secreted products. The production of cytokines by resting or activated NK cells such as IFN-γ or chemokines such as IL-8 are found to be decreased in healthy elderly subjects.
Thus, phagocytic and NK cells play a crucial role in the alteration of the immune response with aging not only by presenting intrinsic changes but also by the dysregulation, via the APC, of the adaptive immune response. The further study of TLRs is expected to unravel many unsuspected interactions during immunosenescence.
Clinical importance of immunosenescence
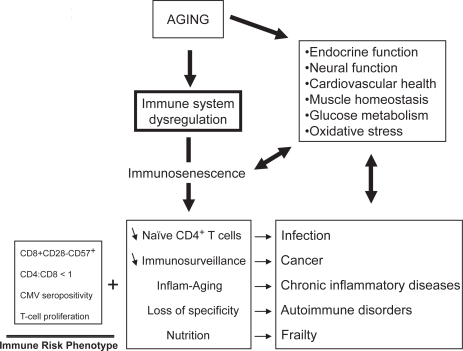
The exact consequences of immunosenescence are very difficult to assess with certitude. However, the clinical observations tend to indicate that the alteration of the immune response with aging has very serious clinical consequences (Figure 2). This deregulated immune response may be responsible for the increased incidence of infections, cancers, autoimmune disorders, chronic inflammatory diseases, as well as subsequently decreased longevity (Castle 2000; Wick et al 2000; Gavazzi and Krauze 2002; DeVeale et al 2004). A direct effect of immunosenescence on infections, cancers, and autoimmune disorders can be easily conceptualized and many experimental data seem to support this contention. The relationship between immunosenescence and the increased incidence of infections with aging can be primary. As the number of naïve CD4+ T cells decreases, the possibility of mounting an effective adaptive immune response against a new pathogen also decreases (Bryl et al 2000, 2004). The deregulation of the immune system leads to a general alteration of the T cell response, a decrease in specificity and loss of memory. In cases of failure to clear tumors, it is hypothesized that one reason may be decreased immunosurveillance. This idea is derived from the findings that tumor regression was observed in immunocompetent hosts while cancer incidence increased in immunocompromised hosts (Salih and Nussler 2001). Nevertheless, it is still an important challenge to be able to link the age-related increase of cancers to decreased immune responses. This would mean that the modulation of the dysregulated immune response with aging could have some influence on cancer occurrence (Pawelec, Koch, Griessemann, et al 2006). Recently, it has been shown that the increased tumor incidence with aging was associated with decreased cytotoxic T lymphocytes (CTL) activity in mice immunized with DCOVA together with an increase of NK1.1+CD3+NKT cells suppressing the proliferation of T cells (Shi et al 2005). These results may explain why the efficiency of DC vaccination is decreased in aged mice. Moreover, immune parameters specifically related to the age of colorectal cancer patients, such as higher levels of C3, C4 and immunosuppressive acidic protein (IAP), and lower T cell stimulation index could contribute to cancer development and explain why they respond to a lesser extent to adjuvant immunochemotherapy than immunologically intact young subjects (Munemoto et al 2004). However, a direct demonstration of a causal link between immunosenescence and infections or tumors is still missing.
Figure 2.
The aging process and its clinical consequences.
The relationship between immunosenescence and the chronic inflammatory processes leading to age-related diseases is more difficult to understand. However, recent reports suggest that changes occurring in immunosenescence including the accumulation of anergic CD8+ CD28− cells are also observed in diseases such as ischemic heart disease, neurodegeneration, and other syndromes (Jonasson et al 2003). Moreover, the deregulation of cytokine production is also observed in these chronic inflammatory conditions, ie, an increase in proinflammatory cytokines such as TNF-α, IL-6, and IL-1 as well as an increase of the antiinflammatory cytokines such as IL-10, IL-4, and IL-15, as posited by the InflammAging hypothesis (Franceschi et al 2000b). A specific association has been demonstrated between elevated TNF-α and dementia (Cacquevel et al 2004). An identical association between TNF-α and the endothelial dysfunction which is the first step initiating the atherosclerotic process has also been demonstrated (Zhang et al 2006). Vascular wall inflammation is important in plaque formation, progression, and plaque rupture. Thus, age-related immune deregulation associated with chronic inflammation and the suppression of the adaptive immune response leads to these chronic inflammatory processes. The increasing prevalence of this inflammation over decades leads to the development of clinically significant pathological conditions such as cardiovascular diseases, dementia, diabetes mellitus, osteoporosis, Parkinson’s disease, and arthritis (Tracy 2003). It was also demonstrated, albeit in a limited number of subjects, that a decrease in T cell functions significantly correlated with a higher mortality after 24 months of follow up in these persons (De Martinis et al 2005). This proinflammatory status was also linked to the frailty syndrome of elderly subjects (Schmaltz et al 2005). This suggests that the decreased T cell functions are correlated with a decreased longevity probably via the occurrence of life threatening diseases such as infections and cancer. The chronic inflammation has important nutritional consequences such as the increased secretion of cortisol (Sapolsky et al 1986, 1987) and thus an attrition of the muscular mass. This latter is also due to the decrease of muscular synthesis from the 5th decade. Muscular attrition is directly linked to the decreased amount of substrate for protein synthesis of the immune response which in turn leads to a decreased efficiency of the immune response. The chronic inflammation is a result of the persistence of the pathogen combined with muscular attrition.
Immune risk phenotype
If the decrease of the immune response has such devastating clinical consequences, can we identify immunologically frail subjects at risk of morbidity and mortality? We believe so and have identified an immune risk phenotype (IRP), derived from the OCTO-NONA longitudinal studies (Wikby et al 1994; Ferguson et al 1995; Olsson et al 2000). The IRP includes increased levels of CD8+ CD28− CD57+ cells, CD4:CD8 ratio <1, decreased mitogen-stimulated T cell proliferation and CMV seropositivity, suggesting that chronic CMV antigen stimulation can drive CD8+ cells into a state of replicative senescence, with parallel homeostatic decreases in the number of CD4+ cells and CD4/CD8 ratio in IRP individuals (Wikby et al 2005). There are also other parameters which might be relevant to refinement of the IRP in future. Possible candidates are cytokines such as IL-6 and TNF-α which also predict morbidity and mortality in some circumstances. They are already strongly linked to frailty in elderly subjects (Ferrucci et al 2002). The measurement of C reactive protein (CRP) is also a useful tool since CRP synthesis is dependent on IL-6 (Park et al 2005).
Interventions to restore the dysregulated immune response with aging
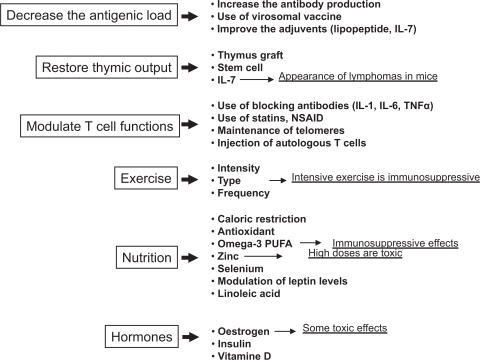
Having completed the brief wide-ranging survey of age-associated immune alterations, with emphasis on what is known in humans, and having in places alluded to possibilities for therapeutic interventions, we are now finally in a position to consider the latter in more detail. So we must ask whether immunosenescence is subject to modulation and whether effective immunosupportive therapies exist. What makes the answer difficult to find in humans is the fact that the development of immunosenescence takes decades and its clinical manifestations are progressive and could be mingled with other manifestations of aging. Moreover, as dysregulated immunity predominates in the elderly, it is often difficult to know which changes are really beneficial or harmful. Biomarkers of immunosenescence are unreliable and therefore monitoring interventions is problematic. Nevertheless, considering the alterations and the putative causes of immunosenescence, we would suggest that three major objectives are emerging: decrease the antigenic load either acute or chronic, but especially the latter, with the concomitant restoration of changes in T cell subpopulations; immunorestoration of the thymic environment to increase the naïve cell output; maintain or enhance T cell function (activation) of remaining intact cells (Figure 3). Some interventions can be considered as preventive, such as nutrition and exercise, while others could be curative/supportive in helping to circumvent damage already done. It must be clearly stated at the outset: no well-established therapy beneficially impacting on the very complex nature of immunosenescence is yet known due to the absence of knowledge of the real primum movens. Nonetheless, we will start with those which could have a general preventive effect, and continue with those which are more specific for a given alteration.
Figure 3.
Preventive/curative strategies to counteract the aging immune system. The strategies depicted are those under current investigation or those proposed in this chapter.
Abbreviations: NSAID, nonsteroidal antiinflammatory drugs; PUFA, polyunsaturated fatty acids.
Overview of possible interventions
It is well known that physical exercise as well as a good nutrition is related to a conserved robust immune system in aged persons. Hormonal products may also influence the activities of the immune system, among which thymic peptides and thyroid hormones were shown to modulate and even restore deficient NK function in old mice. The use of some cytokines, such as IL-2, IL-7, IL-12, or IFN-γ, may be promising to improve immune cell functions. The use of stem cells and thymic reconstitution could have beneficial effects by restoring the naïve cell compartment. The expansion and banking of autologous T cells for immunorestoration purposes is currently under intense investigation. The maintenance of telomere length in T cells despite aging could help to restore their proliferative capacity. Manipulation of lipid metabolism and modulating the cholesterol content of T cell membrane could also be helpful. Vaccination for eradication of chronic infections might help to reduce anergic CD8+ CD28− T cell numbers and increase the naïve CD4+ T cell number. Manipulations aimed at selective reduction of dysfunctional cells or their restoration to full function may be other options.
Nutrition
It is well accepted that nutrition can influence or even play a leading role in the development of various diseases such as infections, cancer, and cardiovascular diseases. All these diseases are linked directly or indirectly to the immune response. Many studies have therefore been devoted to examining the effects of nutrition on the various aspects of the immune response in various age-groups and diseases (de Castro and Stroebele 2002). Indeed, some of the age-related changes considered in our brief survey above could simply reflect nutritional alterations occurring with aging, the most frequent of which is PEM. Even in SENIEUR (Ligthart 2001) and nutritionally near-SENIEUR subjects, alterations showing nutritional variables contributing to immunosenescence, but not explaining all the changes, were identified (Lesourd 1999; Lesourd and Mazari 1999). These results are consistent with studies in calorie-restricted rodents which live longer and have better immune responses than animals fed adlibitum. The use of “functional” foods seems to influence many cellular parameters which can help to decrease the deleterious effect of the aging process (Kilara and Panyam, 2003). In this context, macronutrients such as antioxidants, dietary fibre, omega-3 polyunsaturated fatty acids (PUFAs), as well as micronutrients such as vitamin E, zinc, iron, copper, and selenium are of particular interest (Reinhold et al 1989; Mocchegiani et al 2000b; Ahluwalia 2004). Proteins in PEM or micronutrients may be of importance, but the equilibrium needed between those able to slow down the ageing process is still unknown (Lesourd et al 2002).
Thus, nutritional interventions could be beneficial for the prevention, retardation or even reversal of established immunosenescence. The best example is caloric restriction without undernutrition in animals, which prolongs the maximal life span and has beneficial effects by maintaining a normal immune response even in aged animals. Until now, with a very few notable exceptions (Walford et al 2002), this intervention could not be implemented and systematically studied in humans, but groups of enthusiasts are currently performing heroic, some would say foolish, studies on themselves. In contrast, large numbers of supplementation studies have been performed, and some beneficial effects in aged individuals have been obtained by using vitamins and minerals and/or antioxidants (Lesourd 1997). In Zn-deprived mice, Zn supplementation led to a normalization of thymic architecture and functions (Mocchegiani et al 2004). Peripherally, the Zn supplementation was also beneficial as by increasing T cell and NK cell activities. Zinc and other trace elements, such as selenium, showed some benefit also in elderly subjects by increasing T lymphocyte proliferation and DTH (Santos-Neto et al 1992). However, their efficacy in preventing or reducing morbidity due to immune-related pathologies could not be demonstrated in elderly persons (Provinciali et al 1998). Of the common vitamins, vitamin E has been widely used and has shown several relatively clear beneficial effects, eg, it restores depressed Th1 responses, downregulating prostaglandin E2 synthesis, increasing response to influenza vaccination, decreasing oxidative stress measured by lipid peroxidation products, and increasing NK cell activities (Meydani et al 1997, 2005). It is of note that vitamin B12, folic acid, and Zn administered in vivo could also beneficially influence neutrophil functions (Moroni et al 2005).
Recently, it has been shown that the levels of leptin, a peptide hormone, secreted by adipocytes are elevated with aging more in men than in women. This may be related in some way to the observed loss of appetite evolving to anorexia during aging (Di Francesco et al 2006). Moreover, this hyperleptinemia associated with PEM can contribute to the increased susceptibility to infections via reduced Th1, DTH responses and increased TH2 response (Amati et al 2003). Thus modulation of the leptin levels by nutritional or pharmacological interventions could restore the appetite and consequently the shift from Th1 to Th2 response.
Lipids could have powerful antiinflammatory effects that could be used advantageously in combating the low grade inflammation of aging and in the chronic inflammatory processes of some age-related diseases such as autoimmune diseases, atherosclerosis, or Alzheimer’s disease. One possibility is using conjugated linoleic acid (CLA). This has been shown to have anticarcinogenic, antiatherogenic, and antidiabetic properties, correlating with a favorable effect on certain aspects of immune function even in healthy humans, such as increased lymphocyte proliferation, and decreased proinflammatory cytokine secretion (Tricon et al 2005). It was also shown that it could increase the response to hepatitis B vaccination even in the elderly (Albers et al 2003). The n-3 PUFA have been shown to have potent anti-inflammatory properties when used as adjuvant therapies in autoimmune diseases, osteoporosis and cognitive decline (Fernandes et al 2003; Calder 2003; Kelley et al 2005). Nevertheless, their most important effect is the decrease of the risk and severity of cardiovascular diseases originating from atherosclerosis, a chronic inflammatory condition (Kris-Etherton et al 2003). Their activities are related to the modulation of proinflammatory cytokines. Thus, their use in the prevention or modulation of established immunosenescence could be promising and deserves further study.
Lipids can also have an immunomodulating activity through their capacity to modulate the membrane micro environment. The cell membrane is not as homogeneous as previously believed, but, as previously mentioned, contains lipid rafts enriched in cholesterol, gangliosides, and signaling molecules (Simons and Ikonen 1997). It was shown that with aging in various immune cells there is an alteration in the membrane fluidity rendering these lipid rafts less functional (Rivnay et al 1980, Larbi, Douziech, et al 2004). Since the 80s, preparations called active lipids (AL 721) (Rabinowich et al 1987; Provinciali et al 1990) have been used to increase immune cell functions. This has led to some success as NK cell activities could be increased in vivo. More recently, the use of human high-density lipoprotein (HDL) to modulate T cell functions has been tested. This lipid is able to extract the accumulated cholesterol from lipid rafts, resulting in increased signal transduction via TCR (Duffy and Rader 2005). Preliminary results showed that T cell proliferation and IL-2 production in T cells of the elderly could be enhanced by in vitro administered HDL (Fülöp et al unpublished data). More research is needed to establish whether the statin-induced decreased low-density lipoprotein (LDL) in vivo and corresponding potential HDL increase could have similar results.
Together, data obtained from experimental animals and from human studies indicate that appropriate nutrition is a powerful but in principle very safe immunorestorative means to modulate immunosenescence. However, specific supplementation seems only needed where there is a deficiency resulting in concomitant pathologies. Furthermore, each separate intervention has only partial effects as not all immune functions are restored and over-supplementation can of course even be harmful (Bogden et al 1990; Chandra 2002; Bogden 2004; Stephensen and Kelley 2006). Thus, the best way to use nutrition as a modulator of immunosenescence is to have an optimal diet from the very beginning throughout the entire lifespan, combined with other interventions. In this way nutrition could play a real preventive role.
Exercise
The interaction of physical activity and the immune response is very complex (Albers et al 2005). This depends on the current status of the immune system as well on the intensity, the type, and the frequency of exercise. Nevertheless, exercise has been proposed as a potential means to modulate dysregulated immune responses with aging. Although studies have resulted in contradictory results, it seems that exercise may have some immunorestorative properties on the decreased immune response with aging (Kohut and Senchina 2004; Smith et al 2004; Kohut et al 2005). Long-term moderate exercise may be the best candidate for this purpose. Acute and heavy exercise, in contrast, seems to have immunosuppressive effects. The beneficial effects of exercise have been noted for T cell function, antibody production, and macrophage responses. Moreover, the imbalance between the Th1 and Th2 responses in the elderly also benefited, as well as the naïve and memory cell imbalance (Karanfilov et al 1999). However, many questions remain to be answered before exercise can be considered as a bonafide immunorestorative intervention in aging. The most important of these is related to the intensity and the dose of exercise and to the duration of the effects. The long-term clinical effects should be evaluated by future controlled trials. The identification of the mechanisms by which the appropriate exercise regimen exerts beneficial effects may help us better understand the problems contributing to the immunosenescent state.
Cytokines and hormones
One of the most striking functional alterations observed in T cells is the decrease of the production of IL-2. This cytokine is essential for the clonal proliferation of antigen-reactive T cells and for antibody production. One study was designed to enhance antibody production to influenza vaccination using low does IL-2 as an adjuvant. It was reported that low dose IL-2 treatment was an effective means of enhancing the antibody response to influenza virus antigens in elderly subjects, without serious toxicity (Provinciali et al 1994). Unfortunately, there are only a few studies advocating the success of such intervention, perhaps solely this one.
Other means to intervene in dysregulated immune responses may be by hormonal modulation rather than cytokine application (Arlt and Hewison 2004). It is now accepted that the existence of a neuro-endocrine-immune network implies that hormonal replacement can have beneficial effects on immunity in the elderly as well. It seems that estrogen can have powerful immunomodulating effects, albeit mainly during stress, by modulating the innate immune response (Kovacs et al 2004; Kovacs 2005). Another hormone which could play an important role in modulating and maintaining the immune system is insulin, which can directly influence innate immunity via shared signaling pathways involving PI3K (DeVeale et al 2004). Thus, insulin resistance developing with aging even at the level of innate immunity could have antiinflammatory effects (Pickup and Crook 1998). Restoring insulin sensitivity by drugs such as glitazone could therefore have a beneficial immunorestorative effect in the elderly via the modulation of peroxisome proliferator-activated receptor (PPAR)γ (Berstein et al 2005). Insulin-like growth factor-1 (IGF-1) has also been shown to promote the survival and function of peripheral T cells as well as increasing the function of B cells, NK cells and macrophages. It is well known that the IGF-1 level decreases with age, so future trials could be warranted to establish whether its supplementation or increase through other hormones (ie, growth hormones) would be an effective restorative strategy (Bonkowski et al 2006). However, the potentially toxic effects including glucose intolerance, edema, and arthralgia may limit this approach.
Dehydroepiandrosterone (DHEA) has been considered for immunorestoration, because it dramatically decreases with age in both sexes. The direct action of DHEA is still questionable as no specific receptor has been identified. It seems that it acts by its intracrinologic transformation to sex steroids (Labrie et al 2000). Nevertheless, DHEA has been shown to increase IL-2 production and NK cell activity and to decrease circulating IL-6 levels (Suzuki et al 1991; Gordon et al 2001). These effects can be highly beneficial in elderly individuals, but no consistent in vivo data on immune effects of DHEA supplementation in healthy elderly humans have been reported. DHEA has been suggested to be able to antagonize many of the immunosuppressing effects of cortisol (Rook et al 1994). Thus the administration of DHEA for immunorestoration is still hazardous and needs more well-controlled longitudinal studies.
Other hormones with immune regulatory effects including melatonin, growth hormone, and IGF-1 have various but controversial effects on the aging immune system. The hormone-like vitamin, vitamin D (1,25(OH)2D3), may also be included in this group: it has been suggested that it could intervene in the interaction between APCs and T cells, and as such modulate T cell activation (Hewison et al 2003). Vitamin D insufficiency, a common problem in apparently healthy elderly people, could lead to the disruption of immune tolerance and play a role in the development of chronic inflammatory disease and the autoimmune disorders often seen with aging. This could be related to the failed up-regulation of T regulatory cells for suppressing autoreactive T cells (Adorini 2003). As most elderly people nowadays are under Vitamin D treatment for osteoporosis, it would be worth evaluating the long-term effects of this therapy on immunity.
Strategies to reduce the infectious antigenic load
In this context it is not only vital to recognize the role of acute infections that can further exhaust a declining immune system, and attempt to reduce them by vaccination strategies. It could be even more important to recognize the existence of chronic latent infections, mainly Herpes viruses, providing a continuous antigenic load and chronic stimulation resulting in exhaustion of the T cells involved (Pawelec et al 2004). Not only persistent viruses, but also the presence of subclinical bacterial infections might also contribute to chronic antigenic stress, as well as the tumors that are probably very common but not clinically dominant in the majority of the elderly. Thus, strategies combating these sources of chronic stimulation could have a general immunorestorative role on age-associated immune dysfunction.
Vaccinations are critically important to maintain good health in the elderly, in the face of their declining immune competence. Even in the immunocompromised elderly, vaccinations can be beneficial in preventing pneumococcal pneumonia, influenza, and tetanus. The elderly may not achieve an adequate antibody response, but most are able to mount some level of response (McElhaney 2005). The problem with the present form of conventional split or subunit vaccines is that they are not optimal for stimulation of cell-mediated immunity, in particular, CTL activity. There is an ongoing research effort to develop strategies for enhancing antibody production in the elderly by vaccines targeting both the humoral and the cellular immune response. New strategies seem to be promising, mainly virosomal vaccines tested primarily for influenza so far. Virosomes are lipid-based antigen delivery systems using phospholipids to build reconstituted virus envelopes which can be used for vaccination, closely mimicking the natural virus (Huckriede et al 2005). Thus, functionally reconstituted influenza virosomes preserve the receptor binding and membrane fusion activity of the viral hemagglutinin. Because virosomes lack viral RNA, binding and fusion to cells does not result in their infection. Virosomes are able to activate the humoral and the cellular immune system with great efficacy (Bayes et al 2006). Furthermore, the incorporation of lipopeptide adjuvant activating TLRs would be of great usefulness as in this way DC activation can be controlled via the induced cytokine profile and respectively the nature and the magnitude of the adaptive immune response. Virosomal influenza vaccine has been shown to confer a high degree of serological protection in healthy elderly subjects and subjects at risk for influenza with low pre-vaccination antibody titers, comparable with protection in young adults (De Bruijn et al 2004).
The combination of improved vaccines, such as described above, with immuno-enhancing agents could be of further benefit in the elderly. In this context, mainly the use of IL-2 and IL-7 has been considered (Sin et al 2000). IL-7 could increase the maintenance or even expansion of effector memory helper T cells responding to influenza. General adjuvants have also been used for many years, such as the alum-based human vaccines (Cox and Coulter 1997). However, the adjuvant properties of alum are only minimally effective in elderly compared with young subjects. Therefore there is a great need to develop more effective adjuvants. In an aged mouse model, prokaryotic DNA containing unmethylated cytidine phosphate guanosine (CpG) motifs yielded strong enhancement of the immunization effects of hepatitis B vaccine (Qin et al 2004).
An increased frequency of re-vaccination has also been suggested as a way of improving outcome in the elderly, eg, for tetanus (McElhaney 2005). Moreover, in the future it can be imagined that a vaccine could be developed against chronic, nondirectly lethal viral infections which can cause persisting antigenic stimulation, thereby exhausting the immune repertoire. These viruses are mainly the CMV, HSV, EBV and to lesser extent in the elderly perhaps HIV. The possibility of using these vaccines would dramatically increase the immunocompetence of elderly subjects mainly if we consider the “infectious nature” of immunosenescence as discussed above (Koch et al 2006). At this moment there is no major viable anti-CMV vaccine (Pawelec, Koch, Gouttefangeas, et al. 2006). However, Oxman and colleagues (2005) published a large clinical trial on the utilization of a vaccine to prevent Herpes Zoster and post-herpetic neuralgia in older adults. This is not a vaccine aimed to eradicate the latent herpes zoster (VZV) infection but to significantly decrease VZV clinical infections. The effectiveness of the high potency Oka vaccine probably results from the restoration to some extent of VZV-specific T cells to a level above the threshold for herpes zoster clinical manifestations either by reversing a gradual decline from the original expansion of VZV-specific memory T cells or by substituting immunization for exogenous or endogenous re-exposures that could boost immunity (Arvin 2006). This implies that immunization against VZV, even if it cannot boost the immune system sufficiently to eradicate the virus, could still be strong enough to enhance acquired immunity to control the clinical manifestations of a persistent pathogen. This could, however, diminish the immune system-exhausting effect of this persistent virus. This could open new avenues for other types of vaccination against latent immunosuppressing viruses. Finally, the reduction of the viral load, as in HIV infections as well as in herpes zoster, can be used. The newer generations of antiviral drugs could be used with an acceptable safety profile in the elderly, such as acyclovir and famcyclovir (Scheinfeld 2005). These agents could reduce viral load and extend the time to exhaustion for viral antigen-specific T cells.
Identification and treatment of subclinical infections could be valuable targets for clinical interventions with beneficial effects on immunity as well, for example, the very common urinary tract infections in the elderly. The search for and eradication of these continuous sources of antigenic stimulation could also have an impact on immune response maintenance with aging. The same argument regarding occult sources of antigen applies to tumors, which, while being immunogenic, remain indolent in the elderly.
Restoration of the thymus
The rejuvenation of a functional thymus is likely to be another necessity for maintaining or restoring effective immunity with ageing. This would require that thymic activity is increased without compromising positive and negative thymic selection and that the newly produced naïve thymic cells are released to the periphery (and not stopped by homeostatic feedback from a “full” periphery). Several possibilities can be envisaged to help to maintain the function of the thymus (Sutherland et al 2005). The first is physical grafting of functionally intact thymus to old subjects but this approach clearly has ethical and logistic problems in elderly people. However, transplantation of cultured thymic fragments to neonatal patients with DiGeorge syndrome has been quite successful (Markert et al 1999). Second, rapidly progressing stem cell research could also eventually result in the generation of thymic epithelial progenitor cells for reconstitution. Third, the use of IL-7 has been explored. IL-7 is a cytokine playing a crucial role in the development and maintenance of the peripheral T cell pool. Thymus IL-7 content decreases with age, leading to decreased thymic output of naïve cells. This was the rationale for treatment strategies administering IL-7, which induced some degree of thymic rejuvenation in mice (Aspinall 2006). There are currently many unresolved questions on IL-7 therapy before it could be used in elderly humans, first and foremost being the lymphoma genesis seen in mice. This could be overcome by more efficient targeting of the cytokine to the thymus, rather than systemically using high doses. Thus, the advance of gene therapy designing vectors specific for the thymus would be a major breakthrough in the thymus rejuvenation trials.
Antiinflammatory agents
As discussed above, aging is accompanied by low grade inflammation, held responsible for many of the pathologies developing in the elderly and termed “Inflammaging” (Franceschi et al 2000b). There is an unbalance between the functioning of the innate and the adaptive immune systems manifested by the increase of soluble mediators of Th1 type such as IL-6, TNF-α, and IL-1. In several autoimmune diseases such as rheumatoid arthritis (RA) or multiple sclerosis (MS), blocking antibodies and soluble receptors specific for inflammatory cytokines such TNF-α have had notable therapeutic efficacy (Owens 2002). Thus, the age-associated increase in proinflammatory cytokines and increased autoimmune disease in the elderly, raise the possibility that some sort of proinflammatory cytokine blocking antibodies or soluble receptors could also be beneficial for the elderly. Here, anti-TNF-α, anti-IL-1, anti-IL-6, and anti-IL-15 chimerized monoclonal antibodies could be applied. Soluble recombinant cytokine receptor-Ig fusion proteins could be also useful. However, there are clear drawbacks to general antiinflammatory therapy, as this response is appropriate at times; indeed, in cardiac disease and sepsis their use was detrimental, and they could also increase the susceptibility of individuals to some infections such as tuberculosis.
There are other less potent well-known inflammation-modulating drugs that have few side effects and can be used with safety even in very old subjects. Such drugs include statins, and NSAIDs. These are pleitropic drugs having multiple targets in the inflammatory response. They could act in preventing several age-related immune-associated diseases such as atherosclerosis, dementia, and sarcopenia. Large longitudinal studies were already conducted and prove the beneficial effect of such drugs (Foody et al 2006). The statins developed and used primarily for atherosclerosis prevention and treatment have also shown significant beneficial effects on susceptibility to a wide variety of cancers (Pahan 2006). There are several other drugs which could have anti-inflammatory effects, such as those targeting either the selectins (pan-selectin inhibitors), TLR, the NF-κB pathway by inhibiting inhibitor κB kinase (IKK) activation, or the matrix metalloproteinases (MMPs). These strategies would not aim to completely restore the altered immune response with aging but could attenuate some of the consequences due to the low grade inflammation and its clinical sequele.
Modulating telomere loss: Expression of the catalytic component of telomerase
CD8+ T cells show the greatest telomere length erosion, a sign of proliferation due to chronic antigenic stimulation (Effros 2004). It is tempting to suggest interventions aimed at restoring the telomere repeats. An obvious approach could be telomerase gene therapy. However, there are multiple problems with this approach including eventual carcinogenesis. A possibly safer target of intervention could be the repression of telomere binding protein (TRF1). This has not yet been sufficiently explored for use in therapy. It is also conceivable that the erosion of telomeres could be prevented in the first place. Some data using antioxidants suggest that this might be possible even after several cell division cycles (Zhang et al 2002). Vaccination to decrease chronic viral load exhausting the immune system would be also beneficial for telomere length maintenance. Thus, an effective approach to diminish telomere loss would be of great importance to avoid the accumulation of replicative senescent T cells with aging.
In vitro expansion of immunocompetent T cells and their reintroduction in the aging organism
T cells of patients with immune deficiency cannot efficiently proliferate in vivo by the usual antigenic stimulation. These T cells, however, can be expanded in vitro efficiently in the presence of immobilized anti-CD3 Ab and IL-2. The ideal T cells for in vitro expansion are obtained and stored in liquid nitrogen, when individuals are young and healthy. When individuals need immunocompetent T cells in later life, the frozen T cells can be thawed and expanded in vitro 500- to 1000-fold or more. Roughly speaking, 1 × 1010 T cells can be obtained from 20 ml of peripheral blood, stored in liquid nitrogen. These autologous activated T cells propagated in vitro could be infused into patients or frail elderly people and immunological restoration can be expected. A recent paper reported that immunotherapy with activated T cells propagated in this way did not enhance or promote autoimmune phenomena (Yamaguchi et al 2004). This procedure could be very promising however clearly more research is needed before its routine clinical use.
Modulation of the glycosylation of membrane proteins
As previously mentioned, the group led by Miller (Garcia and Miller 2003; Garcia et al 2005) has shown a deleterious role of glycosylated membrane proteins including CD43 in CD4+ T cells of old animals. They have also shown that the function of mouse CD4+ T cells can be augmented by treatment with an enzyme, O-sialoglycoprotein endopeptidase (OSGE), which cleaves surface CD43, suggesting the idea that the high levels of glycosylated CD43 found on T cells from aged mice may contribute to immunosenescence. Recently they have also obtained data to support the notion that glycosylated surface proteins hinder CD8 T cell activation and function in both young and old mice, and raise the possibility of significantly improving CD8 T cell function in older individuals through enzymatic alteration of surface glycoproteins (Sadighi et al 2006). New results from the same group (Berger et al 2006) now show that OSGE improves T cell function even in mice lacking CD43, indicating that other glycoproteins must contribute to the OSGE effect on function. Modulation of the glycosylation status of the membrane proteins participating in T cell activation could be another promising new avenue for the modulation of the deregulated aging immune system.
Conclusion
There is a large corpus of experimental data suggesting that the adaptive immune response, mainly the T cell response, is deregulated with aging. Evidence is also accumulating to suggest that the innate immune response is altered as well. The exact causes of this deregulation are not known but for T cells, thymic involution, changes in the distribution of subpopulations, and defective T cell signal transduction are likely to be major contributors. The latter two alterations are likely to be the consequences of T cell clonal exhaustion either by chronic antigenic stimulation or by metabolic alterations. It is of note that despite a wealth of data, the exact changes in the immune system with aging are still controversial because of the confounding influence of the physiological aging process and genetic as well as epigenetic factors. Thus, the field urgently needs to agree upon a set of biomarkers of immunosenescence to be applied preferably in careful longitudinal studies. However, what we do know is that the changes in the immune response lead to various diseases. The incidence of disease is dramatically increased with aging. These diseases are primarily of an infectious nature, but probably also include cancer, autoimmunity, and chronic inflammatory diseases. The definition and clinical use of an IRP to predict those at risk of incipient mortality could facilitate a major breakthrough in the prevention and modulation of immune-related morbidity and mortality. Some means to intervene in compensating for immune dysregulation which could be applied in the elderly without ethical or regulatory problems do already exist. These include balanced nutrition in macro and micronutrients, including foods such as vegetables and fruit, functional foods such as probiotic-containing yoghurt, sustained moderate aerobic exercise regimens, and could be relatively easily extended to include better vaccination against different pathogens especially against influenza, pneumococcus pneumoniae, and in the near future against varicella zoster, possibly CMV, and possibly application of certain antiviral drugs and low-dose cytokines. Nevertheless, there is still a long way to go to implement more specific and effective safe immunorestorative therapies, even at the level of specific nutrients or drugs. Thus, a better understanding of immunosenescence by intensive research and the development of new methods and strategies to intervene in its evolution are essential for improving the quality of life of the increasingly large elderly population.
Acknowledgments
This work was partly supported by a grant-in aid from the Canadian Institute of Health Research (No 63149), ImAginE (EU contract QLK6-CT-1999-02031), ZINCAGE project (EU contract n. FOOD-CT-2003-506850), T cells and Aging “T-CIA” (QLK6-CT-2002-02283) and the Deutsche Forsc-hungsgemeinschaft (SFB 685-B4).
References
- Adorini L. Tolerogenic dendritic cells induced by vitamin D receptor ligands enhance regulatory T cells inhibiting autoimmune diabetes. Ann N Y Acad Sci. 2003;987:258–61. doi: 10.1111/j.1749-6632.2003.tb06057.x. [DOI] [PubMed] [Google Scholar]
- Agematsu K, Hokibara S, Nagumo H, et al. CD27: a memory B cell marker. Immunol Today. 2000;21:204–6. doi: 10.1016/s0167-5699(00)01605-4. [DOI] [PubMed] [Google Scholar]
- Ahluwalia N. Aging, nutrition and immune function. J Nutr Health Aging. 2004;8:2–6. [PubMed] [Google Scholar]
- Albers R, Antoine JM, Bourdet-Sicard R, et al. Markers to measure immunomodulation in human nutrition intervention studies. Br J Nutr. 2005;94:452–81. doi: 10.1079/bjn20051469. [DOI] [PubMed] [Google Scholar]
- Albers R, van der Wielen RP, Brink EJ, et al. Effects of cis-9, trans-11 and trans-10, cis-12 conjugated linoleic acid (CLA) isomers on immune function in healthy men. Eur J Clin Nutr. 2003;57:595–603. doi: 10.1038/sj.ejcn.1601585. [DOI] [PubMed] [Google Scholar]
- Amati L, Cirimele D, Pugliese V, et al. Nutrition and immunity: laboratory and clinical aspects. Curr Pharm Des. 2003;9:1924–31. doi: 10.2174/1381612033454252. [DOI] [PubMed] [Google Scholar]
- Arlt W, Hewison M. Hormones and immune function: implications of aging. Aging Cell. 2004;3:209–16. doi: 10.1111/j.1474-9728.2004.00109.x. [DOI] [PubMed] [Google Scholar]
- Arvin A. Aging, immunity, and the varicella-zoster virus. N Engl J Med. 2005;352:2266–7. doi: 10.1056/NEJMp058091. [DOI] [PubMed] [Google Scholar]
- Aspinall R. T cell development, ageing and Interleukin-7. Mech Ageing Dev. 2006;127:572–8. doi: 10.1016/j.mad.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Aspinall R. Longevity and the immune response. Biogerontology. 2000;1:273–8. doi: 10.1023/a:1010046532657. [DOI] [PubMed] [Google Scholar]
- Aydar Y, Balogh P, Tew JG, et al. Altered regulation of Fc gamma RII on aged follicular dendritic cells correlates with immunoreceptor tyrosine-based inhibition motif signaling in B cells and reduced germinal center formation. J Immunol. 2003;171:5975–87. doi: 10.4049/jimmunol.171.11.5975. [DOI] [PubMed] [Google Scholar]
- Banerjee M, Mehr R, Belelovsky A, et al. Age- and tissue-specific differences in human germinal center B cell selection revealed by analysis of IgVH gene hypermutation and lineage trees. Eur J Immunol. 2002;32:1947–57. doi: 10.1002/1521-4141(200207)32:7<1947::AID-IMMU1947>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Bayes M, Rabasseda X, Prous JR. Gateways to clinical trials. Methods Find Exp Clin Pharmacol. 2006;28:121–42. [PubMed] [Google Scholar]
- Ben Yehuda A, Szabo P, Dyall R, et al. Bone marrow declines as a site of B cell precursor differentiation with age: relationship to thymus involution. Proc Natl Acad Sci U S A. 1994;91:11988–92. doi: 10.1073/pnas.91.25.11988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger SB, Sadighi Akha AA, Miller RA, et al. CD43-independent augmentation of mouse T cell function by glycoprotein cleaving enzymes. Immunology. 2006;119:178–86. doi: 10.1111/j.1365-2567.2006.02419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berstein LM, Tsyrlina EV, Vasilyev DA, et al. The phenomenon of the switching of estrogen effects and joker function of glucose: similarities and relation to age-associated pathology and approaches to correction. Ann N Y Acad Sci. 2005;1057:235–46. doi: 10.1196/annals.1356.018. [DOI] [PubMed] [Google Scholar]
- Berzins SP, Boyd RL, Miller JF. The role of the thymus and recent thymic migrants in the maintenance of the adult peripheral lymphocyte pool. J Exp Med. 1998;187:1839–48. doi: 10.1084/jem.187.11.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blander JM, Medzhitov R. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature. 2006;440:808–12. doi: 10.1038/nature04596. [DOI] [PubMed] [Google Scholar]
- Boehmer ED, Meehan MJ, Cutro BT, et al. Aging negatively skews macrophage TLR2- and TLR4-mediated pro-inflammatory responses without affecting the IL-2-stimulated pathway. Mech Ageing Dev. 2005;126:1305–13. doi: 10.1016/j.mad.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Bogden JD, Oleske JM, Lavenhar MA, et al. Effects of one year of supplementation with zinc and other micronutrients on cellular immunity in the elderly. J Am Coll Nutr. 1990;9:214–25. doi: 10.1080/07315724.1990.10720372. [DOI] [PubMed] [Google Scholar]
- Bogden JD. Influence of zinc on immunity in the elderly. J Nutr Health Ageing. 2004;8:48–54. [PubMed] [Google Scholar]
- Bonkowski MS, Rocha JS, Masternak MM, et al. Targeted disruption of growth hormone receptor interferes with the beneficial actions of calorie restriction. Proc Natl Acad Sci U S A. 2006;103:7901–5. doi: 10.1073/pnas.0600161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrego F, Alonso MC, Galiani MD, et al. NK phenotypic markers and IL2 response in NK cells from elderly people. Exp Gerontol. 1999;34:253–65. doi: 10.1016/s0531-5565(98)00076-x. [DOI] [PubMed] [Google Scholar]
- Borthwick NJ, Lowdell M, Salmon M, et al. Loss of CD28 expression on CD8(+) T cells is induced by IL-2 receptor gamma chain signalling cytokines and type I IFN, and increases susceptibility to activation-induced apoptosis. Int Immunol. 2000;12:1005–13. doi: 10.1093/intimm/12.7.1005. [DOI] [PubMed] [Google Scholar]
- Bryl E, Gazda M, Foerster J, et al. Age-related increase of frequency of a new, phenotypically distinct subpopulation of human peripheral blood T cell expressing lowered levels of CD4. Blood. 2000;98:1100–7. doi: 10.1182/blood.v98.4.1100. [DOI] [PubMed] [Google Scholar]
- Bryl E, Witkowski JM. Decreased proliferative capability of CD4+ cells of elderly people is associated with faster loss of activation-related antigens and accumulation of regulatory T cells. Exp Gerontol. 2004;39:587–95. doi: 10.1016/j.exger.2003.10.029. [DOI] [PubMed] [Google Scholar]
- Burns EA. Effects of aging on immune function. J Nutr Health Aging. 2004;8:9–18. [PubMed] [Google Scholar]
- Cacquevel M, Lebeurrier N, Cheenne S, et al. Cytokines in neuroinflammation and Alzheimer’s disease. Curr Drug Targets. 2004;5:529–34. doi: 10.2174/1389450043345308. [DOI] [PubMed] [Google Scholar]
- Calder PC. N-3 polyunsaturated fatty acids and inflammation: from molecular biology to the clinic. Lipids. 2003;38:343–52. doi: 10.1007/s11745-003-1068-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrard G, Bulteau AL, Petropoulos I, et al. Impairment of proteasome structure and function in aging. Int J Biochem Cell Biol. 2002;34:1461–74. doi: 10.1016/s1357-2725(02)00085-7. [DOI] [PubMed] [Google Scholar]
- Carreno BM, Collins M. The B7 family of ligands and its receptors: new pathways for costimulation and inhibition of immune responses. Annu Rev Immunol. 2002;20:29–53. doi: 10.1146/annurev.immunol.20.091101.091806. [DOI] [PubMed] [Google Scholar]
- Castle SC. Clinical relevance of age-related immune dysfunction. Clin Infect Dis. 2000;31:578–85. doi: 10.1086/313947. [DOI] [PubMed] [Google Scholar]
- Chandra RK. Nutrition and the immune system from birth to old age. Eur J Clin Nutr. 2002;56(Suppl 3):S73–6. doi: 10.1038/sj.ejcn.1601492. [DOI] [PubMed] [Google Scholar]
- Chomarat P, Banchereau J, Davoust J, et al. IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nature Immunol. 2000;1:510–14. doi: 10.1038/82763. [DOI] [PubMed] [Google Scholar]
- Cossarizza A, Ortolani C, Paganelli R, et al. CD45 isoforms expression on CD4+ and CD8+ T cells throughout life, from newborns to centenarians: implications for T cell memory. Mech Ageing Dev. 1996;86:173–95. doi: 10.1016/0047-6374(95)01691-0. [DOI] [PubMed] [Google Scholar]
- Cox JC, Coulter AR. Adjuvants—a classification and review of their modes of action. Vaccine. 1997;15:248–56. doi: 10.1016/s0264-410x(96)00183-1. [DOI] [PubMed] [Google Scholar]
- Dailey RW, Eun SY, Russell CE, et al. B cells of aged mice show decreased expansion in response to antigen, but are normal in effector function. Cell Immunol. 2001;214:99–109. doi: 10.1006/cimm.2001.1894. [DOI] [PubMed] [Google Scholar]
- De Bruijn IA, Nauta J, Gerez L, et al. Virosomal influenza vaccine: a safe and effective influenza vaccine with high efficacy in elderly and subjects with low pre-vaccination antibody titers. Virus Res. 2004;103:139–45. doi: 10.1016/j.virusres.2004.02.026. [DOI] [PubMed] [Google Scholar]
- de Castro JM, Stroebele N. Food intake in the real world: implications for nutrition and aging. Clin Geriatr Med. 2002;18:685–97. doi: 10.1016/s0749-0690(02)00056-3. [DOI] [PubMed] [Google Scholar]
- De Martinis MM, Franceschi C, Monti D, et al. Inflammageing and lifelong antigenic load as major determinants of ageing rate and longevity. FEBS Lett. 2005;579:2035–9. doi: 10.1016/j.febslet.2005.02.055. [DOI] [PubMed] [Google Scholar]
- Dejaco C, Duftner C, Schirmer M. Are regulatory T cells linked with aging? Exp Gerontol. 2006;41:339–45. doi: 10.1016/j.exger.2006.01.008. [DOI] [PubMed] [Google Scholar]
- DeVeale B, Brummer T, Seroude L. Immunity and aging: the enemy within? Aging Cell. 2004;3:195–208. doi: 10.1111/j.1474-9728.2004.00106.x. [DOI] [PubMed] [Google Scholar]
- Di Francesco V, Zamboni M, Zoico E, et al. Unbalanced serum leptin and ghrelin dynamics prolong postprandial satiety and inhibit hunger in healthy elderly: another reason for the “anorexia of aging”. Am J Clin Nutr. 2006;83:1149–52. doi: 10.1093/ajcn/83.5.1149. [DOI] [PubMed] [Google Scholar]
- Donnini A, Argentati K, Mancini R, et al. Phenotype-antigen presenting capacity and migration of antigen-presenting cells in young and old age. Exp Gerontol. 2002;37:1097–112. doi: 10.1016/s0531-5565(02)00087-6. [DOI] [PubMed] [Google Scholar]
- Duffy D, Rader DJ. Drugs in development: targeting high-density lipoprotein metabolism and reverse cholesterol transport. Curr Opin Cardiol. 2005;20:301–6. doi: 10.1097/01.hco.0000168532.69342.26. [DOI] [PubMed] [Google Scholar]
- Effros RB, Pawelec G. Replicative senescence of T cells: does the Hayflick Limit lead to immune exhaustion? Immunol Today. 1997;18:450–4. doi: 10.1016/s0167-5699(97)01079-7. [DOI] [PubMed] [Google Scholar]
- Effros RB. Replicative senescence of CD8 T cells: effect of human aging. Exp Gerontol. 2004;39:517–24. doi: 10.1016/j.exger.2003.09.024. [DOI] [PubMed] [Google Scholar]
- Fagnoni FF, Vescovini R, Passeri G, et al. Shortage of circulating naive CD8(+) T cells provides new insights on immunodeficiency in aging. Blood. 2000;95:2860–8. [PubMed] [Google Scholar]
- Fas SC, Fritzsching B, Suri-Payer E, et al. Death receptor signaling and its function in the immune system. Curr Dir Autoimmun. 2006;9:1–17. doi: 10.1159/000090767. [DOI] [PubMed] [Google Scholar]
- Ferguson FG, Wikby A, Maxson P, et al. Immune parameters in a longitudinal study of a very old population of Swedish people: a comparison between survivors and non survivors. J Gerontol A Biol Med Sci. 1995;50:B378–82. doi: 10.1093/gerona/50a.6.b378. [DOI] [PubMed] [Google Scholar]
- Fernandes G, Lawrence R, Sun D. Protective role of n-3 lipids and soy protein in osteoporosis. Prostaglandins Leukot Essent Fatty Acids. 2003;68:361–72. doi: 10.1016/s0952-3278(03)00060-7. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Cavazzini C, Corsi A, et al. Biomarkers of frailty in older persons. J Endocrinol Invest. 2002;25:10–15. [PubMed] [Google Scholar]
- Foody JM, Rathore SS, Galusha D, et al. Hydroxymethylglutaryl-CoA reductase inhibitors in older persons with acute myocardial infarction: evidence for an age-statin interaction. J Am Geriatr Soc. 2006;54:421–30. doi: 10.1111/j.1532-5415.2005.00635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin CF, Larbi A, Lesur O, et al. Impairment of SHP-1 down-regulation in the lipid rafts of human neutrophils under GM-CSF stimulation contributes to their age-related, altered functions. J Leukoc Biol. 2006;79:1061–72. doi: 10.1189/jlb.0805481. [DOI] [PubMed] [Google Scholar]
- Frasca D, Van der Put E, Landin AM, et al. RNA stability of the E2A-encoded transcription factor E47 is lower in splenic activated B cells from aged mice. J Immunol. 2005;175:6633–44. doi: 10.4049/jimmunol.175.10.6633. [DOI] [PubMed] [Google Scholar]
- Friedl P, Gunzer M. Interaction of T cells with APCs: the serial encounter model. Trends Immunol. 2001;22:187–91. doi: 10.1016/s1471-4906(01)01869-5. [DOI] [PubMed] [Google Scholar]
- Fulop T, Jr, Douziech N, Jacob MP, et al. Age-related alterations in the signal transduction pathways of the elastin-laminin receptor. Pathol Biol. 2001;49:339–48. doi: 10.1016/s0369-8114(01)00143-2. [DOI] [PubMed] [Google Scholar]
- Fulop T, Foris G, Worum I, et al. Age-dependent changes of the Fc gamma-receptor-mediated functions of human monocytes. Int Arch Allergy Appl Immunol. 1984;74:76–9. doi: 10.1159/000233520. [DOI] [PubMed] [Google Scholar]
- Fulop T, Larbi A, Douziech N, et al. Signal transduction and functional changes in neutrophils with aging. Aging Cell. 2004;3:217–226. doi: 10.1111/j.1474-9728.2004.00110.x. [DOI] [PubMed] [Google Scholar]
- Fulop T, Larbi A, Linteau A, et al. Role of Mcl-1 and Bax expression alterations in the decreased rescue of human neutrophils from apoptosis by GM-CSF with aging. Ann N Y Acad Sci. 2002;973:305–8. doi: 10.1111/j.1749-6632.2002.tb04656.x. [DOI] [PubMed] [Google Scholar]
- Fulop T, Larbi A, Wikby A, et al. Dysregulation of T cell function in the elderly: scientific basis and clinical implications. Drugs Aging. 2005;22:589–603. doi: 10.2165/00002512-200522070-00005. [DOI] [PubMed] [Google Scholar]
- Garcia GG, Berger SB, Sadighi Akha AA, et al. Age-associated changes in glycosylation of CD43 and CD45 on mouse CD4 T cells. Eur J Immunol. 2005;35:622–31. doi: 10.1002/eji.200425538. [DOI] [PubMed] [Google Scholar]
- Garcia GG, Miller RA. Age-related defects in CD4+ T cell activation reversed by glycoprotein endopeptidase. Eur J Immunol. 2003;33:3464–72. doi: 10.1002/eji.200324310. [DOI] [PubMed] [Google Scholar]
- Gavazzi G, Krauze KH. Ageing and infection. Lancet Infect Dis. 2002;2:659–66. doi: 10.1016/s1473-3099(02)00437-1. [DOI] [PubMed] [Google Scholar]
- Gordon CM, LeBoff MS, Glowacki J. Adrenal and gonadal steroids inhibit IL-6 secretion by human marrow cells. Cytokine. 2001;16:178–86. doi: 10.1006/cyto.2001.0962. [DOI] [PubMed] [Google Scholar]
- Gregg RK, Jain R, Schoenleber SJ, et al. A sudden decline in active membrane-bound TGF-beta impairs both T regulatory cell function and protection against autoimmune diabetes. J Immunol. 2004;173:7308–16. doi: 10.4049/jimmunol.173.12.7308. [DOI] [PubMed] [Google Scholar]
- Gupta S, Chiplunkar S, Kim C, et al. Effect of age on molecular signalling of TNF-alpha-induced apoptosis in human lymphocytes. Mech Age Dev. 2003;124:503–9. doi: 10.1016/s0047-6374(03)00028-9. [DOI] [PubMed] [Google Scholar]
- Gupta S, Kim C, Yel L, et al. A role of fas-associated death domain (FADD) in increased apoptosis in aged humans. Clin Immunol. 2004;242:4–9. doi: 10.1023/B:JOCI.0000018059.56924.99. [DOI] [PubMed] [Google Scholar]
- Gupta S, Su H, Bi R, et al. Life and death of lymphocytes: a role in immunesenescence. Immun Ageing. 2005;23:2–12. doi: 10.1186/1742-4933-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S. Molecular and biochemical pathways of apoptosis in lymphocytes and aged humans. Vaccine. 2000;18:1596–601. doi: 10.1016/s0264-410x(99)00492-2. [DOI] [PubMed] [Google Scholar]
- Hadden JW. Thymic endocrinology. Ann N Y Acad Sci. 1998;840:352–8. doi: 10.1111/j.1749-6632.1998.tb09574.x. [DOI] [PubMed] [Google Scholar]
- Hamerman JA, Lanier LL.2006Inhibition of immune responses by ITAM-bearing receptors0, 320 [DOI] [PubMed]
- He HT, Lellouch A, Marguet D. Lipid rafts and the initiation of T cell receptor signaling. Semin Immunol. 2005;17:23–33. doi: 10.1016/j.smim.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Hermiston ML, Xu Z, Majeti R, et al. Reciprocal regulation of lymphocyte activation by tyrosine kinases and phosphatases. J Clin Invest. 2002;109:9–14. doi: 10.1172/JCI14794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewison M, Freeman L, Hughes SV, et al. Differential regulation of vitamin D receptor and its ligand in human monocyte-derived dendritic cells. J Immunol. 2003;170:5382–90. doi: 10.4049/jimmunol.170.11.5382. [DOI] [PubMed] [Google Scholar]
- High KP. Nutritional strategies to boost immunity and prevent infections in the elderly individuals. Clin Infect Dis. 2001;33:1892–900. doi: 10.1086/324509. [DOI] [PubMed] [Google Scholar]
- Hirokawa K, Utsuyama M. Combined grafting of bone marrow and thymus, and sequential multiple thymus graftings in various strains of mice. The effect on immune functions and life span. Mech Ageing Dev. 1989;49:49–60. doi: 10.1016/0047-6374(89)90067-5. [DOI] [PubMed] [Google Scholar]
- Howard WA, Gibson KL, Dunn-Walters DK. Antibody quality in old age. Rejuvenation Res. 2006;9:117–25. doi: 10.1089/rej.2006.9.117. [DOI] [PubMed] [Google Scholar]
- Hsu HC, Scott DK, Mountz JD. Impaired apoptosis and immune senescence - cause or effect? Immunol Rev. 2005;205:130–46. doi: 10.1111/j.0105-2896.2005.00270.x. [DOI] [PubMed] [Google Scholar]
- Hsu HC, Shi J, Yang P, et al. Activated CD8(+) T cells from aged mice exhibit decreased activation-induced cell death. Mech Ageing Dev. 2001;122:1663–84. doi: 10.1016/s0047-6374(01)00279-2. [DOI] [PubMed] [Google Scholar]
- Huckriede A, Bungener L, Stegmann T, et al. The virosome concept for influenza vaccines. Vaccine. 2005;23(S1):S26–38. doi: 10.1016/j.vaccine.2005.04.026. [DOI] [PubMed] [Google Scholar]
- Johnson SA, Cambier JC. Ageing, autoimmunity and arthritis: senescence of the B cell compartment - implications for humoral immunity. Arthritis Res Ther. 2004;6:131–9. doi: 10.1186/ar1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonasson L, Tompa A, Wikby A. Expansion of peripheral CD8+ T cells in patients with coronary artery disease: relation to cytomegalovirus infection. J Intern Med. 2003;254:472–8. doi: 10.1046/j.1365-2796.2003.01217.x. [DOI] [PubMed] [Google Scholar]
- Kaltoft K. Cytokine-driven immortalization of in vitro activated human T lymphocytes. CD28 expression correlates inversely with cell population doublings. Exp Clin Immunogenet. 1998;15:84–9. doi: 10.1159/000019058. [DOI] [PubMed] [Google Scholar]
- Kane LP, Weiss A. The PI-3 kinase/Akt pathway and T cell activation: pleiotropic pathways downstream of PIP3. Immunol Rev. 2003;192:7–20. doi: 10.1034/j.1600-065x.2003.00008.x. [DOI] [PubMed] [Google Scholar]
- Karanfilov CI, Liu B, Fox CC, et al. Age-related defects in Th1 and Th2 cytokine production by human T cells can be dissociated from altered frequencies of CD45RA+ and CD45RO+ T cell subsets. Mech Ageing Dev. 1999;109:97–112. doi: 10.1016/s0047-6374(99)00030-5. [DOI] [PubMed] [Google Scholar]
- Kelley DS, Hubbard NE, Erickson KL. Regulation of human immune and inflammatory responses by dietary fatty acids. Adv Food Nutr Res. 2005;50:101–38. doi: 10.1016/S1043-4526(05)50004-4. [DOI] [PubMed] [Google Scholar]
- Khan N, Shariff N, Cobbold M, et al. Cytomegalovirus seropositivity drives the CD8 T cell repertoire toward greater clonality in healthy elderly individuals. J Immunol. 2002;169:1984–92. doi: 10.4049/jimmunol.169.4.1984. [DOI] [PubMed] [Google Scholar]
- Kilara A, Panyam D. Peptides from milk proteins and their properties. Crit Rev Food Sci Nutr. 2003;43:607–33. doi: 10.1080/10408690390251138. [DOI] [PubMed] [Google Scholar]
- Kirchhoff S, Muller WW, Li-Weber M, et al. Up-regulation of c-FLIPshort and reduction of activation-induced cell death in CD28-costimulated human T cells. Eur J Immunol. 2000;30:2765–74. doi: 10.1002/1521-4141(200010)30:10<2765::AID-IMMU2765>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Koch S, Solana R, Rosa OD, et al. Human cytomegalovirus infection and T cell immunosenescence: A mini review. Mech Ageing Dev. 2006;127:538–43. doi: 10.1016/j.mad.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Kohut ML, Lee W, Martin A, et al. The exercise-induced enhancement of influenza immunity is mediated in part by improvements in psychosocial factors in older adults. Brain Behav Immun. 2005;19:357–66. doi: 10.1016/j.bbi.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Kohut ML, Senchina DS. Reversing age-associated immunosenescence via exercise. Exerc Immunol Rev. 2004;10:6–41. [PubMed] [Google Scholar]
- Kovacs JE, Plackett TP, Wittle PL. Estrogen replacement, aging, and cell mediated immunity after injury. J Leuk Biol. 2004;76:36–41. doi: 10.1189/jlb.1103538. [DOI] [PubMed] [Google Scholar]
- Kovacs JE. Aging, traumatic injury, and estrogen treatment. Exp Gerontol. 2005;40:549–55. doi: 10.1016/j.exger.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Kris-Etherton PM, Harris WS, Appel LJ, AHA Nutrition Committee, American Heart Association Omega-3 fatty acids and cardiovascular disease: new recommendations from the American Heart Association. Atheroscler Throm Vasc Biol. 2003;23:151–2. doi: 10.1161/01.atv.0000057393.97337.ae. [DOI] [PubMed] [Google Scholar]
- Kyriazis M. Clinical anti-aging hormetic strategies. Rejuvenation Res. 2005;8:96–100. doi: 10.1089/rej.2005.8.96. [DOI] [PubMed] [Google Scholar]
- Labrie F, Luu-The V, Lin SX, et al. Role of 17 beta-hydroxysteroid dehydrogenases in sex steroid formation in peripheral intracrine tissues. Trends Endocrinol Metab. 2000;11:421–7. doi: 10.1016/s1043-2760(00)00342-8. [DOI] [PubMed] [Google Scholar]
- Lacorazza HD, Guevara Patino JA, Weksler ME, et al. Failure of rearranged TCR transgenes to prevent age-associated thymic involution. J Immunol. 1999;163:4262–8. [PubMed] [Google Scholar]
- Larbi A, Douziech N, Dupuis G, et al. Age-associated alterations in the recruitment of signal transduction proteins to lipid rafts in human T lymphocytes. J Leuk Biol. 2004;75:373–81. doi: 10.1189/jlb.0703319. [DOI] [PubMed] [Google Scholar]
- Larbi A, Douziech N, Fortin C, et al. The role of the MAPK pathway alterations in GM-CSF modulated human neutrophil apoptosis with aging. Immun Ageing. 2005;2:6–16. doi: 10.1186/1742-4933-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larbi A, Dupuis G, Douziech N, et al. Low-grade inflammation with aging has consequences for T-lymphocyte signaling. Ann N Y Acad Sci. 2004;1030:125–33. doi: 10.1196/annals.1329.016. [DOI] [PubMed] [Google Scholar]
- Larbi A, Dupuis G, Khalil A, et al. Differential role of lipid rafts in the functions of CD4+ and CD8+ human T lymphocytes with aging. Cell Signal. 2006;18:1017–30. doi: 10.1016/j.cellsig.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Larbi A, Fulop T. Apoptosis and aging. In: Straub RH, Mocchegiani E, editors. Neuroimmune biology, Vol 4, Neuroendocrine Immune Network in Ageing. Ireland: Elsevier; Ireland: 2004. pp. 57–72. [Google Scholar]
- Larbi A, Muti E, Giacconi R, et al. Role of lipid rafts in activation-induced cell death: the Fas pathway in aging. Adv Exp Med Biol. 2006;584:137–55. doi: 10.1007/0-387-34132-3_11. [DOI] [PubMed] [Google Scholar]
- Laude AJ, Prior IA. Plasma membrane microdomains: organization, function and trafficking. Mol Membr Biol. 2004;21:193–205. doi: 10.1080/09687680410001700517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazuardi L, Jenewein B, Wolf AM, et al. Age-related loss of naïve T cells and dysregulation of T cell/B cell interactions in human lymph nodes. Immunology. 2005;114:37–43. doi: 10.1111/j.1365-2567.2004.02006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMaoult J, Szabo P, Weksler ME. Effect of age on humoral immunity, selection of the B cell repertoire and B cell development. Immunol Rev. 1997;160:115–26. doi: 10.1111/j.1600-065x.1997.tb01032.x. [DOI] [PubMed] [Google Scholar]
- Lesourd BM, Mazari L. Nutrition and immunity in the elderly. Proc Nutr Soc. 1999;58:685–95. doi: 10.1017/s0029665199000907. [DOI] [PubMed] [Google Scholar]
- Lesourd BM, Raynaud-Simon A, Mazari M. Nutrition and ageing of the immune system In Nutrition and immune function. Abingdon, UK: Calder Ph Ed CABI; 2002. pp. 357–74. [Google Scholar]
- Lesourd BM. Nutrition and immunity in the elderly: modification of immune responses with nutritional treatments. Am J Clin Nutr. 1997;66:478S–484S. doi: 10.1093/ajcn/66.2.478S. [DOI] [PubMed] [Google Scholar]
- Lesourd BM. Immune response during disease and recovery in the elderly. Proc Nutr Soc. 1999;58:85–98. doi: 10.1079/pns19990013. [DOI] [PubMed] [Google Scholar]
- Ligthart GH. The SENIEUR protocol after 16 years: the next step is to study the interaction of ageing and disease. Mech Ageing Dev. 2001;122:136–40. doi: 10.1016/s0047-6374(00)00242-6. [DOI] [PubMed] [Google Scholar]
- Maczek C, Bock G, Jurgens G, et al. Environmental influence on age-related changes of human lymphocyte membrane viscosity using severe combined immunodeficiency mice as an in vivo model. Exp Gerontol. 1998;33:485–98. doi: 10.1016/s0531-5565(98)00011-4. [DOI] [PubMed] [Google Scholar]
- Markert ML, Boeck A, Hale LP, et al. Transplantation of thymus tissue in complete DiGeorge syndrome. N Engl J Med. 1999;341:1180–9. doi: 10.1056/NEJM199910143411603. [DOI] [PubMed] [Google Scholar]
- McElhaney JE. The unmet need in the elderly: Designing new influenza vaccines for older adults. Vaccine. 2005;23S1:10–25. doi: 10.1016/j.vaccine.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Janeway C. Innate immunity. N Engl J Med. 2000;343:338–44. doi: 10.1056/NEJM200008033430506. [DOI] [PubMed] [Google Scholar]
- Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–45. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- Meydani SN, Han SN, Wu D. Vitamin E and immune response in the aged: molecular mechanisms and clinical implications. Immunol Rev. 2005;205:269–84. doi: 10.1111/j.0105-2896.2005.00274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meydani SN, Meydani M, Blumberg JB, et al. Vitamin E supplementation and in vivo immune response in healthy elderly subjects. A randomized controlled trial. JAMA. 1997;277:1380–6. doi: 10.1001/jama.1997.03540410058031. [DOI] [PubMed] [Google Scholar]
- Meyer KC. Aging. Proc Am Thorac Soc. 2005;2:433–9. doi: 10.1513/pats.200508-081JS. [DOI] [PubMed] [Google Scholar]
- Miller C, Kelsoe G, Han S. Lack of B7-2 expression in germinal centers of aged mice. Aging Immunol Infect Dis. 1994;5:249–56. [Google Scholar]
- Miller RA. The aging immune system: primers and prospectus. Science. 1996;273:70–4. doi: 10.1126/science.273.5271.70. [DOI] [PubMed] [Google Scholar]
- Mocchegiani E, Giacconi R, Cipriano C, et al. Are zinc-bound metallothionein isoforms (I+II and III) involved in impaired thymulin production and thymic involution during ageing? Immun Aging. 2004;1:5. doi: 10.1186/1742-4933-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocchegiani E, Muzzioli M, Giacconi R. Zinc, metallothioneins, immune responses, survival and ageing. Biogerontology. 2000a;1:133–43. doi: 10.1023/a:1010095930854. [DOI] [PubMed] [Google Scholar]
- Mocchegiani E, Muzzioli M, Giacconi R. Zinc and immunoresistance to infection in aging: new biological tools. Trends Pharmacol Sci. 2000b;21:205–8. doi: 10.1016/s0165-6147(00)01476-0. [DOI] [PubMed] [Google Scholar]
- Montes CL, Maletto BA, Acosta Rodriguez EV, et al. B cells from aged mice exhibit reduced apoptosis upon B cell antigen receptor stimulation and differential ability to up-regulate survival signals. Clin Exp Immunol. 2006;143:30–40. doi: 10.1111/j.1365-2249.2005.02969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroni F, Di Paolo ML, Rigo A, et al. Interrelationship among neutrophil efficiency, inflammation, antioxidant activity and zinc pool in very old age. Biogerontology. 2005;6:271–81. doi: 10.1007/s10522-005-2625-0. [DOI] [PubMed] [Google Scholar]
- Mortensen RF, Zhong W. Regulation of phagocytic leukocyte activities by C-reactive protein. J Leukoc Biol. 2000;67:495–500. doi: 10.1002/jlb.67.4.495. [DOI] [PubMed] [Google Scholar]
- Munemoto Y, Iida Y, Ohata K, et al. Significance of postoperative adjuvant immunochemotherapy after curative resection of colorectal cancers: identification of responders incorporating the age factor. Oncol Rep. 2004;11:623–35. [PubMed] [Google Scholar]
- Nel AE, Slaughter N. T cell activation through the antigen receptor. Part 2: role of signaling cascades in T cell differentiation, anergy, immune senescence, and development of immunotherapy. J Allergy Clin Immunol. 2002;109:901–15. doi: 10.1067/mai.2002.124965. [DOI] [PubMed] [Google Scholar]
- Olsson J, Wikby A, Johannson B, et al. Age-related change in peripheral blood T-lymphocyte subpopulations and cytomegalovirus infection in the very old: the Swedish longitudinal OCTO immune study. Mech Age Dev. 2000;121:187–201. doi: 10.1016/s0047-6374(00)00210-4. [DOI] [PubMed] [Google Scholar]
- O’Neill LA. How Toll-like receptors signal: what we know and what we don’t know. Curr Opin Immunol. 2006;18:3–9. doi: 10.1016/j.coi.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Ouyang Q, Wagner WM, Voehringer D, et al. Age-associated accumulation of CMV-specific CD8+ T cells expressing the killer cell lectine-like receptor G1 (KLRG-1) Exp Gerontol. 2003;38:911–20. doi: 10.1016/s0531-5565(03)00134-7. [DOI] [PubMed] [Google Scholar]
- Owens T. Identification of new therapeutic targets for prevention of CNS inflammation. Expert Opin Ther Targets. 2002;6:203–15. doi: 10.1517/14728222.6.2.203. [DOI] [PubMed] [Google Scholar]
- Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271–84. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- Pahan K. Biomedicine and diseases: lipid-lowering drugs. Cell Mol Life Sci. 2006;63:1165–78. doi: 10.1007/s00018-005-5406-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahlavani MA, Harris MD, Richardson A. Activation of p21(ras)/ MAPK signal transduction molecules decreases with age in mitogen-stimulated T cells from rats. Cell Immunol. 1995;165:84–91. doi: 10.1006/cimm.1998.1274. [DOI] [PubMed] [Google Scholar]
- Pahlavani MA, Harris MD. Effect of in vitro generation of oxygen free radicals on T cell function in young and old rats. Free Radic Biol Med. 1998;25:903–13. doi: 10.1016/s0891-5849(98)00124-5. [DOI] [PubMed] [Google Scholar]
- Park HS, Park JY, Yu R. Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-alpha and IL-6. Diabetes Res Clin Pract. 2005;69:29–35. doi: 10.1016/j.diabres.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Pawelec G, Akbar A, Caruso C, et al. Is immunosenescence infectious? Trends Immunol. 2004;25:406–10. doi: 10.1016/j.it.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Pawelec G, Hirokawa K, Fulop T. T cell signalling with aging. Mech Age Dev. 2001;22:1613–37. doi: 10.1016/s0047-6374(01)00290-1. [DOI] [PubMed] [Google Scholar]
- Pawelec G, Koch S, Gouttefangeas C, et al. Immunorejuveation in the elderly. Rejuvenation Research. 2006;9:111–116. doi: 10.1089/rej.2006.9.111. [DOI] [PubMed] [Google Scholar]
- Pawelec G, Koch S, Griessemann H, et al. Immunosenescence, suppression and tumour progression. Cancer Immunol Immunother. 2006;55:981–6. doi: 10.1007/s00262-005-0109-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawelec G, Sansom D, Rehbein A, et al. Decreased proliferative capacity and increased susceptibility to activation-induced cell death in late-passage human CD4+ TCR2+ cultured T cell clones. Exp Gerontol. 1996;31:655–62. doi: 10.1016/s0531-5565(96)00097-6. [DOI] [PubMed] [Google Scholar]
- Pawelec G, Solana R. Immunosenescence. Trend Immunol. 1997;11:514–16. doi: 10.1016/s0167-5699(97)01145-6. [DOI] [PubMed] [Google Scholar]
- Pawelec G. Hypothesis: loss of telomerase inducibility and subsequent replicative senescence in cultured human T cells is a result of altered costimulation. Mech Ageing Dev. 2000;121:181–5. doi: 10.1016/s0047-6374(00)00209-8. [DOI] [PubMed] [Google Scholar]
- Pawelec G. Immunosenescence and human longevity. Biogerontology. 2003;4:167–70. doi: 10.1023/a:1024185405946. [DOI] [PubMed] [Google Scholar]
- Petropoulos I, Friguet B. Protein maintenance in aging and replicative senescence: a role for the peptide methionine sulfoxide reductases. Biochim Biophys Acta. 2005;1703:261–6. doi: 10.1016/j.bbapap.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Pickup JC, Crook MA. Is type II diabetes mellitus a disease of the innate immune system? Diabetologia. 1998;41:1241–8. doi: 10.1007/s001250051058. [DOI] [PubMed] [Google Scholar]
- Poggioli S, Mary J, Bakala H, et al. Evidence of preferential protein targets for age-related modifications in peripheral blood lymphocytes. Ann N Y Acad Sci. 2004;1019:211–14. doi: 10.1196/annals.1297.034. [DOI] [PubMed] [Google Scholar]
- Ponnappan U, Trebilcock GU, Zheng MZ. Studies into the effect of tyrosine phosphatase inhibitor phenylarsine oxide on NFkappaB activation in T lymphocytes during aging: evidence for altered IkappaB-alpha phosphorylation and degradation. Exp Gerontol. 1999;34:95–107. doi: 10.1016/s0531-5565(98)00059-x. [DOI] [PubMed] [Google Scholar]
- Ponnappan U, Zhong M, Trebilcock GU. Decreased proteasome-mediated degradation in T cells from the elderly: A role in immune senescence. Cell Immunol. 1999;192:167–74. doi: 10.1006/cimm.1998.1418. [DOI] [PubMed] [Google Scholar]
- Provinciali M, Di Stefano G, Colombo M, et al. Adjuvant effect of low-dose interleukin-2 on antibody response to influenza virus vaccination in healthy elderly subjects. Mech Ageing Dev. 1994;77:75–82. doi: 10.1016/0047-6374(94)90016-7. [DOI] [PubMed] [Google Scholar]
- Provinciali M, Fabris N, Pieri C. Improvement of natural killer cell activity by in vitro active lipids (AL 721) administration in old mice. Mech Ageing Dev. 1990;52:245–54. doi: 10.1016/0047-6374(90)90128-3. [DOI] [PubMed] [Google Scholar]
- Provinciali M, Montenovo A, Di Stefano G, et al. Effect of zinc or zinc plus arginine supplementation on antibody titre and lymphocyte subsets after influenza vaccination in elderly subjects: a randomized controlled trial. Age Ageing. 1998;27:715–22. doi: 10.1093/ageing/27.6.715. [DOI] [PubMed] [Google Scholar]
- Provinciali M, Smorlesi A. Immunoprevention and immunotherapy of cancer in ageing. Cancer Immunol Immunother. 2005;54:93–106. doi: 10.1007/s00262-004-0539-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin W, Jiang J, Chen Q, et al. CpG ODN enhances immunization effects of hepatitis B vaccine in aged mice. Cell Mol Immunol. 2004;1:148–52. [PubMed] [Google Scholar]
- Rabinowich H, Lyte M, Steiner Z, et al. Augmentation of mitogen responsiveness in the aged by a special lipid diet AL 721. Mech Ageing Dev. 1987;40:131–8. doi: 10.1016/0047-6374(87)90012-1. [DOI] [PubMed] [Google Scholar]
- Reinhold U, Pawelec G, Enczmann J, et al. Class-specific effects of selenium on PWM-driven human antibody synthesis in vitro. Biol Trace Elem Res. 1989;20:45–58. doi: 10.1007/BF02919097. [DOI] [PubMed] [Google Scholar]
- Rijhsinghani AG, Thompson K, Bhatia SK, et al. Estrogen blocks early T cell development in the thymus. Am J Reprod Immunol. 1996;36:269–77. doi: 10.1111/j.1600-0897.1996.tb00176.x. [DOI] [PubMed] [Google Scholar]
- Riley JL, June CH. The CD28 family: a T cell rheostat for therapeutic control of T cell activation. Blood. 2005;105:13–21. doi: 10.1182/blood-2004-04-1596. [DOI] [PubMed] [Google Scholar]
- Rink L, Cakman I, Kirchner H. Altered cytokine production in the elderly. Mech Ageing Dev. 1998;102:199–209. doi: 10.1016/s0047-6374(97)00153-x. [DOI] [PubMed] [Google Scholar]
- Rivnay B, Bergman S, Shinitzky M, et al. Correlations between membrane viscosity, serum cholesterol, lymphocyte activation and aging in man. Mech Ageing Dev. 1980;12:119–26. doi: 10.1016/0047-6374(80)90088-3. [DOI] [PubMed] [Google Scholar]
- Roberts SB, Fuss P, Heyman MB, et al. Control of food intake in older men. JAMA. 1994;272:1601–6. doi: 10.1001/jama.1994.03520200057036. [DOI] [PubMed] [Google Scholar]
- Rook GA, Hernandez-Pando R, Lightman SL. Hormones, peripherally activated prohormones and regulation of the Th1/Th2 balance. Immunol Today. 1994;15:301–3. doi: 10.1016/0167-5699(94)90075-2. [DOI] [PubMed] [Google Scholar]
- Rubin RL, Kretz-Rommel A. A nondeletional mechanism for central T cell tolerance. Crit Rev Immunol. 2001;21:29–40. [PubMed] [Google Scholar]
- Sadighi Akha AA, Berger SB, Miller RA. Enhancement of CD8 T cell function through modifying surface glycoproteins in young and old mice. Immunology. 2006;119:187–94. doi: 10.1111/j.1365-2567.2006.02420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadighi Akha AA, Miller RA. Signal transduction in the aging immune system. Curr Opin Immunol. 2005;17:486–91. doi: 10.1016/j.coi.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Salih HR, Nussler V. Commentary: Immune escape versus tumor tolerance: how do tumors evade immune surveillance? Eur J Med Res. 2001;6:323–32. [PubMed] [Google Scholar]
- Salvioli S, Capri M, Scarcella E, et al. Age-dependent changes in the susceptibility to apoptosis of peripheral blood CD4+ and CD8+ T lymphocytes with virgin or memory phenotype. Mech Ageing Dev. 2003;24:409–18. doi: 10.1016/s0047-6374(03)00016-2. [DOI] [PubMed] [Google Scholar]
- Sansoni P, Cossarizza A, Brianti V, et al. Lymphocytes subsets and natural killer cell activity in healthy old people and centenarians. Blood. 1993;82:2767–73. [PubMed] [Google Scholar]
- Santos-Neto L, Tosta CE, Dorea JG. Zinc reverses the increased sensitivity of lymphocytes from aged subjects to the antiproliferative effect of prostaglandin E2. Clin Immunol Immunopathol. 1992;64:184–7. doi: 10.1016/0090-1229(92)90198-w. [DOI] [PubMed] [Google Scholar]
- Sapolsky R, Armanini M, Packan D, et al. Stress and glucocorticoids in aging. Endocriniol Metab Clin North Am. 1987;16:965–80. [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocr Rev. 1986;7:284–301. doi: 10.1210/edrv-7-3-284. [DOI] [PubMed] [Google Scholar]
- Scapini P, Lapinet-Vera JA, Gasperini S, et al. The neutrophil as a cellular source of chemokines. Immunol Rev. 2000;177:195–203. doi: 10.1034/j.1600-065x.2000.17706.x. [DOI] [PubMed] [Google Scholar]
- Scheinfeld N. Infections in the elderly. Dermatol Online J. 2005;11:8. [PubMed] [Google Scholar]
- Schindowski K, Frohlich L, Maurer K, et al. Age-related impairment of human T lymphocytes’ activation: specific differences between CD4(+) and CD8(+) subsets. Mech Ageing Dev. 2002;123:375–90. doi: 10.1016/s0047-6374(01)00396-7. [DOI] [PubMed] [Google Scholar]
- Schmaltz HN, Fried LP, Xue QL, et al. Chronic cytomegalovirus infection and inflammation are associated with prevalent frailty in community-dwelling older women. J Am Geriatr Soc. 2005;53:747–54. doi: 10.1111/j.1532-5415.2005.53250.x. [DOI] [PubMed] [Google Scholar]
- Shi M, Bi X, Xu S, et al. Increased susceptibility of tumorigenicity and decreased anti-tumor effect of DC vaccination in aged mice are potentially associated with increased number of NK1.1+CD3+ NKT cells. Exp Oncol. 2005;27:125–9. [PubMed] [Google Scholar]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–72. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Simons K, Ikonen E. How cells handle cholesterol. Science. 2000;290:1721–6. doi: 10.1126/science.290.5497.1721. [DOI] [PubMed] [Google Scholar]
- Sin JI, Kim J, Pachuk C, et al. Interleukin 7 can enhance antigen-specific cytotoxic-T-lymphocyte and/or Th2-type immune responses in vivo. Clin Diagn Lab Immunol. 2000;7:751–8. doi: 10.1128/cdli.7.5.751-758.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TP, Kennedy SL, Fleshner M. Influence of age and physical activity on the primary in vivo antibody and T cell-mediated responses in men. J Appl Physiol. 2004;97:491–8. doi: 10.1152/japplphysiol.01404.2003. [DOI] [PubMed] [Google Scholar]
- Stephensen CB, Kelley DS. The innate immune system: friend and foe. Am J Clin Nutr. 2006;83:187–8. doi: 10.1093/ajcn/83.2.187. [DOI] [PubMed] [Google Scholar]
- Sudeghi HM, Schnelle JF, Thoma JK, et al. Phenotypic and functional characteristics of circulating monocytes of elderly persons. Exp Gerontol. 1999;34:959–65. doi: 10.1016/s0531-5565(99)00065-0. [DOI] [PubMed] [Google Scholar]
- Sutherland JS, Goldberg GL, Hammett MV, et al. Activation of thymic regeneration in mice and humans following androgen blockade. J Immunol. 2005;175:2741–53. doi: 10.4049/jimmunol.175.4.2741. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Suzuki N, Daynes RA, et al. Dehydroepiandrosterone enhances IL2 production and cytotoxic effector function of human T cells. Clin Immunol Immunopathol. 1991;61:202–11. doi: 10.1016/s0090-1229(05)80024-8. [DOI] [PubMed] [Google Scholar]
- Szabo P, Li F, Mathew J, et al. Evolution of B cell clonal expansions with age. Cell Immunol. 2004;231:158–67. doi: 10.1016/j.cellimm.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Taner SB, Onfelt B, Pirinen NJ, et al. Control of immune responses by trafficking cell surface proteins, vesicles and lipid rafts to and from the immunological synapse. Traffic. 2004;5:651–61. doi: 10.1111/j.1600-0854.2004.00214.x. [DOI] [PubMed] [Google Scholar]
- Tavano R, Gri G, Molon B, et al. CD28 and lipid rafts coordinate recruitment of Lck to the immunological synapse of human T lymphocytes. J Immunol. 2004;173:5392–7. doi: 10.4049/jimmunol.173.9.5392. [DOI] [PubMed] [Google Scholar]
- Tracy RP. Emerging relationships of inflammation, cardiovascular disease and chronic diseases of aging. Int J Obes Relat Metab Disord. 2003;27:S29–34. doi: 10.1038/sj.ijo.0802497. [DOI] [PubMed] [Google Scholar]
- Tricon S, Burdge GC, Williams CM, et al. The effects of conjugated linoleic acid on human health-related outcomes. Proc Nutr Soc. 2005;64:171–82. doi: 10.1079/pns2005418. [DOI] [PubMed] [Google Scholar]
- Valenzuela HF, Effros RB. Divergent telomerase and CD28 expression patterns in human CD4 and CD8 T cells following repeated encounters with the same antigenic stimulus. Clin Immunol. 2002;105:117–25. doi: 10.1006/clim.2002.5271. [DOI] [PubMed] [Google Scholar]
- Vallejo AN, Brandes JC, Weyand CM, et al. Modulation of CD28 expression: distinct regulatory pathways during activation and replicative senescence. J Immunol. 1999;162:6572–9. [PubMed] [Google Scholar]
- Vasto S, Malavolta M, Pawelec G. Age and immunity. Immun Ageing. 2006;3:2. doi: 10.1186/1742-4933-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virts EL, Phillips JA, Thoman ML. A novel approach to thymic rejuvenation in the aged. Rejuvenation Res. 2006;9:134–42. doi: 10.1089/rej.2006.9.134. [DOI] [PubMed] [Google Scholar]
- Voorrips LE, van Acker TM, Deurenberg P, et al. Energy expenditure at rest and during standardized activities: a comparison between elderly and middle-aged women. Am J Clin Nutr. 1993;58:15–20. doi: 10.1093/ajcn/58.1.15. [DOI] [PubMed] [Google Scholar]
- Walford RL, Mock D, Verdery R, et al. Calorie restriction in biosphere 2: alterations in physiologic, hematologic, hormonal, and biochemical parameters in humans restricted for a 2-year period. J Gerontol A Biol Sci Med Sci. 2002;57:B211–24. doi: 10.1093/gerona/57.6.b211. [DOI] [PubMed] [Google Scholar]
- Wang D, Matsumoto R, You Y, et al. CD3/CD28 costimulation-induced NF-kappaB activation is mediated by recruitment of protein kinase C-theta, Bcl10, and IkappaB kinase beta to the immunological synapse through CARMA1. Mol Cell Biol. 2004;24:164–71. doi: 10.1128/MCB.24.1.164-171.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner AB, de Vries E, Tait SW, et al. TRAIL receptor and CD95 signal to mitochondria via FADD, caspase-8/10, Bid, and Bax but differentially regulate events downstream from truncated Bid. J Biol Chem. 2002;277:40760–7. doi: 10.1074/jbc.M204351200. [DOI] [PubMed] [Google Scholar]
- Whisler RL, Grants IS. Age-related alterations in the activation and expression of phosphotyrosine kinases and protein kinase C (PKC) among human B cells. Mech Ageing Dev. 1993;71:31–46. doi: 10.1016/0047-6374(93)90033-n. [DOI] [PubMed] [Google Scholar]
- Whisler RL, Williams JW, Jr, Newhouse YG. Human B cell proliferative responses during aging. Reduced RNA synthesis and DNA replication after signal transduction by surface immunoglobulins compared to B cell antigenic determinants CD20 and CD40. Mech Ageing Dev. 1991;61:209–22. doi: 10.1016/0047-6374(91)90018-u. [DOI] [PubMed] [Google Scholar]
- Wick G, Jansen-Dürr P, Berger P, et al. Diseases of aging. Vaccine. 2000;18:1567–83. doi: 10.1016/s0264-410x(99)00489-2. [DOI] [PubMed] [Google Scholar]
- Wikby A, Ferguson F, Forsey R, et al. An immune risk phenotype, cognitive impaiment and survival in very late life: impact of allostatic load in Swedish octogenarian and nonsagenarian humans. J Gerontol Biol Sci. 2005;60A:556–65. doi: 10.1093/gerona/60.5.556. [DOI] [PubMed] [Google Scholar]
- Wikby A, Johansson B, Ferguson F, et al. Age-related changes in immune parameters in a very old population of Swedish people a longitudinal study. Exp Gerontol. 1994;29:531–41. doi: 10.1016/0531-5565(94)90036-1. [DOI] [PubMed] [Google Scholar]
- Witkowski JM, Bryl E. Paradoxical age-related cell cycle quickening of human CD4+ lymphocytes; a role for cyclin D1 and calpain. Exp Gerontol. 2004;39:577–85. doi: 10.1016/j.exger.2003.10.028. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Bamba K, Kitayama A, et al. Long-term intravenous administration of activated autologous lymphocytes for cancer patients does not induce antinuclear antibody and rheumatoid factor. Anticancer Res. 2004;24:242. [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Hemmi H, et al. Essential role for TIRAP in activation of the signalling cascade shared by TLR2 and TLR4. Nature. 2002;420:324–9. doi: 10.1038/nature01182. [DOI] [PubMed] [Google Scholar]
- Zhang C, Xu X, Potter BJ, et al. TNF-alpha contributes to endothelial dysfunction in ischemia/reperfusion injury. Arterioscler Thromb Vasc Biol. 2006;26:475–80. doi: 10.1161/01.ATV.0000201932.32678.7e. [DOI] [PubMed] [Google Scholar]
- Zhang F, Jia Z, Deng Z, et al. In vitro modulation of telomerase activity, telomere length and cell cycle in MKN45 cells by verbascoside. Planta Med. 2002;68:115–18. doi: 10.1055/s-2002-20255. [DOI] [PubMed] [Google Scholar]