Abstract
Transcription of ribosomal RNA genes by RNA polymerase (pol) I oscillates during the cell cycle, being maximal in S and G2 phase, repressed during mitosis, and gradually recovering during G1 progression. We have shown that transcription initiation factor (TIF)-IB/SL1 is inactivated during mitosis by cdc2/cyclin B-directed phosphorylation of TAFI110. In this study, we have monitored reactivation of transcription after exit from mitosis. We demonstrate that the pol I factor UBF is also inactivated by phosphorylation but recovers with different kinetics than TIF-IB/SL1. Whereas TIF-IB/SL1 activity is rapidly regained on entry into G1, UBF is reactivated later in G1, concomitant with the onset of pol I transcription. Repression of pol I transcription in mitosis and early G1 can be reproduced with either extracts from cells synchronized in M or G1 phase or with purified TIF-IB/SL1 and UBF isolated in the presence of phosphatase inhibitors. The results suggest that two basal transcription factors, e.g., TIF-IB/SL1 and UBF, are inactivated at mitosis and reactivated by dephosphorylation at the exit from mitosis and during G1 progression, respectively.
Keywords: transcription initiation factor IB, SL1, repression, phosphorylation
Cell cycle-dependent fluctuations of transcription have been observed for genes involved in many cellular processes. A key role in the regulation of the cell cycle is played by cyclin-dependent kinases (cdks), a family of serine/threonine protein kinases whose activity is modulated by association with cell cycle-regulated cyclins, stage-specific association with cdk–cyclin inhibitors, and phosphorylation or dephosphorylation of their catalytic subunits. Distinct cdks phosphorylate specific target proteins to positively or negatively modulate their activity. Several studies have demonstrated that silencing of gene expression that accompanies M phase is brought about by transient phosphorylation of numerous proteins by maturation-promoting factor (MPF), a heterodimeric cdk consisting of cdc2 and cyclin B. Phosphorylation by MPF structurally and functionally changes a wide range of targets to shut down important cellular processes.
Accumulating evidence indicates that the global repression of transcription during mitosis involves the direct inactivation of key components of the transcription machinery (1). For class II genes, multiple components of the basal transcription apparatus are inactivated by mitotic phosphorylation, the TATA box-binding protein (TBP)-associated factor (TAF) subunits of transcription factor (TF)IID (2), the cdk7 subunit of TFIIH (3, 4,), and the heptapeptide repeats present in the carboxyl-terminal domain of the largest subunit of RNA polymerase (pol) II (5). For class III genes, inactivation of the TBP-containing complex TFIIIB has been demonstrated to cause repression of pol III transcription (6–8). On exit from mitosis, transcription is reactivated during G1 progression (9). Whether dephosphorylation of mitotically phosphorylated protein(s) is sufficient or whether de novo synthesis or phosphorylation(s) by G1-specific cdks are required for transcriptional activation is unknown.
rRNA-encoding genes (rDNA) represent an attractive model for investigating such fundamental processes, because their transcriptional activity oscillates during the cell cycle. Pol I-directed rDNA transcription is maximal in S and G2, shuts down in mitosis and recovers in G1. The molecular mechanisms underlying these fluctuations in transcriptional activity are poorly characterized. Recent work has established that silencing of cellular pre-rRNA synthesis during mitosis is caused by inactivation of the pol I-specific transcription initiation factor TIF-IB/SL1 by cdc2/cyclin B-mediated phosphorylation (10). TIF-IB/SL1 is a multiprotein complex containing TBP and three pol I-specific TAFs (TAFIs) (11–13). Binding of TIF-IB/SL1 to the core element of the ribosomal gene promoter is enhanced by the upstream binding factor (UBF), a member of the family of high mobility group (HMG) box proteins (14). Mitotic phosphorylation has been demonstrated to impair the capability of TIF-IB/SL1 to interact with UBF (10), indicating that phosphorylation of the pol I-specific TBP–TAF complex is used as a molecular switch to prevent preinitiation complex formation and rDNA transcription at mitosis.
Practically nothing is known about the mechanisms that reactivate rDNA transcription at the exit from mitosis. In this communication, we have investigated the molecular mechanism underlying transcriptional repression in early G1. We demonstrate that despite TIF-IB/SL1 activity resuming on exit from mitosis, overall transcriptional activity remains low. Recovery of transcription during G1 progression is brought about by reactivation of UBF. The results suggest that the activities of both basal DNA binding factors, e.g., TIF-IB/SL1 and UBF, are regulated in a cell cycle-dependent fashion.
MATERIALS AND METHODS
Cell Lines, Synchronization, and Extract Preparation.
FT210 cells (15) were cultured at the permissive temperature (33°C) in RPMI 1640 medium containing 5% newborn calf serum. For synchronization, cells were arrested at mid-to-late G2 by incubation for 18 h at the nonpermissive temperature (39°C) and allowed to proceed into mitosis or G1 by shifting to 33°C and culturing for 1 or 3 h, respectively. NIH 3T3 fibroblasts overexpressing Flag epitope-tagged mUBF1 were maintained in DMEM supplemented with 10% FCS. For synchronization, cells were cultured in low serum (0.2% FCS) for 36 h, stimulated by adding fresh medium containing 10% FCS, and harvested after 3 h. Whole-cell extracts were prepared according to Manley et al. (16).
Isolation of Nascent Pre-rRNA.
FT210 cells (1 × 106) were lysed in 1 ml of buffer (20 mM Hepes⋅KOH, pH 7.6/7.5 mM MgCl2/0.2 mM EDTA/0.3 M NaCl/1M urea/1% Nonidet P-40/1 mM DTT/0.1 mg/ml yeast tRNA) and incubated on ice for 10 min, and chromatin was sedimented by centrifuging (30 min, 15,000 × g, 4°C). After resuspension in 100 μl of 15 mM Tris⋅HCl pH 7.6/2.5 mM CaCl2/10 mM EDTA/0.1 mg/ml yeast tRNA, DNA was digested by incubation for 15 min at 37°C with 80 μg/ml DNase I (Worthington). Proteinase K and SDS (1%) were added to a final concentration of 0.2 mg/ml, and digestion was continued for 15 min. RNA was extracted, treated once more with DNase I, dotted onto a nylon membrane, and hybridized to a labeled probe covering murine rDNA sequences from −168 to +3,101.
In Vitro Transcription Assays.
Standard transcription reactions (25 μl) contained 25 μg of whole-cell extract proteins, 25 ng of pMr600 (a pUC9-derivative containing 5′-terminal murine rDNA sequences from −312 to +292 with respect to the transcription start site) linearized with EcoRI/12 mM Tris⋅HCl, pH 7.9/0.1 mM EDTA/0.5 mM dithioerythritol/5 mM MgCl2/80 mM KCl/12% glycerol/0.66 mM each ATP, CTP, GTP/0.01 mM UTP/1 μCi of [α-32P]UTP (3,000 Ci/mmol; 1 Ci = 37 GBq). After incubation for 60 min at 30°C, transcripts were isolated and analyzed on 4.5% polyacrylamide gels. The fractionation scheme for purification of murine pol I and pol I-specific transcription factors has been described (17). The reconstituted transcription system contained 5–10 ng of pMr600/EcoRI, 4 μl of a pol I-containing fraction (H-400), 2 μl of TIF-IA/TIF-IC (poly-l-lysine–agarose fraction), 3 μl of TIF-IB (CM-400 fraction) and 5 ng of recombinant hUBF1. To prevent de novo phosphorylation during transcription, ATP and GTP were replaced by adenosine 5′-[β,γ-imido]triphosphate (AMP-PNP) and guanosine 5′-[β,γ-imido]triphosphate (GMP-PNP). In addition, the assays contained 2 mM of dimethylaminopurine (DMAP) and phosphatase inhibitors (2 mM 2-glycerophosphate, 0.2 mM sodium orthovanadate).
Purification of UBF.
Extracts were prepared from exponentially growing or mitotic FT210 cells. To minimize dephosphorylation of UBF, all buffers contained nonspecific phosphatase inhibitors (10 mM 2-glycerophosphate/10 mM KF/1 mM sodium orthovanadate). Proteins were fractionated on a DEAE-Sepharose column equilibrated in 40 mM Hepes⋅KOH, pH 7.9/50 mM (NH4)2SO4/5 mM MgCl2/0.2 mM EDTA/0.5 mM DTT/20% glycerol (18). UBF was eluted at 500 mM (NH4)2SO4, dialyzed against buffer AM-100 (100 mM KCl/20 mM Tris⋅HCl, pH 7.9/5 mM MgCl2/0.1 mM EDTA/10% glycerol/0.5 mM dithioerythritol) and further purified on a Resource-Q column (Amersham Pharmacia). After washing with buffer AM-300 (same as AM-100 but with 300 mM KCl), UBF was eluted at 500 mM KCl. Flag-tagged human UBF1 was purified from either Escherichia coli or Sf9 cells infected with recombinant baculovirus as described (19). Alternatively, UBF was immunopurified from NIH 3T3 cells overexpressing Flag-tagged mUBF1.
Purification of TIF-IB.
For affinity purification of TIF-IB, anti-mTAFI95 antibodies (20) were coupled to Dynabeads containing sheep anti-rabbit IgG (Dynal). Resuspended beads (50 μl) were incubated with 500 μl of FT210 cell extracts (5 mg of protein) in buffer AM-300 for 2 h at 4°C, washed with buffers AM-1000/0.1% Nonidet P-40 and AM-700/0.1% Nonidet P-40 and AM-300/0.1% Nonidet P-40 and resuspended in AM-100.
Western Blots.
Cells were lysed in sample buffer and sonicated for 10 sec. Proteins were separated by SDS/PAGE and transferred to nitrocellulose. The membrane was blocked in PBS containing 5% milk powder and 0.2% Tween 20 for 1 h and probed with specific antibodies, and proteins were visualized by using enhanced chemiluminescence (ECL) (Amersham Pharmacia). Antibodies against UBF (19), TBP (21), mTAFI95 (20) and the Flag epitope (M2, Kodak) were used.
RESULTS
Pre-rRNA Synthesis Fluctuates During the Cell Cycle.
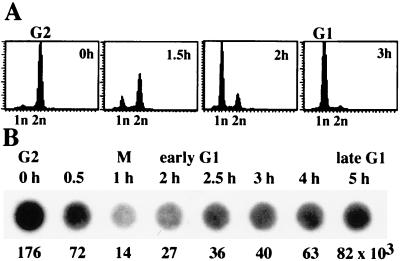
To monitor pre-rRNA synthesis during cell cycle progression, we have used FT210 cells, a murine mammary cancer cell line carrying a temperature-sensitive mutant of cdc2 (15). FT210 cells were arrested at the mid-to-late G2 phase by culturing at the restrictive temperature (39°C), and then allowed to reenter the cell cycle by shifting to the permissive temperature (33°C). Aliquots of cells were analyzed by using flow cytometry to verify that efficient synchronization had occurred (Fig. 1A). To measure rRNA synthetic activity of synchronized cells during cell cycle progression, we made use of the fact that nascent RNA can be separated from bulk nuclear RNA by isolating nascent pre-rRNA chains from ternary pol I complexes (22). Like pol II, ternary pol I complexes resist treatment with moderate concentrations of urea in conjunction with Nonidet P-40 and 0.3 M NaCl. Under the conditions used, histones and ternary elongation complexes are not dissociated from DNA and therefore, the relative amount of nascent pre-rRNA chains can be estimated by dot hybridization (Fig. 1B) to a 5′-terminal mouse rDNA probe (from −168 to +3,101). As shown in Fig. 1B, the strongest hybridization signal was observed in G2 cells. Transcriptional activity dropped during M phase (1 h) and slowly recovered during G1 phase. Three hours after release from the G2 arrest, most cells were in early G1. Significantly, the overall transcriptional activity of early G1 cells was impaired, being only slightly higher than in M phase cells. Transcriptional activity gradually recovered during G1 progression. Thus, pre-rRNA synthesis is rapidly shut off at the entry of mitosis and is slowly reactivated during the G1 phase.
Figure 1.
Measurement of pre-rRNA synthesis during the cell cycle. (A) FACS analysis. FT210 cells were synchronized in G2 by shifting to 39°C for 18 h and released from the G2 block by shifting to the permissive temperature (33°C). Aliquots of cells were subjected to FACS analysis at the times indicated. (B) Quantitation of nascent pre-rRNA chains. Nascent RNA was extracted from synchronized cells at the times indicated, dotted onto a membrane, and hybridized to a labeled murine rDNA probe covering nucleotides from −168 to +3,101. Quantitation of the hybridization signals by a PhosphorImager is shown below the blots.
Cell Cycle-Dependent Regulation of rDNA Transcription Can Be Reproduced in Vitro.
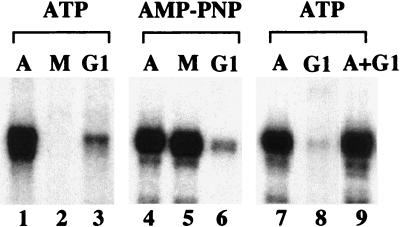
To determine whether cell cycle-dependent regulation of pol I transcription can be reproduced in vitro, extracts were prepared from asynchronous cell cultures or from cells that were synchronized in M or G1 phase, respectively. For synchronization, FT210 cells were first arrested in G2 by culturing at the nonpermissive temperature (39°C) and then released for cell cycle progression by shifting for 1 or 3 h to the permissive temperature (33°C). Similar to results in HeLa cells (23), extracts from asynchronous FT210 cells exhibited high transcriptional activity, whereas extracts from mitotic cells were almost inactive (Fig. 2, lanes 1 and 2). Moreover, consistent with the in vivo data, the transcriptional activity of extracts from early G1 cells was also severely impaired (lane 3).
Figure 2.
In vitro reproduction of transcriptional repression in extracts from M and G1 phase cells. Standard transcription assays contained 25 μg of extract from asynchronous (A), mitotic (M), or G1 cells and either ATP and GTP (lanes 1–3, 7–9) or AMP-PNP/GMP-PNP (lanes 4–6). To exclude that early G1 extracts contain a dominant repressor of pol I transcription, equal amounts of asynchronous and G1 extract were assayed alone (lanes 7, 8) or simultaneously (lane 9).
To analyze whether transcriptional repression in early G1 is brought about by the same mechanism(s) that mediate(s) mitotic silencing of rRNA synthesis, transcriptions were performed in the presence of the nucleotide analogs AMP-PNP and GMP-PNP, which are not substrates for protein kinases (Fig. 2, lanes 4–6). Mitotic repression of pol I transcription was not observed in the presence of the nucleotide analogs (lane 5). This is consistent with previous data showing that in mitotic extracts, phosphorylation of the target protein(s) by cdc2/cyclin B is required for transcriptional repression, because cellular phosphatases can dephosphorylate and thus activate the target protein(s) (1, 23). Significantly, transcription in early G1 extracts was not restored in the presence of the nucleotide analogs (lane 6), indicating that different mechanisms are involved in transcriptional repression in M and G1 cell extracts. When extracts from early G1 cells were mixed with extracts from asynchronous cells, no reduction of the activity of the asynchronous cell extract was observed, demonstrating that the impaired activity of G1 cell extract is not caused by the presence of (a) dominant repressor(s) (lanes 7–9).
TIF-IB Is Reactivated During M/G1 Transition.
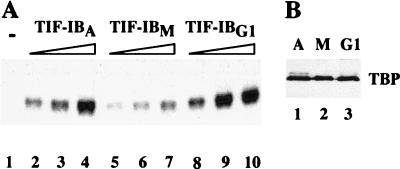
The low transcriptional activity of early G1 extracts suggests that factor(s) that are required for rDNA transcription initiation are missing or inactive. We previously demonstrated that mitotic repression of human pol I transcription is mediated by inactivation of SL1 by cdc2/cyclin B-directed phosphorylation of the largest subunit, e.g., hTAFI110 (10). Thus, the persistence of mitotically phosphorylated, inactive TIF-IB/SL1 could account for the low transcriptional activity of G1 cells. To test this, we precipitated the murine factor TIF-IB from whole-cell extracts with polyclonal antibodies directed against mTAFI95, the largest subunit of TIF-IB, and assayed immunoprecipitated TBP-TAFI complexes in a reconstituted transcription system lacking TIF-IB (20). In the absence of TIF-IB, this system is transcriptionally inactive (Fig. 3A, lane 1). Addition of TIF-IB from asynchronous cells strongly augments transcription (lanes 2–4), whereas the same amount of TIF-IB from mitotic cells is much less active (lanes 5–7). Of note, the activity of TIF-IB was fully recovered after exit from mitosis as revealed by the high transcriptional activity of TIF-IB from early G1 cells (lanes 8–10). Thus, impaired rDNA transcription in early G1 cells is not caused by limited TIF-IB activity.
Figure 3.
TIF-IB/SL1 is reactivated after exit from mitosis. (A) In vitro transcription. Extracts from asynchronous (A), mitotic (M), and early G1 FT210 cells were preincubated with 2.5 μM adenosine 5′-[γ-thio]triphosphate for 30 min at 30°C, and then TIF-IB was immunoprecipitated with α-mTAFI95 antibodies immobilized on sheep anti-rabbit IgG Dynabeads. After stringent washing, bead-bound TIF-IB activity was monitored in a reconstituted transcription system. Lane 1 shows transcription in the absence of TIF-IB, lanes 2–4 in the presence of increasing amounts (50–200 pg) of TIF-IB from asynchronous cells (TIF-IBA), lanes 5–7 from mitotic cells (TIF-IBM), and lanes 8–10 from G1 cells (TIF-IBG1). (B) Western blot. Bead-bound TIF-IB precipitated from asynchronous (lane 1), mitotic (lane 2) and G1 cell extracts (lane 3) were subjected to Western blot analysis by using anti-TBP antibodies.
Transcription in G1 Extracts Can Be Rescued by Exogenous UBF.
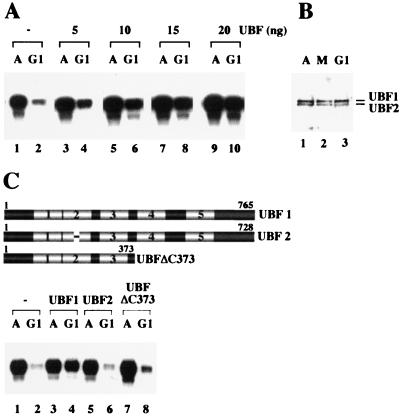
The fact that transcriptional activity in early G1 is low, despite TIF-IB activity being fully restored, suggests that different repressive mechanisms appear to down-regulate rDNA transcription at mitosis and early G1, respectively. To investigate which of the components required for pol I transcription are deficient or inactive in early G1 phase extracts, partially purified or recombinant transcription factors and pol I were added to asynchronous or G1 extracts, respectively, and assayed for their capability to activate transcription. Of several protein fractions tested (data not shown), only UBF was capable of selectively stimulating transcription in G1 extracts in a dose-dependent manner. Complementation with UBF only slightly increased transcription in asynchronous extracts (Fig. 4A, lanes 3, 5, 7, and 9), but strongly stimulated transcription in G1 extracts (lanes 4, 6, 8, and 10).
Figure 4.
Transcription in early G1 cell extracts is rescued by UBF. (A) Exogenous UBF stimulates transcriptional activity of early G1 extracts. Transcription assays contained 25 μg of protein from asynchronous (A) and G1 cells (G1) and increasing amounts of recombinant UBF1 added at the onset of transcription (lanes 3–10). (B) The amount of cellular UBF does not change throughout the cell cycle. Protein (100 μg) extracted from asynchronous (A, lane 1), mitotic (M, lane 2), and early G1 cells (lane 3) were analyzed on immunoblots by using anti-UBF antiserum. (C) Rescue of transcription in G1 extracts requires transcriptionally active UBF. Asynchronous and G1 extracts were assayed in the absence of UBF (lanes 1, 2) and in the presence of 20 ng of UBF1 (lanes 3, 4), UBF2 (lanes 5, 6), or UBFΔC373 (lanes 7, 8). A scheme representing UBF1, UBF2, and the deletion mutant UBFΔC373 is shown above. The HMG-box motifs are numbered.
The observation that UBF is capable of rescuing transcription in G1 extracts suggests that either the amount or activity of UBF is decreased in G1 phase cells. A quantitative Western blot analysis revealed no significant difference in the level of endogenous UBF in asynchronous, mitotic and G1 extracts (Fig. 4B). In G1 cells, we consistently observed a slight increase in the ratio of the two splice variants UBF1 and UBF2 (Fig. 4B, lane 3). Because, however, both the DNA-binding and transcriptional activity of UBF2 is about one order of magnitude lower than UBF1 (24), an increase in the ratio of UBF1 vs. UBF2 cannot account for the impaired transcriptional activity of G1 extracts.
Inactivation of UBF can be brought about either by changes in the phosphorylation pattern or by association with proteins that interfere with UBF function (19, 25–28). To distinguish between these possibilities, the rescue experiment was also performed with transcriptionally inactive forms of UBF, e.g., UBF2 and a deletion mutant, UBFΔC373, that lacks the activation domain within the carboxyl-terminal part and therefore is transcriptionally inactive. When added to extracts from early G1 cells, only UBF1, but not UBF2 or UBFΔC373, stimulated transcription (Fig. 4C). Thus, although both UBF2 and UBFΔC373 are capable of executing all known UBF–protein interactions (29, 30), they fail to activate transcription of early G1 extracts. This indicates that transcriptional repression in early G1 is not caused by associated protein(s) that may interfere with UBF function and suggests that UBF is inactive in early G1 cells.
UBF from G1 Cells Is Transcriptionally Inactive.
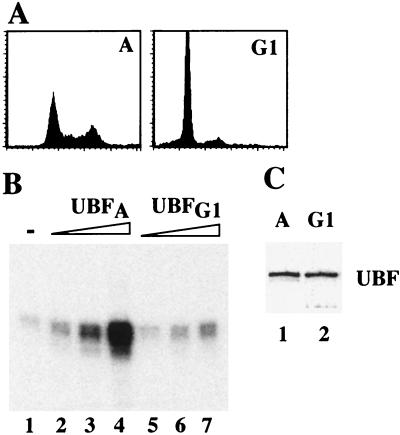
To demonstrate that the activity of UBF is impaired after exit from mitosis, a stable NIH 3T3 cell line expressing Flag epitope-tagged UBF1 was established. In the experiment shown in Fig. 5, cells were synchronized by serum starvation and released into cell-cycle progression by serum stimulation for 3 h. Synchronization and G1 progression was monitored by fluorescence-activated cell sorting (FACS) analysis (Fig. 5A) and immunoblots visualizing cyclin D expression (data not shown). Flag-tagged UBF1 was affinity-purified from asynchronous and G1 cells, respectively, and tested in a UBF-responsive reconstituted transcription system. To preserve phase-specific phosphorylations, phosphatase inhibitors were included both during UBF isolation and transcription. In addition, transcription was performed in the presence of AMP-PNP and GMP-PNP to exclude de novo phosphorylation of UBF during transcription. As shown in Fig. 5B, UBF1 from asynchronous cells mediated a concentration-dependent activation of transcription (lanes 2–4), whereas the same amounts of UBF1 from G1 cells were virtually inactive (lanes 5–7). Thus, inactivation of UBF appears to be causally responsible for the low level of rDNA transcription in early G1 cells.
Figure 5.
Impaired transcriptional activity of UBF isolated from G1 cells. (A) FACS analysis. Serum-starved NIH 3T3 cells were synchronized in early G1 by serum stimulation for 3 h. (B) In vitro transcription. UBF was immunopurified from asynchronous (UBFA, lanes 2–4) or early G1 phase (UBFG1, lanes 5–7) NIH 3T3 cells expressing Flag-epitope tagged UBF1. Purified UBF (1, 2.5, and 4 ng) was assayed in an UBF-responsive reconstituted transcription system. (C) Western blot. Flag-UBF1 (10 μl; 15 ng) isolated from asynchronous (lane 1) and G1 cells (lane 2) were subjected to Western blot analysis by using anti-Flag antibodies (mAb M2).
An Okadaic Acid-Sensitive Phosphatase Activates Mitotic UBF.
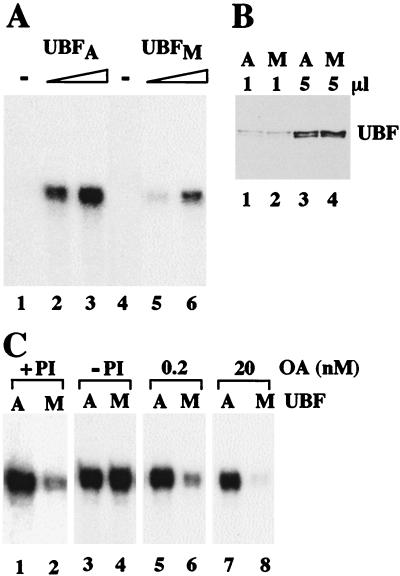
Inactivation of UBF could occur either at the exit from or—like TIF-IB/SL1—at the onset of mitosis. To test whether UBF is also inactivated during mitosis, the transcriptional activity of UBF isolated from mitotic FT210 cells was compared with that of UBF from asynchronous cells. Again, increasing amounts of UBF from asynchronous cells strongly augmented transcription in a reconstituted transcription system (Fig. 6A, lanes 2 and 3). In contrast, the ability of UBF prepared from mitotic cells to activate pol I transcription was strongly impaired (lanes 4, 5). Mixing of UBF from mitotic and asynchronous cells did not prevent UBF-mediated transcription activation (data not shown), indicating that the reduced transcriptional activity of UBF from mitotic cells is not due to copurifying inhibitory proteins. Thus, two basal pol I factors, e.g., TIF-IB/SL1 and UBF, appear to be inactivated at the onset of mitosis.
Figure 6.
UBF from mitotic cells is inactive. (A) Comparison of the transcriptional activity of UBF from asynchronous and mitotic FT210 cells. UBF was isolated by chromatography on DEAE- and Q-Sepharose and assayed for transcriptional activity. Shown is transcription in the absence of UBF (lanes 1 and 4) and in the presence of 4 (lanes 2 and 3) or 8 (lanes 5 and 6) ng of UBF from asynchronous (UBFA) and mitotic cells (UBFM). Reactions contained AMP-PNP, GMP-PNP, and nonspecific phosphatase inhibitors to prevent modification of UBF during transcription. (B) Western blot. Q-Sepharose fractions (1 and 5 μl) purified from asynchronous (lanes 1, 3) and mitotic (lanes 2, 4) extracts were subjected to Western blot analysis by using anti-UBF serum. (C) An okadaic acid-sensitive phosphatase counteracts mitotic inactivation of UBF. Equal amounts (10 ng) of UBF purified from asynchronous (A) and mitotic (M) FT210 cells were assayed in a partially purified transcription system either in the presence of 2 mM 2-glycerophosphate/0.2 mM sodium orthovanadate (+PI; lanes 1 and 2), in the absence of phosphatase inhibitors (−PI; lanes 3 and 4) or in the presence of 0.2 nM (lanes 5 and 6) and 20 nM okadaic acid (lanes 7 and 8). The assays were performed in the presence of AMP-PNP and GMP-PNP to prevent de novo phosphorylation of UBF during transcription.
Importantly, inactivation of UBF was only observed when phosphatase inhibitors were included in all buffers used for UBF extraction, fractionation, and dialysis, as well as in the reconstituted transcription system (Fig. 6C, lanes 1 and 2). In the absence of phosphatase inhibitors, equal amounts of UBF from both asynchronous or mitotic cells displayed the same transcriptional activity (lanes 3, 4), indicating that mitotic UBF can be activated by dephosphorylation. To identify the phosphatase that counteracts mitotic phosphorylation of UBF, we tested the effect of okadaic acid on UBF-mediated transcription. Okadaic acid is a potent inhibitor of protein phosphatases 1 and 2A (PP1 and PP2A). At low concentrations, okadaic acid inhibits PP2A but not PP1 (31) and therefore, can be used as a tool to distinguish between both phosphatases. In the presence of 0.2 nM okadaic acid, mitotic UBF was almost inactive (lane 6). At higher concentrations, the activity of mitotic UBF was completely impaired (lane 8), whereas UBF from asynchronous cells was not significantly affected (lanes 5 and 7). This result demonstrates that mitotic repression of UBF activity is relieved by an okadaic acid-sensitive phosphatase, presumably PP2A.
DISCUSSION
The numerous biological events that are associated with the periodicity of the cell cycle make it an attractive model for the study of regulatory mechanisms that link gene activity to cell cycle control. The most pronounced effects are observed during mitosis, when transcription of most nuclear genes is shut off. Consistent with conserved regulatory mechanisms shared by all three classes of nuclear RNA polymerases, the respective TBP-containing factors, e.g., TIF-IB/SL1, TFIID, and TFIIIB, which impart promoter selectivity and regulatory potential to the respective RNA polymerases, are inactivated by mitotic phosphorylation (2, 6–8, 10). In each case, TAFs are targets for inhibitory phosphorylation. Gottesfeld’s group was the first to show (7) that mitotic arrest of pol III transcription was due to phosphorylation of TFIIIB. Likewise, repression of activator-dependent but not basal pol II transcription correlates with mitotic inactivation of TFIID (2). For pol I, we have recently demonstrated that the shut-off of rDNA transcription during M phase is due to cdc2/cyclin B-directed phosphorylation of TAFI110, the largest subunit of human SL1 (10).
Very little is known about the mechanisms that activate transcription at the exit from mitosis. As demonstrated in this and earlier studies (10, 23), transcriptional activity in mitotic extracts was recovered by using nonhydrolyzable analogs of ATP and GTP. This result suggests that when hydrolyzable nucleotides are absent from the reaction, phosphatases can restore activity. Indeed, consistent with specific phosphatases counteracting the action of regulatory kinases, the activity of mitotic SL1 could be restored by phosphatase treatment in vitro (10). In contrast to M phase extracts, transcription in early G1 extracts was not elevated in the presence of kinase inhibitors. Thus, different mechanisms mediate transcriptional repression in M and early G1 phase.
We now demonstrate that TIF-IB/SL1 from early G1 cells is fully active, indicating that inhibitory mitotic phosphorylation(s) have been removed at the exit from mitosis. Transcriptional repression in early G1 extracts could be overcome by addition of exogenous UBF. This observation, together with the finding that UBF can be reactivated by an okadaic-sensitive phosphatase, suggests that transcriptional repression in early G1 is due to inactivation of UBF.
Inactivation of UBF could be brought about by any of the following possibilities, which are not mutually exclusive. First, interaction with early G1 phase-specific protein(s) could impair UBF activity. Second, positive phosphorylation(s) could be removed from UBF at the onset of mitosis and restored during G1 progression. Finally, UBF could be inactivated by mitotic phosphorylation and reactivated later (presumably by a different phosphatase) than TIF-IB/SL1. Although the present data do not definitely exclude any of these possibilities, we favor the latter one for the following reasons. UBF has been shown to interact with a number of proteins, including the retinoblastoma protein pRb (19, 28), the third largest subunit of pol I (29, 30), and p53 (unpublished data). All of these interactions are mediated by the amino-terminal part of UBF. If transcriptional repression during early G1 was brought about by association of UBF with pRb, then both UBF2 and the carboxyl-terminal deletion mutant UBFΔC373, which efficiently interact with pRb, should be capable of rescuing transcriptional activity. This, however, was definitely not the case. Only UBF1, and none of the transcriptionally inactive variants of UBF, augmented transcription in G1 extracts. The available data suggest that the two basal factors that bind to the rDNA promoter, e.g., TIF-IB/SL1 and UBF, are inactivated by mitotic phosphorylation. At the exit from mitosis, TIF-IB/SL1 is immediately reactivated, whereas UBF activity recovers when cells pass through G1.
The finding that UBF is inactivated at mitosis was unexpected, because in a previous study SL1 immunoprecipitated from interphase cells was sufficient to relieve transcriptional repression of mitotic extracts, suggesting that TIF-IB/SL1 is the only factor that is targeted by mitotic phosphorylation (10). In this former study, we have used SL1 that was immunoprecipitated from crude extracts. Because, however, UBF interacts and coprecipitates with TIF-IB/SL1 (32), this SL1 preparation very likely contained trace amounts of contaminating UBF that was not detectable on Western blots. Consequently, we have missed mitotic inactivation of UBF in these earlier studies. Subsequent experiments with highly purified TIF-IB/SL1 revealed that transcriptional rescue of mitotic extracts requires addition of both TIF-IB/SL1 and UBF (unpublished results). Consistent with UBF being targeted by one or more mitotic kinases, UBF isolated from mitotic cells is inactive, but can be activated by an okadaic acid-sensitive phosphatase present in the reconstituted transcription system. Although the phosphatase that counteracts mitotic inactivation of UBF has yet to be identified, the sensitivity toward low concentrations of okadaic acid suggests that PP2A may be the enzyme that relieves mitotic inactivation of UBF. The slow restoration of pol I transcription during G1 may reflect the time the phosphatase requires to reactivate UBF. Consistent with this result, there is increasing experimental evidence demonstrating an important role of PP2A in cell cycle control (33). Reduction of PP2A activity accompanies entry into mitosis, and reactivation during G1 appears to correlate with the kinetics of pol I transcription activation.
Our finding that during mitosis, two basal pol I transcription initiation factors are inactivated that recover at different times during G1 progression adds another level of complexity to cell cycle-dependent regulation of rDNA transcription. As changes in the phosphorylation pattern of UBF have been shown to correlate with UBF activity (25–27), UBF may be the central target for different kinase-driven signal-transduction pathways. In an attempt to identify the cellular protein kinases that phosphorylate UBF during the cell cycle, we have mapped one serine residue (S-484) that is phosphorylated by G1-specific cdk–cyclin complexes (34). Together, the available data reveal that the activity of UBF is both positively and negatively modulated by phosphorylation. UBF-mediated regulation occurs mainly during early G1 when cells decide if they exit the cycle to terminally differentiate, stay quiescent, or undergo a further division cycle. Because this decision is triggered by external signals, which in turn activate cellular protein kinases, changing patterns of UBF phosphorylation constitute an efficient means of adapting cellular rRNA synthetic activity to cell proliferation.
Acknowledgments
We thank B. Dörr for preparation and fractionation of cell extracts, P. Schül for technical assistance, F. Hanaoka for providing FT210 cells, L. Tora for anti-TBP antibodies, and H. Beckmann for baculovirus stocks expressing Flag-tagged human UBF. This work was supported by the Deutsche Forschungsgemeinschaft (Priority program “Cell Cycle”), the European Union (TMR program HMG-box proteins), and the Fond der Chemischen Industrie.
ABBREVIATIONS
- pol
RNA polymerase
- TBP
TATA box-binding protein
- TAFI
pol I-specific TBP-associated factor
- UBF
upstream binding factor
- TIF
transcription initiation factor
- PP
protein phosphatase
- cdk
cyclin-dependent kinase
- TF
transcription factor
- rDNA
rRNA-encoding DNA
- FACS
fluorescence-activated cell sorting
- AMP-PNP
adenosine 5′-[β,γ-imido]triphosphate
- GMP-PNP
guanosine 5′-[β,γ-imido]triphosphate
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Gottesfeld J M, Forbes D J. Trends Biochem Sci. 1997;22:197–202. doi: 10.1016/s0968-0004(97)01045-1. [DOI] [PubMed] [Google Scholar]
- 2.Segil N, Guermah M, Hoffmann A, Roeder R G, Heintz N. Genes Dev. 1996;10:2389–2400. doi: 10.1101/gad.10.19.2389. [DOI] [PubMed] [Google Scholar]
- 3.Long J L, Leresche A, Kriwacki R W, Gottesfeld J M. Mol Cell Biol. 1998;18:1467–1476. doi: 10.1128/mcb.18.3.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akoulitechev S, Reinberg D. Genes Dev. 1998;12:3541–3550. doi: 10.1101/gad.12.22.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cisek L J, Corden J L. Nature (London) 1989;339:679–684. doi: 10.1038/339679a0. [DOI] [PubMed] [Google Scholar]
- 6.Hartl P, Gottesfeld J, Forbes D J. J Cell Biol. 1993;120:613–624. doi: 10.1083/jcb.120.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gottesfeld J M, Wolf V J, Dang T, Forbes D J, Hartl P. Science. 1994;263:81–84. doi: 10.1126/science.8272869. [DOI] [PubMed] [Google Scholar]
- 8.White R J, Gottlieb T M, Downes C S, Jackson S P. Mol Cell Biol. 1995;15:1983–1992. doi: 10.1128/mcb.15.4.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.White R J, Gottlieb T M, Downes C S, Jackson S P. Mol Cell Biol. 1995;15:6653–6662. doi: 10.1128/mcb.15.12.6653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heix J, Vente A, Voit R, Budde A, Michaelidis T M, Grummt I. EMBO J. 1998;17:7373–7381. doi: 10.1093/emboj/17.24.7373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Comai L, Tanese N, Tjian R. Cell. 1992;68:965–976. doi: 10.1016/0092-8674(92)90039-f. [DOI] [PubMed] [Google Scholar]
- 12.Eberhard D, Tora L, Egly J M, Grummt I. Nucleic Acids Res. 1993;21:4180–4186. doi: 10.1093/nar/21.18.4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rudloff U, Eberhard D, Tora L, Stunnenberg H, Grummt I. EMBO J. 1994;13:2611–2616. doi: 10.1002/j.1460-2075.1994.tb06551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jantzen H M, Admon A, Bell S P, Tjian R. Nature (London) 1990;344:830–836. doi: 10.1038/344830a0. [DOI] [PubMed] [Google Scholar]
- 15.Yasuda H, Kamijo M, Honda R, Nakamura M, Hanaoka F, Ohba Y. Cell Struct Funct. 1991;16:105–112. doi: 10.1247/csf.16.105. [DOI] [PubMed] [Google Scholar]
- 16.Manley J L, Fire A, Sharp P A, Gefter M L. Proc Natl Acad Sci USA. 1980;77:3855–3859. doi: 10.1073/pnas.77.7.3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schnapp A, Grummt I. Methods Enzymol. 1996;273:346–359. doi: 10.1016/s0076-6879(96)73023-9. [DOI] [PubMed] [Google Scholar]
- 18.Smith S D, Oriahi E, Lowe D, Yang-Yen H F, O’Mahony D, Rose K, Chen K, Rothblum L I. Mol Cell Biol. 1990;10:3105–3116. doi: 10.1128/mcb.10.6.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Voit R, Schäfer K, Grummt I. Mol Cell Biol. 1997;17:4230–4237. doi: 10.1128/mcb.17.8.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heix J, Zomerdijk J C M B, Ravanpay A, Tjian R, Grummt I. Proc Natl Acad Sci USA. 1997;94:1733–1738. doi: 10.1073/pnas.94.5.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brou C, Wu H, Ali S, Scheer E, Lang C, Davidson I, Cahmbon P, Tora L. EMBO J. 1993;12:489–499. doi: 10.1002/j.1460-2075.1993.tb05681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wuarin J, Schibler U. Mol Cell Biol. 1994;14:7219–7225. doi: 10.1128/mcb.14.11.7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuhn A, Vente A, Dorée M, Grummt I. J Mol Biol. 1998;284:1–5. doi: 10.1006/jmbi.1998.2164. [DOI] [PubMed] [Google Scholar]
- 24.Voit R, Stefanovsky V, Evers R, Bianchi M, Grummt I. EMBO J. 1994;13:416–424. doi: 10.1002/j.1460-2075.1994.tb06276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Mahony D J, Smith S D, Xie W, Rothblum L I. Nucleic Acids Res. 1992;20:1301–1308. doi: 10.1093/nar/20.6.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Voit R, Schnapp A, Kuhn A, Rosenbauer H, Hirschmann P, Stunnenberg H G, Grummt I. EMBO J. 1992;11:2211–2218. doi: 10.1002/j.1460-2075.1992.tb05280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Voit R, Kuhn A, Sander E E, Grummt I. Nucleic Acids Res. 1995;23:2593–2599. doi: 10.1093/nar/23.14.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cavanaugh A, Hempel W M, Taylor L J, Rogalsky V M, Todorov G, Rothblum L I. Nature (London) 1995;374:177–180. doi: 10.1038/374177a0. [DOI] [PubMed] [Google Scholar]
- 29.Schnapp G, Santori F, Carles C, Riva M, Grummt I. EMBO J. 1994;13:190–199. doi: 10.1002/j.1460-2075.1994.tb06248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hanada K, Song C Z, Yamamoto K, Yano K, Maeda Y, Yamaguchi K, Muramatsu M. EMBO J. 1996;15:2217–2226. [PMC free article] [PubMed] [Google Scholar]
- 31.Bialojan C, Takai A. Biochem J. 1988;15:283–290. doi: 10.1042/bj2560283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hempel W M, Cavanaugh A H, Hannan R D, Taylor L, Rothblum L I. Mol Cell Biol. 1996;16:557–563. doi: 10.1128/mcb.16.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wera S, Hemmings B A. Biochem J. 1995;311:17–29. doi: 10.1042/bj3110017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Voit R, Hoffmann M, Grummt I. EMBO J. 1999;18:1891–1899. doi: 10.1093/emboj/18.7.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]