Abstract
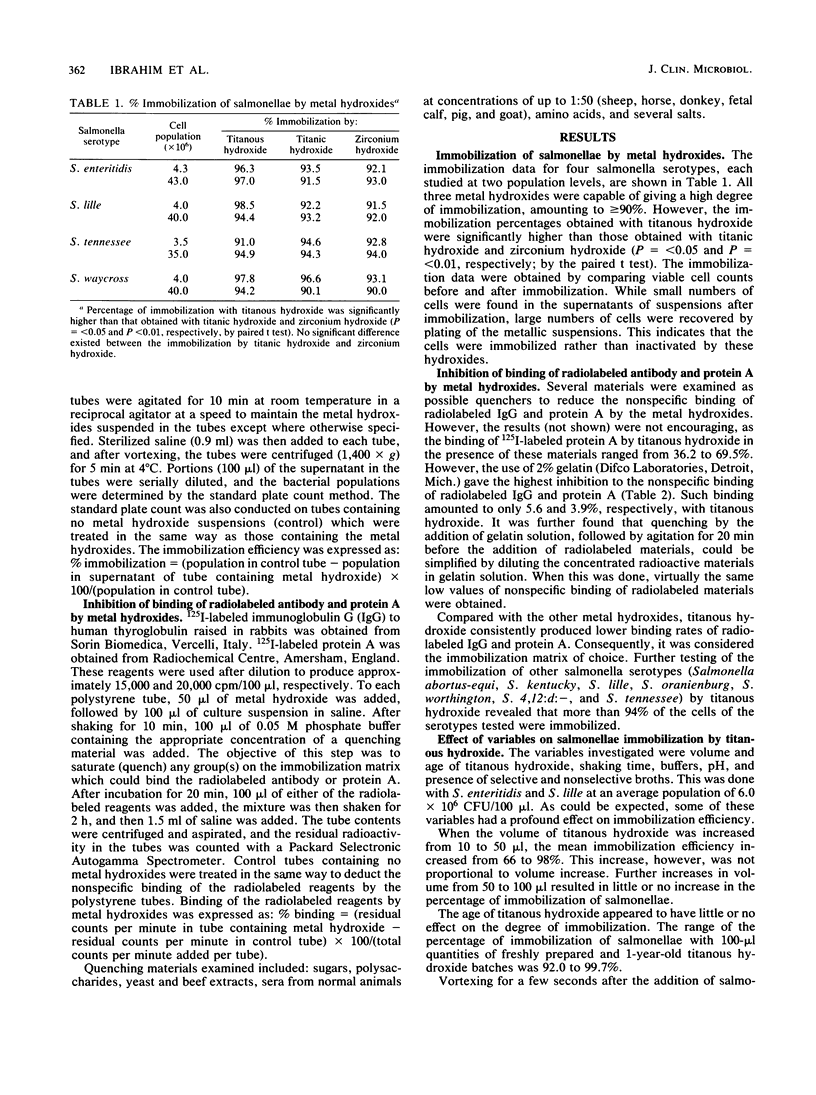
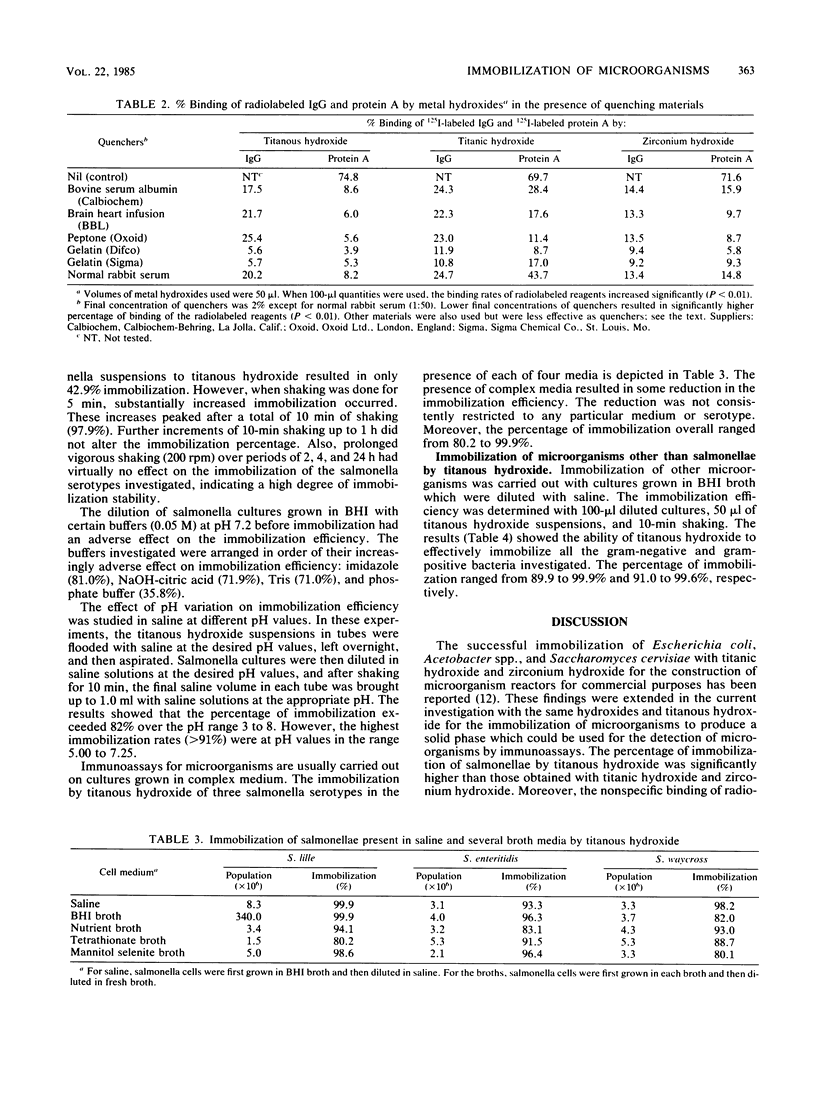
Several cultures of gram-negative and gram-positive bacteria were successfully immobilized with titanous hydroxide. The immobilization efficiency for the microorganisms investigated in saline and broth media ranged from 80.2 to 99.9%. The immobilization of salmonellae was effective over a wide pH range. The presence of buffers, particularly phosphate buffer, drastically reduced the immobilization rate. However, buffers may be added to immunoassay systems after immobilization of microorganisms. The immobilization process involved only one step, i.e., shaking 100 microliter of culture with 50 microliter of titanous hydroxide suspension in polystyrene tubes for only 10 min. The immobilized cells were so tenaciously bound that vigorous agitation for 24 h did not result in cell dissociation. The nonspecific binding of 125I-labeled antibody from rabbits and 125I-labeled protein A by titanous hydroxide was inhibited in the presence of 2% gelatin and amounted to only 5.6 and 3.9%, respectively. We conclude that this immobilization procedure is a potentially powerful tool which could be utilized in solid-phase immunoassays concerned with the diagnosis of microorganisms.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cantarero L. A., Butler J. E., Osborne J. W. The adsorptive characteristics of proteins for polystyrene and their significance in solid-phase imunoassays. Anal Biochem. 1980 Jul 1;105(2):375–382. doi: 10.1016/0003-2697(80)90473-x. [DOI] [PubMed] [Google Scholar]
- Fox J., Hechemy K. Coupling of Escherichia coli lipopolysaccharide to epoxy-activated Sepharose 6B. Infect Immun. 1978 Jun;20(3):867–868. doi: 10.1128/iai.20.3.867-868.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee A. P., Langone J. J. Immunoassay using 125I- or enzyme-labeled protein A and antigen-coated tubes. Anal Biochem. 1981 Sep 15;116(2):524–530. doi: 10.1016/0003-2697(81)90397-3. [DOI] [PubMed] [Google Scholar]
- Granfors K., Viljanen M. K., Ahvonen P., Toivanen P. Measurement of IgM and IgG antibodies to Yersinia by solid-phase radioimmunoassay. J Infect Dis. 1978 Aug;138(2):232–236. doi: 10.1093/infdis/138.2.232. [DOI] [PubMed] [Google Scholar]
- Habeeb A. J., Hiramoto R. Reaction of proteins with glutaraldehyde. Arch Biochem Biophys. 1968 Jul;126(1):16–26. doi: 10.1016/0003-9861(68)90554-7. [DOI] [PubMed] [Google Scholar]
- Hughes R. C., Thurman P. F. Cross-linking of bacterial cell walls with glutaraldehyde. Biochem J. 1970 Oct;119(5):925–926. doi: 10.1042/bj1190925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack T. R., Zajic J. E. The enzymatic conversion of L-histidine to urocanic acid by whole cells of Micrococcus luteus immobilized on carbodiimide activated carboxymethylcellulose. Biotechnol Bioeng. 1977 May;19(5):631–648. doi: 10.1002/bit.260190503. [DOI] [PubMed] [Google Scholar]
- Jacobson B. S., Branton D. Plasma membrane: rapid isolation and exposure of the cytoplasmic surface by use of positively charged beads. Science. 1977 Jan 21;195(4275):302–304. doi: 10.1126/science.831278. [DOI] [PubMed] [Google Scholar]
- Kennedy J. F., Barker S. A., Humphreys J. D. Microbial cells living immobilised on metal hydroxides. Nature. 1976 May 20;261(5557):242–244. doi: 10.1038/261242a0. [DOI] [PubMed] [Google Scholar]
- Kohler R. B., Zimmerman S. E., Wilson E., Allen S. D., Edelstein P. H., Wheat L. J., White A. Rapid radioimmunoassay diagnosis of Legionnaires' disease: detection and partial characterization of urinary antigen. Ann Intern Med. 1981 May;94(5):601–605. doi: 10.7326/0003-4819-94-5-601. [DOI] [PubMed] [Google Scholar]
- Langone J. J. [125I]protein A: a tracer for general use in immunoassay. J Immunol Methods. 1978;24(3-4):269–285. doi: 10.1016/0022-1759(78)90131-x. [DOI] [PubMed] [Google Scholar]
- Leinonen M., Frasch C. E. Class-specific antibody response to group B Neisseria meningitidis capsular polysaccharide: use of polylysine precoating in an enzyme-linked immunosorbent assay. Infect Immun. 1982 Dec;38(3):1203–1207. doi: 10.1128/iai.38.3.1203-1207.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards F. M., Knowles J. R. Glutaraldehyde as a protein cross-linkage reagent. J Mol Biol. 1968 Oct 14;37(1):231–233. doi: 10.1016/0022-2836(68)90086-7. [DOI] [PubMed] [Google Scholar]
- Sing V. O., Schroth M. N. Bacteria--plant cell surface interactions: active immobilization of saprophytic bacteria in plant leaves. Science. 1977 Aug 19;197(4305):759–761. doi: 10.1126/science.197.4305.759. [DOI] [PubMed] [Google Scholar]


