Abstract
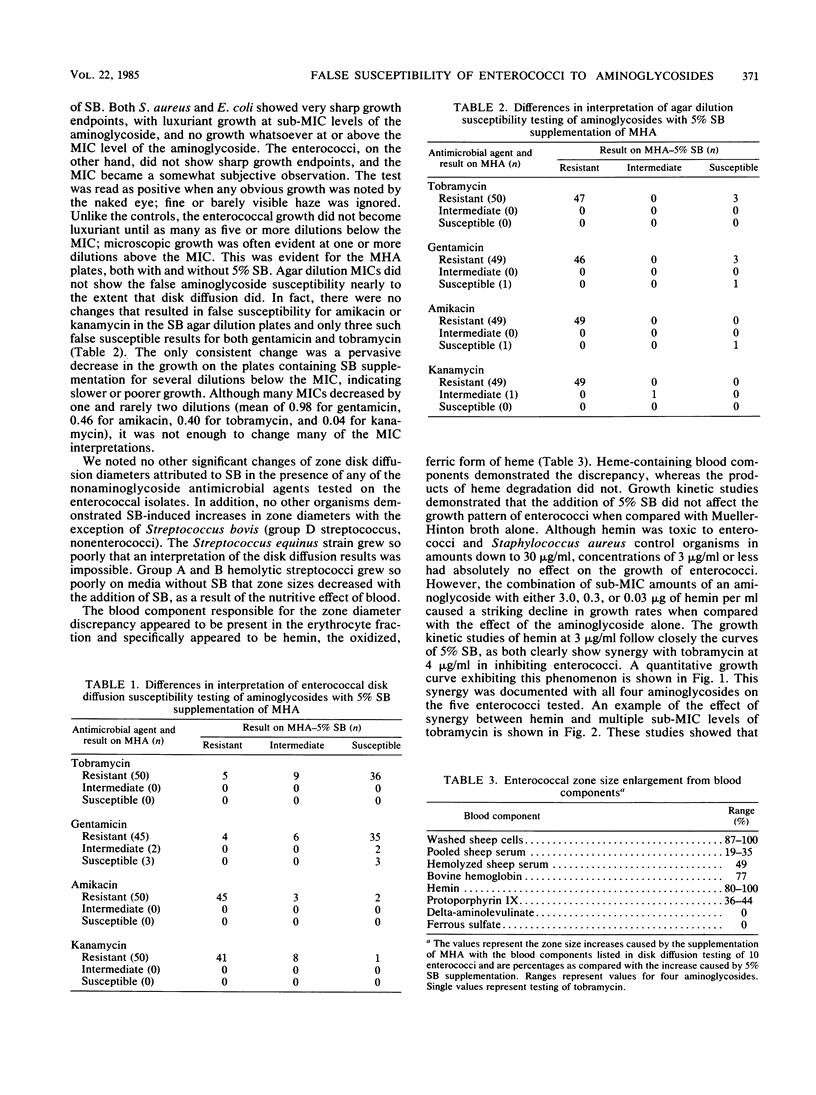
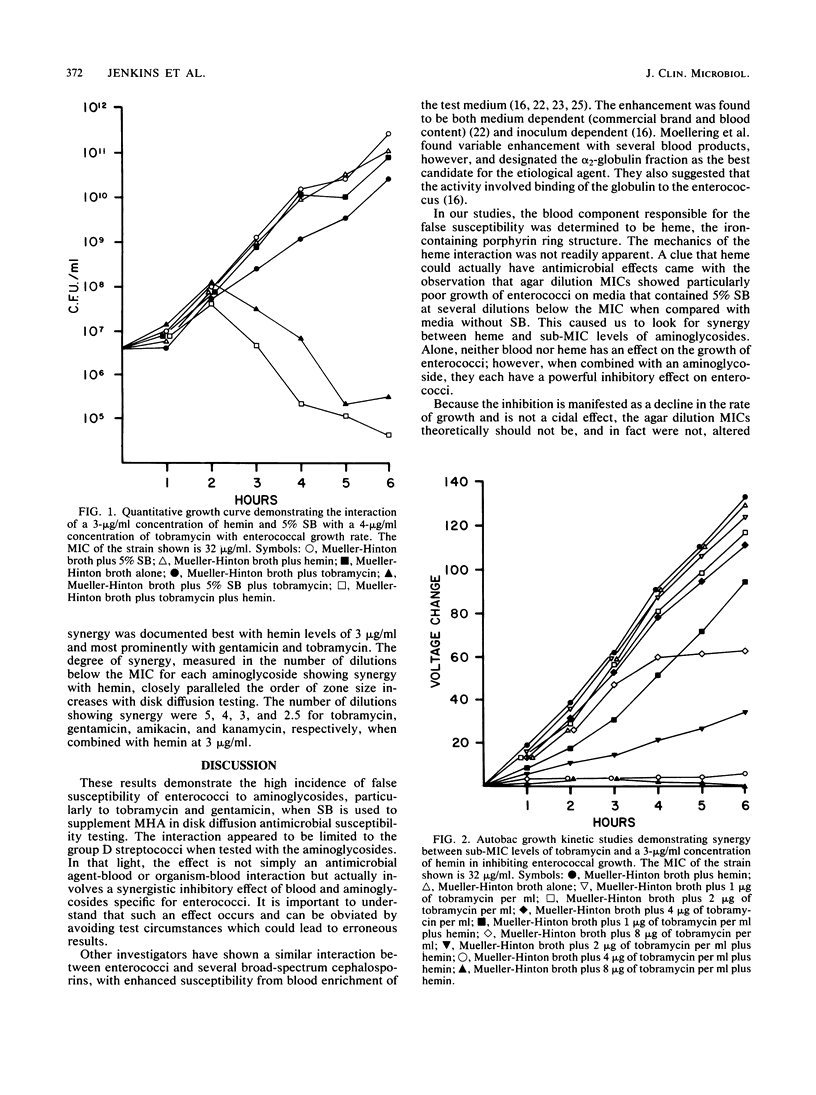
Disk diffusion susceptibility tests for enterococci are frequently modified by adding 5% sheep blood (SB) to Mueller-Hinton agar; the performance standards from the National Committee for Clinical Laboratory Standards sanction this addition. Susceptibility testing of aminoglycoside antibiotics is not recommended for enterococci; in actual practice, however, some laboratories do include aminoglycoside antibiotics routinely, and others may test upon request or in selected situations. In examining 50 clinical isolates of enterococci, SB-enriched Mueller-Hinton agar frequently gave enlarged zone sizes that falsely indicated susceptibility (72% for gentamicin and tobramycin), with the average increase in zone size being 6.3 and 7.6 mm, respectively. Comparison agar dilution MICs demonstrated uniform resistance, with or without added SB. The effect was shown to be caused by heme in concentrations as low as 0.03 micrograms/ml, which, when combined with aminoglycoside antibiotics, caused a synergistic growth inhibition of the enterococci, resulting in larger aminoglycoside antibiotic zones. We postulate that the heme effect is related to a catalytic cleavage of intracellular H2O2 and resultant lipid peroxidation. No other organism or antimicrobial agent tested demonstrated a similar effect, although other investigators have shown a similar phenomenon with the broad-spectrum cephalosporins. Because enterococci grow well and give accurate susceptibility results on Mueller-Hinton agar without SB supplementation and because of the spectrum of definable problems with a number of antimicrobial agents, we recommend that enterococci routinely be tested without SB.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arbeit R. D., Karakawa W. W., Vann W. F., Robbins J. B. Predominance of two newly described capsular polysaccharide types among clinical isolates of Staphylococcus aureus. Diagn Microbiol Infect Dis. 1984 Apr;2(2):85–91. doi: 10.1016/0732-8893(84)90002-6. [DOI] [PubMed] [Google Scholar]
- Bauer A. W., Kirby W. M., Sherris J. C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966 Apr;45(4):493–496. [PubMed] [Google Scholar]
- Brenner V. C., Sherris J. C. Influence of different media and bloods on results of diffusion antibiotic susceptibility tests. Antimicrob Agents Chemother. 1972 Feb;1(2):116–122. doi: 10.1128/aac.1.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderwood S. A., Wennersten C., Moellering R. C., Jr, Kunz L. J., Krogstad D. J. Resistance to six aminoglycosidic aminocyclitol antibiotics among enterococci: prevalence, evolution, and relationship to synergism with penicillin. Antimicrob Agents Chemother. 1977 Sep;12(3):401–405. doi: 10.1128/aac.12.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou A. C., Chevli R., Fitch C. D. Ferriprotoporphyrin IX fulfills the criteria for identification as the chloroquine receptor of malaria parasites. Biochemistry. 1980 Apr 15;19(8):1543–1549. doi: 10.1021/bi00549a600. [DOI] [PubMed] [Google Scholar]
- Chou A. C., Fitch C. D. Hemolysis of mouse erythrocytes by ferriprotoporphyrin IX and chloroquine. Chemotherapeutic implications. J Clin Invest. 1980 Oct;66(4):856–858. doi: 10.1172/JCI109925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FULTON J. D., RIMINGTON C. The pigment of the malaria parasite Plasmodium berghei. J Gen Microbiol. 1953 Feb;8(1):157–159. doi: 10.1099/00221287-8-1-157. [DOI] [PubMed] [Google Scholar]
- Kosower N. S., Kosower E. M., Wertheim B., Correa W. S. Diamide, a new reagent for the intracellular oxidation of glutathione to the disulfide. Biochem Biophys Res Commun. 1969 Nov 6;37(4):593–596. doi: 10.1016/0006-291x(69)90850-x. [DOI] [PubMed] [Google Scholar]
- McCay P. B., Gibson D. D., Hornbrook K. R. Glutathione-dependent inhibition of lipid peroxidation by a soluble, heat-labile factor not glutathione peroxidase. Fed Proc. 1981 Feb;40(2):199–205. [PubMed] [Google Scholar]
- Meshnick S. R., Chang K. P., Cerami A. Heme lysis of the bloodstream forms of Trypanosoma brucei. Biochem Pharmacol. 1977 Oct 15;26(20):1923–1928. doi: 10.1016/0006-2952(77)90167-8. [DOI] [PubMed] [Google Scholar]
- Orjih A. U., Banyal H. S., Chevli R., Fitch C. D. Hemin lyses malaria parasites. Science. 1981 Nov 6;214(4521):667–669. doi: 10.1126/science.7027441. [DOI] [PubMed] [Google Scholar]
- Sahm D. F., Baker C. N., Jones R. N., Thornsberry C. Influence of growth medium on the in vitro activities of second- and third-generation cephalosporins against Streptococcus faecalis. J Clin Microbiol. 1984 Sep;20(3):561–567. doi: 10.1128/jcm.20.3.561-567.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahm D. F., Baker C. N., Jones R. N., Thornsberry C. Medium-dependent zone size discrepancies associated with susceptibility testing of group D streptococci against various cephalosporins. J Clin Microbiol. 1983 Oct;18(4):858–865. doi: 10.1128/jcm.18.4.858-865.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornsberry C., Sahm D. F. Effect of media and blood on the antimicrobial activity of cephalosporins on serogroup D streptococci: a review. Diagn Microbiol Infect Dis. 1984 Jun;2(3 Suppl):75S–84S. [PubMed] [Google Scholar]


