Abstract
Alendronate (ALN) and risedronate (RIS) are bisphosphonates effective in reducing bone loss and fractures associated with postmenopausal osteoporosis. However, it is uncertain how long it takes bone turnover to be re-established after treatment withdrawal, and whether this differs between the two drugs. The objective of this study was to determine the time required to re-establish normal bone turnover after the discontinuation of ALN and RIS treatment in an animal model of estrogen-deficiency osteoporosis. Two hundred ten, 6-mo-old female Sprague-Dawley rats were ovariectomized and 6 wk later were randomized into baseline controls (n = 10) and four treatment groups (n = 50/group): vehicle-treated controls (CON; 0.3 ml sterile water), ALN (2.4 μg/kg), low-dose RIS (RIS low; 1.2 μg/kg), and high-dose RIS (RIS high; 2.4 μg/kg). Treatments were administered 3 times/wk by subcutaneous injection. Baseline controls were killed at the initiation of treatment. Other groups were treated for 8 wk, and subgroups (n = 10/ treatment group) were killed 0, 4, 8, 12, and 16 wk after treatment was withdrawn. Static and dynamic histological analyses were performed for cortical (tibial diaphysis) and trabecular (proximal tibia and L4 vertebrae) bone. DXA and mechanical testing was performed on the L5 vertebra. After 8 wk of treatment, trabecular bone turnover rates were significantly suppressed in all drug-treated animals. Trabecular bone formation rate (BFR/BS) remained significantly lower than vehicle in bisphosphonate-treated animals through 12 wk. Sixteen weeks after treatment withdrawal, trabecular BFR/BS in the proximal tibia was re-established in animals treated with RIS but not in animals treated with ALN compared with controls. BMD of the fifth lumbar vertebra remained significantly higher than controls 16 wk after treatment withdrawal in ALN-treated animals but not in RIS-treated animals. Despite reductions in BMD and increases in bone turnover, ultimate force of the fifth lumbar vertebra remained significantly higher in all drug-treated animals through 16 wk after withdrawal.
Key words: bisphosphonates, ovariectomy, alendronate, risedronate, treatment withdrawal
INTRODUCTION
The bisphosphonates alendronate (ALN) and risedronate (RIS) are commonly prescribed anti-remodeling agents for preventing and treating postmenopausal osteoporosis.(1) Both drugs have a high affinity for bone mineral and function by reducing bone turnover by inhibiting osteoclast-mediated resorption.(2–4) Clinical studies have shown clinical efficacy of ALN and RIS in reducing bone loss and vertebral and nonvertebral fractures in postmenopausal women.(5–13) Although effective in this role, questions remain regarding the long-term skeletal consequences of bisphosphonates, including what happens when therapy is discontinued. Whereas there are some DXA and turnover marker data available from clinical off-response studies,(8,14–22) it is unclear how long it takes for bone turnover to be re-established after discontinuation of therapy and whether this differs depending on the bisphosphonate used. This is important in light of uncertainties regarding the consequences of prolonged suppression of bone remodeling with these drugs,(4,23) which may include impaired fracture healing(24,25) and interference with simultaneous or sequential therapies that affect bone remodeling.(26–28)
Both ALN and RIS are potent inhibitors of osteoclast-mediated bone resorption; however, these drugs have different 3D chemical structures,(29,30) skeletal uptakes and retentions,(4,14,31,32) binding affinities for hydroxyapatite,(33–35) and inhibitory potencies for the target enzyme.(36) The overall in vivo efficacy of bisphosphonate therapy results from a combination of these properties, and it is plausible that ALN and RIS have different skeletal recovery rates when therapy is discontinued because of their structural and pharmacokinetic differences. However, there are no head-to-head clinical or animal studies comparing skeletal recovery rates after discontinuation of ALN and RIS. Some clinical off-response data have shown reductions in bone mass after discontinuation of treatment with either ALN or RIS, with rates of loss similar to controls for both drugs.(6,15,18,19,22,37) Animal studies have shown continued suppression of bone turnover after the discontinuation of RIS in both OVX and aged rats when the treatment and withdrawal periods were equivalent(38–40); however, there are no published animal data relating to the discontinuation of ALN therapy. The aim of this study was to compare the skeletal response to the discontinuation of ALN and RIS therapy in the OVX rat, an established model for studying the skeletal effects of estrogen deficiency.(41)
MATERIALS AND METHODS
Animals
Two hundred ten 6-mo-old virgin female Sprague-Dawley rats (∼300 g) were purchased from Harlan Laboratories (Indianapolis, IN, USA). Rats underwent ovariectomy (OVX) at Harlan Laboratories using a bilateral dorsal approach. One week after surgery, rats were received by Indiana University's Laboratory Animal Resource Center, where they acclimatized for 7 days. OVX was evaluated at necropsy by confirming that the uterine horns had atrophied with no ovarian tissue. Six-month-old female rats were used because skeletal growth is minimal, and combined with OVX, they provide a model for postmenopausal osteoporosis.(41–43) Rats were pair-housed in plastic cages under standard environmental conditions (12-h light/dark cycle) and received Harlan Teklad 2018 rat chow (18% protein, 1.01% calcium, 0.65% phosphorus) and water ad libitum at all times during acclimatization and experimental treatment periods. All procedures performed in this experiment were approved by the Indiana University Animal Care and Use Committee.
Experimental treatment and design
After acclimating for 7 days, rats were randomized by body weight into four treatment groups: vehicle-treated controls (CON: 0.3 ml, sterile water), alendronate (ALN: 2.4 μg/kg), low-dose risedronate (RIS low: 1.2 μg/kg), or high-dose risedronate (RIS high: 2.4 μg/kg). Starting 6 wk after surgery, rats were treated 3 times/wk by subcutaneous injection for 8 wk (Fig. 1). Animals were weighed weekly to determine the appropriate treatment dosage. Drugs were administered at the same time on all treatment days.
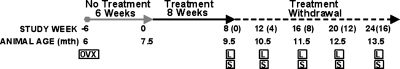
FIG. 1.
Study timeline. Treatment was initiated 6 wk after OVX surgery in female Sprague-Dawley rats. Animals were treated 3 times/wk for 8 wk with either 0.3 ml saline (CON), ALN (2.4 μg/kg), RIS low (1.2 μg/kg), or RIS high (2.4 μg/kg). Ten animals per treatment group were killed at 0, 4, 8, 12, and 16 wk after treatment was withdrawn. OVX, ovariectomized; L, bone label calcein administered 10 and 4 days before death; S, 10 rats per treatment group were killed.
Sterile water was used as the vehicle for both ALN and RIS, and allowances were made for the moisture content of each drug. ALN and RIS drug substances were supplied by Procter and Gamble Pharmaceuticals (Mason, OH, USA). Bisphosphonate dosages for ALN and RIS were based on clinical dose levels (on an oral mg/kg basis), which have been found to inhibit bone loss in OVX rats based on a subcutaneous route of administration.(38,44–46) The 1.2-μg/kg dose of RIS is comparable to the clinical dose of 5 mg/d prescribed for the treatment and prevention of postmenopausal osteoporosis, and the 2.4-μg/kg dose of ALN is comparable to the clinical dose of 10 mg/d prescribed for the treatment of postmenopausal osteoporosis. Two different doses of RIS were administered to allow for clinical comparisons (2.4 μg/kg ALN versus 1.2 μg/kg RIS) and dose equivalent comparisons (2.4 μg/kg ALN versus 2.4 μg/kg RIS). These doses assume an oral bioavailability of ∼0.5% based on a total weekly clinical dose of 35 mg for RIS and 70 mg for ALN. ALN and RIS were administered by subcutaneous injection to ensure that 100% of the administered drug was absorbed and to reduce the interference of the drug with food that can occur with oral drug administration, which reduces the bioavailability of both drugs.(47) Animals (n = 10 per treatment group) were killed 0, 4, 8, 12, and 16 wk after treatment was discontinued. Ten rats served as baseline OVX controls and were killed at the start of treatment. All rats received an intraperitoneal injection of the fluorochrome dye calcein (10 mg/kg body mass; Sigma-Alrich, St Louis, MO, USA) 10 and 4 days before death for dynamic histomorphometric measurements.
Processing and preparation of bone samples
Rats were killed by an intraperitoneal injection of 0.4 ml ketamine/xylazine, after which the right tibia (proximal end and mid-diaphysis) and L4 and L5 vertebra were removed and cleaned of soft tissue. Immediately after necropsy, the tibia and L4 vertebra were fixed in 10% phosphate-buffered formalin for 48 h and transferred to 70% ethanol, and the L5 vertebra was wrapped in saline-soaked gauze and stored at −80°C until analysis. The right proximal tibia, right tibial mid-diaphysis, and L4 vertebra were dehydrated in a graded series of ethanol (70–100%) and embedded, undecalcified, and unstained in methyl-methacrylate (MMA; Sigma-Aldrich) with 3% dibutyl phthalate (DBP; Sigma-Aldrich). The proximal tibia and L4 vertebra were cut longitudinally at a thickness of ∼6 μm using a Reichert-Jung supercut microtome (model 2050). The tibial mid-diaphysis was cut perpendicular to the long axis of the bone 1 mm proximal to the tibiofibular junction at a thickness of ∼70 μm using a diamond wire saw (Histo-saw; Delaware Diamond Knives, Wilmington, DE, USA). Thick sections were ground wet to a thickness of ∼50 μm using 800-grit sandpaper. All sections (thin and thick) were mounted on standard microscope slides and coverslipped with Eukitt.
Static and dynamic histomorphometric analyses
Histomorphometric measurements were made using a Nikon Optiophot 2 fluorescent microscope (Nikon, Garden City, NY, USA) with Bioquant image analysis system (R&M Biometrics, Nashville, TN, USA). For the proximal tibia and L4 vertebra, ∼8 mm2 of bone tissue from two sections (separated by ∼18 μm) were examined. Trabecular bone measurements were made in the secondary spongiosa, 1 mm distal from the growth plate and 0.5 mm from the endocortical surface of the cortical wall. For the tibial mid-diaphysis, periosteal and endocortical bone surfaces were measured from two sections separated by ∼70 μm. Measurements were made at ×200 magnification for both trabecular and cortical bone surfaces. Static parameters for trabecular bone included total tissue volume (TV), trabecular bone area (Tb.Ar), and bone perimeter (Tb.Pm). Dynamic parameters for trabecular bone included single-label perimeter (sL.Pm), double-label perimeter (dL.Pm), and interlabel thickness (Ir.L.Th). For cortical bone, static and dynamic parameters included cortical bone area (Ct.Ar), sL.Pm, dL.Pm, and Ir.L.Th for both periosteal (Ps) and endocortical (Ec) surfaces. From these primary data, the following measurements were calculated for both trabecular and cortical bone: bone volume (BV/TV; %), mineralizing surface (MS/BS = [1/2sL.Pm + dL.Pm]/B.Pm; %), mineral apposition rate (MAR = Ir.L.Th/6 d; μm/d), and bone formation rate (BFR/BS = MAR × MS/BS × 3.65; μm3/ μm2/yr). TRACP staining (kit 387A-1KT; Sigma-Aldrich) was performed on trabecular bone of the proximal tibial metaphysis and L4 vertebrae. To calculate osteoclast surface normalized by bone surface (Oc.S/BS), ∼8 mm2 of bone tissue from one section was examined. Sections were counterstained with hematoxylin (387A-1KT; Sigma-Aldrich). Multinucleated cells that were TRACP+ and adjacent to a trabecular bone surface were counted as osteoclasts. All histomorphometric measurements and calculations were performed according to the guidelines of the American Society for Bone and Mineral Research.(48)
DXA of the fifth lumbar vertebra
BMD (g/cm2) of the L5 vertebra was measured with a PIXImus II mouse densitometer (Lunar, Madison, WI, USA) with ultra-high resolution (0.18 × 0.18 mm/pixel).
Mechanical testing of the fifth lumbar vertebra
Bone strength of the L5 vertebra was assessed in compression using an MTS hydraulic mechanical testing devise (MTS Systems, Eden Prairie, MN, USA). The vertebra was prepared by cutting off the end plates from the vertebral body to create parallel planar surfaces using a diamond wafer saw. Before testing, bones were thawed at room temperature in vials of physiological saline at room temperature for 2 h. A static preload of 1 N was applied to the bone before testing. The vertebral body was loaded to failure in compression with a cross-head speed of 5 mm/min with a yield strain of 0.2 mm/mm. Ultimate force (N), energy to failure (mJ), and stiffness (N/mm) were calculated from force versus displacement curves as data.
Statistical analyses
All data are presented as mean ± SE. Statistical analyses were performed using SPSS version 13.0.1. Differences between treatment groups were evaluated at each time point using a one-way ANOVA. When a significant overall F value (F < 0.05) was present, differences between individual group means were tested using Fisher's protected least significant difference (PLSD) posthoc test. For all tests, differences were considered significant at p < 0.05 on a two-tailed test.
RESULTS
Animals
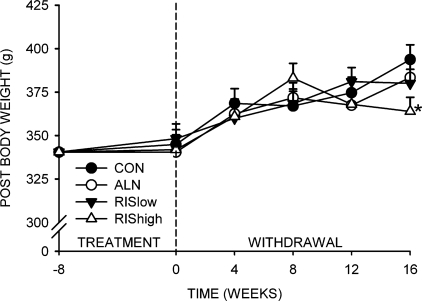
There were no significant differences among groups for body weight at either the beginning or end of the 8-wk treatment period (Fig. 2). All animals gained weight through 8 wk after the withdrawal of treatment. There were no differences among groups at 0, 4, 8, or 12 wk after treatment withdrawal. However, body weight of the RIS high group did not increase between 8 and 16 wk, resulting in significantly lower body weight at 16 wk after treatment withdrawal compared with the CON, ALN, and RIS low groups (Fig. 2).
FIG. 2.
Body weight changes with treatment withdrawal. *Significantly different from vehicle-treated CON (p < 0.05).
Dynamic histology of the proximal tibia and fourth lumbar vertebra
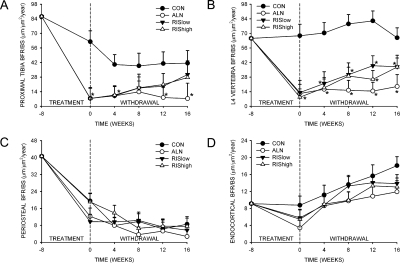
After 8 wk of treatment, trabecular bone turnover was significantly suppressed in the proximal tibial metaphysis and L4 vertebra in all drug-treated groups compared with the CON group (Figs. 3A and 3B). After treatment was discontinued, trabecular bone turnover in RIS low and RIS high groups increased steadily for the proximal tibial metaphysis and L4 vertebrae. At the proximal tibia metaphysis in both RIS-treated groups, BFR/BS was not different from CON by 16 wk of treatment withdrawal (Figs. 3A). In contrast, BFR/BS in the proximal tibia in the ALN-treated group remained significantly lower than the CON group at all times after treatment was withdrawn. For the L4 vertebra, although BFR/BS in the RIS low and RIS high groups tended to increase toward CON, BFR/BS in all drug-treated groups remained significantly lower than CON 16 wk after treatment withdrawal.
FIG. 3.
Effect of bisphosphonate treatment and withdrawal on BFR/BS of the proximal tibial metaphysis (A), fourth lumbar vertebra (B), periosteal surface of the tibial mid-diaphysis (C), and endocortical surface of the tibial mid-diaphysis (D). *Significantly different from vehicle-treated CON (p < 0.05).
After 8 wk of treatment, periosteal BFR/BS had significantly decreased in all groups (Fig. 3C). Because this also occurred in CON, this seems to be an age-related change rather than a response to bisphosphonate treatment. There was no recovery of BFR/BS at any time during treatment withdrawal, and there were no differences among groups (Fig. 3C). For the endocortical surface, after 8 wk of treatment, there were no differences between groups for the suppression of BFR/BS. Between 0 and 16 wk of treatment withdrawal, BFR/BS of the endocortical surface increased steadily in all groups, with no significant differences between CON and drug-treated groups (Fig. 3D).
Bone resorption of the proximal tibia and fourth lumbar vertebra
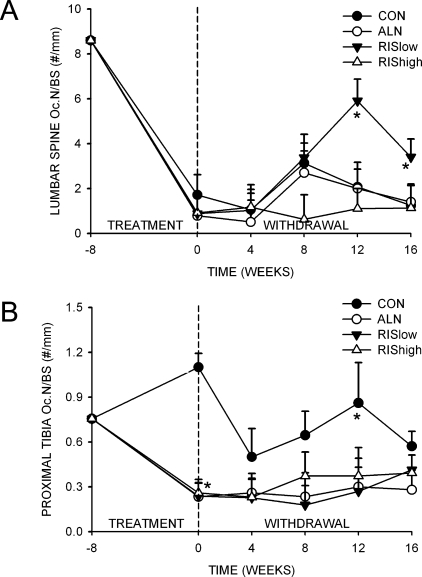
In the fourth lumbar vertebrae, N.Oc/BS was not different among groups after 8 wk of treatment; however, N.Oc/BS increased significantly by 12 wk in the RIS low–treated group compared with the CON group (Fig. 4A). In contrast, in the proximal tibia, drug treatment resulted in a significant reduction in N.Oc/BS in all treated animals compared with the CON (Fig. 4B). By 16 wk of treatment withdrawal, there were no differences among bisphosphonate-treated groups during the 16 wk of treatment withdrawal.
FIG. 4.
Effect of bisphosphonate treatment and withdrawal on N.Oc/BS for the (A) fourth lumbar vertebra and (B) proximal tibial metaphysis. *Significantly different from vehicle-treated CON (p < 0.05).
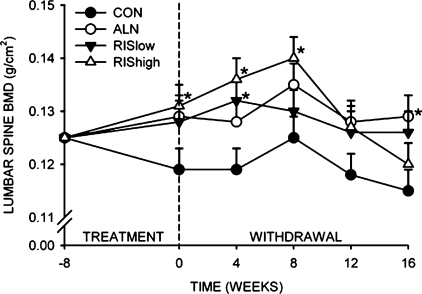
BMD of the fifth lumbar vertebra
After 8 wk of treatment, the RIS high–treated animals had significantly greater BMD of the fifth lumbar vertebra compared with controls (Fig. 5). After 16 wk of treatment withdrawal, ALN-treated animals maintained significantly higher BMD of the fifth lumbar vertebra compared with controls (Fig. 5). There was no difference in BMD at the lumbar spine in RIS-treated groups compared with CON after 16 wk of treatment withdrawal.
FIG. 5.
Effect of bisphosphonate treatment and withdrawal on BMD of the fifth lumbar vertebra. *Significantly different from vehicle-treated CON (p < 0.05).
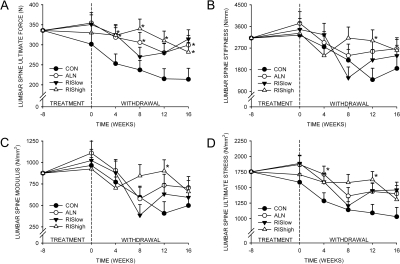
Bone strength of the fifth lumbar vertebra
Although there was not a significant difference among groups in ultimate force of the fifth lumbar vertebra after 8 wk of treatment, ultimate force was significantly higher in all drug-treated groups after treatment was discontinued (Fig. 6A). This was primarily because of an age-related decrease in strength in control vertebrae. There was no difference in ultimate force between ALN- and RIS-treated animals at any time point. Stiffness, modulus, and ultimate stress were not significantly different among any of the groups 16 wk after treatment withdrawal.
FIG. 6.
Effect of bisphosphonate treatment and withdrawal on bone strength of the L5 vertebrae. (A) Ultimate force. (B) Stiffness. (C) Modulus. (D) Ultimate stress. *Significantly different from vehicle-treated CON (p < 0.05).
DISCUSSION
This study examined differences in the skeletal response to the discontinuation of ALN or RIS in OVX rats, an animal model of estrogen-deficiency osteoporosis. The data showed that normal trabecular bone turnover tends to be re-established sooner in animals treated with RIS than those treated with ALN. Specifically, bone formation rates remained significantly lower in trabecular bone of the proximal tibia and lumbar spine in ALN-treated rats compared with nontreated CON rats 16 wk after treatment was discontinued. In contrast, rats treated with either dose of RIS showed a gradual recovery of normal bone formation rates at these skeletal sites. After 16 wk of treatment withdrawal, bone turnover at the proximal tibia was not significantly different between RIS-treated and CON rats, and N.Oc/BS for the proximal tibia tended to increase in the RIS animals compared with ALN-treated animals.
There was an age-related decrease in BFR/BS, particularly on the trabecular and periosteal surfaces of the proximal tibia. We attempted to minimize age-related changes by starting with 6-mo-old rats, which are generally considered to be adult. Although growth has slowed by this age, remodeling rates continue to slow independent of treatment as the animals continue to age. We used age-matched controls, an accepted manner of controlling for such effects. Even though there was an age-related drift, for BFR/BS in the proximal tibia (but not for the lumbar vertebra), BFR/BS was not different from age-matched controls for RIS-treated animals but was different for ALN-treated animals after 16 wk of treatment withdrawal. This shows a difference in the recovery of trabecular bone remodeling at this site between RIS-treated and ALN-treated animals.
A recent study in aged rats showed that risedronate treatment blunted the stimulatory effect of subsequent PTH on bone remodeling.(49) However, with a 10-wk withdrawal period after an 8-wk treatment period with RIS, the PTH stimulation of bone formation was not blunted. This, together with our data, suggests that after ∼10–16 wk of treatment withdrawal, the amount of RIS cleared from the skeleton seems to be sufficient to allow for the administration of alternative therapies and for normal bone remodeling to resume.
Our study is the first animal study to examine differences between ALN and RIS after the withdrawal of treatment using an animal model of estrogen deficiency osteoporosis. Our finding that the skeletal effects of 8 wk of RIS treatment began to diminish after 16 wk of treatment withdrawal differ from the findings of Wronski et al.(38) and Jee et al.(40) Wronski et al. treated 3-mo-old OVX rats with RIS (5 μg/kg), 2 times/wk for 180 days, followed by discontinuation of treatment for 35, 90, 180, and 360 days. After 360 days of treatment withdrawal, trabecular bone volume within the proximal tibia was not significantly different between RIS-treated and OVX controls; however, BFR of the proximal tibia remained suppressed. Thus, whereas trabecular BFR remained suppressed in the RIS-treated animals over a withdrawal period twice the length of the treatment period, bone volume was not preserved. It is important to note that the study by Wronski et al.(38) was performed in 3-mo-old OVX rats, and the total dosage of RIS was higher (10 μg/kg/wk) than the two dosages used in our study (3.6 and 7.2 μg/kg/wk). In addition, the animals in that study were treated for 25 wk compared with 8 wk in our study, which might have an impact on the time required to resume normal remodeling. In a separate study, Jee et al.(40) treated 9-mo-old virgin female rats for 60 days with either 1 or 5 μg/kg RIS twice weekly and observed a sustained suppression of bone turnover in the proximal tibia, even after 540 days of treatment withdrawal. A caveat of this study is that the rats were not OVX, and the amount of bone loss in the aged controls was less than that expected after 540 days of OVX in rats. Thus, it is not known if estrogen plays a protective role against bone loss after treatment is discontinued.
For cortical bone, Li et al.(39) treated 3-mo-old OVX rats with twice weekly RIS (5 μg/kg) for 60 days, followed by treatment withdrawal for 21 and 90 days. Periosteal bone formation was suppressed, and remained suppressed for up to 90 days of treatment withdrawal. This supports our data, which showed a continued suppression of periosteal bone formation through 16 wk of treatment withdrawal. However, the fact that we observed recovery of endocortical bone formation during 16-wk treatment withdrawal suggests that there are surface-specific differences in the persistence of the drug response when bisphosphonate therapy is discontinued. We postulate these differences are caused by differences in how these drugs are distributed and incorporated into the bone matrix.(32) Specifically, studies using 14C-alendronate to examine the elimination of drug from different cortical and trabecular skeletal regions found ALN to bind preferentially to sites of high bone turnover.(50,51) Although baseline bone formation rate on the periosteal surface was ∼2.5 times that on the endocortical surface, this occurs through direct apposition of new bone in a modeling mode; because it does not involve coupled resorption and formation in a remodeling mode, we would expect less drug to bind to the periosteal surface. The gradual decline in periosteal bone formation even in the controls suggests an age-related decline in bone formation on this surface, without significant effects of the bisphosphonate treatment.
The different recovery rates between the two drugs at trabecular and endocortical surfaces is most likely related to the surface area to which the drug is able to bind. Whereas cortical bone represents a greater proportion of the skeleton (∼80%), trabecular bone has greater surface area to which the drug can bind. Thus, we would expect more drug to bind to the trabecular surfaces compared with endocortical surfaces, resulting in greater suppression on trabecular surfaces and a longer recovery period. This, together with the higher mineral binding affinity of ALN, would result in greater drug loading and slower release of ALN compared with RIS at all surfaces, but most evident at trabecular surfaces.
Although there are clinical trials examining the withdrawal of bisphosphonate therapy,(8,15,19,21,52) there are no head-to-head trials comparing the effects of discontinuing ALN and RIS treatment. The results from non–head-to-head studies are difficult to interpret comparatively because the skeletal response to treatment withdrawal is likely related to the study population (younger early postmenopausal women versus older postmenopausal osteoporotic women), treatment duration, drug dose, skeletal regions examined, availability of a placebo group during treatment withdrawal, and intake of calcium (500–1000 mg) and vitamin D after discontinuation of bisphosphonate therapy. A common finding in the withdrawal studies with ALN and RIS is a significant reduction in bone mass within a year after the discontinuation of therapy. The rate of loss was similar to women given placebo for both ALN (dosages = 2.5–20 mg/d)(14–16,19–21,37) and RIS (5 mg/d).(18,52) After withdrawal, markers of bone resorption increased toward baseline levels in women treated with ALN(14,15,19–22,37) and RIS(18) compared with controls. With respect to other treatment agents, the skeletal effects of ALN are preserved longer after treatment withdrawal than with the withdrawal of estrogen therapy(16,21); however, this comparison has not been made for RIS.
Bisphosphonates have unique structural features that arise from two side chains off a central carbon atom in a P-C-P backbone, where each P represents a phosphate group.(34,53) ALN and RIS are nitrogen-containing bisphosphonates that have a similar hydroxyl (−OH) R1 side chain; however, they differ in their R2 side chains, which contain different functional nitrogen groups. ALN has an alkyl nitrogen chain, whereas RIS has a nitrogen atom contained in a pyridyl ring. Until recently, differences in binding affinities between bisphosphonates have not been attributed to the R2 side chain. However, recent work using a constant composition potentiostatic method by Nancollas et al.(33) showed ALN to have a 35% greater binding affinity than RIS for hydroxyapatite, a difference attributed to interactions between the R2 side chain and hydroxyapatite. Differences in binding affinities between ALN and RIS may play a role in how these drugs are retained in the skeleton, and thereby may influence the persistence of the drug effect. Thus, these structural differences provide a plausible mechanistic explanation for our observation of continued suppression of bone turnover with ALN but not with RIS.
Currently there are limited pharmacokinetic data evaluating the skeletal retention of ALN and RIS. Published data suggest ALN is retained longer in the skeleton than RIS; however, clinical study designs vary considerable in their patient populations, dose levels, and study durations, which likely influence the measured pharmacokinetic parameters such as the terminal half-life for bone.(4) For example, the half-life of ALN has been reported to be ∼10 yr based on a study 540 days in duration(54) compared with 20 days for RIS based on a study 28 days in duration.(55) In one head-to-head clinical study in postmenopausal osteopenic women, a greater amount of ALN was retained in the skeleton compared with RIS after 1, 3, and 28 days,(14) a finding consistent with reported differences in mineral binding affinities.(24)
Data from our study might have clinical implications for design and use of effective therapies for osteoporosis. At clinical dose levels, ALN seems to have a more persistent effect on suppressing bone turnover than RIS after treatment is withdrawn. This suggests that patients recommended for a “drug holiday” may continue to experience benefits from ALN treatment resulting from its lasting residual effects on bone turnover and BMD after treatment is discontinued. Conversely, the data also indicate that RIS may be the preferred drug for patients who need a quicker return of bone remodeling, such as those who need to switch to an alternative therapy. These observations need to be confirmed in clinical populations before current prescription practices are altered because the use of an animal model may not necessarily predict the clinical scenario. Although the OVX rat is widely accepted as a model for postmenopausal bone loss, it does not replicate all aspects of the human condition.
ACKNOWLEDGMENTS
We thank Keith Condon and Lauren Waugh for help in preparing bone tissue for histological analysis, and Andrew Koivuniemi and Mark Koivuniemi for help with DXA scanning and the mechanical testing measurements. The authors thank Procter and Gamble Pharmaceuticals and the Alliance for Better Bone Health for providing the drug powders (ALN and RIS) for this study and for providing financial support to execute the study. This study was also supported by NIH Training Grant T32 AR-7581-09.
Footnotes
Dr Phipps is employed by Procter and Gamble Pharmaceuticals. Dr Burr is a consultant for Eli Lilly, Procter and Gamble Pharmaceuticals, and Amgen. Dr Fuchs states that she has no conflicts of interest.
REFERENCES
- 1.Close P, Neuprez A, Reginster JY. Developments in the pharmacotherapeutic management of osteoporosis. Expert Opin Pharmacother. 2006;7:1603–1615. doi: 10.1517/14656566.7.12.1603. [DOI] [PubMed] [Google Scholar]
- 2.Sato M, Grasser W, Endo N, Akins R, Simmons H, Thompson DD, Golub E, Rodan GA. Bisphosphonate action. Alendronate localization in rat bone and effects on osteoclast ultrastructure. J Clin Invest. 1991;88:2095–2105. doi: 10.1172/JCI115539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breuil V, Cosman F, Stein L, Horbert W, Nieves J, Shen V, Lindsay R, Dempster DW. Human osteoclast formation and activity in vitro: Effects of alendronate. J Bone Miner Res. 1998;13:1721–1729. doi: 10.1359/jbmr.1998.13.11.1721. [DOI] [PubMed] [Google Scholar]
- 4.Rodan GA, Fleisch HA. Bisphosphonates: Mechanisms of action. J Clin Invest. 1996;97:2692–2696. doi: 10.1172/JCI118722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosen CJ, Hochberg MC, Bonnick SL, McClung M, Miller P, Broy S, Kagan R, Chen E, Petruschke RA, Thompson DE, de Papp AE. Treatment with once-weekly alendronate 70 mg compared with once-weekly risedronate 35 mg in women with postmenopausal osteoporosis: A randomized double-blind study. J Bone Miner Res. 2005;20:141–151. doi: 10.1359/JBMR.040920. [DOI] [PubMed] [Google Scholar]
- 6.Watts NB, Josse RG, Hamdy RC, Hughes RA, Manhart MD, Barton I, Calligeros D, Felsenberg D. Risedronate prevents new vertebral fractures in postmenopausal women at high risk. J Clin Endocrinol Metab. 2003;88:542–549. doi: 10.1210/jc.2002-020400. [DOI] [PubMed] [Google Scholar]
- 7.Pols HA, Felsenberg D, Hanley DA, Stepan J, Munoz-Torres M, Wilkin TJ, Qin-sheng G, Galich AM, Vandormael K, Yates AJ, Stych B. Multinational, placebo-controlled, randomized trial of the effects of alendronate on bone density and fracture risk in postmenopausal women with low bone mass: Results of the FOSIT study. Fosamax International Trial Study Group. Osteoporos Int. 1999;9:461–468. doi: 10.1007/pl00004171. [DOI] [PubMed] [Google Scholar]
- 8.Bone HG, Hosking D, Devogelaer JP, Tucci JR, Emkey RD, Tonino RP, Rodriguez-Portales JA, Downs RW, Gupta J, Santora AC, Liberman UA. Ten years' experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med. 2004;350:1189–1199. doi: 10.1056/NEJMoa030897. [DOI] [PubMed] [Google Scholar]
- 9.Hosking D, Adami S, Felsenberg D, Andia JC, Valimaki M, Benhamou L, Reginster JY, Yacik C, Rybak-Feglin A, Petruschke RA, Zaru L, Santora AC. Comparison of change in bone resorption and bone mineral density with once-weekly alendronate and daily risedronate: A randomised, placebo-controlled study. Curr Med Res Opin. 2003;19:383–394. doi: 10.1185/030079903125002009. [DOI] [PubMed] [Google Scholar]
- 10.Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T, Keller M, Chesnut CH, III, Brown J, Eriksen EF, Hoseyni MS, Axelrod DW, Miller PD. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: A randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA. 1999;282:1344–1352. doi: 10.1001/jama.282.14.1344. [DOI] [PubMed] [Google Scholar]
- 11.Cranney A, Tugwell P, Adachi J, Weaver B, Zytaruk N, Papaioannou A, Robinson V, Shea B, Wells G, Guyatt G. Meta-analyses of therapies for postmenopausal osteoporosis. III. Meta-analysis of risedronate for the treatment of postmenopausal osteoporosis. Endocr Rev. 2002;23:517–523. doi: 10.1210/er.2001-3002. [DOI] [PubMed] [Google Scholar]
- 12.Cranney A, Wells G, Willan A, Griffith L, Zytaruk N, Robinson V, Black D, Adachi J, Shea B, Tugwell P, Guyatt G. Meta-analyses of therapies for postmenopausal osteoporosis. II. Meta-analysis of alendronate for the treatment of postmenopausal women. Endocr Rev. 2002;23:508–516. doi: 10.1210/er.2001-2002. [DOI] [PubMed] [Google Scholar]
- 13.Marcus R, Wong M, Heath H, III, Stock JL. Antiresorptive treatment of postmenopausal osteoporosis: Comparison of study designs and outcomes in large clinical trials with fracture as an endpoint. Endocr Rev. 2002;23:16–37. doi: 10.1210/edrv.23.1.0453. [DOI] [PubMed] [Google Scholar]
- 14.Bagger YZ, Tanko LB, Alexandersen P, Ravn P, Christiansen C. Alendronate has a residual effect on bone mass in postmenopausal Danish women up to 7 years after treatment withdrawal. Bone. 2003;33:301–307. doi: 10.1016/s8756-3282(03)00112-1. [DOI] [PubMed] [Google Scholar]
- 15.Ensrud KE, Barrett-Connor EL, Schwartz A, Santora AC, Bauer DC, Suryawanshi S, Feldstein A, Haskell WL, Hochberg MC, Torner JC, Lombardi A, Black DM. Randomized trial of effect of alendronate continuation versus discontinuation in women with low BMD: Results from the Fracture Intervention Trial long-term extension. J Bone Miner Res. 2004;19:1259–1269. doi: 10.1359/JBMR.040326. [DOI] [PubMed] [Google Scholar]
- 16.Wasnich RD, Bagger YZ, Hosking DJ, McClung MR, Wu M, Mantz AM, Yates JJ, Ross PD, Alexandersen P, Ravn P, Christiansen C, Santora AC., II Changes in bone density and turnover after alendronate or estrogen withdrawal. Menopause. 2004;11:622–630. doi: 10.1097/01.gme.0000123641.76105.b5. [DOI] [PubMed] [Google Scholar]
- 17.Tonino RP, Meunier PJ, Emkey R, Rodriguez-Portales JA, Menkes CJ, Wasnich RD, Bone HG, Santora AC, Wu M, Desai R, Ross PD. Skeletal benefits of alendronate: 7-year treatment of postmenopausal osteoporotic women. Phase III Osteoporosis Treatment Study Group. J Clin Endocrinol Metab. 2000;85:3109–3115. doi: 10.1210/jcem.85.9.6777. [DOI] [PubMed] [Google Scholar]
- 18.Mortensen L, Charles P, Bekker PJ, Digennaro J, Johnston CC., Jr Risedronate increases bone mass in an early postmenopausal population: Two years of treatment plus one year of follow-up. J Clin Endocrinol Metab. 1998;83:396–402. doi: 10.1210/jcem.83.2.4586. [DOI] [PubMed] [Google Scholar]
- 19.Stock JL, Bell NH, Chesnut CH, III, Ensrud KE, Genant HK, Harris ST, McClung MR, Singer FR, Yood RA, Pryor-Tillotson S, Wei L, Santora AC., II Increments in bone mineral density of the lumbar spine and hip and suppression of bone turnover are maintained after discontinuation of alendronate in postmenopausal women. Am J Med. 1997;103:291–297. doi: 10.1016/s0002-9343(97)00130-7. [DOI] [PubMed] [Google Scholar]
- 20.Ravn P, Bidstrup M, Wasnich RD, Davis JW, McClung MR, Balske A, Coupland C, Sahota O, Kaur A, Daley M, Cizza G. Alendronate and estrogen-progestin in the long-term prevention of bone loss: Four-year results from the early postmenopausal intervention cohort study. A randomized, controlled trial. Ann Intern Med. 1999;131:935–942. doi: 10.7326/0003-4819-131-12-199912210-00005. [DOI] [PubMed] [Google Scholar]
- 21.Greenspan SL, Emkey RD, Bone HG, Weiss SR, Bell NH, Downs RW, McKeever C, Miller SS, Davidson M, Bolognese MA, Mulloy AL, Heyden N, Wu M, Kaur A, Lombardi A. Significant differential effects of alendronate, estrogen, or combination therapy on the rate of bone loss after discontinuation of treatment of postmenopausal osteoporosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2002;137:875–883. doi: 10.7326/0003-4819-137-11-200212030-00008. [DOI] [PubMed] [Google Scholar]
- 22.Black DM, Schwartz AV, Ensrud KE, Cauley JA, Levis S, Quandt SA, Satterfield S, Wallace RB, Bauer DC, Palermo L, Wehren LE, Lombardi A, Santora AC, Cummings SR. Effects of continuing or stopping alendronate after 5 years of treatment: The Fracture Intervention Trial Longterm Extension (FLEX): A randomized trial. JAMA. 2006;296:2927–2938. doi: 10.1001/jama.296.24.2927. [DOI] [PubMed] [Google Scholar]
- 23.Arlot M, Meunier PJ, Boivin G, Haddock L, Tamayo J, Correa-Rotter R, Jasqui S, Donley DW, Dalsky GP, Martin JS, Eriksen EF. Differential effects of teriparatide and alendronate on bone remodeling in postmenopausal women assessed by histomorphometric parameters. J Bone Miner Res. 2005;20:1244–1253. doi: 10.1359/JBMR.050309. [DOI] [PubMed] [Google Scholar]
- 24.Cao Y, Mori S, Mashiba T, Westmore MS, Ma L, Sato M, Akiyama T, Shi L, Komatsubara S, Miyamoto K, Norimatsu H. Raloxifene, estrogen, and alendronate affect the processes of fracture repair differently in ovariectomized rats. J Bone Miner Res. 2002;17:2237–2246. doi: 10.1359/jbmr.2002.17.12.2237. [DOI] [PubMed] [Google Scholar]
- 25.Odvina CV, Zerwekh JE, Rao DS, Maalouf N, Gottschalk FA, Pak CY. Severely suppressed bone turnover: A potential complication of alendronate therapy. J Clin Endocrinol Metab. 2005;90:1294–1301. doi: 10.1210/jc.2004-0952. [DOI] [PubMed] [Google Scholar]
- 26.Black DM, Greenspan SL, Ensrud KE, Palermo L, McGowan JA, Lang TF, Garnero P, Bouxsein ML, Bilezikian JP, Rosen CJ. The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med. 2003;349:1207–1215. doi: 10.1056/NEJMoa031975. [DOI] [PubMed] [Google Scholar]
- 27.Bilezikian JP, Rubin MR, Finkelstein JS. Parathyroid hormone as an anabolic therapy for women and men. J Endocrinol Invest. 2005;28(8 Suppl):41–49. [PubMed] [Google Scholar]
- 28.Delmas P, Watts NB, Miller P, Cahall D, Bilezikian J, Lindsay R. Bone turnover markers demonstrate greater earlier responsiveness to teriparatide following treatment with risedronate compared with alendronate. J Bone Miner Res. 2007;S27:1092. [Google Scholar]
- 29.Papapoulos SE. Bisphosphonate actions: Physical chemistry revisited. Bone. 2006;38:613–616. doi: 10.1016/j.bone.2006.01.141. [DOI] [PubMed] [Google Scholar]
- 30.Dunford JE, Thompson K, Coxon FP, Luckman SP, Hahn FM, Poulter CD, Ebetino FH, Rogers MJ. Structure-activity relationships for inhibition of farnesyl diphosphate synthase in vitro and inhibition of bone resorption in vivo by nitrogen-containing bisphosphonates. J Pharmacol Exp Ther. 2001;296:235–242. [PubMed] [Google Scholar]
- 31.Lin JH, Russell G, Gertz B. Pharmacokinetics of alendronate: An overview. Int J Clin Pract Suppl. 1999;101:18–26. [PubMed] [Google Scholar]
- 32.Lin JH. Bisphosphonates: A review of their pharmacokinetic properties. Bone. 1996;18:75–85. doi: 10.1016/8756-3282(95)00445-9. [DOI] [PubMed] [Google Scholar]
- 33.Nancollas GH, Tang R, Phipps RJ, Henneman Z, Gulde S, Wu W, Mangood A, Russell RG, Ebetino FH. Novel insights into actions of bisphosphonates on bone: Differences in interactions with hydroxyapatite. Bone. 2006;38:617–627. doi: 10.1016/j.bone.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 34.van Beek ER, Lowik CW, Ebetino FH, Papapoulos SE. Binding and antiresorptive properties of heterocycle-containing bisphosphonate analogs: Structure-activity relationships. Bone. 1998;23:437–442. doi: 10.1016/s8756-3282(98)00120-3. [DOI] [PubMed] [Google Scholar]
- 35.Leu CT, Luegmayr E, Freedman LP, Rodan GA, Reszka AA. Relative binding affinities of bisphosphonates for human bone and relationship to antiresorptive efficacy. Bone. 2006;38:628–636. doi: 10.1016/j.bone.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 36.Cremers SC, Pillai G, Papapoulos SE. Pharmacokinetics/pharmacodynamics of bisphosphonates: Use for optimisation of intermittent therapy for osteoporosis. Clin Pharmacokinet. 2005;44:551–570. doi: 10.2165/00003088-200544060-00001. [DOI] [PubMed] [Google Scholar]
- 37.McClung M, Clemmesen B, Daifotis A, Gilchrist NL, Eisman J, Weinstein RS, Fuleihan Ge-H, Reda C, Yates AJ, Ravn P. Alendronate prevents postmenopausal bone loss in women without osteoporosis. A double-blind, randomized, controlled trial. Alendronate Osteoporosis Prevention Study Group. Ann Intern Med. 1998;128:253–261. doi: 10.7326/0003-4819-128-4-199802150-00001. [DOI] [PubMed] [Google Scholar]
- 38.Wronski TJ, Dann LM, Qi H, Yen CF. Skeletal effects of withdrawal of estrogen and diphosphonate treatment in ovariectomized rats. Calcif Tissue Int. 1993;53:210–216. doi: 10.1007/BF01321840. [DOI] [PubMed] [Google Scholar]
- 39.Li QN, Liang NC, Huang LF, Wu T, Hu B, Mo LE. Skeletal effects of constant and terminated use of sodium risedronate in ovariectomized rats. Zhongguo Yao Li Xue Bao. 1998;19:160–163. [PubMed] [Google Scholar]
- 40.Jee WS, Lin BY, Ma YF, Ke HZ. Extra cancellous bone induced by combined prostaglandin E2 and risedronate administration is maintained after their withdrawal in older female rats. J Bone Miner Res. 1995;10:963–970. doi: 10.1002/jbmr.5650100618. [DOI] [PubMed] [Google Scholar]
- 41.Thompson DD, Simmons HA, Pirie CM, Ke HZ. FDA Guidelines and animal models for osteoporosis. Bone. 1995;17(4 Suppl):125S–133S. doi: 10.1016/8756-3282(95)00285-l. [DOI] [PubMed] [Google Scholar]
- 42.Martin EA, Ritman EL, Turner RT. Time course of epiphyseal growth plate fusion in rat tibiae. Bone. 2003;32:261–267. doi: 10.1016/s8756-3282(02)00983-3. [DOI] [PubMed] [Google Scholar]
- 43.Hansson LI, Menander-Sellman K, Stenstrom A, Thorngren KG. Rate of normal longitudinal bone growth in the rat. Calcif Tissue Res. 1972;10:238–251. doi: 10.1007/BF02012553. [DOI] [PubMed] [Google Scholar]
- 44.Lin BY, Jee WS, Ma YF, Ke HZ, Kimmel DB, Li XJ. Effects of prostaglandin E2 and risedronate administration on cancellous bone in older female rats. Bone. 1994;15:489–496. doi: 10.1016/8756-3282(94)90272-0. [DOI] [PubMed] [Google Scholar]
- 45.Li QN, Liang NC, Huang LF, Wu T, Hu B, Mo LE. Skeletal effects of constant and terminated use of risedronate on cortical bone in ovariectomized rats. J Bone Miner Metab. 1999;17:18–22. doi: 10.1007/s007740050058. [DOI] [PubMed] [Google Scholar]
- 46.Ogawa K, Hori M, Takao R, Sakurada T. Effects of combined calcitonin and alendronate treatment on the architecture and strength of bone in ovariectomized rats. J Bone Miner Metab. 2005;23:351–358. doi: 10.1007/s00774-005-0612-9. [DOI] [PubMed] [Google Scholar]
- 47.Uludag H. Bisphosphonates as a foundation of drug delivery to bone. Curr Pharm Des. 2002;8:1929–1944. doi: 10.2174/1381612023393585. [DOI] [PubMed] [Google Scholar]
- 48.Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone histomorphometry: Standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 49.Yao W, Su M, Zhang Q, Tian X, Setterberg RB, Blanton C, Lundy MW, Phipps R, Jee WS. Risedronate did not block the maximal anabolic effect of PTH in aged rats. Bone. 2007;41:813–819. doi: 10.1016/j.bone.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 50.Lin JH, Chen IW, Duggan DE. Effects of dose, sex, and age on the disposition of alendronate, a potent antiosteolytic bisphosphonate, in rats. Drug Metab Dispos. 1992;20:473–478. [PubMed] [Google Scholar]
- 51.Lin JH, Duggan DE, Chen IW, Ellsworth RL. Physiological disposition of alendronate, a potent anti-osteolytic bisphosphonate, in laboratory animals. Drug Metab Dispos. 1991;19:926–932. [PubMed] [Google Scholar]
- 52.Watts NB, Chines A, Olszynski WP, McKeever CD, McClung MR, Zhou X, Grauer A. Fracture risk remains reduced one year after discontinuation of risedronate. Osteoporos Int. 2008;19:365–372. doi: 10.1007/s00198-007-0460-7. [DOI] [PubMed] [Google Scholar]
- 53.Russell RG, Xia Z, Dunford JE, Oppermann U, Kwaasi A, Hulley PA, Kavanagh KL, Triffitt JT, Lundy MW, Phipps RJ, Barnett BL, Coxon FP, Rogers MJ, Watts NB, Ebetino FH. Bisphosphonates: An update on mechanisms of action and how these relate to clinical efficacy. Ann N Y Acad Sci. 2007;1117:209–257. doi: 10.1196/annals.1402.089. [DOI] [PubMed] [Google Scholar]
- 54.Khan SA, Kanis JA, Vasikaran S, Kline WF, Matuszewski BK, McCloskey EV, Beneton MN, Gertz BJ, Sciberras DG, Holland SD, Orgee J, Coombes GM, Rogers SR, Porras AG. Elimination and biochemical responses to intravenous alendronate in postmenopausal osteoporosis. J Bone Miner Res. 1997;12:1700–1707. doi: 10.1359/jbmr.1997.12.10.1700. [DOI] [PubMed] [Google Scholar]
- 55.Mitchell DY, Heise MA, Pallone KA, Clay ME, Nesbitt JD, Russell DA, Melson CW. The effect of dosing regimen on the pharmacokinetics of risedronate. Br J Clin Pharmacol. 1999;48:536–542. doi: 10.1046/j.1365-2125.1999.00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]