Abstract
Alteration of p53 is an early event in the development of endometrial serous carcinoma (ESC). We have recently identified a group of benign-looking endometria with p53 overexpression, designated “p53 signatures.” In this study, we investigated these p53 signatures and evaluated whether they represented “latent” precancers for ESC. The p53 signatures were specifically associated with ESC, frequently found in the benign-appearing endometrium adjacent to the ESC and only rarely in either the endometrium adjacent to endometrioid carcinomas or in non-cancerous uteri. Forty-two percent of the p53 signature samples showed at least one p53 gene mutation. There were eight ESC uteri with p53 signatures that revealed p53 gene mutations. In four (50%) of these cases, at least one identical p53 gene mutation was found in the signature glands, precancerous regions, and cancerous areas within the same uterus. We concluded that p53 gene mutations apparently precede the morphological changes in affected endometrial cells. The finding of identical p53 mutations in the p53 signatures, precancerous regions, and ESCs in a subset of the uteri provides further evidence of a probable shared lineage between these lesions and suggests that the epithelia that display these p53 signatures are likely latent ESC precancerous regions. These findings underscore the significance of the p53 signature as a target for further research in the early detection and prevention of ESC.
Endometrial cancer is the most common malignancy of the female genital tract.1 A dualistic model of endometrial carcinogenesis has been proposed since the 1980s, based on light microscopic appearance, clinical behavior, and epidemiology.2,3 Although type I cancers, the majority of which are of the endometrioid histotype, comprise approximately 80% of all endometrial carcinomas, type II cancers, most of which are of the serous and clear cell histotypes contribute a disproportionate percentage of patient deaths for the whole group.3 The advanced stage at which many Type II carcinomas present is undoubtedly at least one factor that contributes to the comparatively unfavorable patient outcomes that has been observed in this group. The recognition of the precursors for type II endometrial cancers may therefore be of significant clinical value, both as a morphological marker that potentially portends a heightened cancer risk, and possibly, a biological target for ablation.
In the last decade, progress has been greatest in molecular and histological resolution of precursors of type I carcinomas, resulting in a cohesive model of endometrial carcinogenesis encompassing both genetic and hormonal factors, revisions in the diagnostic criteria for precancers, and novel prevention strategies.4 In contrast, until recently, there have been relatively few comprehensive studies on the potential type II precursor lesions. Serous endometrial intraepithelial carcinoma (EIC) was previously considered the putative precancerous lesion of endometrial serous carcinoma (ESC), the prototype of type II endometrial cancer. However, serous EIC is now considered as an early form of ESC,5 since it is not infrequently associated with extrauterine disease.5,6,7,8,9,10,11,12,13 In a series of clinicopathologic studies published over the past few years, our group has shown that the spectrum of lesions to which we have applied the designation endometrial glandular dysplasia (EmGD), represent the most likely morphologically recognizable precancers for both serous and clear cell carcinoma of the endometrium.14,15,16,17
Lesions of EmGD are readily visible on routinely stained histological sections due to their increased degree of nuclear atypia relative to the background endometrium.16 Nonetheless, while studying these EmGD cases, we noticed that there were endometrial epithelia that were morphologically unremarkable but displayed diffuse and strong p53 nuclear staining. Similar staining patterns have subsequently been reported in tubal epithelia within the context of pelvic serous carcinogenesis, and have been designated the “p53 signatures.”18,19 We hypothesized that the epithelia that constitute these p53 signatures represent a “latent form” of ESC precancer. The aims of this study are to characterize the p53 mutational status of these p53 signatures lesions, their relationship to synchronous cancers, and their frequency in non-cancerous uteri.
Materials and Methods
Case Selection
All cases in this study were retrospectively obtained from the Departments of Pathology at the University of Arizona, Stony Brook Medical Center, and Yale University. One hundred and eighty two (182) hysterectomy specimens were studied. These included 46 ESC, 16 serous EIC, 60 endometrial endometrioid carcinoma (EEC), and 60 uteri that were resected for a variety of benign indications (control). Only cases in which slides of adjacent non-cancerous endometrium were available for review were included. Case review was accomplished under the light microscopy (Olympus BX41). The age of patients and histological types of the tumors at diagnosis were collected. No patient with a history of prior radiation or chemotherapy was included in the group of cancer patients. In patients with benign diseases, no personal history of cancer or transplantation therapy was included. A history of hormone replacement was not studied, but any patient with a known history of hormone replacement was excluded. The study was approved by Human Investigative Committee in all participating institutions.
Tissue Handling and Pathological Analysis
Tissue was obtained from the hysterectomy specimens, which was formalin fixed and paraffin embedded. We processed the histological sections containing both benign looking resting endometrium (RE) including inactive, weakly proliferative, and proliferative endometria and cancerous areas for all of the cancer cases. The diagnosis and histological classification of the endometrial carcinomas was made using the criteria proposed by The World Health Organization.20
p53 Immunohistochemical Analyses and the Definition of p53 Signatures
Immunohistochemical analysis for p53 protein expression was performed as described previously.13 Assessment of immunohistochemical results for p53 was based on distinct nuclear staining.13 Every endometrial gland or epithelium stained that had more than half of the nuclei with at least a moderate degree of staining intensity was considered as positive. Occasional cytoplasmic p53 staining was considered as negative.
“p53 signatures” were defined by the endometrial epithelial cells, with either glandular or surface growth patterns, which are morphologically unremarkable but display diffuse and strong p53 nuclear staining.
Laser Capture Microdissection and DNA Sequence Analysis for p53 Exons
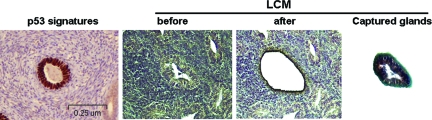
Laser capture microdissection (LCM) samples obtained from the study cases were subject to DNA sequence analysis for p53 exons 5 to 8. The method has been recently described.21 Approximately 300–500 cells of each target area were procured for the DNA sequence analysis. Touchdown PCR was performed to amplify exons 5 to 8 of the p53 gene using tailed primers (Table 1). An example of the LCM procedure for a p53 signature gland is illustrated in Figure 1. Candidate p53 gene mutations were examined by comparing the p53 mutations database through the following link: http://p53.free.fr/Database/p53_database.html. The data on p53 mutations in p53 signature glands and concurrent endometrial samples were compared.
Table 1.
Primer Sequences for PCR Amplification of p53 Exons 5 to 8
| Exon | Primer sequence |
|---|---|
| 5F | 5′-GCGTACTAGCGTACCACGTGTCGACTACTCTGTCTCCTTCCTCTTCCTAC-3′ |
| 5R | 5′-GACGATACGACGGGCGTACTAGCGTAGCAACCAGCCCTGTCGTCT-3′ |
| 6F | 5′-GCGTACTAGCGTACCACGTGTCGACTAAGCAGTCACAGCACATGACGGAG-3′ |
| 6R | 5′-GACGATACGACGGGCGTACTAGCGTAACTGACAACCACCCTTAACCC-3′ |
| 7F | 5′-GCGTACTAGCGTACCACGTGTCGACTTTGCCACAGGTCTCCCCAAG-3′ |
| 7R | 5′-GACGATACGACGGGCGTACTAGCGTATGGAAGAAATCGGTAAGAGGTGG-3′ |
| 8F | 5′-GCGTACTAGCGTACCACGTGTCGACTAGGTAGGACCTGATTTCCTTACTGC-3′ |
| 8R | 5′-GACGATACGACGGGCGTACTAGCGTATGAATCTGAGGCATAACTGCACC-3′ |
Figure 1.
Example of laser capture microdissection (LCM) from a p53 signature gland. Diffuse nuclear staining of p53 by immunohistochemistry from case 11 was seen in a morphologically benign endometrial gland (p53 signature in the far left; magnification = original ×100). Subsequent section of the same gland was then subject to LCM (right three panels).
Statistical Analysis
The patients’ age and pathological features in each of the endometrial carcinoma types was compared using the χ2 test. The difference between cases with p53 signature and those without p53 signature was assessed by Fisher’s exact test and the P value was two-sided.
Results
Selected Clinical and Pathological Features
The age of the patients undergoing hysterectomy ranged from 47 to 89 years with a mean of 65 years. The mean age of patients with ESC including serous EIC was 65 years (ranged: 48 to 89), as compared with 57 years (ranged: 47 to 77) for women with EEC (P < 0.001). The age of patients with lesions designated as p53 signature ranged from 57 to 79 years with a mean of 65 years. All but 23 cancer patients presented with postmenopausal bleeding. Among the 23 patients who did not have postmenopausal bleeding, 15 had an abnormal pap smear, 6 had an increased endometrial stripe thickness on ultrasound, and 2 had a pelvic mass on routine pelvic examinations. For the benign control group, the patient age ranged from 56 to 80, with an average age of 62 years.
p53 Signature Was Significantly Associated with ESC Uteri
Based on the immunohistochemical criteria for p53 staining as described above, we recorded the number of p53 signature glands from each uterus. p53 signatures were found in 18 (39.1%) of 46 ESC, 6 (37.5%) of 16 serous EIC, 2 (3.3%) of 60 EEC, and 1 (1.7%) of 60 benign uteri. Compared with EEC uteri, the frequency of p53 signatures was 11.8-fold higher in uteri with ESC (P < 0.001) and 11.4-fold higher in uteri with EIC (P < 0.001). Since there was only one p53 signature identified in the benign control group, an average of a 24-fold increase in p53 signatures was found in ESC and EIC group as compared with the benign uteri (P < 0.0001). The rate of p53 signatures was not significantly different between uteri with ESC and those with EIC. Overall, p53 signatures were significantly associated with uteri with ESC including serous EIC. The data are summarized in Table 2.
Table 2.
Correlation of p53 Signatures with Noncancerous Background Endometrium in All Studied Cases
| Uteri | Number (%)
|
|||
|---|---|---|---|---|
| ESC (n = 46) | EIC (n = 16) | EEC (n = 60) | BU (n = 60) | |
| # p53 signatures | 18 (39.1) | 6 (37.5) | 2 (3.3) | 1 (1.7) |
| Background endometrial findings | ||||
| Atrophic endometrium | 9 (50) | 3 (50) | 2 (100) | 1 (100) |
| Proliferative endometrium | 5 (28) | 1 (17) | 0 (0) | 0 (0) |
| Endometrial hyperplasia | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Atypical endometrial hyperplasia | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Endometrial polyp | 4 (22) | 2 (23) | 0 (0) | 0 (0) |
The stated benign endometrial histology represented the background finding of the uteri studied. The epithelia with p53 signatures showed no morphologic difference from background endometrial.
Pathological Characteristics of p53 Signature Glands
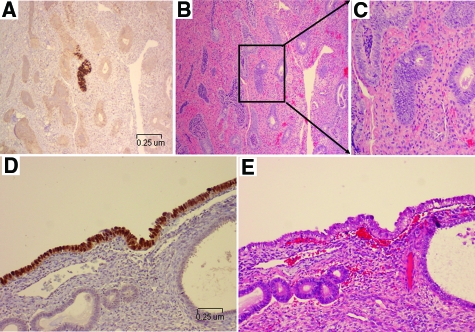
The following microscopic patterns were observed on immunohistochemically-stained slides: (1) a single or a group of p53 signature glands in the endometrium or within an endometrial polyp, (2) a flat single layer of the epithelium on the surface of the endometrium or on a polyp. Both patterns of the p53 signature glands were composed of morphologically benign-appearing epithelia. The majority of p53 signature glands showed positive p53 nuclear staining in the entire gland. Foci of p53 signature glands were usually smaller than 1 mm in size. There were a total of 18 uteri with p53 signatures. Uteri with a single p53 signature gland was seen in 2 (11%), with 2 to 5 foci within a single uterus was seen in 13 (72%), while more than 5 were identified in 3 (17%) of the 18 uteri. Two (11%) of 18 uteri with 2 or more p53 signature glands concurrently had p53 signature gland(s) on the endometrial surface. Representative morphological pictures of p53 signature glands are presented in Figure 2, A–E.
Figure 2.
p53 signatures and their corresponding H&E endometrial glands. Lower panel showed representative endometrial glands from case 2 with p53 signatures (A, glandular pattern), the corresponding H&E section (B), and the morphologically magnified p53 signature gland (C). Lower panel showed pictures from case 9 with a surface pattern of p53 signature (D, surface pattern) and the corresponding area of H&E section (E). Morphologically, nuclear atypia of the epithelia with p53 signatures was inconspicuous. (Magnification of A, B, D, and E = original ×200).
There were 2 cases of p53 signatures in EEC group, which actually showed focal serous differentiation (5% and 10%, respectively). A single p53 signature was found among 60 benign control uteri. There were 6 p53 signatures in endometrial polyp, 4 in ESC, and 2 in EIC uteri. No p53 signatures were found in endometrial polyps of EEC or benign uteri. The detailed data of p53 signatures and their association to non-cancerous endometria are summarized in Table 2.
p53 Signatures with Frequent p53 Gene Mutations
Among the 18 uteri containing p53 signatures, we obtained a total of 116 informative LCM samples. These included 92 samples from 14 cancerous uteri and 24 from 12 benign controls. The 92 samples were taken from p53 signatures (n = 38), EmGD (n = 16), ESC including EIC (n = 20), EEC (n = 6), and RE with negative p53 immunoreactivity (n = 12). The 38 p53 signature LCM samples were derived from ESC or EIC uteri (n = 35) and EEC (n = 3). Among the 38 LCM samples with p53 signatures, 16 (42%) showed at least one p53 gene mutation. The 16 p53 gene mutations were from 8 uteri with either ESC or EIC. Of the 16 p53 mutations, 4 (25%) were in exon 5, 1 (6%) in exon 6, 9 (56%) in exon 7, and 2 (13%) in exon 8, respectively. No mutations were found in samples of EEC and benign uteri or cancer uteri with p53 negative RE. The detailed mutation data from p53 signature glands with corresponding amino acid changes are summarized in Table 3 (negative results were not included).
Table 3.
Summary of All p53 Signatures with Positive p53 Gene Mutations
| Case # | Diagnosis of the uteri | Nucleotide change | Exon (codon) | Amino acid change | Mutation effect |
|---|---|---|---|---|---|
| 2 | EIC | GTT>GCT | 5 (172) | Val>Ala | Missense |
| ATG>GTG | 7 (243) | Met>Val | Missense | ||
| 3 | ESC | CGG>TGG | 7 (248) | Arg>Trp | Missense |
| 5 | ESC | GTT>GCT | 5 (147) | Val>Ala | Missense |
| CGG>TGG | 7 (248) | Arg>Trp | Missense | ||
| 6 | ESC | CGG>TGG | 7 (248) | Arg>Trp | Missense |
| 7 | ESC | GAG>AAG | 6 (221) | Glu>Lys | Missense |
| CGG>TGC | 7 (248) | Arg>Gln | Missense | ||
| 11 | ESC | GTT>GCT | 5 (147) | Val>Ala | Missense |
| CGG>TGG | 7 (248) | Arg>Trp | Missense | ||
| 15 | EIC | CAT>CTT | 6 (193) | His>Leu | Missense |
| ATG>ATA | 7 (237) | Met>Ile | Missense | ||
| CGG>TGG | 7 (248) | Arg>Trp | Missense | ||
| 18 | ESC | GCC>GTC | 5 (161) | Ala>Val | Missense |
| ATG>ATA | 7 (237) | Met>Ile | Missense | ||
| CGG>TGG | 8 (282) | Arg>Trp | Missense |
High Concordant p53 Mutations in p53 Signature and in Endometrial Serous Neoplastic Lesions
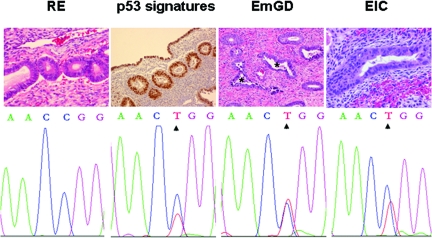
It is known that p53 gene mutations are commonly present in lesions of EmGD and ESC or EIC.21,22 Since a significant number of p53 mutations were detected in p53 signatures, we studied whether there are identical gene mutations present between p53 signatures and lesions of EmGD and ESC or EIC within the same uteri. The eight uteri containing p53 gene mutations associated with their p53 signatures all had coexisting lesions of EmGD and ESC or serous EIC. At least one identical p53 gene mutation was found in p53 signatures, EmGD, EIC, and ESC within the same uterus in four (50%) of the eight uteri (case # 5, 7, 15, and 18). Representative pictures for those lesions with identical mutations are shown in Figure 3. Among the remaining four uteri, two (cases# 2 and 11) showed at least one identical mutations between p53 signatures and lesions of either EmGD or EIC or ESC. Cases 3 and 6 showed different p53 gene mutations in lesions of p53 signatures, EmGD, serous EIC, and ESC.
Figure 3.
P53 gene sequencing results from laser capture microdissected (LCM) samples. Top row shows representative images of H&E staining of resting endometrium (RE), endometrial glandular dysplasia (EmGD), and serous endometrial intraepithelial carcinoma (EIC) and p53 immunohistochemical staining (p53 signatures). Magnification = original ×200. Noticeable degree of nuclear atypia in EmGD (asterisks) clearly exceeds the RE but falls short of EIC. Samples of p53 signatures, EmGD, and EIC from DNA sequence analyses showed identical p53 gene mutations of exon 7 at codon 248 from CGG to TGG (Arg to Trp), while no mutation was found in the corresponding RE sample. These samples were obtained from case 5. Identical mutation was also observed in endometrial serous carcinoma area (not shown) in the same uterus.
Discussion
This study was initiated following the incidental finding of p53-positive normal appearing endometrial epithelia during examinations of precancerous lesions in uteri with ESC.16 To maintain a nomenclatural congruence with a conceptually identical finding in the medical literature, we have designated these immunophenotypically distinctive endometrial epithelia as “p53 signature.” Simply, endometrial p53 signatures are characterized by benign appearing endometrial glandular cells with diffuse moderate to strong nuclear staining for p53. The term “p53 signature” has been recently applied in fallopian tube by Dr. Christopher Crum’s group when they studied the cell origins of pelvic serous cancers.18,19
Functional p53 gene and protein are required to maintain integrity of cell cycle control mainly via repairing damaged DNA. Cells with p53 signature, particularly those with a mutated p53 gene are expected to have impaired DNA repair function, ultimately leading to loss of cell cycle control.23 Impaired DNA is actually present in tubal p53 signature cells, which were identified by H2AX staining.18,19 Although p53 mutations occur more frequently in many well-developed human cancers, it may also be seen earlier in precancerous lesions including EmGD.21,24 Like EmGD, the p53 signature is found mainly in ESC uteri.16 The p53 signature is also more commonly associated with EmGD and serous EIC, but is uncommonly seen in uteri with EEC and is extremely rare in age-controlled benign uteri, which suggests that the p53 signature has a strong tendency to occur in uteri harboring serous neoplasms and/or its preceding lesions. The most common mutations in p53 signature are missense, just like p53 mutations in EmGD as we recently reported.21 In this study, the p53 signature was present in an average of 38% of ESC/EIC uteri and 42% showed p53 gene mutations, which is similar to that of EmGD (43%).21 Furthermore, the current study showed 50% of the mutations were concordant in specific codons among p53 signature, EmGD, and ESC or EIC lesions in the same uteri. All these findings suggest that the p53 signature is the subpopulation of endometrial epithelia serving as a latent precancer that preferentially will give rise to EmGD, which may eventually develop into EIC and/or ESC under appropriate conditions.
p53 immunohistochemistry is able to recognize what is perhaps the earliest stage of endometrial serous carcinogenesis before histological change is manifest. Many well-known examples of mutation-bearing precancers, including EmGD, endometrial intraepithelial neoplasia, and colonic adenomatous polyps have morphological changes recognizable under light microscopy, which indicates a significant change in phenotype. It is understandable that genetic changes precede the phenotypic changes. It is known that successful carcinogenesis requires multiple “hits” in a step-wise process.25,26 Mutations of p53 gene in normal appearing endometria in the postmenopausal uteri may represent one of the possible early “hits,” which is not, in of itself, enough to cause phenotypic changes. It would be interesting to know what other genetic events in addition to p53 mutation are required to change from p53 signature to EmGD, the earliest identifiable lesion in endometrial serous carcinogenesis.17
Compared with EEC, ESC occurs mostly in postmenopausal women whose average age is 10 years older. ESC used to be considered arising in atrophic endometrium in a de novo process, which has led to the earlier conception that background atrophic endometrium is part of the criteria for the pathological diagnosis of ESC.3,12,27 However, ESC as well as its precancer, EmGD, may also be present in proliferative endometrial background.16 In this study, about a quarter of p53 signatures were found in resting endometrium including inactive or weakly and proliferative phases of the endometria. This is probably related to the hormonal status of individual patient. Hormonal factors are attractive as putative modulators for endometrium including those postmenopausal uteri. Unlike women in the reproductive age, the endometrium in postmenopausal women lacks cyclic shedding when cyclic hormone replacement is not used. It is unknown what the fate of the p53 signatures will be and how long it will take for the p53 signatures to transform into a morphologically recognizable precancer. However, once a p53 mutation is initiated, genomic instability of the p53 signature cells increase, and it is expected to confer a mutator phenotype that may accelerate subsequent progression to serous carcinoma. Expansion or contraction of mutant clones would also be anticipated to modify the additional genetic mutations that may occur in a cell already having an initial mutation.28 This is consistent with our findings that majority of p53 signatures were multifoci in a single uterus. Although a p53 mutation in p53 signatures is unlikely induced by hormones, it is of great interest if hormonal factors by inducing endometrial shedding are able to modify the risk of ESC.
Clonal proliferation of a single cell carrying specific mutations in cancer related genes is a well accepted model of carcinogenesis. Although a high concordance of p53 mutation in specific codons found in this study indicates that at least some of ESC/EIC is derived from lesions of p53 signatures, probably through EmGD, multiple non-concordant individual p53 gene mutations were identified among the remaining 50% of p53 signature glands. An alternative interpretation is “field effect,” which postulates that the entire tissue “field” simultaneously is predisposed to neoplasia because of prolonged exposure to the same carcinogen(s). This would result in tumor growth from multiple centers containing precursor lesions within a single organ.29 This model is supported by cases 15 and 18, in which separate foci of p53 signature showed different p53 codon mutations. Therefore, in addition to clonal expansion, synchronous growth of p53 signatures may also represent an important process of ESC carcinogenesis. Overall, the findings in this study, together with our previous studies on the precancers further support that ESC may develop from p53 signature glandular epithelia through EmGD to serous EIC and to ESC.16,21,30 This proposed model is similar to that proposed for endometrioid (type I) cancers where the Mutter group has found that morphologically normal glands with phosphatase and tensin homolog mutations are relatively common in cycling and anovulatory endometria. Such rare glands persist between menstrual cycles and eventually progress to form distinctive clusters with clonal growth.31 These data are consistent with a progression model in which initial mutation is not rate limiting.
In summary, this study showed that p53 signatures are frequently seen in uteri with serous neoplasms and are only rarely seen in association with EEC or benign endometrium. Furthermore, we demonstrated identical p53 mutations in the p53 signatures, EmGD, EIC, and/or ESC in a subset of the uteri studied. These data suggest that the overexpression of p53, or altered p53 function, is a marker of the earliest phase of endometrial serous carcinogenesis, and that progression model, mediated by altered p53 function, that includes a latent precancerous phase (p53 signatures), a premalignant phase (EmGD), and a carcinoma phase (ESC including EIC) may be operational. Although it is premature for us to suggest a routine staining of endometrial biopsy with p53, the application of such staining in a certain clinical setting may be informative in identifying latent or premalignant lesions that are likely to progress to the aggressive type of endometrial cancer. Finding a way to eradicate the latent precancers may offer an effective method for the cancer prevention.
Footnotes
Address reprint requests to Wenxin Zheng, M.D., F.C.A.P. Professor of Pathology and Gynecology, Department of Pathology, University of Arizona, 1501 N. Campbell Avenue, #5224A, Tucson, AZ 85724, E-mail: zhengw@email.arizona.edu or Beihua Kong, M.D, Ph.D, Professor of Obstetrics & Gynecology, Qilu Hospital, Shandong University, 107 Wenhuaxi Road, Ji’nan, Shandong, China 250012, E-mail: kongbeihua@sdu.edu.cn.
Supported in part by Shandong University School of Medicine, China, Women’s Cancer Division of Arizona Cancer Center, Arizona Cancer Center Core Grant (P30 CA23074), and Arizona Cancer Center Better Than Ever grant.
The views expressed in this article are those of the authors and do not reflect the official policy of the Department of Defense or other Departments of the United States Government.
References
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer. J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15:10–17. doi: 10.1016/0090-8258(83)90111-7. [DOI] [PubMed] [Google Scholar]
- Sherman ME. Theories of endometrial carcinogenesis: a multidisciplinary approach. Mod Pathol. 2000;13:295–308. doi: 10.1038/modpathol.3880051. [DOI] [PubMed] [Google Scholar]
- Hecht JL, Mutter GL. Molecular and pathologic aspects of endometrial carcinogenesis. J Clin Oncol. 2006;10:4783–4791. doi: 10.1200/JCO.2006.06.7173. [DOI] [PubMed] [Google Scholar]
- Zheng W, Schwartz PE. Serous EIC as an early form of uterine papillary serous carcinoma: recent progress in understanding its pathogenesis and current opinions regarding pathologic and clinical management. Gynecol Oncol. 2005;96:579–582. doi: 10.1016/j.ygyno.2004.12.045. [DOI] [PubMed] [Google Scholar]
- Ambros RA, Sherman ME, Zahn CM, Bitterman P, Kurman RJ. Endometrial intraepithelial carcinoma: a distinctive lesion specifically associated with tumors displaying serous differentiation. Hum Pathol. 1995;26:1260–1267. doi: 10.1016/0046-8177(95)90203-1. [DOI] [PubMed] [Google Scholar]
- Carcangiu ML, Tan LK, Chambers JT. Stage IA uterine serous carcinoma: a study of 13 cases. Am J Surg Pathol. 1997;21:1507–1514. doi: 10.1097/00000478-199712000-00015. [DOI] [PubMed] [Google Scholar]
- Goff BA, Kato D, Schmidt RA, Ek M, Ferry JA, Muntz HG, Cain JM, Tamimi HK, Figge DC, Greer BE. Uterine papillary serous carcinoma: patterns of metastatic spread. Gynecol Oncol. 1994;54:264–268. doi: 10.1006/gyno.1994.1208. [DOI] [PubMed] [Google Scholar]
- Lee KR, Belinson JL. Recurrence in noninvasive endometrial carcinoma. Relationship to uterine papillary serous carcinoma. Am J Surg Pathol. 1991;15:965–973. doi: 10.1097/00000478-199110000-00007. [DOI] [PubMed] [Google Scholar]
- Sherman M, Bur ME, Kurman RJ. p53 in endometrial cancers and its putative precursors: evidence for diverse pathways of tumorigenesis. Hum Pathol. 1995;26:1268–1274. doi: 10.1016/0046-8177(95)90204-x. [DOI] [PubMed] [Google Scholar]
- Silva EG, Jenkins R. Serous carcinoma in endometrial polyps. Mod Pathol. 1990;3:120–128. [PubMed] [Google Scholar]
- Sherman ME, Bitterman P, Rosenshein NB, Delgado G, Kurman RJ. Uterine serous carcinoma: a morphologically diverse neoplasm with unifying clinicopathologic features. Am J Surg Pathol. 1992;16:600–610. doi: 10.1097/00000478-199206000-00008. [DOI] [PubMed] [Google Scholar]
- Zheng W, Khurana R, Farahmand S, Wang Y, Zhang ZF, Felix JC. p53 immunostaining as a significant adjunct diagnostic method for uterine surface carcinoma: precursor of uterine papillary serous carcinoma. Am J Surg Pathol. 1998;22:1463–1473. doi: 10.1097/00000478-199812000-00003. [DOI] [PubMed] [Google Scholar]
- Fadare O, Liang SX, Ulukus EC, Chambers SK, Zheng W. Precursors of endometrial clear cell carcinoma. Am J Surg Pathol. 2006;30:1519–1530. doi: 10.1097/01.pas.0000213296.88778.db. [DOI] [PubMed] [Google Scholar]
- Liang SX, Chambers SK, Cheng L, Zhang S, Zhou Y, Zheng W. Endometrial glandular dysplasia: a putative precursor lesion of uterine papillary serous carcinoma. Part II: molecular features. Int J Surg Pathol. 2004;12:319–331. doi: 10.1177/106689690401200405. [DOI] [PubMed] [Google Scholar]
- Zheng W, Liang SX, Yu H, Rutherford T, Chambers SK, Schwartz PE. Endometrial glandular dysplasia: a newly defined precursor lesion of uterine papillary serous carcinoma. Part I: morphologic features. Int J Surg Pathol. 2004;12:207–223. doi: 10.1177/106689690401200302. [DOI] [PubMed] [Google Scholar]
- Yi X, Zheng W. Endometrial glandular dysplasia and endometrial intraepithelial neoplasia. Curr Opin Obstet Gynecol. 2008;20:20–25. doi: 10.1097/GCO.0b013e3282f2fd50. [DOI] [PubMed] [Google Scholar]
- Crum CP, Drapkin R, Miron A, Ince TA, Muto M, Kindelberger DW, Lee Y. The distal fallopian tube: a new model for pelvic serous carcinogenesis. Curr Opin Obstet Gynecol. 2007;19:3–9. doi: 10.1097/GCO.0b013e328011a21f. [DOI] [PubMed] [Google Scholar]
- Lee Y, Miron A, Drapkin R, Nucci MR, Medeiros F, Saleemuddin A, Garber J, Birch C, Mou H, Gordon RW, Cramer DW, McKeon FD, Crum CP. A candidate precursor to serous carcinoma that originates in the distal fallopian tube. J Pathol. 2007;211:26–35. doi: 10.1002/path.2091. [DOI] [PubMed] [Google Scholar]
- Silverberg SG, Mutter GL, Kurman RJ, Kubik-Huch RA, Nogales F, Tavassoli FA. Tumors of the uterine corpus. Epithelial tumors and related lesions. Lyon: IARC Press; World Health Organization Classification of Tumors, Third Series, Edited by Tavassoli, F.A. and Devilee, P. Pathology and genetics: Tumors of the breast and female genital organs. 2003:pp 224–225. [Google Scholar]
- Jia L, Liu Y, Yi X, Miron A, Crum CP, Kong B, Zheng W. Endoemtrial glandular dysplasia with frequent p53 gene mutation: a genetic evidence supporting its precancer nature for endometrial serous carcinoma. Clin Cancer Res. 2008;14:2263–2269. doi: 10.1158/1078-0432.CCR-07-4837. [DOI] [PubMed] [Google Scholar]
- Tashiro H, Isacson C, Levine R, Kurman RJ, Cho KR, Hedrick L. p53 gene mutations are common in uterine serous carcinoma and occur early in their pathogenesis. Am J Pathol. 1997;150:177–185. [PMC free article] [PubMed] [Google Scholar]
- Gorgoulis VG, Vassiliou LV, Karakaidos P, Zacharatos P, Kotsinas A, Liloglou T, Venere M, Ditullio RA, Jr, Kastrinakis NG, Levy B, Kletsas D, Yoneta A, Herlyn M, Kittas C, Halazonetis TD. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature. 2005;434:907–913. doi: 10.1038/nature03485. [DOI] [PubMed] [Google Scholar]
- Orlando FA, Tan D, Baltodano JD, Khoury T, Gibbs JF, Hassid VJ, Ahmed BH, Alrawi SJ. Aberrant crypt foci as precursors in colorectal cancer progression. J Surg Oncol. 2008;98:207–213. doi: 10.1002/jso.21106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns J. Mutation and cancer: the antecedents to our studies of adaptive mutation. Genetics. 1998;148:1433–1440. doi: 10.1093/genetics/148.4.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolgavkar SH, Kundson AG. Mutation and cancer: a model for human carcinogenesis. J Natl Caner Inst (Bethesda) 1981;66:1037–1052. doi: 10.1093/jnci/66.6.1037. [DOI] [PubMed] [Google Scholar]
- Hendrickson M, Ross J, Eifel P, Martinez A, Kempson R. Uterine papillary serous carcinoma: a highly malignant form of endometrial adenocarcinoma. Am J Surg Pathol. 1982;6:93–108. doi: 10.1097/00000478-198203000-00002. [DOI] [PubMed] [Google Scholar]
- Jackson AL, Loeb LA. The mutation rate and cancer. Genetics. 1998;148:1483–1490. doi: 10.1093/genetics/148.4.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong MS, Incze J, Vaughan CW. Field cancerization in the aerodigestive tract–its etiology, manifestation, and significance. J Otolaryngol. 1984;13:1–6. [PubMed] [Google Scholar]
- Zheng W, Liang SX, Yi X, Ulukus EC, Davis J, Chambers SK. Occurrence of endometrial glandular dysplasia precedes uterine papillary serous carcinoma. Int J Gynecol Pathol. 2007;26:38–52. doi: 10.1097/01.pgp.0000228138.56222.4e. [DOI] [PubMed] [Google Scholar]
- Mutter GL, Ince TA, Baak JPA, Kust G, Zhou X, Eng C. Molecular identification of latent precancers in histologically normal endometrium. Cancer Res. 2001;61:4311–4314. [PubMed] [Google Scholar]